Abstract
Management of glioblastoma in the elderly population is challenging. In the near future, more than half of patients with this tumor will be over the age of 65. Clinicians have been historically reluctant to treat such patients with the same intensity as younger patients. Due to upper age limits or poor accrual of elderly patients in clinical trials, randomized data for this patient population have been relatively sparse until recently. In this review, we will discuss the concept of an elderly patient population, describe evidence for molecular differences in glioblastoma of elderly versus young patients, evaluate recent first-line trials studying glioblastoma in elderly patients, and discuss best therapeutic practices including the value of molecular testing.
Keywords: elderly, frailty, glioblastoma, IDH, MGMT, radiotherapy and temozolomide, resilience, surgery
Managing elderly patients is conceptually and clinically challenging. While “elderly” patients are usually defined according to a specific age threshold, this threshold is increasing in Western societies and can vary with geographical, social, and cultural factors. Only recently has it become routine to factor age and potentially associated changes in physiology, pharmacokinetics, the impact of polypharmacy, and evolving values in patients’ perspectives into treatment decisions.1 Standard treatment approaches are clearly influenced by age, and the generally dismal prognosis for elderly patients may result from their less intense treatment.2 Clinical trials in glioblastoma have traditionally used upper age limits and thereby excluded elderly patients, which has limited progress for decades. Recently, trials conducted by the Scandinavian Neuro Oncology Network, the Neurooncology Working Group of the German Cancer Society (NOA), as well as the Canadian Cancer Trials Group (CCTG) and the European Organisation for Research and Treatment of Cancer (EORTC) have provided randomized data for the treatment of elderly glioblastoma patients. These trials support maximal safe surgery in elderly patients, provide evidence that hypofractionated radiotherapy over 3 weeks is equivalent to longer treatment,3 and most recently, demonstrate that chemoradiation with temozolomide is superior to radiotherapy alone.4 This last study establishes a new paradigm for the treatment of patients older than 65 years with Karnofsky performance status (KPS) >70. Furthermore, molecular testing has revealed a low prevalence of favorable prognostic markers in elderly patients.5 The virtual absence of isocitrate dehydrogenase (IDH) mutations in patients over the age of 65, according to the new World Health Organization (WHO) classification, suggests that differential prognosis is not based solely on age but rather on the fact that separate entities exist with a distinct age distribution.6 In contrast, the distribution of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation is similar to that in younger patients. The potential use of MGMT as a predictive biomarker is suggested by the most recent trial data.4,7 In contrast to the newly diagnosed setting, there are no controlled trials focusing on elderly patients at recurrence. Here, the data need to be extrapolated from the general population and adapted according to the first-line management. This review provides a critical evaluation of the evidence for the treatment of glioblastoma in elderly patients with management recommendations and a synopsis on the use of molecular markers in elderly patients.
An Elderly Population in Neuro-Oncology
The Central Brain Tumor Registry of the United States (CBTRUS) statistical report comprises data on patients with 112458 malignant primary brain and central nervous system tumors between 2006 and 2010. Glioblastomas comprise 45.2%8 of these tumors and patients have a median age at diagnosis of 64. The average age-adjusted incidence rate per year is 3.19 (3.16–3.21) per 100000 with a peak incidence of 14.93/100000 between 75 and 84 years. The incidence of glioblastoma therefore increases with age and shows the highest incidence in the 75- to 84-year-old age group.8 As with other neoplasms, this age-related increase in glioblastoma incidence has been attributed to immunological deficits, but DNA repair deficits may also be contributory. These factors together with an aging population in most countries account for a larger number of diagnoses of glioblastoma. Importantly, survival markedly decreases with age. The probability of surviving 12 months with a glioblastoma is 9.2% for patients ≥75 years compared with 40.7% for patients between 55 and 64 years of age.8 These data do not allow one to differentiate between the impact of age, tumor biology, and treatment on prognosis, as age is one of the factors clinicians may use when deciding on treatment intensity.9,10 A population-based evaluation of patterns of care in 4137 patients ≥65 years of age with glioblastoma revealed that comorbidities and age influence the probability of receiving a resection, radiotherapy, or chemotherapy.2
Although maximal safe surgery and radiotherapy have been standards in the treatment of glioblastoma for many years, the management of elderly patients has remained challenging, since treatment-related toxicities and slower recovery rates are more common. Consequently, survival times are short, with a median of less than 4 months in a population-based survey.2 For decades, these patients have been understudied and excluded from trials despite being the most prominent age group. This has now changed and it is deemed unethical to limit treatment based on age alone. With this in mind, we aim to discuss the most salient topics in the care of elderly patients. Of utmost importance is the clinical definition of these patients. Elements for this discussion are provided below.
Frailty Score
Frailty may be associated with increased rates of perioperative complications, including infections, difficulties in terminating ventilation, and prolonged recovery.11 It is not clear that old age alone is associated with such complications, and maximal safe resection should be considered in elderly glioblastoma patients.12 Postoperative management can incorporate symptom control with corticosteroids and antiseizure medication, as required. Early introduction of palliative care may have a role in many patients. Management should be based upon the fitness of the patient, performance status, and MGMT promoter methylation status.13 Frailty is commonly defined as unintentional weight loss (5 kg in the previous year), self-reported exhaustion, weakness of grip, slow walking speed, and low physical activity.14 Clinical frailty scales rely on energy levels and motivation as surrogates for age-related health status. Scales commonly focus on ambulatory abilities and need for assistance, thereby effectively building a better KPS.15 Others have developed scales based on the EORTC 30-item quality of life assessment scale (EORTC-QLQ-C30), and a cancer-specific comprehensive geriatric assessment. Whereas the EORTC-QLQ-C30 is an excellent tool to follow patients over time, it does not provide levels to decide on the fitness of a patient and at a given time point will be influenced by the disease (ie, less well to assess the past prior to a therapy decision).
