Abstract
The blood–brain barrier (BBB) excludes the vast majority of cancer therapeutics from normal brain. However, the importance of the BBB in limiting drug delivery and efficacy is controversial in high-grade brain tumors, such as glioblastoma (GBM). The accumulation of normally brain impenetrant radiographic contrast material in essentially all GBM has popularized a belief that the BBB is uniformly disrupted in all GBM patients so that consideration of drug distribution across the BBB is not relevant in designing therapies for GBM. However, contrary to this view, overwhelming clinical evidence demonstrates that there is also a clinically significant tumor burden with an intact BBB in all GBM, and there is little doubt that drugs with poor BBB permeability do not provide therapeutically effective drug exposures to this fraction of tumor cells. This review provides an overview of the clinical literature to support a central hypothesis: that all GBM patients have tumor regions with an intact BBB, and cure for GBM will only be possible if these regions of tumor are adequately treated.
Keywords: blood brain barrier, drug therapy, glioblastoma, magnetic resonance imaging
The prognosis for newly diagnosed glioblastoma (GBM) remains dire despite years of intensive basic, translational, and clinical research. Over the past 30 years, there have been only 4 FDA approvals for systemically administered therapies for GBM: lomustine, carmustine, temozolomide, and bevacizumab. The first 3 drugs are simple alkylating agents approved over a decade ago with partial brain penetration, while bevacizumab is a monoclonal antibody that binds vascular endothelial growth factor (VEGF) and prevents VEGF receptor activation on capillary endothelial cells. Bevacizumab is effective at controlling edema in some GBM patients, but clinical trials have not demonstrated a convincing impact on patient survival.1–3 In contrast, in the last decade there has been tremendous progress in developing highly effective, targeted therapies for most other non-CNS solid malignancies. Specifically, 28 molecularly targeted agents have gained FDA marketing approval since 2006 for breast (n = 8), lung (n = 13), and melanoma (n = 7) (https://www.cancer.gov/about-cancer/treatment/drugs/cancer-type). While there are many potential factors that contribute to the striking lack of progress in developing effective therapies for GBM, we propose that limited and heterogeneous drug delivery across the blood–brain barrier (BBB) is a major cause of treatment failure for otherwise promising novel therapies in GBM.
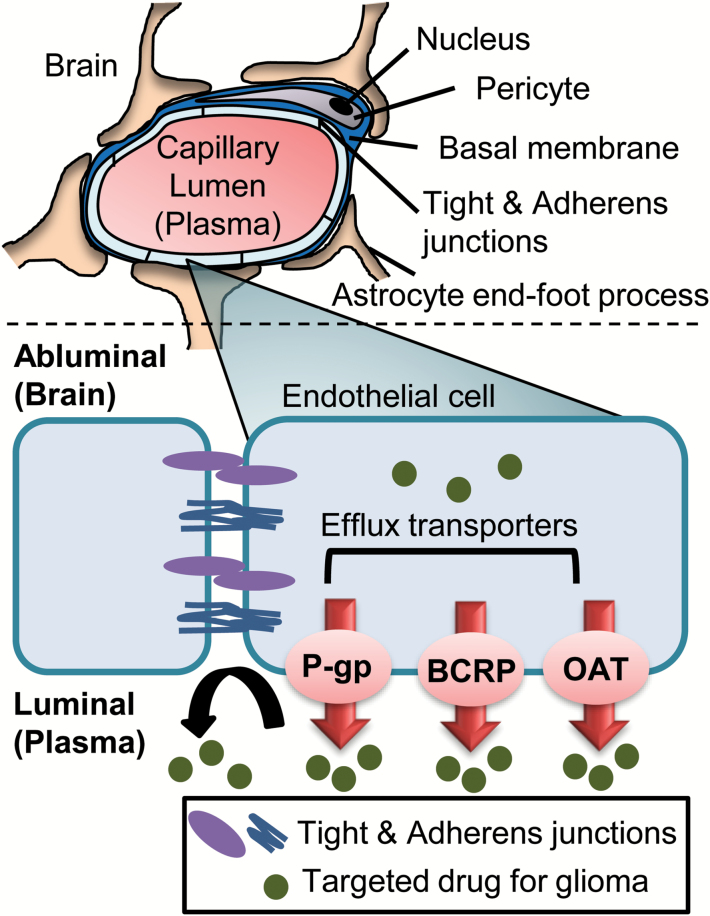
The BBB provides both physical and biochemical barriers to drug delivery into normal brain (Fig. 1).4,5 Continuous tight and adherens junctions between brain capillary endothelial cells prevent paracellular diffusion, and as a result, molecules in the bloodstream can enter the brain only by transiting across endothelial cell luminal and abluminal plasma membranes.6,7 This physical barrier markedly limits brain distribution of many oncologic drugs, including monoclonal antibodies, antibody-drug conjugates, and hydrophilic molecules that do not readily cross lipid bilayers. For lipophilic molecules that readily diffuse across plasma membranes, various transmembrane efflux transporters in endothelial cells function as a biochemical barrier by actively transporting drugs into the capillary lumen. P-glycoprotein, breast cancer resistance protein, and organic anion transporters are especially important efflux pumps within the BBB that limit accumulation of small-molecule targeted therapies.7,8 Between the biochemical and physical barriers presented by the normal BBB, many anticancer agents have significantly impaired distribution into normal brain parenchyma (Table 1). Thus, the BBB is unequivocally important for exclusion of the vast majority of approved and experimental oncologic drugs from normal brain.
