Abstract
Background
Recent studies have questioned the value of adding whole-brain radiotherapy (WBRT) to stereotactic radiosurgery (SRS) for brain metastasis treatment. Neurotoxicity, including radiation-induced brain volume reduction, could be one reason why not all patients benefit from the addition of WBRT. In this study, we quantified brain volume reduction after WBRT and assessed its prognostic significance.
Methods
Brain volumes of 91 patients with cerebral metastases were measured during a 150-day period after commencing WBRT and were compared with their pretreatment volumes. The average daily relative change in brain volume of each patient, referred to as the “brain volume reduction rate,” was calculated. Univariate and multivariate Cox regression analyses were performed to assess the prognostic significance of the brain volume reduction rate, as well as of 3 treatment-related and 9 pretreatment factors. A one-way analysis of variance was used to compare the brain volume reduction rate across recursive partitioning analysis (RPA) classes.
Results
On multivariate Cox regression analysis, the brain volume reduction rate was a significant predictor of overall survival after WBRT (P < 0.001), as well as the number of brain metastases (P = 0.002) and age (P = 0.008). Patients with a relatively favorable prognosis (RPA classes 1 and 2) experienced significantly less brain volume decrease after WBRT than patients with a poor prognosis (RPA class 3) (P = 0.001). There was no significant correlation between delivered radiation dose and brain volume reduction rate (P = 0.147).
Conclusion
In this retrospective study, a smaller decrease in brain volume after WBRT was an independent predictor of longer overall survival.
Keywords: adverse effects, brain metastases, neurotoxicity, prognosis, whole brain radiation therapy
Importance of the study
Currently, the optimal treatment for patients with brain metastases is unclear. There is evidence that adjuvant WBRT in addition to SRS leads to increased intracranial tumor control rates in patients with <5 brain metastases, but not to a better overall survival. Additionally, the negative impact of adjuvant WBRT on neurocognition and quality of life has been confirmed in recent studies. This raises the question of whether adverse effects are the reason adjuvant use of WBRT does not lead to longer overall survival; moreover, it is unknown which patients are at particular risk for these adverse effects. Therefore, we quantified brain volume reduction after WBRT and analyzed its role as an independent prognostic factor.
Since the initial report on the utility of whole-brain radiotherapy (WBRT) by Chao et al in 1954,1 WBRT has become a mainstay treatment for cerebral metastases. However, growing concerns regarding this modality’s toxicity profile have emerged. Possible WBRT-associated toxicities include neurocognitive impairment and morphological brain changes such as leukoencephalopathy and radiation necrosis.2 Brain volume reduction is another important phenomenon frequently described after WBRT3; however, its effect on overall survival has not been investigated to date, nor has the causal relationship with WBRT been definitely shown.
Such possible WBRT-associated toxicities are of particular interest because studies have questioned the value of adding WBRT to stereotactic radiosurgery (SRS). In previous studies of patients with up to 3 or 4 brain metastases, the addition of WBRT to SRS provided better intracranial tumor control but failed to improve overall survival and led to significantly more severe neurocognitive decline.4–7 On the other hand, Aoyama et al found that WBRT could improve survival in a subgroup of patients who had favorable prognoses.8 This raises the question of whether the potential adverse effects of WBRT, including radiation-induced brain volume reduction, can explain why the adjuvant administration of WBRT does not result in longer survival in all patient subgroups.9
Other, albeit controversial, studies have suggested that radiation-associated neurotoxicity may compromise survival. A randomized clinical trial by Le Péchoux et al compared the effects of standard (25 Gy) versus high (36 Gy) doses of prophylactic cranial irradiation in 720 patients with limited-stage small-cell lung cancer.10 Surprisingly, the higher dose did not lead to a reduction in the total incidences of brain metastases but was associated with increased mortality. Even though differences in radiotherapy techniques, imbalances between the 2 treatment groups, an increased cancer-related mortality in the higher-dose group, and other factors were considered, a satisfactory explanation for the increased mortality in the high-dose group remained lacking.
To better understand the significance of brain volume loss following WBRT, we aimed to quantify brain volume reduction after WBRT and determine its role as an independent prognostic factor.
Materials and Methods
Ethics Statement
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments. For this retrospective study, formal consent was not required.
Subjects, Radiation Therapy, and Image Acquisition
We identified all patients who received WBRT at our institution between 2003 and 2015. From this group of 662 patients, we selected those who fulfilled the inclusion criteria of our retrospective study: pathological proof of a malignant tumor, WBRT administered for cerebral metastasis, pretreatment MRI performed no more than 60 days before commencing WBRT, at least one MRI scan obtained more than 60 days after commencing WBRT, and the availability of image data in the local neuroradiology department. Ninety-one patients met these criteria and were enrolled.
WBRT was administered with 6 or 15 MV photon beams from a Siemens Oncor or Primart linear accelerator (for fractionation schemes, see Table 1). Sixty-eight patients received additional SRS treatment with 16 or 18 Gy prescribed to the 80% or 90% isodose line.
Table 1.