Implementing age-specific methodology into clinical trials has been advocated by the EORTC.16 Critical elements that should be incorporated into trials include the Oncodage G8 questionnaire (Table 1), the Instrumental Activities of Daily Living (IDAL) questionnaire,18 the Charlson Comorbidity Index (CCI),19 and data about whether a patient lives independently, with support from others, or in an institution (Table 1). The IDAL questionnaire assesses ability and motivation to use the phone, go shopping, prepare food, do household work, use transportation, correctly and independently take medication, and manage the financial budget.18 The CCI employs a scoring system for the presence of various diseases and then prognosticates lethality according to age with and without particular comorbidities. Deficiencies of the CCI include limited abilities to differentiate between diseases and between disease severity. Future studies should implement these assessments to better define the target population and answer specific questions within this population.
Table 1.
Oncodage screening geriatric eight questions (G8)17
| Items | Possible Answers | |
|---|---|---|
| A | Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0: severe loss of appetite 1: moderate loss of appetite 2: normal appetite |
| B | Weight loss during the past 3 months? | 0: weight loss > 3 kg 1: does not know 2: weight loss between 1–3 kg 3: no weight loss |
| C | Mobility? | 0: bed or chair bound 1: able to get out of bed chair, but does not go out 2: goes out |
| E | Neuropsychological problems? | 0: severe dementia or depression 1: mild dementia or depression 2: no psychological problems |
| F | Body mass index (BMI) (weight in kg)/(height in m2)? | 0: BMI < 19 1: 19 ≥ BMI < 21 2: 21 ≥ BMI < 23 3: BMI ≥ 23 |
| H | Takes more than 3 medications per day? | 0: yes 1: no |
| P | In comparison with other people of the same age, how does the patient consider her/his health status? | 0: not so good 1: does not know 2: as good 3: better |
| Age? | 0: >85 y 1: 80–85 y 2: <80 y |
The scales summarized contain age as one element, but not as a full surrogate for being elderly. An elderly patient population could be defined by a lower age corridor of 65–70 years, an upper KPS of 80, and additional elements of frailty, for example, in the CCI. Most trials conducted so far focused mainly on age. A challenge will be to use the proposed treatments, mainly longer or shorter chemoradiation (6 or 12 cycles maintenance temozolomide), in the appropriate population, although key elements of “young” and “elderly” have not been part of the inclusion criteria.
Molecular Specifics of Glioblastoma in Elderly Patients
Older patients with glioblastoma were viewed not to benefit to the same extent from the addition of temozolomide to the standard of care.20 The prognosis of elderly patients even considering comorbidities is worse compared with that of younger patients.21 With the emergence of precision medicine, molecular diagnostics may be utilized to explain these differences and to define a specific profile for glioblastoma in elderly patients. Already some years ago, biomarkers such as TP53 mutation, 1p deletion, cyclin dependent kinase 2 alpha (CDKN2A)/p16 deletion, and epidermal growth factor receptor (EGFR) amplification had been described to be associated with age. TP53 mutations and EGFR amplification had a differential prognostic value when samples were stratified for age. TP53 mutation was a positive prognostic marker in patients ≤70 years and a negative marker in patients >70 years, whereas EGFR amplification was a negative prognostic marker in patients ≤70 years and a positive marker in older patients.22 Also microenvironmental factors like vascular endothelial growth factor (VEGF) expression have been correlated with age.23 Older patients appear to have higher VEGF expression and therefore may respond better to VEGF inhibition.23 Along these lines, some have proposed that edema is worse in elderly patients. However, an MRI-based analysis did not support age at diagnosis as a determinant of peritumoral edema. Rather, tumor localization in the white matter and a larger area of necrosis were associated with greater extent of edema. This study concluded that glioblastomas in elderly patients do not have a distinct radiographic appearance at diagnosis.24
Positive prognostic biomarkers, like mutations of IDH, are virtually absent in glioblastoma of elderly patients.5 Lower-grade IDH wildtype diffuse astrocytomas particularly in elderly patients can follow a more aggressive course, resembling glioblastoma.6,13
Increased age is associated with a decrease in general methylation levels in the brain.25 Consistent with this, the general methylation levels in glioblastomas are low. Despite this and despite earlier reports that elderly patients tend to have MGMT promoter hypermethylation,26 recent studies reveal that the frequency of MGMT promoter methylation does not in fact vary with age.5 Gene expression and methylation analyses have been used to categorize glioblastoma into subtypes. Verhaak et al proposed proneural, neural, classical, and mesenchymal subtypes based on expression and genomic abnormalities.27 On the basis of an earlier expression classification, Lee et al suggested that the prognostic effect of age may be a reflection of less favorable subtypes occurring in older patients.28 The proneural, IDH-mutated subtype occurs more often in younger patients and is associated with longer survival.28 Moreover, analyses of methylation patterns29,30 and other integrated data30 propose that age correlates with distinct glioblastoma clusters.
An evaluation focusing on patients with an overall survival (OS) of >36 months (compared with patients dying a tumor-related death <12 mo) did not provide evidence that any distinct gene expression profile (proneural, classical, or mesenchymal) was associated with outcome. Rather, patients with long-term survival were younger and more often had tumors with IDH1/2 mutation and MGMT promoter methylation.31
Update on Randomized Trials—10 Years of Progress
It has taken more than a decade to confirm that combined chemoradiation with temozolomide is as effective in elderly patients as in young patients. With similar paradigms now being used for essentially all patient populations with diffuse gliomas, it is now a perfect time to implement new concepts for elderly patients.