Fig. 1.
Illustration of key components of the BBB that provide physical (tight and adherens junctions) and biochemical (transporter-mediated efflux) barriers to brain penetration of antiglioma agents.
Table 1.
Heterogeneous brain distribution of antiglioma agents in clinical and preclinical studies*
| Drug | Tumor Tissue-to-Plasma Ratioa | Efflux Transporter Substrate Status (e.g., P-gp, Bcrp) | References | ||
|---|---|---|---|---|---|
| Contrast Enhancing | Non-Enhancing | Normal (“distant”) Brain | |||
| Temozolomide | — | 0.20 | (0.41) | Yes | 65–67 |
| Methotrexate | 0.30 | 0.063 | (0.11) | Yes | 68,69 |
| Carboplatin | 0.054 to 0.49 | — | 0.17 (0.031) | — | 49,70,71 |
| Cilengitide | 3.39 | — | — | — | 72 |
| Erlotinib | 0.35 (0.51) | (0.10) | (0.02 to 0.09) | Yes | 73,74 |
| Imatinib | 1.35 | — | (0.16) | Yes | 75,76 |
| Gefitinib | 26.4 | — | (0.10) | Yes | 77,78 |
| Estramustine | 15.8 (4.6) | — | (3.5) | — | 79,80 |
| Idarubicin | 15.6 | 3.75 | — | Yes | 81,82 |
| Ranimustine (MCNU) | 2.54 | — | 0.16 | — | 70 |
| Tauromustine (TCNU) | 0.51 | 0.56 | — | — | 83 |
| Liposomal daunorubicin | 2.16 to 7.11 | 2.02 to 7.88 | 1.1 to 4.55 | — | 84,85 |
| Mitoxantrone | 34 | — | (0.25) | Yes | 86,87 |
| Paclitaxel | 7.35 | — | (0.5) | Yes | 88–90 |
| Etoposide | 0.19 to 0.36 | 0.13 | — | Yes | 91–94 |
| Teniposide | 0.19 to 2.39 | 0.029 to 0.19 | — | — | 91,95,96 |
| Temsirolimus | 1.43 | — | — | Yes | 97,98 |
*The review article by Pitz et al64 previously reported the tissue-to-plasma ratios observed in human brain tumors (high-grade glioma). This table provides additional data, including the preclinical brain penetration data (in parentheses) and efflux transporter substrate status.
Abbreviations: P-gp, P-glycoprotein; Bcrp, breast cancer resistance protein. Blank areas (—), not reported in the literature.
aDetermined from the area under the concentration-time curve ratio and/or a single-time concentration ratio. Data provided are clinical or preclinical.
A variety of pathologic conditions, including brain tumors, can disrupt the integrity of the BBB. This BBB dysfunction is most commonly detected on conventional contrast-enhanced MRI following intravenous administration of gadolinium-based contrast agents. In regions of physically disrupted BBB, the hydrophilic contrast molecules diffuse out of the vessel lumen and accumulate within the extravascular extracellular space, manifesting as contrast-enhancing hyperintense regions on T1-weighted (T1W) sequences in nearly all GBM.9 These contrast-enhancing regions are associated with dense tumor and are the typical target for surgical resection. However, beyond the contrast-enhancing region, essentially all GBM have a region of non-enhancing edema that is evident on imaging as increased signal intensity on T2-weighted (T2W) or T2W fluid attenuation inversion recovery (FLAIR) imaging. This imaging feature reflects a combination of cellular infiltration and vasogenic edema.10–13 The presence of vasogenic edema reflects a more subtle dysregulation of the BBB that allows abnormal accumulation of fluid within the brain parenchyma but is insufficient to allow accumulation of contrast. As discussed in detail below, there is unequivocal evidence that all GBM have tumor cells infiltrating this edema volume and that these cells have a profound influence on the ultimate efficacy of therapy.