Characteristics of 91 patients with brain metastases
| Variables (No. Cases) | Median Survival Time, mo (95% CI) | P-value (log-rank test) |
|---|---|---|
| Age | 0.011 | |
| <65 y (53) | 13.4 (6.9–19.9) | |
| ≥65 y (38) | 8.1 (5.2–11.0) | |
| Sex | 0.565 | |
| Male (50) | 10.4 (5.9–14.9) | |
| Female (41) | 13.1 (5.0–21.2) | |
| Histology | 0.237 | |
| Non–small cell lung cancer (41) | 10.1 (4.9–15.3) | |
| Malignant melanoma (19) | 9.6 (5.0–14.2) | |
| Breast cancer (12) | 13.4 (0–34.3) | |
| Small cell lung cancer (8) | 23.3 (4.4–42.2) | |
| Renal cell cancer (3) | 8.1 (3.1–13.1) | |
| Other tumors (8) | 22.6 (5.4–39.8) | |
| ECOG performance status | 0.009 | |
| 0 (23) | 19.7 (11.4–28.0) | |
| 1 (48) | 10.4 (5.8–15.0) | |
| 2 (19) | 7.1 (6.5–7.7) | |
| 3 (1) | 5.0 | |
| Primary tumor | 0.006 | |
| Controlled (52) | 15.9 (6.4–25.4) | |
| Uncontrolled (39) | 9.9 (5.4–14.4) | |
| Extracranial metastases | 0.038 | |
| No extracranial metastases (35) | 17.5 (12.5–22.5) | |
| Present extracranial metastases (56) | 8.3 (5.1–11.5) | |
| RPA class | 0.002 | |
| 1 (8) | 52.1 (0.4–103.8) | |
| 2 (63) | 12.1 (9.0–15.2) | |
| 3 (20) | 7.1 (6.4–7.8) | |
| Number of brain metastases | 0.009 | |
| ≤3 brain metastases (51) | 17.3 (9.6–25.0) | |
| >3 brain metastases (40) | 7.3 (6.0–8.6) | |
| Fractionation | 0.006 | |
| 36 Gy in 12 fractions (22) | 7.0 (5.6–8.4) | |
| Other fractionation (69) | 13.4 (6.7–20.1) | |
| Stereotactic radiosurgery | <0.001 | |
| Yes (68) | 15.9 (9.2–22.6) | |
| No (23) | 6.8 (5.8–7.8) | |
| Surgery of brain metastases | 0.007 | |
| Yes (18) | 23.3 (15.3–31.3) | |
| No (73) | 9.9 (7.4–12.4) | |
| Interval of brain metastases | 0.737 | |
| Metachronous (53) | 10.1 (6.1–14.1) | |
| Synchronous (38) | 12.3 (4.6–20.0) |
Images were collected on different Siemens 1.5 Tesla MRI scanners (Magnetom Aera or Magnetom Avanto) at our institution. All analyzed images consisted of 160 or 192 contiguous, sagittal, or transversal planes of 3-dimensional T1-weighted magnetization-prepared rapid gradient-echo images with 1 × 1 × 1 mm isotropic resolution (repetition time [TR] = 1900 ms, echo time [TE] = 3.02 ms, inversion time [TI] = 1100 ms, matrix = 256 × 265, field of view [FoV] = 250, flip angle = 15 degrees or TR = 2200 ms, TE = 2.67 ms, TI = 900 ms, matrix = 256 × 246, FoV = 250, flip angle = 8 degrees) after intravenous application of 0.2 mL/kg Dotarem (Guerbet) or 0.1 mL/kg Gadovist (Bayer), respectively.
Image Analysis
Volumetric analysis was performed by uploading the anonymized compressed T1-weighted images in NIfTI format to the online MRI brain volumetry system “volBrain,” version 1.0. The volBrain pipeline sets the images to the specific geometry and intensity space of the template library. A detailed description of the volBrain pipeline was published by Manjón and Coupé.11 When the automatic process is complete, a PDF report with the volumes of brain tissue and subcortical structures is generated, along with NIfTI files containing the segmentations of the uploaded image in native or standard stereotactic space (Montreal Neurological Institute).
Manjón and Coupé, as well as Naess-Schmidt et al, demonstrated that volBrain is not inferior to other brain MRI analysis software such as Freesurfer, FSL-FIRST, or SPM with respect to reproducibility and accuracy.11,12
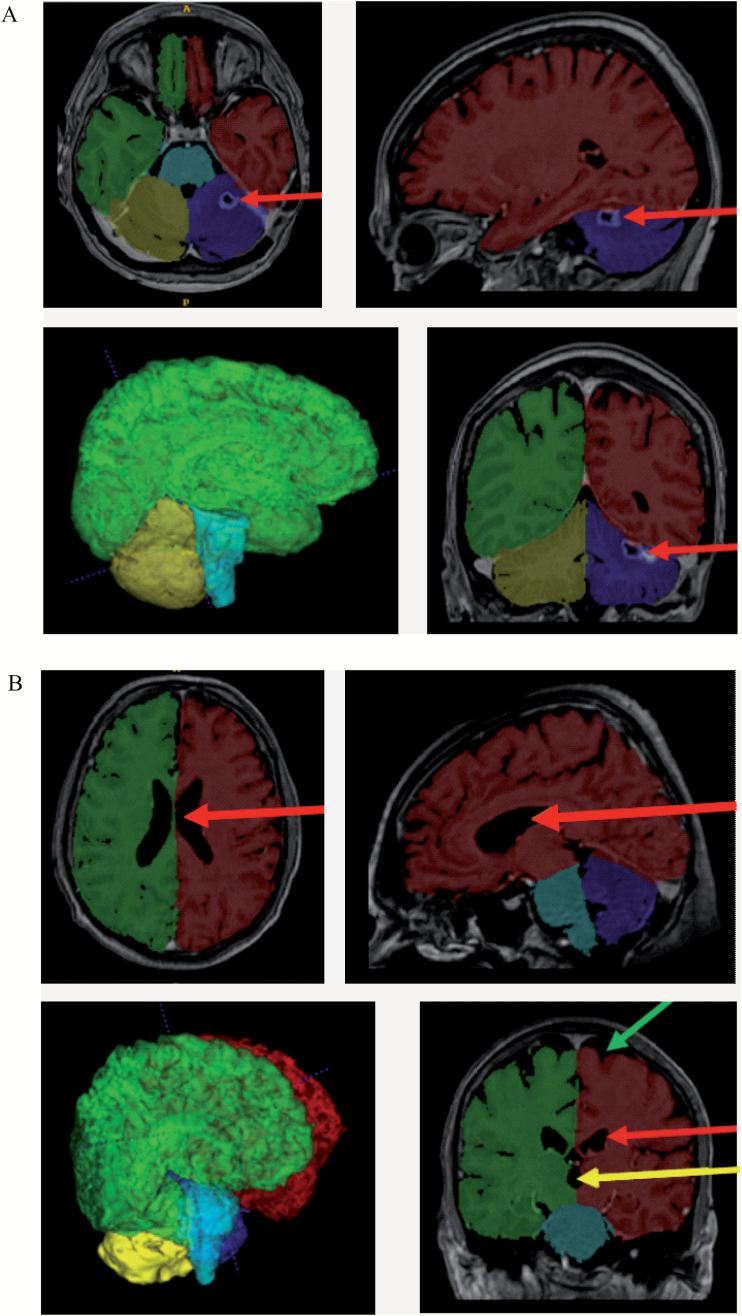
To verify the quality of the labeling process, we visually checked that the area identified as brain tissue by volBrain in all patients was correct. We opened the MRI scan in ITK-SNAP version 3.4.0 software and overlaid it with its respective segmented brain tissue images. By this method, we also confirmed that pathological changes like resection cavities and large brain metastases did not interfere with the segmentation process and that volBrain accurately differentiated between brain tissue and cerebrospinal fluid (Fig. 1).
Fig. 1.