Despite the common use of radiotherapy, chemotherapy, and the combination across all age groups, it was only in 2007 that a French trial provided evidence for postsurgical radiotherapy being effective in elderly patients. A regimen of 50 Gy in 1.8-Gy fractions was superior to best supportive care in patients 70 years or older with good KPS32 (Table 2). Due to the considerable time burden of radiation and the impression of a better ratio of treatment to survival time, patients with unfavorable prognostic factors defined by age or performance status are commonly treated with hypofractionated radiotherapy (eg, 40.05 Gy in 15 fractions).3 In elderly patients, this is the standard of care for patients with tumors lacking MGMT promoter methylation.33,34 Further hypofractionation to 5 × 5 Gy may be feasible without compromising survival35 but may not be as well tolerated in terms of neurocognitive function, which will assume more relevance once other treatment options allow longer survival in elderly patients, although the study provided no decline in health-related quality of life (HRQoL) between this regimen and the 40.05 Gy.35
Table 2.
Randomized clinical trials for elderly glioblastoma patients
| Trial | Surgery12 | ANOCEF32 | Canadian Phase II3 | NOA-0834 | Nordic33 | CCTG CE.6/EORTC 260624 |
|---|---|---|---|---|---|---|
| Question | Biopsy vs resection | BSC vs RT plus BSC | RT in 60 Gy vs hypofractionated RT | RT in 60 Gy vs TMZ | RT in 60 Gy vs hypofractionated RT vs TMZ | Hypofractionated RT vs hypofractionated RT plus TMZ |
| Groups, n | 16 | 42 | 47 | 178 | 100 | 281 |
| 14 | 39 | 48 | 195 | 98 | 281 | |
| 93 | ||||||
| Age, y | 72 (67–79) | 73 (70–85) | Mean 72.4 (SD 5.4) | 71 (66–82) | 70 y (60–80) | 73 y (range 65–90) |
| 70 (66 – 80) | 75 (70–84) | Mean 71 (SD 5.5) | 72 (66–84) | 70 (60–83) | ||
| 70 (60–88) | ||||||
| Treatment | Stereotactic biopsy | BSC | 60 Gy in 2 Gy fractions over 6 wk | 60 Gy in 1.8–2 Gy fractions | 60 Gy in 2 Gy fractions over 6 wk | 40 Gy in 2.67 Gy fractions over 3 wk |
| Open craniotomy/ resection | 50 Gy in 28 fractions with BSC | 40 Gy in 2.67 Gy fractions over 3 wk | TMZ 100 mg/m2 given on days 1–7 of 1 wk every 14 days until progression | 34 Gy in 3.4 Gy fractions over 2 wk | 40 Gy in 2.67 Gy fractions over 3 wk plus 75 mg/m2 TMZ plus 12 cycles TMZ | |
| TMZ 200 mg/m2 on days 1–5 of every 28 days for up to 6 cycles | ||||||
| Outcome (OS), mo | 2.8 (95% CI, 1.8–5.1) | 3.8 (95% CI, 3–4.8) | 5.1 | 9.6 (8.2–10.8) | 6.0 (95% CI 5.1–6.8) | 7.6 |
| 5.6 (95% CI, 2.4–9.1) (P = 0.035) |
6.5 (95% CI, 5.6–7.8) HR 0.47 (95% CI, 0.29–0.76; P = 0.002) |
5.6 (HR 0.89; 95% CI, 0.59–1.36; P = 0.57) |
8.6 (95% CI 7.3–10.2) (HR 1·09, 95% CI 0·84–1·42, p non inferiority = 0·033) |
7.5 (95% CI 6.5–8.6) 8.3 (95% CI 7.1–9.5) |
9.3 (HR 0.67, 95% CI, 0.56–0.80, P < 0.001) |
|
| MGMT | Predictive for TMZ | Predictive for TMZ | Not clear; benefit of TMZ also in the unmeth. tumors |
Abbreviations: RT, radiotherapy; TMZ, temozolomide; BSC, best supportive care.
In addition to the concerns regarding adverse effects, radiation oncologists have some reservations against the use of hypofractionated regimens with nominally but also biologically lower doses. The commonly used elderly regimen provides a higher daily fraction but lower total exposure, resulting in an equivalent dose of about 50 Gy. Despite this principle, the trial data from both the Canadian phase II trial3 as well as the comparisons between the 2 radiotherapy groups from the Nordic trial,33 2 Gy × 30 fractions versus 3.4 Gy × 10 fractions, revealed the hypofractionated regimen to be more effective (Table 2). However, comparisons in the Nordic trial were limited, especially since it had 3 arms. In this trial of patients ≥60 years of age, subjects were randomized to 2 different radiotherapy regimens versus temozolomide alone (200 mg/m2 days 1–5 every 28 days for 6 cycles). Standard radiotherapy yielded inferior results, and temozolomide appeared to be superior in patients >70 years of age. MGMT promoter methylation was associated with better OS in temozolomide-treated patients (9.7 vs 6.8 mo, 95% CI: 8.0–11.4 vs 5.9–7.7; hazard ratio [HR] 0.56; 95% CI: 0.34–0.93, P = 0.03), but not in radiation-treated patients (8.2 vs 7.0 mo, 95% CI: 6.6–9.9 vs 5.7–8.3; HR 0.97; 0.69–1.38, P = 0.88).