Numerous studies support the importance of maximal surgical resection to prolong survival of patients with GBM.13–16 Indeed, some have proposed resecting a margin of surrounding “normal” brain from non-eloquent regions, if clinically feasible, to minimize the residual disease burden.13,17 Nevertheless, GBM is ultimately not a surgically curable disease. Even with complete resection of all radiographic abnormality (both T1W contrast-enhancing and T2W FLAIR volumes), recurrence is inevitable. This sobering reality has been appreciated since the early twentieth century, when pioneering work by Scherer demonstrated infiltration of glioma cells into otherwise normal brain,18,19 and Dandy and colleagues demonstrated that even removal of the entire ipsilateral hemisphere (hemispherectomy) was followed by recurrence in the contralateral hemisphere.20,21 In conjunction with this natural history, several image-guided surgical sampling studies have demonstrated significant tumor cell infiltrates present in 80%–100% of biopsies obtained in regions of T2W/T2W FLAIR abnormality (reviewed by Matsuo et al22). Sampling one or more centimeters beyond the T2W/T2W FLAIR abnormality also demonstrates a smaller fraction of biopsies containing tumor cells.11,12 Collectively, these surgical data provide indisputable evidence that a clinically meaningful tumor burden exists beyond tumor volume defined radiographically by contrast enhancement and support the concept of GBM as a whole-brain disease.23 To achieve significant improvement in progression-free survival, and especially to achieve a cure, at least some component of a multifactorial approach to therapy must address the non-contrast-enhancing tumor burden infiltrating the brain.
While conventional T1W contrast-enhanced MRI provides gross qualitative assessment of BBB disruption, this technique fails to resolve the degree to which the BBB is disrupted, which can vary from patient to patient and within different regions of the same tumor. Instead, advanced techniques such as dynamic contrast enhanced (DCE)-MRI can quantitatively measure the transport constant of contrast molecules across different contrast-enhancing regions using pharmacokinetic modeling and dynamic imaging acquisition to estimate vascular permeability.9,24 Numerous studies have shown extensive intratumoral heterogeneity of DCE parameters within T1W contrast-enhancing tumor regions reflecting varying degrees of BBB disruption and vascular permeability.25–27 For tumor regions that are devoid of contrast, other advanced and emerging MRI techniques are needed to characterize tumor extent using tumoral imaging phenotypes that are independent of BBB integrity,28 such as tumor cell density on diffusion-weighted imaging (DWI),29 white matter infiltration on diffusion tensor imaging (DTI),30,31 or diffusion kurtosis imaging (DKI),32 metabolic profiling on MR spectroscopy (MRS),33,34 and microvessel volume on dynamic susceptibility-weighted contrast-enhanced (DSC) MRI.35,36 Highlighting the issue of non-contrast-enhancing tumor volumes, an analysis of 21 previously untreated GBM patients by DWI demonstrated a significant volume of hypercellularity extending beyond regions of T1W contrast enhancement (mean volume 7.3 cc, minimum volume 0.2 cc, maximum volume 59.8 cc) in all patients.37 Similar radiotherapy planning studies using MRS demonstrate significant extension of metabolically detectable tumor beyond T1W contrast-enhancing regions.38–40 Finally, consistent with the Response Assessment in Neuro-Oncology guidelines, isolated progression of the T2 signal on serial head MRI is a strong predictor of subsequent radiographic progression within the contrast-enhancing volume.41,42 Collectively, these observations reinforce the fact that contrast enhancement is not an accurate delimiter of gross tumor burden in GBM.
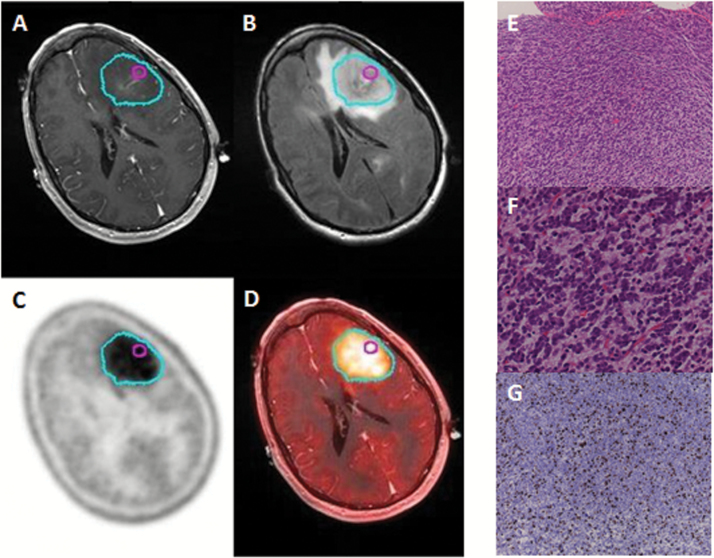
Complementary PET techniques have been used to delineate the tumor extent in GBMs. Several large neutral amino acids (11C-methionine; 18F-fluoro-ethyl tyrosine [FET]; 3,4-dihydroxy-6-18F-fluoro-l-phenylalanine [FDOPA]) are actively transported into the brain across the BBB and preferentially accumulate in brain tumor tissue.43,44 Using PET imaging of these tracers, several studies demonstrate that a significant fraction of the PET-defined tumor volume (59%–71%) extends beyond the contrast-enhancing lesion in the majority (68%–100%) of GBM patients (Fig. 2).22,45,46 Collectively, these MR and PET imaging studies demonstrate that a majority of GBMs have gross tumor burden with an intact BBB that extends beyond the contrast-enhancing tumor volume. Combined with the surgical experience, these data support our central contention that all GBM have a clinically significant tumor burden “protected” by an intact BBB.