Visualization of the results of the volBrain segmentation process with ITK-SNAP. (A) MRI scan of a 45-year-old woman 129 days after commencing WBRT. VolBrain correctly identified the resection cavity in the left cerebellar hemisphere as not being brain tissue (red arrow). The image in the lower-left corner depicts a 3-dimensional reconstruction of the segmentation result created by volBrain. (B) MRI scan of a 68-year-old man 96 days after commencing WBRT. VolBrain exactly differentiated between brain tissue on the one hand and cerebrospinal fluid in the ventricles (third ventricle: yellow arrow; lateral ventricles: red arrow) and subarachnoid space (green arrow) on the other. Description of the segmentation labels: red, left cerebral hemisphere; green, right cerebral hemisphere; blue, left cerebellar hemisphere; yellow, right cerebellar hemisphere; turquoise, brainstem; gray tones, area identified as nonbrain tissue.
Patient Characteristics
Forty-one women and 50 men were included in our retrospective study, with a mean age of 59.1 ± 11.9 years (median, 60 y; range, 25–83 y). Additional characteristics are presented in Table 1.
Brain metastasis was defined as synchronous if it appeared before or within 3 months following the diagnosis of the primary tumor; otherwise, it was classified as metachronous. Control of the primary tumor was defined as remission or stable disease without any clinical or radiological findings suggestive of primary tumor progression at the beginning of WBRT. The number of brain metastases was based on the report of radiological examinations (CT or MRI). Radiographic response to WBRT was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.13 The final survival data were based on information compiled until February 25, 2017.
Study Endpoints
Aside from the brain volume reduction rate (BVRR), defined as the average daily change in brain volume during the first 150 days after starting WBRT, 3 treatment-related factors and 9 pretreatment factors were analyzed for their ability to predict overall survival after WBRT. Patients were further stratified according to the 3 recursive partitioning analysis (RPA) classes developed by the Radiation Therapy Oncology Group. Patients aged <65 years and with KPS ≥70, a controlled primary tumor, and no extracranial metastases were classified as RPA class 1; patients with KPS <70 were classified as RPA class 3; and all other patients were designated to RPA class 2.14 The major study endpoint was overall survival, which was defined as the time from starting WBRT to the date of death or last follow-up.
Statistical Analysis
As the intervals between WBRT and follow-up scans varied among individual patients, the following method was used to obtain a time-independent parameter for brain volume reduction.
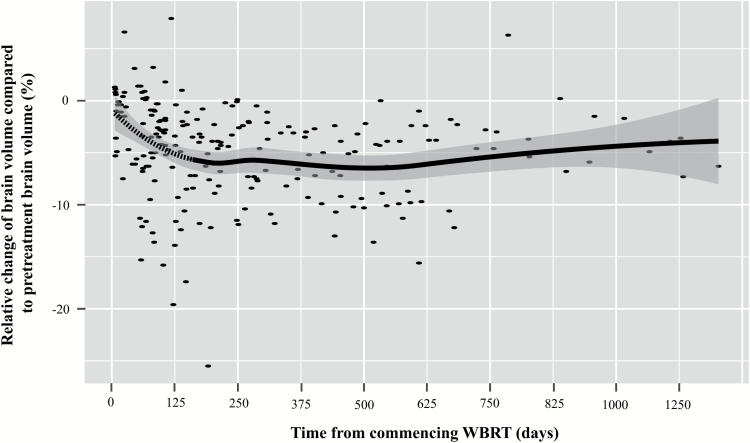
The pretreatment MRI scan and all available MRI scans acquired after commencing WBRT were analyzed with volBrain. A scatter plot was created to display the relationship between the time since commencing WBRT and the relative change of brain volume. This scatter plot suggested a nonlinear relationship between brain volume change and time; hence, a suitable fit curve was unachievable with these data points using conventional linear least squares regression. As the true relationship between brain volume change and time after WBRT was unknown, we performed a locally weighted LOESS regression analysis to obtain a smooth curve (Fig. 2). LOESS is a nonparametric regression method that finds the best fit curve without assuming a certain distribution of the underlying data.15 As LOESS regression requires a kernel (ie, a weighting function), we chose the Gaussian kernel function for its greater efficacy in minimizing the mean integrated squared error compared with other weighting functions.16 The optimal smoothing parameter for LOESS regression was calculated using an improved Akaike information criterion.17 The smooth curve indicated a linear time-related relative decrease in brain volume only during the first 150 days after initiating WBRT; this implied that the average daily relative change in brain volume did not depend on the interval between WBRT and brain volume measurement during this period. Therefore, only brain volume measurements acquired during the first 150 days after starting WBRT were statistically analyzed.
