Abstract
Background
Cabozantinib is a tyrosine kinase inhibitor with activity against vascular endothelial growth factor receptor 2 (VEGFR2) and MET that has demonstrated clinical activity in advanced solid tumors. This open-label, phase II trial evaluated cabozantinib in patients with recurrent or refractory glioblastoma (GBM).
Methods
Patients were initially enrolled at a starting dose of 140 mg/day, but the starting dose was amended to 100 mg/day because of toxicity. Treatment continued until disease progression or unacceptable toxicity. The primary endpoint was objective response rate assessed by an independent radiology facility using modified Response Assessment in Neuro-Oncology criteria. Additional endpoints included duration of response, 6-month and median progression-free survival, overall survival, and safety.
Results
Among 152 patients naive to prior antiangiogenic therapy, the objective response rate was 17.6% and 14.5% in the 140 mg/day and 100 mg/day groups, respectively, which did not meet the predefined statistical target for success. The proportions of patients alive and progression free at 6 months were 22.3% and 27.8%, respectively. Median progression-free survival was 3.7 months in both groups, and median overall survival was 7.7 months and 10.4 months, respectively. The incidence of grade 3/4 adverse events (AEs) was 79.4% and 84.7% in the 140 mg/day and 100 mg/day groups, respectively, and dose reductions due to AEs were experienced by 61.8% and 72.0%, respectively. Common grade 3/4 AEs included fatigue, diarrhea, and palmar-plantar erythrodysesthesia syndrome.
Conclusions
Cabozantinib showed evidence of clinical activity in patients with recurrent GBM naive to antiangiogenic therapy, although the predefined statistical target for success was not met. At the starting doses assessed, AEs were frequently managed with dose reductions.
Clinical Trials Registration Number
NCT00704288 (https://www.clinicaltrials.gov/ct2/show/NCT00704288)
Keywords: antiangiogenic, cabozantinib, naive, progressive glioblastoma, recurrent
Importance of the study
GBM is the most common brain tumor in adults. Treatment options are limited for recurrent disease and include repeated surgical resection, irradiation, chemotherapy, tumor treating fields, or bevacizumab therapy. Despite demonstration of some efficacy with agents that target the VEGF pathway such as bevacizumab, the 5-year survival rate for patients with GBM remains low, and additional therapies are needed. Cabozantinib is an inhibitor of tyrosine kinases including VEGFR2, MET, and AXL. The MET pathway has been implicated in the pathogenesis of GBM and resistance to bevacizumab therapy. In the current study, 152 patients with recurrent GBM who were naive to prior antiangiogenic therapy were treated with cabozantinib at either 140 mg or 100 mg orally daily. Cabozantinib showed evidence of clinical activity in patients with recurrent GBM naive to prior antiangiogenic therapy.
Among primary brain and central nervous system tumors diagnosed in the United States between 2006 and 2010, glioblastoma (GBM) accounted for 45.6% of malignant brain and central nervous system tumors and is the most common malignant brain tumor in adults.1 The current standard of care for patients with newly diagnosed GBM includes surgical resection of the tumor to the extent possible, followed by adjuvant radiotherapy plus temozolomide2,3; nevertheless, the median 5-year survival rate remains less than 5%.1
Treatment options for patients with recurrent disease are limited and may include repeat surgical resection, reirradiation, systemic chemotherapy, tumor treating fields, bevacizumab, or best supportive care.3 Bevacizumab monotherapy is approved in the United States for patients with recurrent GBM based on a clinically meaningful and durable objective response rate (ORR) of 19.6% and a median duration of response ranging from 3.9 months to 4.2 months based on World Health Organization (WHO) radiographic criteria.3–5 In heavily pretreated patients with GBM, the median overall survival (OS) with single-agent bevacizumab ranges from 7.8 months6 to 9.2 months.7 The Dutch BELOB trial in patients with GBM at first recurrence reported 9-month OS rates of 38% for bevacizumab, 43% for lomustine, 59% for bevacizumab/lomustine 90 mg/m2, and 88% for bevacizumab/lomustine 110 mg/m2.8 In a phase III trial of bevacizumab plus lomustine compared with lomustine alone in patients with GBM at first recurrence, median progression-free survival (PFS) was 4.2 months versus 1.5 months and median OS was 9.1 months versus 8.6 months, respectively.9 GBMs are highly vascularized neoplasms, and agents such as bevacizumab that target vascular endothelial growth factor (VEGF) have demonstrated some efficacy in this setting. In addition to the VEGF pathway, the MET/hepatocyte growth factor pathway has been implicated as an important mediator in the pathogenesis of GBM.10–13 In particular, MET overexpression is associated with poor response to treatment and shorter survival.12 Furthermore, preclinical data suggest that MET overexpression may contribute to bevacizumab resistance and that targeting MET may offer a mechanism to prevent or overcome resistance.13,14 The receptor tyrosine kinase AXL has also been implicated in GBM pathogenesis in preclinical studies and associated with a poor prognosis in patients with GBM.15–18 However, bevacizumab only inhibits circulating VEGF and has no direct effect on the other angiogenic factors or molecular targets expressed by the tumor that are known to play a role in the pathobiology of GBM. Furthermore, in vitro evidence suggests that targeting VEGF alone may drive a pro-invasive phenotype.19 As a result, there is a need for therapies that target multiple pathways involved in GBM pathogenesis and that may overcome mechanisms of resistance.