The NOA-08 trial of the Neurooncology Working Group of the NOA randomized patients with glioblastoma (n = 331) and anaplastic astrocytoma (n = 40) ≥66 years of age to radiotherapy (30 × 2 Gy) or temozolomide (dose-intensified one week on/one week off).36 Median progression-free survival (PFS) and OS were similar in both arms. Patients with MGMT promoter-methylated tumors had longer PFS when treated with temozolomide compared with radiotherapy of 8.4 versus 4.6 months (P < 0.0001). In contrast, patients with tumors without MGMT promoter methylation had longer PFS when treated with radiotherapy rather than temozolomide of 4.6 versus 3.3 months (P < 0.01). Overall survival showed a similar trend—however, it was diluted by “cross-over” in approximately 50% of the patients.34
Based on this series of trials, the standard of care differentiated postoperative therapy according to MGMT status. Patients without hypermethylation of the MGMT promoter were commonly treated with radiotherapy alone. Many sites also adopted the hypofractionated 3-weekly course of radiotherapy. The standard for patients above the age of 65–70 years with hypermethylated MGMT promoter was less clear. On the one hand, the Nordic and NOA-08 trials provided evidence for the efficacy of temozolomide alone; however, comparative data between chemoradiation with temozolomide and temozolomide alone were lacking and remain absent today. The centers used temozolomide alone or standard chemoradiation according to the EORTC 26981 (CCTG CE.3) trial. However, the latter study restricted enrollment to patients ≤70 years and used a regimen of 60 Gy radiotherapy in 2-Gy fractions. Exploratory subgroup analyses of this trial suggested a diminishing impact from the addition of temozolomide to patients with increasing age, with the survival benefit for temozolomide losing statistical significance for patients aged 65–70 (HR = 0.78 [0.50–1.24], P = 0.29).20,37 The uncertainty surrounding the value of temozolomide for elderly patients generated by the age-based analysis of EORTC 26981/CCTG CE.3 coupled with the knowledge that elderly glioblastoma patients often receive abbreviated courses of radiotherapy and that chemoradiotherapy poses a greater burden in this elderly patient group served as the backdrop for the CCTG CE.6/EORTC 26062 trial. In order to show superiority of temozolomide added to an abbreviated course of radiotherapy (15 × 2.67 Gy to 40.05 Gy) with up to 12 cycles maintenance temozolomide versus radiotherapy alone, 281 patients were randomized to each arm. The median age was 73 years (range, 65–90). Combined chemoradiation improved OS compared with radiotherapy alone (median 9.3 mo vs 7.6 mo, providing an HR of 0.67, 95% CI: 0.56–0.80, P < 0.0001). Combined chemoradiation also improved PFS as documented by a median of 5.3 months with chemoradiotherapy versus 3.9 months with radiotherapy alone. The risk reduction was 50% as documented by an HR of 0.50 with 95% CI of 0.41–0.60. As in the previous trials, patients with glioblastoma harboring a hypermethylated MGMT promoter (n = 165) benefited the most from the addition of alkylating chemotherapy. The median OS for chemoradiation in MGMT hypermethylated patients was 13.5 months compared with 7.7 months for the radiotherapy cohort (HR = 0.53, 95% CI: 0.38–0.73, P = 0.0001). Unexpectedly, for patients with MGMT unmethylated tumors (n = 189) there was also a strong trend toward benefit from temozolomide. The HR was 0.75 (95% CI: 0.56–1.01, P = 0.055) for chemoradiotherapy versus radiotherapy, with median OS of 10.0 versus 7.9 months. Similar to NOA-08,34 which employed the same frailty instrument, quality-of-life assessments did not reveal any relevant differences in both arms, indicating that the combination did not adversely impact daily living and that radiotherapy, although less efficacious, provides the same quality of life, though over a shorter period. This practice-influencing trial demonstrates that the addition of temozolomide to short-course (hypofractionated) radiotherapy improves survival for elderly patients with glioblastoma. However, patients with MGMT methylated tumors benefit the most, with a near doubling of median OS.4 Of note, median OS in the best-performing group of NOA-08, patients with MGMT methylated tumors treated with temozolomide, had not been reached at the initial report and may well be in a similar range.34Table 2 provides an overview of the most important randomized trials over the past 15 years. Cross-trial comparisons are generally limited but may be even more so in these trials, since concepts for eligibility but also the philosophy of post-progression treatment (NOA-08 vs Nordic) strongly varied. In the next section, we will provide an interpretation of the impact of these and other data on best practices for diagnosis, surgery, radiotherapy, and chemotherapy, as well as the limitations, a provisional treatment algorithm (Fig. 1), and future perspectives.
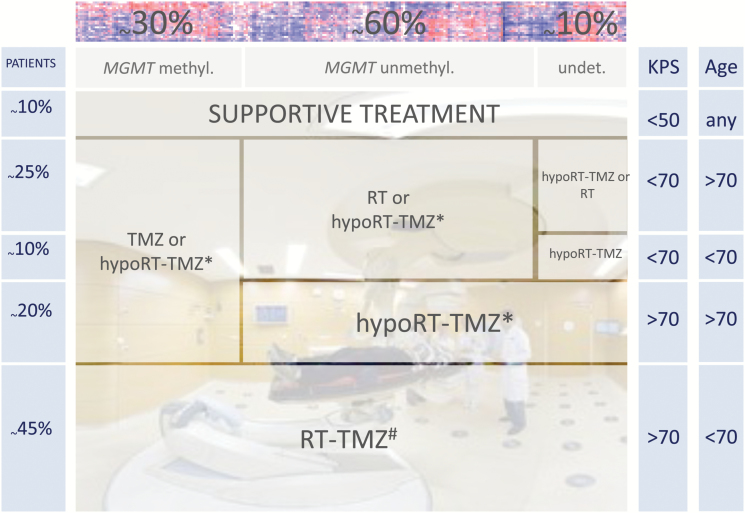
Fig. 1.