Fig. 2.
Time-dependent change of brain volume after WBRT. For every patient, all available MRI scans after WBRT were analyzed with respect to the relative change of brain volume compared with the pretreatment volume. Every point represents one MRI scan. The gray-shaded area represents the 95% CI for the LOESS smooth curve. The smooth curve suggests a linear decline in brain volume in the first 150 days after commencing WBRT (dashed section of the curve).
For every patient in our cohort, we fitted the individual linear regression line for the correlation between days since commencing WBRT and relative change in brain volume, up to 150 days, with the pretreatment brain volume as the reference value. The value of the slope of the regression line was considered the patient’s average daily relative change in brain volume, or the BVRR. The greater a patient’s BVRR, the greater the daily relative decline in brain volume during the measurement period. For example, a BVRR of 0.55‰ meant that the patient’s brain volume declined by a mean of 0.55‰ per day during the measurement period.
Overall survival was calculated by the Kaplan–Meier method from the date of WBRT commencement to the date of death. Patients who remained alive were censored from the date of their last follow-up visit. The log-rank test was used to compare the survival distributions between 2 groups.
Cox regression analysis was performed to assess the ability of different parameters to predict overall survival after WBRT. To identify independent prognostic factors, all significant variables on univariate Cox regression analysis (P ≤ 0.05) were subjected to multivariate Cox regression analysis. A forward selection procedure with a cutoff of P = 0.10 was used. The assumption of proportional hazards was verified by examining log-minus-log plots.
An optimal cutoff value for the BVRR was determined by maximally selected rank statistics for survival data.18 The P-value for the optimal cutoff value was approximated using the “HL” method described by Hothorn and Lausen.19
One-way ANOVA was chosen to compare the BVRR across RPA classes. Post hoc analysis was performed with Tukey’s honest significant difference (HSD) test.
The biologically effective doses (BEDs) were selected to compare the biological effects of different fractionation schedules and were calculated by using the linear quadratic model. Brain tissue is considered late-responding owing to its slow rate of cellular turnover; hence, an α/β ratio of 2 Gy was chosen for estimating the delivered BED.20 Spearman’s rank correlation coefficient was used to assess the relationship between BEDs and BVRRs.
The Mann–Whitney U-test was used to compare BVRRs between patients with complete or partial responses and those with stable or progressive disease.
Continuous data are presented as mean ± standard deviation, unless otherwise noted. P-values < 0.05 were considered statistically significant.
All statistical analyses were performed using SPSS v23 and R v3.3.0.
Results
Data of 3 treatment-related factors, 9 pretreatment factors, and BVRRs were available for all patients (Table 1). The median follow-up period was 10.4 months (range, 2.3–111.2). The 6-month and 1-, 2-, and 3-year survival rates of the entire cohort were 77.7%, 49.8%, 25.1%, and 15.3%, respectively. The median overall survival after WBRT was 11.3 months (95% CI: 8.4–14.2). Seventy-one patients had died by the time of our analysis. The average number of MRI scans per patient used for calculating the BVRR was 2.76 ± 0.7 (median, 3; range, 2–5).
Development of Brain Atrophy Following WBRT
Before commencing WBRT, the average brain volume was 1257.9 ± 127.6 cm3 (median, 1249 cm3; range, 922–1697 cm3). The median interval between starting WBRT and the final MRI scan used for volume measurement was 97 days (range, 60–149). An average decline of 48.2 ± 64.6 cm3 was observed during the measurement period (ie, the first 150 days after commencing WBRT). At the end of the measurement period, the average brain volume was 1209.7 ± 129.3 cm3 (median, 1203 cm3; range, 821–1570). The median BVRR was 0.25‰ (range, −0.67‰ to 2.12‰), with positive values indicating a decrease in brain volume and negative values indicating an increase (eg, due to cerebral edema).
Figure 2 shows that brain volume reduction mostly developed in the first 150 days after commencing WBRT, after which the rate of brain volume decrease effectively plateaued.
We evaluated the radiographic cerebral response to WBRT at the end of the study period for each patient. Fifteen patients (16%) experienced a complete response and 35 (38%) a partial response. Twenty-eight patients (31%) had stable disease and 13 (14%) experienced progressive disease. There was no significant difference in BVRR between patients with complete or partial response (median, 0.28‰) and patients with stable or progressive disease (median, 0.23‰) (P = 0.311). Moreover, the median total pretreatment brain metastasis volume was 2 cm3, which was minuscule compared with the observed median brain volume loss of 35 cm3. This indicated that the measured brain volume decrease after WBRT was largely unrelated to tumor shrinkage following radiotherapy.
Univariate Analysis of Factors Influencing Survival
On univariate Cox regression analysis, a higher BVRR was a strong predictor of shorter overall survival after WBRT (P < 0.001, hazard ratio [HR] 3.34, 95% CI: 1.97–5.68).