Fig. 2.
Illustrative case for a patient with significant tumor burden beyond contrast-enhancing regions. Sequential imaging with (A) T1W+contrast, (B) T2W FLAIR, (C) FDOPA PET (cyan contour), and (D) PET/CT fusion demonstrate significant regions of an FDOPA-positive GBM without contrast enhancement on MRI. Location of a stereotactic biopsy is marked with a magenta contour. Samples were processed for photomicroscopy of hematoxylin and eosin at (E) 100x magnification and (F) 400x magnification showing hypercellularity. (G) Ki-67 staining of the same sample, imaged at 100x magnification shows a high proliferative index (>20%).
In conjunction with evidence that all GBM have regions of microscopic and gross tumor burden with an intact BBB, there are significant clinical data demonstrating the negative impact of inadequate drug distribution on control of microscopic tumor burden in the context of brain metastases. Small-cell lung cancer is highly sensitive to doublet chemotherapy with cisplatin and etoposide, and in limited stage disease treated with cisplatin/etoposide and localized chest irradiation, approximately 25% of patients can be cured.47 However, small-cell lung cancer has an exceptionally high propensity to metastasize to the brain,48 and because neither cisplatin nor etoposide has significant distribution into normal brain,49–51 the ultimate brain failure rate is as high as 80%. As a result, several large randomized clinical trials have demonstrated that prophylactic cranial irradiation in patients with no evidence of disease in the chest or brain at completion of chemo/radiotherapy reduces the risk of failure in the brain by over 50%.52,53 Similarly, cranial or craniospinal irradiation has been used in pediatric patients with high-risk acute lymphoblastic leukemia to prevent central nervous system tumor recurrences before modern intrathecal chemotherapy regimens were developed.54–56 In the context of precision medicine strategies, similar patterns of brain-only failure have been observed in patients with human epidermal growth factor receptor 2–amplified breast cancer and anaplastic lymphoma kinase–translocation lung cancers treated with highly effective but brain impenetrant targeted therapies.57–59 These clinical experiences, with both cytotoxic and targeted therapies, demonstrate that poor brain distribution can result in an inadequate treatment of subclinical deposits of tumor cells in the brain. While there are no direct clinical data demonstrating the impact of poor drug delivery on patterns of failure in GBM, extrapolation of the clinical data in brain metastases suggests that poor drug delivery into regions of GBM with an intact BBB will limit efficacy of therapy in these regions. Stated differently, a central tenet of oncology is that a cure is possible only if an effective therapy is delivered with adequate exposure to the entire population of targeted cells, and failure to adequately deliver therapies into regions of GBM that have an intact BBB will preclude a chance for cure.
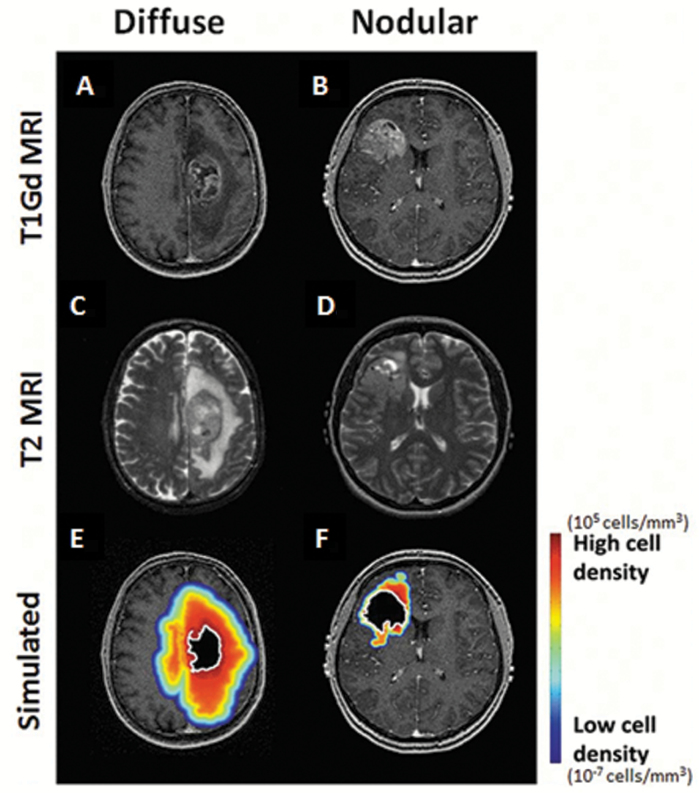
In this context, is there any role for testing drugs with poor penetration across an intact BBB in patients with GBM? Following standard of care surgery, radiation, and temozolomide therapy, the predominant failure pattern for GBM patients is within the high-dose radiation volume that is centered on the region of contrast enhancement.60–62 Anecdotal reports of drugs with very limited brain distribution (vemurafenib, ABT-414) demonstrate that at least a subset of GBM patients may benefit from such therapies (Gan et al, ASCO meeting 2015).63 While we would predict that these agents would be ineffective in lesions with extensive tumor infiltration beyond the contrast-enhancing region, there are a subset of “nodular” GBM with a dominant contrast-enhancing lesion and limited surrounding edema volume (Fig. 3).11,13 If therapeutic levels of a poorly brain penetrant but otherwise effective drug are effectively delivered into tumor regions with a disrupted BBB, we might speculate that those patients with a nodular imaging phenotype, with the dominant tumor burden contained within the contrast-enhancing region, may derive greater tumor control benefits than those patients who have a greater tumor burden outside of the enhancing region. However, these strategies ultimately will fail without an effective approach to address the non-contrast-enhancing portion of the tumor.