Compilation of therapy options and recommendations for patients with glioblastoma in different age groups according to functional status (KPS). Prevalence (according to CBTRUS8) is depicted by the size of the boxes. Regimens are depicted hypofractionated radiotherapy (RT)–temozolomide (TMZ) according to Perry et al4 or RT-TMZ according to Stupp et al.20
Best Therapeutic Practice
Diagnosis
The updated WHO classification provides a clear definition of glioblastoma irrespective of age.6 Beneficial prognostic markers are absent in glioblastomas of elderly patients, but this has not impacted therapy.5 The absence of IDH mutations is a general feature of a bona fide glioblastoma that has not progressed from a lower-grade lesion. Absence of IDH mutation appears to be more prevalent in the elderly population.
We currently are unable to use trial results directly for decision making in our individual patients. This is in part trivial and the same in all trials, but is more pronounced in the elderly population, as in trials we manage to exclude several factors that are more prevalent with age, comorbidities, comedications, different social situations, and different levels of independence. To determine optimal approaches, KPS and a simple frailty tool (Table 1) should be assessed, and chronological age alone should not be used for categorizing patients as elderly or not. Importantly, age-specific burdens should be inquired about. Imaging is done by MRI, and currently we do not have indications for specific protocols in elderly patients, as long as kidney function is preserved, which may be hampered by the contrast agents. Presence of an implanted device may preclude MRI and mandate imaging with cranial CT or PET.
Surgery
Resection is superior to biopsy at least in selected patients.12 Although criticized for KPS bias in the patient groups and the limited group size as well as limitations of adjuvant options to radiotherapy, the Vuorinen trial is unique in its attempt to provide randomized information and indication of a benefit for a more radical surgical approach. The data are supported by larger though nonrandomized series. Resection showed a longer median OS than biopsy (5.7 vs 4.0 mo, P = 0.02) in a case-control study involving 2 × 40 patients matched for age, KPS, tumor localization, and subsequent therapy.38
In the CCTG CE.6/EORTC 26062 trial,4 biopsy-only patients had significantly shorter survival than those with tumor resection (HR = 1.67, 95% CI: 1.38–2.02, P < 0.0001), while higher Mini-Mental State Examination score predicted longer survival (HR = 0.96, 95% CI: 0.94–0.98, P < 0.0001).4 Previously, the Nordic and NOA-08 trials also showed that surgical resection is superior to biopsy alone, and hence is a relevant prognostic parameter (NOA-08: risk of biopsy vs resection, 1.84 [1.44–2.35]).34
Despite the general wish for more randomized data, current approaches include the assessment of frailty in the preoperative assessment for outcome of surgery in brain tumor patients. The study specifically aims at determining the predictive impact for the occurrence of neurosurgical complications (NCT02530749).
At present, the totality of all data clearly indicates that maximal safe resection is as important in elderly patients as it is in young patients.
In all patients, but with a higher frequency in the elderly population, there are situations where the clinical situation (KPS or comorbidities) does not allow further tumor-specific measures. Here, best supportive care as outlined below is indicated. In our view, this generally requires a histological diagnosis and should be done based on imaging alone only in very rare, well-discussed exemptions (Fig. 1).
Molecular diagnostics
With the exception of IDH and MGMT31 status, molecular markers do not have a defined role in clinical practice and are considered investigational. Testing of IDH is necessary to comply with the recent update of the WHO classification of central nervous system tumors.6 The presence of an IDH mutation in a glioblastoma most likely defines a separate disease entity. In our view, MGMT testing just for the sake of completeness is not supported; it is a relevant molecular biomarker and should be done to facilitate therapy decisions. Such judgment may include withholding alkylating chemotherapy, adjusting the duration of alkylating treatment in a patient with impaired tolerance, and participating in trials or alternative therapies.
Radiotherapy
For elderly patients with a poor prognosis due to comorbidities or frailty, careful evaluation of postsurgical treatment options is necessary. This evaluation should seek to maximize the value of care rather than reduce treatment based on elderly age alone. In support of this, the ANOCEF trial showed that radiotherapy improved OS relative to best supportive care. Importantly, radiotherapy had no detrimental effects on the quality of life or cognitive function in this trial.32 For patients >70 years of age, conventional radiotherapy (60 Gy over 6 wk) may be less appropriate than hypofractionated schedules, though there may be a minimum number of fractions one should use. Reasons to pursue abbreviated treatment include less frequent visits to the clinic and less time spent on treatment versus OS. The main reasons for conventional radiotherapy frequently discussed are (i) lower long-term toxicity based on the fraction size with the risk of increasing the biologically effective dose to normal tissue to a proportionally greater extent and (ii) the alleviation of concerns about tumor undertreatment both for the radiation as well as for the chemotherapy part with hypofractionation. Assuming an α/β ratio of 2.3 for glioblastoma, the biologically equivalent dose of the most common hypofractionated regimens (40 Gy in 15 fractions, 34 Gy in 10 fractions) is only in the range of 45 Gy in 2-Gy fractions. It remains to be seen whether the shorter temozolomide duration is relevant in a patient population other than that in the initial phase II trial, where median survival was quite low, suggesting a poor prognosis group. In addition to the lower radiation dose, hypofractionated chemoradiation regimens have shorter temozolomide courses. It would be interesting to understand whether this missing temozolomide dose may account for worse overall outcomes of elderly patients in the current CCTG CE.6/EORTC 26062 trial versus other trials using 6-week courses of chemoradiation.