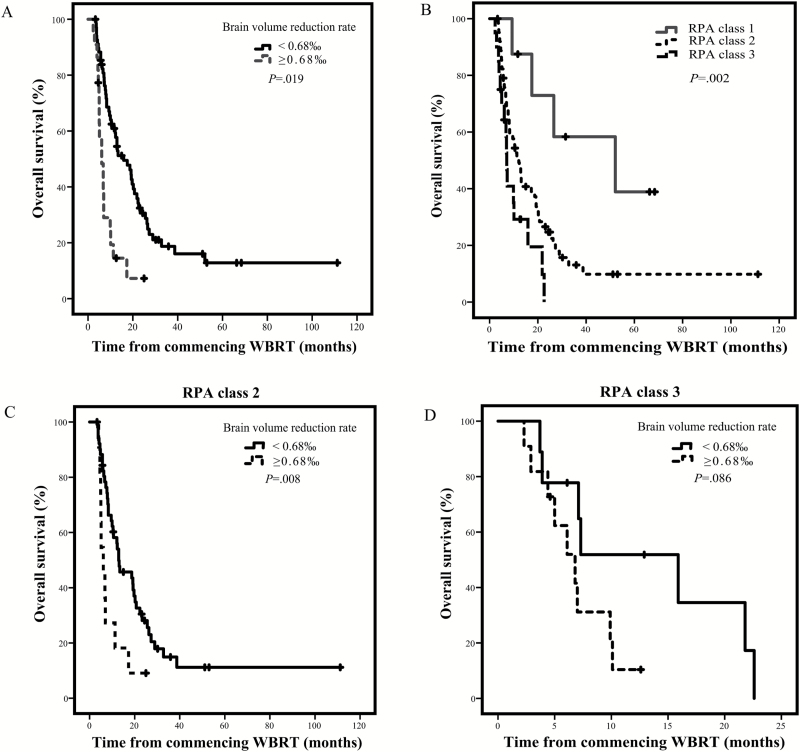
The optimal cutoff value for the BVRR in our cohort was 0.68‰ according to maximally selected rank statistics. The median overall survival was 6.1 months (95% CI: 4.2–8.0) for a BVRR of ≥0.68‰ (22 patients) versus 15.9 months (95% CI: 9.6–22.2) for a BVRR of <0.68‰ (69 patients) (P = 0.019, adjusted for multiple testing) (Fig. 3A).
Fig. 3.
Kaplan–Meier curves depicting survival. (A) Univariate effect of the extent of BVRR on overall survival. (B) Overall survival in patients of each RPA class. (C) Univariate effect of the extent of BVRR on overall survival for RPA class 2 patients. (D) Univariate effect of the extent of BVRR on overall survival for RPA class 3 patients.
Also associated with a shorter survival on univariate Cox regression analysis were a higher RPA class (P < 0.001), the number of brain metastases (P = 0.001), no SRS (P = 0.001), a higher Eastern Cooperative Oncology Group (ECOG) performance status (P = 0.002), no surgery for brain metastases (P = 0.004), an uncontrolled primary tumor (P = 0.007), WBRT with 36 Gy in 12 fractions (P = 0.007), age (P = 0.014), and the presence of extracranial metastasis (P = 0.040). A diagnosis of a primary tumor with synchronous brain metastasis, male sex, and primary tumor histology had no significant prognostic value on univariate Cox regression analysis (Table 2).
Table 2.
Cox proportional hazards analysis of factors affecting overall survival after WBRT
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | B | HR (95% CI) | P | B | HR (95% CI) | P |
| Brain volume reduction rate (per ‰) | 1.207 | 3.34 (1.97–5.68) | <0.001 | 1.059 | 2.88 (1.70–4.89) | <0.001 |
| RPA class | 0.804 | 2.24 (1.42–3.51) | <0.001 | NS | ||
| Number of brain metastases | 0.203 | 1.23 (1.09–1.38) | 0.001 | 0.195 | 1.22 (1.07–1.38) | 0.002 |
| No stereotactic radiosurgery | 0.987 | 2.68 (1.54–4.68) | 0.001 | NS | ||
| ECOG | 0.559 | 1.75 (1.23–2.50) | 0.002 | NS | ||
| No surgery of brain metastases | 0.859 | 2.36 (1.25–4.47) | 0.004 | NS | ||
| Uncontrolled primary tumor | 0.662 | 1.94 (1.20–3.12) | 0.007 | NS | ||
| WBRT with 36 Gy in 12 fractions | 0.729 | 2.07 (1.23–3.51) | 0.007 | NS | ||
| Age (per year) | 0.025 | 1.03 (1.01–1.05) | 0.014 | 0.027 | 1.03 (1.01–1.05) | 0.008 |
| Extracranial metastases | 0.510 | 1.67 (1.02–2.71) | 0.040 | NS | ||
| Synchronous brain metastases | 0.005 | 1.01 (1.00–1.01) | 0.165 | * | ||
| Male sex | 0.140 | 1.15 (0.72–1.84) | 0.558 | * | ||
| Primaries | * | |||||
| NSCLC (reference category) | 0.260 | * | ||||
| SCLC | 0.749 | 2.12 (0.83–5.42) | 0.119 | * | ||
| Malignant melanoma | −0.068 | 0.93 (0.25–3.49) | 0.920 | * | ||
| Breast cancer | 0.526 | 1.69 (0.61–4.68) | 0.311 | * | ||
| Renal cell cancer | 0.167 | 1.18 (0.40–3.53) | 0.764 | * | ||
| Other tumors | 1.092 | 2.98 (0.70–12.63) | 0.138 | * | ||
Abbreviations: NS, not significant; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; * not subjected to multivariate analysis.