Fig. 3.
Patient-specific simulations of tumor cell distribution and density for both a diffuse and a nodular newly diagnosed GBM. T1W + gadolinium (Gd) contrast and T2W MRIs for a diffuse (A, C) or nodular (B, D) tumor. A simulation estimating glioma cell extent is overlaid on the T1Gd MRI with red and blue indicating high and low (but nonzero) glioma cell density, respectively (E, F).
Accepting the importance of drug distribution across an intact BBB into brain is a critical first step in developing effective therapies for GBM and must be a key consideration in any clinical trial design for newly diagnosed or recurrent GBM. Acknowledging interspecies differences in the biochemical functions within the BBB, pharmacokinetic and pharmacodynamic analyses of drug delivery into normal rodent brain and corresponding relevant orthotopic tumor models may provide initial data regarding potential limitations of drug delivery encountered in patients. Within phase I tolerability studies, embedding phase 0 clinical designs to assess drug distribution and pharmacodynamic effects using either image-guided surgical sampling or functional imaging assessments can specifically address drug penetration and efficacy in tumor regions with an intact BBB (T2W FLAIR) versus disrupted BBB (T1W+contrast). Decisions to move forward with clinical efficacy testing in phase II/III trials then can be made based on a combined understanding of mechanism of action, drug potency, and intra- and intertumoral heterogeneity of drug distribution. Especially for drugs with relatively poor brain distribution, consideration of intra- and interpatient heterogeneity in BBB disruption, as approximated by contrast enhancement, and the fractional tumor burden within and beyond regions of contrast enhancement, will be instrumental in failure analysis of negative clinical trials and for identifying regimens that are effective in subsets of patients. Moreover, the clinical realities of the contribution of the BBB to treatment failure in GBM argue for renewed efforts to develop BBB-penetrating agents, optimize BBB-disruption technologies, and refine implantable drug delivery technologies that bypass the BBB and deliver therapeutic concentrations throughout an infiltrating tumor volume.
Key Conclusions
GBM is highly infiltrative and is a whole-brain disease.
All GBM have clinically significant regions of tumor with an intact BBB.
Failure to deliver an effective therapy to all regions of GBM will result in treatment failure.
Funding
This work was supported by the MIT/Mayo Physical Sciences Center for Drug Distribution and Efficacy in Brain Tumors (U54CA210180), the Mayo Clinic SPORE in Brain Cancer (P50CA108961), and RO1NS077921. We gratefully acknowledge the support of the MIT/Mayo Physical Sciences Center for Drug Distribution and Efficacy in Brain Tumors (U54CA210180), the James S. McDonnell Foundation, the Ivy Foundation, the Mayo Clinic SPORE in Brain Cancer (P50CA108961) and the NIH (RO1NS077921, R01 NS060752, R01 CA164371, U54 CA210180, U54 CA143970, U54 CA193489, U01CA220378).
Conflict of interest statement. None.