Chemotherapy
In addition to the controlled data from the Nordic and the NOA-08 trials, data from an uncontrolled French trial with temozolomide alone at 150–200 mg/m2 5/28 days in frail patients >70 years with KPS <70 support the assumption that temozolomide is generally safe (grade 3 and 4 neutropenia and thrombocytopenia in 13% and 14%, respectively). In this trial, temozolomide was also effective, with median PFS of 16 weeks and median OS of 25 weeks in patients who have been excluded from most chemotherapy trials to date. Furthermore, 23/70 patients had a functional improvement determined by KPS, quality of life, and cognitive function. This result demonstrates that effective treatment can be achieved without significant functional loss. In essence, temozolomide seems tolerable in elderly patients with low KPS and should not be withheld, especially from patients with hypermethylated MGMT promoter.39
Chemoradiotherapy
In patients with good (>70) KPS and age >65–70 years, combined chemoradiation is recommended for patients with hypermethylated MGMT promoter. However, data on temozolomide monotherapy versus chemoradiation are lacking, making temozolomide monotherapy a reasonable option when KPS or patient preference suggests that combined treatment may be too exhausting. Further research on patients with unmethylated MGMT is needed, as data in the most recent CCTG CE.6/EORTC 26062 trial4 show that temozolomide is beneficial in these patients as well as in MGMT-methylated patients. Based on the initial temozolomide trial,20 we have good data that temozolomide is beneficial for patients <70 years with good KPS. Avoidance of the MGMT test seems more related to the lack of alternatives than to trust in the temozolomide effect in the patients with tumors harboring an unmethylated MGMT promoter. With the most recent randomized data from CCTG CE.6/EORTC 26062 in patients with age >70 and good KPS, the standard seems well defined as hypofractionated chemoradiation. However, the conclusions are in part built on shifting sand. The lack of comparison to temozolomide alone and to the standard 60 Gy chemoradiotherapy plus the high efficacy of temozolomide alone in NOA-08 in the patients with MGMT methylated tumors make these options valid as well (Fig. 1) and an interesting aspect for a future comparison.
In the future, more comprehensive geriatric workup, MRI with sophisticated radiomics,40 and biological characterization may optimize treatment planning. Besides clinical trials, it will be critical to get a sense of what the recent study data mean in real world practice. In addition to pure efficacy considerations, it will be important to maintain quality of life, preserve functional independence, and minimize therapy-related side effects.
Practical Aspects and Symptomatic Management
Brain tumors may cause a spectrum of symptoms, including fatigue, depression, nausea, seizures, headaches, and focal deficits, many of which result from elevated intracranial pressure. Practical aspects for management may even be more important for elderly patients as premorbid resources, such as mobility, communication, memory, and other intellectual functions, existence of a social network helping to make decisions and interfering with the process, but also allowing for more advanced or time-consuming treatments, may be more limited. In elderly patients, the brain tumor diagnosis may more likely trigger considerations on the ability to maintain an independent household. Resilience may benefit from older age, as lifelong experiences may help us to return from a very stressful situation. As resilience depends on social networks, intellectual capacities, and openness to new projects, the age-related deficits inhibit this adaptation. Although good evidence for the value of rehabilitation exists,41 we may be more active in convincing families and caregivers of the value of maintaining or regaining mobility, communication, swallowing, etc, functions to enhance independence and HRQoL.
With pretumor physical impairments or cognitive deficits as well as other relevant diseases or polypharmacy, the symptom management for elderly patients is not distinct, but potentially more challenging than for younger patients.
This review touches only specific aspects and refers to excellent summaries on practical aspects.42
Increased intracranial pressure resulting from edema can be controlled with corticosteroids. Their benefit must be balanced over time with potential side effects such as proximal myopathy, weight gain, opportunistic infections, steroid-induced diabetes with a higher prevalence in elderly patients,43 and osteoporotic fractures, also more likely, which can be debilitating.44 Furthermore, steroids may reduce the benefit from temozolomide in the most responsive MGMT promoter methylated subgroup45 and potentially even more generally.46 As mentioned above, rehabilitation and long-lasting physical as well as occupational therapies may be key to allow maintaining or regaining independence and ensure the optimal quality of living. Although data exist to show the effects of inpatient rehabilitation not to differ between brain tumor and other neurological patients, the efforts for rehabilitation seem to be less in this group. Randomized data are being generated in an ongoing trial.47,48 Costs are not prohibitive compared with neurological diseases like stroke or traumatic brain injury.49
Seizures may occur as well as cognitive, motor, or sensory deficits in a location-dependent manner. Anticonvulsants are therefore often warranted. However, as in the younger population, there is no indication for primary prophylaxis. Especially in elderly patients, dose restrictions must be considered in patients with renal deficits and in patients with a tendency toward aggressive behavior on levetiracetam or in patients with accentuated cognitive deficits on some of the older anticonvulsants. Restrictions in the ability to drive are the same as in the younger population—however, use of public transportation or social networks allowing for the same level of mobility and access to therapies and supportive measures are more difficult to realize in elderly patients.50
Patients with glioblastoma are among the most highly at-risk individuals for thrombosis and its complications in all medical and surgical practice.51 The incidence is variable. Prospective data suggest 0.015 cases per month (18% annually) despite perioperative heparin thromboprophylaxis.52 In addition to the risk conferred by the tumor, paresis is a risk factor, whereas age is not. Current practice suggests no prophylactic anticoagulation, but management analogous to the non–brain tumor patient.
Randomized data for palliative care in the glioblastoma setting are lacking. In a randomized study in lung cancer patients, however, the addition of palliative care improved quality of life and increased OS.53 Based on this and similar studies, we recommend the early incorporation of palliative care support.