Multivariate Analysis of Factors Influencing Survival
All variables significant on univariate Cox regression analysis (P ≤ 0.05) were subjected to multivariate Cox regression analysis. The BVRR (P ≤ 0.001), the number of brain metastases (P = 0.002), and age (P = 0.008) were independent prognostic factors for survival after WBRT. The remaining factors that were significant prognostic factors on univariate analysis were not significant on multivariate analysis (Table 2).
A second multivariate Cox regression analysis including RPA class, BVRR, and the interaction term (RPA class*BVRR) demonstrated no significant role for the interaction between RPA class and BVRR on overall survival (P interaction = 0.943).
RPA Class and BVRR
The survival rates of patient subgroups as stratified by RPA class were significantly different (P = 0.002) (Fig. 3B). The median survival times were 52.1 months for RPA class 1, 12.1 months for RPA class 2, and 7.1 months for RPA class 3.
Patients in the RPA class 2 and 3 subgroups were further divided by their BVRRs using the calculated cutoff value of 0.68‰. For patients with RPA class 2, those with a BVRR ≥0.68‰ exhibited significantly poorer survival (median, 6.1 mo) than those with rates <0.68‰ (median, 12.9 mo; P = 0.008) (Fig. 3C). For patients with RPA class 3, the difference in survival between the 2 groups was just above the probability threshold for significance (P = 0.086) (Fig. 3D). The median survival was 15.9 months for RPA class 3 patients with a BVRR <0.68‰ and 6.8 months for those with a BVRR ≥0.68‰.
One-way ANOVA showed that the BVRRs were significantly different between RPA classes (P < 0.001) (Table 3). These rates were 0.00 ± 0.23‰, 0.29 ± 0.47‰, and 0.74 ± 0.56‰ for RPA classes 1, 2, and 3, respectively (Supplementary Figure S1). Post hoc analysis using Tukey’s HSD test revealed a significant difference in BVRRs between RPA classes 1 and 3 (P = 0.001) and between classes 2 and 3 (P = 0.001), but not between classes 1 and 2 (P = 0.227).
Table 3.
One-way ANOVA to compare the effect of RPA class on brain atrophy rate
| RPA Class | N | Mean BVRR in ‰ | Standard Deviation | Standard Error | 95% CI | Minimal BVRR in ‰ | Maximal BVRR in ‰ |
|---|---|---|---|---|---|---|---|
| 1 | 8 | −0.0062 | 0.23083 | 0.08161 | −0.1992 to 0.1867 | −0.49 | 0.17 |
| 2 | 63 | 0.2909 | 0.47201 | 0.05947 | 0.1721 to 0.4098 | −0.67 | 20.12 |
| 3 | 20 | 0.7410 | 0.55609 | 0.12435 | 0.4807 to 10.0013 | −0.43 | 10.88 |
| Total | 91 | 0.3637 | 0.52002 | 0.05451 | 0.2554 to 0.4720 | −0.67 | 20.12 |
| Sum of squares | Degrees of freedom | Mean square | F | P | |||
| Between groups | 4.276 | 2 | 2.138 | 9.377 | <0.001 | ||
| Within groups | 20.062 | 88 | 0.228 | ||||
| Total | 24.338 | 90 | |||||
Biologically Effective Doses and BVRR
The BEDs were 68.4 Gy, 75 Gy, 80 Gy, and 90 Gy for 36 Gy in 20 fractions, 30 Gy in 10 fractions, 40 Gy in 20 fractions, and 36 Gy in 12 fractions, respectively. There was no significant correlation between BEDs and BVRRs (P = 0.147).
Discussion
In this study, a lower BVRR after receiving WBRT was an independent positive prognostic factor in patients with brain metastases.
We demonstrated that the BVRR remained a predictive factor even when other prognostic factors were incorporated into the multivariate Cox regression model; in fact, it even outperformed the RPA classification for predicting overall survival after WBRT in this model. Additionally, we ruled out a significant interaction between BVRR and RPA class in terms of effect on overall survival.
Aside from the BVRR, age and the number of brain metastases were also independent prognostic factors in our multivariate Cox regression model. These 2 factors are included in prognostic scoring systems for patients with brain metastases (eg, the diagnosis-specific graded prognostic assessment; the scoring system devised by Rades et al).21
Although functional performance assessment has been shown to correlate with survival in many cancers,22 we did not find ECOG performance status to be a prognostic factor on multivariate Cox regression analysis. This may be due to the fact that only 20 patients had an ECOG performance status of 2 or 3 in our study.
We grouped patients by their RPA class and compared survival curves for low (<0.68‰) and high (≥0.68‰) BVRRs within each RPA class. For RPA class 2, a lower BVRR resulted in a significantly longer survival; for RPA class 3, the difference in survival was only borderline significant, possibly because of the small sample size (20 patients). Subgroup analysis was not possible for RPA class 1 as there were only 8 patients who experienced a low BVRR (<0.68‰)—even though this subgroup analysis demonstrated that considering the BVRR in addition to RPA classification might provide additional prognostic information.
All patients received corticosteroids during the BVRR measurement period; hence, it was important to ensure that the measured volume reduction was not partially a consequence of the alleviation of cerebral edema. In a study of 23 patients with brain tumors, Andersen et al measured the peritumoral edema areas (but not volumes) at their largest dimensions and found the mean area before treatment to be 14.1 cm2.23 After 7 days of treatment with corticosteroids, the mean peritumoral edema area decreased to 12.6 cm2. We attempted to transfer their approach to extrapolate the effect of decreased edema in our population. Therefore, we assumed a spherical shape for the peritumoral edema regarding the data of Andersen et al and calculated the volumes of spheres within a circular area that corresponded to the peritumoral edema areas of 14.1 cm2 and 12.6 cm2. According to these calculations, the average peritumoral edema volume would have decreased from 39.8 to 33.6 cm3 after 7 days of treatment with corticosteroids. Compared with the average decline of brain volume after WBRT in our study (48.2 cm3), the calculated reduction of cerebral edema in the study by Andersen et al (6.2 cm3) is negligible. Hence, we deduced that decreased cerebral edema induced by corticosteroids is not the major cause of brain volume reduction observed in our study.