References
- 1. Gilbert MR, Dignam JJ, Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chinot OL, de La Motte Rouge T, Moore N et al. . AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. [DOI] [PubMed] [Google Scholar]
- 3. Taal W, Oosterkamp HM, Walenkamp AM et al. . Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 4. Fokas E, Steinbach JP, Rödel C. Biology of brain metastases and novel targeted therapies: time to translate the research. Biochim Biophys Acta. 2013;1835(1):61–75. [DOI] [PubMed] [Google Scholar]
- 5. Theodorakis PE, Müller EA, Craster RV, Matar OK. Physical insights into the blood-brain barrier translocation mechanisms. Phys Biol. 2017;14(4):041001. [DOI] [PubMed] [Google Scholar]
- 6. Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64(2):328–363. [DOI] [PubMed] [Google Scholar]
- 7. Parrish KE, Sarkaria JN, Elmquist WF. Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood-brain barrier. Clin Pharmacol Ther. 2015;97(4):336–346. [DOI] [PubMed] [Google Scholar]
- 8. Oberoi RK, Parrish KE, Sio TT, Mittapalli RK, Elmquist WF, Sarkaria JN. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro Oncol. 2016;18(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Y, Sundgren PC, Tsien CI, Chenevert TT, Junck L. Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol. 2006;24(8):1228–1235. [DOI] [PubMed] [Google Scholar]
- 10. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 11. Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463–469. [DOI] [PubMed] [Google Scholar]
- 13. Baldock AL, Ahn S, Rockne R et al. . Patient-specific metrics of invasiveness reveal significant prognostic benefit of resection in a predictable subset of gliomas. PLoS One. 2014;9(10):e99057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lacroix M, Abi-Said D, Fourney DR et al. . A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 15. Stummer W, Reulen HJ, Meinel T et al. ; ALA-Glioma Study Group Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564. [DOI] [PubMed] [Google Scholar]
- 16. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264. [DOI] [PubMed] [Google Scholar]
- 17. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 18. Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63(1):1–35. [Google Scholar]
- 19. Scherer HJ. Structural development in gliomas. Am J Cancer. 1938;34(3):333–351. [Google Scholar]
- 20. Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary report. J Am Med Assoc. 1928;90:823–825. [Google Scholar]
- 21. Bell E Jr, Karnosh LJ. Cerebral hemispherectomy: report of a case 10 years after operation. J Neurosurg. 1949;6(4):285–293. [DOI] [PubMed] [Google Scholar]
- 22. Matsuo M, Miwa K, Tanaka O et al. . Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82(1):83–89. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tofts PS, Brix G, Buckley DL et al. . Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. [DOI] [PubMed] [Google Scholar]
- 25. Choi YS, Kim DW, Lee SK et al. . The added prognostic value of preoperative dynamic contrast-enhanced MRI histogram analysis in patients with glioblastoma: analysis of overall and progression-free survival. AJNR Am J Neuroradiol. 2015;36(12):2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arevalo-Perez J, Thomas AA, Kaley T et al. . T1-weighted dynamic contrast-enhanced MRI as a noninvasive biomarker of epidermal growth factor receptor vIII status. AJNR Am J Neuroradiol. 2015;36(12):2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santarosa C, Castellano A, Conte GM et al. . Dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging for glioma grading: preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur J Radiol. 2016;85(6):1147–1156. [DOI] [PubMed] [Google Scholar]
- 28. Weber MA, Henze M, Tüttenberg J et al. . Biopsy targeting gliomas: do functional imaging techniques identify similar target areas?Invest Radiol. 2010;45(12):755–768. [DOI] [PubMed] [Google Scholar]
- 29. LaViolette PS, Mickevicius NJ, Cochran EJ et al. . Precise ex vivo histological validation of heightened cellularity and diffusion-restricted necrosis in regions of dark apparent diffusion coefficient in 7 cases of high-grade glioma. Neuro Oncol. 2014;16(12):1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price SJ, Young AM, Scotton WJ et al. . Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J Magn Reson Imaging. 2016;43(2):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price SJ, Jena R, Burnet NG et al. . Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol. 2006;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang R, Jiang J, Zhao L et al. . Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget. 2015;6(39):42380–42393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKnight TR, von dem Bussche MH, Vigneron DB et al. . Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg. 2002;97(4):794–802. [DOI] [PubMed] [Google Scholar]
- 34. Croteau D, Scarpace L, Hearshen D et al. . Correlation between magnetic resonance spectroscopy imaging and image-guided biopsies: semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery. 2001;49(4):823–829. [DOI] [PubMed] [Google Scholar]
- 35. Hu LS, Ning S, Eschbacher JM et al. . Multi-parametric MRI and texture analysis to visualize spatial histologic heterogeneity and tumor extent in glioblastoma. PLoS One. 2015;10(11):e0141506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maia AC Jr, Malheiros SM, da Rocha AJ et al. . Stereotactic biopsy guidance in adults with supratentorial nonenhancing gliomas: role of perfusion-weighted magnetic resonance imaging. J Neurosurg. 2004;101(6):970–976. [DOI] [PubMed] [Google Scholar]