Future Development
Glioblastoma is a particularly angiogenic tumor, especially in elderly patients. VEGF is a key molecule driving angiogenesis in glioblastoma, and VEGF inhibitors such as bevacizumab (BEV) have been explored in the multimodality treatment of glioblastoma54–56 and at progression.57 These trials did not improve OS but did prolong PFS, which may be explained in part by the drug’s ability to reduce edema, but can nevertheless improve quality of life in some patients. Certain findings suggest that elderly patients could particularly benefit from anti-VEGF strategies: OS in elderly patients is decreased compared with OS in younger patients. Elderly patients receive less salvage therapy at recurrence than younger patients.2 In recurrent glioblastoma patients, BEV has shown promising activity in particular in elderly patients, and data from the randomized trial of BEV in patients with newly diagnosed glioblastoma (AVAGlio) indicate a significant increase in PFS.58 Only a minority of patients enrolled in AVAGlio qualify as elderly (8%, 73/921 patients ≥70 y). However, there is a trend, demonstrated by a superior HR, for the prolongation of PFS by the addition of BEV particularly in patients with glioblastomas without MGMT promoter methylation. However, comorbidities in elderly patients make it very likely that the triple combination of radiation, temozolomide, and anti-angiogenic agents will be tolerated less well in this population. Data for BEV in elderly patients are provided by the Avastin plus Radiotherapy in Elderly Patients with Glioblastoma (ARTE) trial (NCT01443676) at the World Federation of Neuro-Oncology Societies (WFNOS) meeting, failing to show a survival impact of BEV when combined with hypofractionated radiotherapy at 40.05 Gy. This small but randomized phase II trial included radiotherapy alone as the standard arm and was amended to patients with glioblastoma without hypermethylation of the MGMT promoter.58 In summary, these observations may justify further studies on the role of anti-angiogenic treatments in newly diagnosed glioblastoma of elderly patients, potentially with a stratification based on MGMT promoter methylation status.
Additionally, further research on tumor treating fields59 is needed in elderly glioblastoma patients. Differences in outcome may occur if the tumor treating field is used following hypofractionated chemoradiation, and study of this may be beneficial as well.60
Whether elderly patients should be included in immunotherapy or precision medicine trials should be decided by clinical, social, and personal factors, and not by age. Age leading to a reduction in fitness and activity should be a reason to initiate more careful assessments and support measures; chronological age per se should not be used to exclude patients from trials. Innovative therapies may emerge from the recent discovery that transmembrane nanotubes (TMs) connect glioblastoma cells and are intimately involved in proliferation and therapeutic resistance.61 These TMs are more prevalent and extensive in 1p/19q non-codeleted astrocytic tumors and tend to be more prevalent with higher WHO grade. They may be relevant in the elderly population, although conclusive proof is missing, as TMs cannot be detected well without IDH co-staining and are thus far confined to these tumors for assessment. TMs may provide an Achilles heel for therapeutic intervention (Table 3).62
Table 3.
Transmembrane nanotube (TM) length increases with dismal prognosis*61
| WHO Grade | WHO Grade II Astrocytoma | WHO Grade III Astrocytoma |
WHO Grade IV
Astrocytoma (Glioblastoma) |
|---|---|---|---|
| <50 µm [%] | 10–20 | <10 | <10 |
| 50–100 µm [%] | >50 | >50 | 30–40 |
| >100 µm [%] | 20–30 | 30–40 | >50 |
*Glioma thick sections stained positive for the IDH1 R132H protein have been analyzed for the prevalence of TM in different length. Parallel experiments had revealed that TM length is correlated with therapy resistance.
Conclusions
Treatment principles should be the same for both elderly and young glioblastoma patients. We should be focused on a maximal safe benefit from every intervention. Specific points for elderly patients are the need for a more detailed assessment of resources and function prior to each intervention.
Molecular information helps us to understand the lower frequency of long-term benefit in these patients, as positive prognostic markers are virtually absent. However, we do have a strong predictive marker, MGMT promoter methylation. Its relevance is challenged by the most recent data from the CCTG/EORTC trials, but the totality of data still speaks for a strong impact of MGMT, on which we should build future concepts. Therefore, we are still convinced that management should be based upon the fitness of the patient, performance status, and MGMT promoter methylation status. This approach is incorporated into the current European Association of Neuro-Oncology guidelines.
Beyond molecular parameters, it will be interesting to see how our perception of therapeutic limitations as opposed to lesser benefit impacts outcome in elderly patients.
There is a need to establish cohorts of elderly patients in our trials or do specific trials for elderly patients. Elderly and frail patients may even help us to reconsider approaches in younger patients, which often focus too much on efficacy parameters like PFS or OS rather than functionality and quality of life.
Funding
No funding was received relating to preparation of this manuscript.
Conflict of interest statement. T.T.B. reports consulting for Merck (compensated), Roche (compensated), Amgen (compensated), and Proximagen (compensated). W.W. reports consulting and membership on a trial steering committee for Roche (compensated); consulting for BMS (compensated); consulting and membership on a steering committee for Apogenix (uncompensated); lecture fees from Prime Oncology; and research funding from Boehringer Ingelheim, Eli Lilly, MSD, Pfizer, and Roche.