In our study, brain volume reduction was mainly observed in the first 150 days after commencing WBRT, after which the rate of brain volume decrease effectively plateaued. This suggests that the measured brain volume reduction likely has to be at least partially attributed to WBRT.
Almost all present studies used parameters like ventricular dilatation, expansion of the intracranial cerebrospinal fluid volume, and cortical sulcus enlargement as markers of brain volume reduction after WBRT.24–26 We found only one study in which the loss of brain volume after WBRT was quantified: a retrospective study by Fuentes et al of 15 patients with medulloblastoma.3 After a median period of 154 days, an average brain volume reduction of 3.0% was observed. In contrast, we measured an average brain volume reduction of 3.7% after a median period of only 97 days. These divergent results may be due to the small sample size in Fuentes et al’s study as well as different patient characteristics; the median age of their patients (26 y) was considerably younger than that in our study.
Recurrences of brain metastases after radiation therapy are frequent, with distant failure rates ranging between 34% and 52%.27 Currently, there is no consensus on the optimal treatment for these patients.28 Available treatment options range from supportive care alone to resection, WBRT, SRS, or even interstitial brachytherapy.29 Considering that BVRR plus RPA class provided additional prognostic information compared with RPA class alone in our study, determining the patients’ BVRR after WBRT in addition to their RPA class could help clinicians estimate their prognoses more reliably. In this regard, patients with a high RPA class and an increased BVRR would be best suited for best supportive care alone or treatments with a low side effect profile and a short treatment time like single SRS. In contrast, patients with a low RPA class and a low BVRR should benefit from aggressive or time-consuming treatments like multiple SRS sessions, fractionated stereotactic radiotherapy, neurosurgery, or aggressive CNS-directed systemic agents.30
Upon validation of the prognostic significance of BVRR after WBRT and the identification of risk factors for this process, this novel prognostic marker could also be used for patients with untreated brain metastases.
Aside from treatment of brain metastases alone, the prognostic value of brain volume loss could already today inform oncologic decision making in general. Fortunately, some patients (eg, patients with breast cancer) enjoy an extended survival following WBRT.31 In this group of patients, numerous oncologic treatment decisions have to be made following WBRT, and the prognostic information provided by brain volume loss could be of great importance.
In our study, patients with relatively good prognoses (RPA classes 1 and 2) tended to exhibit less brain volume reduction after WBRT than patients with poor prognoses (RPA class 3). Therefore, it is possible that advanced malignant disease as indicated by a higher RPA class is an important risk factor for brain volume reduction after WBRT. The analysis of risk factors for brain volume reduction was not the aim of the present study, although we plan to test this hypothesis in due course.
Even though one might assume that higher radiation doses lead to a more pronounced brain volume loss after WBRT, we could not find a correlation between BEDs and BVRRs in our study. This supports the hypothesis that toxicity is not different between common fractionation schedules used for WBRT.2,32 In this regard, for example, a systematic review by Gaspar et al did not find a dose-effect relationship with respect to neurological function or overall survival after WBRT.33 On the other hand, Klein et al analyzed the neurocognitive function in 195 patients with low-grade glioma and observed a decline in memory function only in patients receiving fraction doses exceeding 2 Gy.34 However, comparing the neurotoxicities of various fractionation schemes was beyond the scope of this study.
In this hypothesis-generating study, our finding that brain volume reduction after WBRT is an independent prognostic factor for patients with brain metastases introduces the notion that this event may be linked to other factors, such as chemotherapy or cancer progression. The pathophysiology of radiation-induced brain volume loss and how it differs from brain volume loss caused by other factors has not yet been completely elucidated. One frequently proposed mechanism is that radiation leads to inflammation and endothelial damage, resulting in vascular fibrosis with luminal stenosis and fibrinoid vascular necrosis.35 This facilitates hypoxic injury, white matter changes, and parenchymal central nervous system necrosis. Concerning chemotherapy, the proposed etiologies of brain volume loss include neurotoxicity through DNA damage and microvascular injury.36 Today, there is little evidence of a link between tumor burden and brain volume loss; one preclinical study suggested that cerebellar neuronal degeneration might be caused by the tumor itself and subsequent cachexia (eg, due to autoimmune reactions, cytokines such as tumor necrosis factor alpha, and metabolic changes).37 Hence, greater brain volume shrinkage after WBRT may only constitute a surrogate parameter for other circumstances already associated with worsening prognoses in cancer patients. However, the evidence to date does not rule out BVRR after WBRT as an independent prognostic factor.
It has been clearly demonstrated that WBRT can impair memory,38 which has led to new approaches like whole brain irradiation with hippocampal sparing or testing of neuroprotectors (eg, memantine).2,39 The role that brain volume reduction plays in neurocognitive decline after WBRT remains unclear. In a prospective study of 115 patients by Shibamoto et al, no correlation between brain atrophy and Mini Mental State Examination score was observed.24 In contrast, Asai et al found a correlation between radiation-induced brain atrophy and dementia in a 91-patient study.40 Similarly, radiation-induced dementia was accompanied by cortical atrophy and white matter changes in a small retrospective study by DeAngelis et al.26
Limitations of our study include its heterogeneous cohort (owing to its retrospective nature), the absence of a comparison SRS-only cohort, and the lack of neurocognitive tests. Therefore, a correlation between brain volume reduction and cognitive impairment was not investigated.