- 37. Pramanik PP, Parmar HA, Mammoser AG et al. . Hypercellularity components of glioblastoma identified by high b-value diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2015;92(4):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ken S, Vieillevigne L, Franceries X et al. . Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol. 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cordova JS, Shu HK, Liang Z et al. . Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016;18(8):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park I, Tamai G, Lee MC et al. . Patterns of recurrence analysis in newly diagnosed glioblastoma multiforme after three-dimensional conformal radiation therapy with respect to pre-radiation therapy magnetic resonance spectroscopic findings. Int J Radiat Oncol Biol Phys. 2007;69(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen PY, Cloughesy TF, Ellingson BM et al. . Report of the jumpstarting brain tumor drug development coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro Oncol. 2014;16(Suppl 7):vii36–vii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Radbruch A, Lutz K, Wiestler B et al. . Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the response assessment in neurooncology criteria. Neuro Oncol. 2012;14(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasey JS, Koh WJ, Evans ML et al. . Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36(2):417–428. [DOI] [PubMed] [Google Scholar]
- 44. Miyagawa T, Oku T, Uehara H et al. . “Facilitated” amino acid transport is upregulated in brain tumors. J Cereb Blood Flow Metab. 1998;18(5):500–509. [DOI] [PubMed] [Google Scholar]
- 45. Nowosielski M, DiFranco MD, Putzer D et al. . An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS One. 2014;9(4):e95830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pafundi DH, Laack NN, Youland RS et al. . Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turrisi AT III, Kim K, Blum R et al. . Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265–271. [DOI] [PubMed] [Google Scholar]
- 48. Arriagada R, Le Chevalier T, Borie F et al. . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–190. [DOI] [PubMed] [Google Scholar]
- 49. Jacobs S, McCully CL, Murphy RF, Bacher J, Balis FM, Fox E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol. 2010;65(5):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seute T, Leffers P, Wilmink JT, ten Velde GP, Twijnstra A. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. J Clin Oncol. 2006;24(13):2079–2083. [DOI] [PubMed] [Google Scholar]
- 51. Kiya K, Uozumi T, Ogasawara H et al. . Penetration of etoposide into human malignant brain tumors after intravenous and oral administration. Cancer Chemother Pharmacol. 1992;29(5):339–342. [DOI] [PubMed] [Google Scholar]
- 52. Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2014;14:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xie SS, Li M, Zhou CC, Song XL, Wang CH. Prophylactic cranial irradiation may impose a detrimental effect on overall survival of patients with nonsmall cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9(7):e103431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pui CH, Campana D, Pei D et al. . Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. [DOI] [PubMed] [Google Scholar]
- 56. Conter V, Schrappe M, Aricó M et al. . Role of cranial radiotherapy for childhood T-cell acute lymphoblastic leukemia with high WBC count and good response to prednisone. Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Münster groups. J Clin Oncol. 1997;15(8):2786–2791. [DOI] [PubMed] [Google Scholar]
- 57. Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights into brain metastasis in patients with ALK+ lung cancer: is the brain truly a sanctuary?Cancer Metastasis Rev. 2015;34(4):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dempke WC, Edvardsen K, Lu S, Reinmuth N, Reck M, Inoue A. Brain metastases in NSCLC—are TKIs changing the treatment strategy?Anticancer Res. 2015;35(11):5797–5806. [PubMed] [Google Scholar]
- 59. Bendell JC, Domchek SM, Burstein HJ et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 60. Minniti G, Amelio D, Amichetti M et al. . Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97(3):377–381. [DOI] [PubMed] [Google Scholar]
- 61. Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brandes AA, Tosoni A, Franceschi E et al. . Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. [DOI] [PubMed] [Google Scholar]
- 63. Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15(22):7092–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goldwirt L, Beccaria K, Carpentier A et al. . Preclinical impact of bevacizumab on brain and tumor distribution of irinotecan and temozolomide. J Neurooncol. 2015;122(2):273–281. [DOI] [PubMed] [Google Scholar]
- 67. van Tellingen O, de Vries N, Buckle T, Boogerd W, Beijnen J. Inhibition of breast cancer resistance protein and P-glycoprotein enhances the brain penetration of temozolomide [abstract]. Cancer Research. 2008;68(9 suppl):3243.18451150 [Google Scholar]
- 68. Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG; New Approaches to Brain Tumor Therapy (NABTT) Consortium Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sane R, Wu SP, Zhang R, Gallo JM. The effect of ABCG2 and ABCC4 on the pharmacokinetics of methotrexate in the brain. Drug Metab Dispos. 2014;42(4):537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shinohara C, Matsumoto K, Kuriyama M et al. . Clinical pharmacokinetics of carboplatin and MCNU in malignant brain tumor and normal brain tissues. Gan To Kagaku Ryoho. 1994;21(8):1163–1168. [PubMed] [Google Scholar]