References
- 1. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. [DOI] [PubMed] [Google Scholar]
- 2. Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 3. Roa W, Brasher PM, Bauman G et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 4. Perry JR, Laperriere N, O’Callaghan CJ et al. ; Trial Investigators. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 5. Wiestler B, Claus R, Hartlieb SA et al. ; Neuro-oncology Working Group (NOA) of the German Cancer Society. Malignant astrocytomas of elderly patients lack favorable molecular markers: an analysis of the NOA-08 study collective. Neuro Oncol. 2013;15(8):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 7. Wick W, Weller M, van den Bent M et al. MGMT testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 8. Ostrom QT, Gittleman H, Farah P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gately L, Collins A, Murphy M, Dowling A. Age alone is not a predictor for survival in glioblastoma. J Neurooncol. 2016;129(3):479–485. [DOI] [PubMed] [Google Scholar]
- 10. Rusthoven CG, Koshy M, Sher DJ et al. Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era: a national cancer database analysis. JAMA Neurol. 2016;73(7):821–828. [DOI] [PubMed] [Google Scholar]
- 11. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien). 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 13. Weller M, van den Bent M, Tonn JC et al. ; for the European Association for Neuro-Oncology (EANO) Task Force on Gliomas. European Association for Neuro-Oncology guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 14. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 15. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pallis AG, Ring A, Fortpied C et al. ; European Organisation for Research and Treatment of Cancer Elderly Task Force. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol. 2011;22(8):1922–1926. [DOI] [PubMed] [Google Scholar]
- 17. Soubeyran P, Bellera C, Goyard J et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12):e115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 20. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 21. Scoccianti S, Magrini SM, Ricardi U et al. Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology). Neurosurgery. 2010;67(2):446–458. [DOI] [PubMed] [Google Scholar]
- 22. Batchelor TT, Betensky RA, Esposito JM et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228–233. [DOI] [PubMed] [Google Scholar]
- 23. Nghiemphu PL, Liu W, Lee Y et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seidel C, Dörner N, Osswald M et al. Does age matter?—A MRI study on peritumoral edema in newly diagnosed primary glioblastoma. BMC Cancer. 2011;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Christensen BC, Houseman EA, Marsit CJ et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5(8):e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73(18):1509–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verhaak RG, Hoadley KA, Purdom E et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee Y, Scheck AC, Cloughesy TF et al. Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genomics. 2008;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noushmehr H, Weisenberger DJ, Diefes K et al. ; Cancer Genome Atlas Research Network. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sturm D, Witt H, Hovestadt V et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 31. Reifenberger G, Hentschel B, Felsberg J et al. ; German Glioma Network. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–1350. [DOI] [PubMed] [Google Scholar]
- 32. Keime-Guibert F, Chinot O, Taillandier L et al. ; Association of French-Speaking Neuro-Oncologists. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 33. Malmström A, Grønberg BH, Marosi C et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 34. Wick W, Platten M, Meisner C et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 35. Roa W, Kepka L, Kumar N et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 36. Weller M, Tabatabai G, Kästner B et al. ; DIRECTOR Study Group. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 37. Laperriere N, Weller M, Stupp R et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–357. [DOI] [PubMed] [Google Scholar]
- 38. Chaichana KL, Garzon-Muvdi T, Parker S et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18(1):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gállego Pérez-Larraya J, Ducray F, Chinot O et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed] [Google Scholar]
- 40. Kickingereder P, Götz M, Muschelli J et al. Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res. 2016;22(23):5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geler-Kulcu D, Gulsen G, Buyukbaba E, Ozkan D. Functional recovery of patients with brain tumor or acute stroke after rehabilitation: a comparative study. J Clin Neurosci. 2009;16(1):74–78. [DOI] [PubMed] [Google Scholar]
- 42. Mason M, Laperriere N, Wick W et al. Glioblastoma in the elderly: making sense of the evidence. Neurooncol Pract. 2016;3(2):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Welch MR, Grommes C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013;2(3):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23(6):597–602. [DOI] [PubMed] [Google Scholar]
- 45. Weiler M, Blaes J, Pusch S et al. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci U S A. 2014;111(1):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pitter KL, Tamagno I, Alikhanyan K et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hansen A, Rosenbek Minet LK, Søgaard K, Jarden JO. The effect of an interdisciplinary rehabilitation intervention comparing HRQoL, symptom burden and physical function among patients with primary glioma: an RCT study protocol. BMJ Open. 2014;4(10):e005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bartolo M, Zucchella C, Pace A et al. Improving neuro-oncological patients care: basic and practical concepts for nurse specialist in neuro-rehabilitation. J Exp Clin Cancer Res. 2012;31:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCarty S, Keeshin S, Eickmeyer SM, Shahpar S, Semik P, Wong AWK. Evaluation of the cost of comprehensive outpatient therapies in patients with malignant brain tumors. Am J Phys Med Rehabil. 2017;96(5):341–346. [DOI] [PubMed] [Google Scholar]
- 50. Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it?Lancet Oncol. 2012;13(9):e375–e382. [DOI] [PubMed] [Google Scholar]
- 51. Perry JR. Thromboembolic disease in patients with high-grade glioma. Neuro Oncol. 2012;14(Suppl 4): iv73–iv80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brandes AA, Scelzi E, Salmistraro G et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33(10):1592–1596. [DOI] [PubMed] [Google Scholar]
- 53. Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 54. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 55. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wick W, Platten M, Wick A et al. Current status and future directions of anti-angiogenic therapy for gliomas. Neuro Oncol. 2016;18(3):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wick W, Brandes A, Gorlia T et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015;17(suppl 5):LB05. [Google Scholar]
- 58. Wirsching HG, Tabatabai G, Hottinger A et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the ARTE trial. Neuro Oncol. 2017;suppl (OS07.2). [DOI] [PubMed] [Google Scholar]
- 59. Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 60. Wick W. TTFields: where does all the skepticism come from?Neuro Oncol. 2016;18(3):303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Osswald M, Jung E, Sahm F et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. [DOI] [PubMed] [Google Scholar]
- 62. Osswald M, Solecki G, Wick W, Winkler F. A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol. 2016;18(4):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]