To our knowledge, this is the largest study to date in which quantitative measurement of WBRT-associated shrinkage of the whole brain was performed. Additionally, this is the first study to investigate the prognostic value of brain volume reduction after WBRT. The considerations described above demonstrate that further studies are warranted to investigate the role of brain volume reduction after WBRT as an independent prognostic factor for overall survival, and to clarify whether brain volume decrease can explain the non-advantage of adding WBRT to SRS in certain patient subgroups.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sector.
Conflict of interest statement. None.
Supplementary Material
Acknowledgments
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med.”
References
- 1. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682–689. [DOI] [PubMed] [Google Scholar]
- 2. McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4(Suppl 4):S236–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuentes D, Contreras J, Yu J et al. Morphometry-based measurements of the structural response to whole-brain radiation. Int J Comput Assist Radiol Surg. 2015;10(4):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 5. Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoyama H, Shirato H, Tago M et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 7. Kocher M, Soffietti R, Abacioglu U et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1(4):457–464. [DOI] [PubMed] [Google Scholar]
- 9. Sahgal A, Aoyama H, Kocher M et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710–717. [DOI] [PubMed] [Google Scholar]
- 10. Le Péchoux C, Dunant A, Senan S et al. ; Prophylactic Cranial Irradiation (PCI) Collaborative Group Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10(5):467–474. [DOI] [PubMed] [Google Scholar]
- 11. Manjón JV, Coupé P. volBrain: an online MRI brain volumetry system. Front Neuroinform. 2016;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Næss-Schmidt E, Tietze A, Blicher JU et al. Automatic thalamus and hippocampus segmentation from MP2RAGE: comparison of publicly available methods and implications for DTI quantification. Int J Comput Assist Radiol Surg. 2016;11(11):1979–1991. [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 14. Gaspar L, Scott C, Rotman M et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 15. Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Amer Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 16. Soh Y, Hae Y, Mehmood A, Hadi Ashraf R, Kim I. Performance evaluation of various functions for kernel density estimation. Open J Appl Sci. 2013;3(1):58–64. [Google Scholar]
- 17. Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J Royal Stat Soc: Series B (Statistical Methodology). 1998;60(2):271–293. [Google Scholar]
- 18. Hothorn T, Zeileis A. Generalized maximally selected statistics. Biometrics. 2008;64(4):1263–1269. [DOI] [PubMed] [Google Scholar]
- 19. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121–137. [Google Scholar]
- 20. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. [DOI] [PubMed] [Google Scholar]
- 21. Zindler JD, Rodrigues G, Haasbeek CJ et al. The clinical utility of prognostic scoring systems in patients with brain metastases treated with radiosurgery. Radiother Oncol. 2013;106(3):370–374. [DOI] [PubMed] [Google Scholar]
- 22. Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersen C, Astrup J, Gyldensted C. Quantitative MR analysis of glucocorticoid effects on peritumoral edema associated with intracranial meningiomas and metastases. J Comput Assist Tomogr. 1994;18(4):509–518. [DOI] [PubMed] [Google Scholar]
- 24. Shibamoto Y, Baba F, Oda K et al. Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72(4):1168–1173. [DOI] [PubMed] [Google Scholar]
- 25. Sanghera P, Gardner SL, Scora D, Davey P. Early expansion of the intracranial CSF volume after palliative whole-brain radiotherapy: results of a longitudinal CT segmentation analysis. Int J Radiat Oncol Biol Phys. 2010;76(4):1171–1176. [DOI] [PubMed] [Google Scholar]
- 26. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. [DOI] [PubMed] [Google Scholar]
- 27. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. [DOI] [PubMed] [Google Scholar]
- 28. Ammirati M, Cobbs CS, Linskey ME et al. The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romagna A, Schwartz C, Egensperger R et al. Iodine-125 brachytherapy as upfront and salvage treatment for brain metastases: a comparative analysis. Strahlenther Onkol. 2016;192(11):780–788. [DOI] [PubMed] [Google Scholar]
- 30. Kurtz G, Zadeh G, Gingras-Hill G et al. Salvage radiosurgery for brain metastases: prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014;88(1):137–142. [DOI] [PubMed] [Google Scholar]
- 31. Sperduto PW, Kased N, Roberge D et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hindo WA, DeTrana FA III, Lee MS, Hendrickson FR. Large dose increment irradiation in treatment of cerebral metastases. Cancer. 1970;26(1):138–141. [DOI] [PubMed] [Google Scholar]
- 33. Gaspar LE, Mehta MP, Patchell RA et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klein M, Heimans JJ, Aaronson NK et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 35. Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. [DOI] [PubMed] [Google Scholar]
- 36. McDonald BC, Saykin AJ. Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav. 2013;7(4):374–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michalak S, Wender M, Michałowska-Wender G. Cachexia-induced cerebellar degeneration: involvement of serum TNF and MCP-1 in the course of experimental neoplastic disease. Acta Neurobiol Exp (Wars). 2006;66(2):113–122. [DOI] [PubMed] [Google Scholar]
- 38. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 39. Oehlke O, Wucherpfennig D, Fels F et al. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: local tumour control and survival. Strahlenther Onkol. 2015;191(6):461–469. [DOI] [PubMed] [Google Scholar]
- 40. Asai A, Matsutani M, Kohno T et al. Subacute brain atrophy after radiation therapy for malignant brain tumor. Cancer. 1989;63(10):1962–1974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.