- 71. Whittle IR, Malcolm G, Jodrell DI, Reid M. Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br J Neurosurg. 1999;13(2):132–137. [DOI] [PubMed] [Google Scholar]
- 72. Gilbert MR, Kuhn J, Lamborn KR et al. . Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neurooncol. 2012;106(1):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raizer JJ, Abrey LE, Lassman AB et al. ; North American Brain Tumor Consortium A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the blood-brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Holdhoff M, Supko JG, Gallia GL et al. . Intratumoral concentrations of imatinib after oral administration in patients with glioblastoma multiforme. J Neurooncol. 2010;97(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huang L, Li X, Roberts J, Janosky B, Lin MH. Differential role of P-glycoprotein and breast cancer resistance protein in drug distribution into brain, CSF and peripheral nerve tissues in rats. Xenobiotica. 2015;45(6):547–555. [DOI] [PubMed] [Google Scholar]
- 77. Hofer S, Frei K. Gefitinib concentrations in human glioblastoma tissue. J Neurooncol. 2007;82(2):175–176. [DOI] [PubMed] [Google Scholar]
- 78. Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johansson M, Bergenheim AT, D’Argy R et al. . Distribution of estramustine in the BT4C rat glioma model. Cancer Chemother Pharmacol. 1998;41(4):317–325. [DOI] [PubMed] [Google Scholar]
- 80. Bergenheim AT, Gunnarsson PO, Edman K, von Schoultz E, Hariz MI, Henriksson R. Uptake and retention of estramustine and the presence of estramustine binding protein in malignant brain tumours in humans. Br J Cancer. 1993;67(2):358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boogerd W, Tjahja IS, van de Sandt MM, Beijnen JH. Penetration of idarubicin into malignant brain tumor tissue. J Neurooncol. 1999;44(1):65–69. [DOI] [PubMed] [Google Scholar]
- 82. Weiss M, Kang W. P-glycoprotein inhibitors enhance saturable uptake of idarubicin in rat heart: pharmacokinetic/pharmacodynamic modeling. J Pharmacol Exp Ther. 2002;300(2):688–694. [DOI] [PubMed] [Google Scholar]
- 83. Whittle IR, MacPherson JS, Miller JD, Smyth JF. The disposition of TCNU (tauromustine) in human malignant glioma: pharmacokinetic studies and clinical implications. J Neurosurg. 1990;72(5):721–725. [DOI] [PubMed] [Google Scholar]
- 84. Albrecht KW, de Witt Hamer PC, Leenstra S et al. . High concentration of daunorubicin and daunorubicinol in human malignant astrocytomas after systemic administration of liposomal daunorubicin. J Neurooncol. 2001;53(3):267–271. [DOI] [PubMed] [Google Scholar]
- 85. Zucchetti M, Boiardi A, Silvani A, Parisi I, Piccolrovazzi S, D’Incalci M. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol. 1999;44(2):173–176. [DOI] [PubMed] [Google Scholar]
- 86. Green RM, Stewart DJ, Hugenholtz H, Richard MT, Thibault M, Montpetit V. Human central nervous system and plasma pharmacology of mitoxantrone. J Neurooncol. 1988;6(1):75–83. [DOI] [PubMed] [Google Scholar]
- 87. Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333(3):788–796. [DOI] [PubMed] [Google Scholar]
- 88. Heimans JJ, Vermorken JB, Wolbers JG et al. . Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5(10):951–953. [DOI] [PubMed] [Google Scholar]
- 89. Fine RL, Chen J, Balmaceda C et al. . Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12(19):5770–5776. [DOI] [PubMed] [Google Scholar]
- 90. Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O. The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother Pharmacol. 2004;53(2):173–178. [DOI] [PubMed] [Google Scholar]
- 91. Zucchetti M, Rossi C, Knerich R et al. . Concentrations of VP16 and VM26 in human brain tumors. Ann Oncol. 1991;2(1):63–66. [DOI] [PubMed] [Google Scholar]
- 92. Stewart DJ, Richard MT, Hugenholtz H et al. . Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neurooncol. 1984;2(2):133–139. [DOI] [PubMed] [Google Scholar]
- 93. Helms HC, Hersom M, Kuhlmann LB, Badolo L, Nielsen CU, Brodin B. An electrically tight in vitro blood-brain barrier model displays net brain-to-blood efflux of substrates for the ABC transporters, P-gp, Bcrp and Mrp-1. AAPS J. 2014;16(5):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Allen JD, Van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH. Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein. Cancer Res. 2003;63(6):1339–1344. [PubMed] [Google Scholar]
- 95. van Tellingen O, Boogerd W, Nooijen WJ, Beijnen JH. The vascular compartment hampers accurate determination of teniposide penetration into brain tumor tissue. Cancer Chemother Pharmacol. 1997;40(4):330–334. [DOI] [PubMed] [Google Scholar]
- 96. Stewart DJ, Richard MT, Hugenholtz H et al. . Penetration of teniposide (VM-26) into human intracerebral tumors. Preliminary observations on the effect of tumor type, rate of drug infusion and prior treatment with amphotericin B or oral glycerol. J Neurooncol. 1984;2(4):315–324. [DOI] [PubMed] [Google Scholar]
- 97. Kuhn JG, Chang SM, Wen PY et al. ; North American Brain Tumor Consortium and the National Cancer Institute Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res. 2007;13(24):7401–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Minocha M, Khurana V, Qin B, Pal D, Mitra AK. Co-administration strategy to enhance brain accumulation of vandetanib by modulating P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp1/Abcg2) mediated efflux with m-TOR inhibitors. Int J Pharm. 2012;434(1–2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]