Cabozantinib is an oral tyrosine kinase inhibitor with potent activity against MET, VEGF receptors (VEGFRs), and AXL.20,21 Preclinical data indicate that cabozantinib suppresses MET and VEGFR2 signaling and induces apoptosis of endothelial cells and a variety of tumor cell lines. For example, cabozantinib significantly improved survival in a xenograft mouse model of human GBM.22 In addition, several clinical trials in patients with advanced solid tumors have demonstrated that cabozantinib therapy can cause tumor regression.23–25 The current phase II trial sequentially examined the efficacy and safety of 2 doses of cabozantinib in 222 patients with refractory or recurrent GBM. Herein we report the results from the subset of patients who were naive to antiangiogenic therapy at study entry. Results for patients who received prior antiangiogenic therapy are presented in the companion article (Cloughesy et al).
Materials and Methods
Eligibility Requirements
Eligible patients were adults with refractory or recurrent GBM in first or second relapse. Patients were required to have radiographic evidence of recurrent disease by gadolinium-enhanced MRI scan (performed within the past 14 days and while on a fixed or decreasing dose of glucocorticoids for at least 5 days). Some entry criteria differed among patients due to amendments to the protocol (see Supplementary material). Eligible patients received prior temozolomide and radiation therapy (required for patients enrolled at the 100 mg/day dose); had a Karnofsky performance status score of ≥60%; and had adequate hematologic, renal, and liver function. Exclusion criteria included prior anticancer therapy within 28 days before the first dose of cabozantinib (including investigational agents and biologic agents) or mitomycin C or nitrosoureas within 42 days before the first dose of cabozantinib, or prior antiangiogenic therapy (for the group naive to antiangiogenic therapy). All patients provided informed written consent. The protocol was approved by ethics committees or institutional review boards at each investigator’s institution. This trial was conducted in accordance with the principles of the International Conference on Harmonisation (ICH) guideline for Good Clinical Practice, the World Medical Association Declaration of Helsinki, and Title 21 of the United States Code of Federal Regulations.
Study Design and Treatment
This was a phase II, multicenter, open-label, single-agent, noncomparator trial that sequentially explored 2 dose levels of cabozantinib in patients with recurrent or refractory GBM. Patients were defined to be enrolled upon receipt of the first dose of cabozantinib. Patients were initially enrolled to oral cabozantinib 140 mg/day (freebase weight); however, because rates of dose reduction and interruption at 140 mg/day were deemed to be high, a protocol amendment added another cohort (15 antiangiogenic treatment-naive patients planned) at a reduced starting dose of 100 mg/day to allow qualitative assessment of the safety of the dose. The 100 mg/day starting dose was deemed appropriate based on the median average daily dose of the first cohort enrolled and initial assessments suggesting continued efficacy at doses ≤100 mg/day. Another protocol amendment added an additional cohort to be enrolled at the 100 mg/day starting dose (80 antiangiogenic treatment-naive patients planned). Treatment was continued until documented disease progression or unacceptable toxicity. To manage adverse events (AEs), dose could be reduced to 100 mg (for those enrolled at 140 mg/day), 60 mg, and 40 mg; dose could be reduced to lower than 40 mg after consultation with the sponsor. Patients naive to antiangiogenic therapy are the focus of this paper. Further details of the study design and amendments to the protocol are provided in the Supplementary material.
Efficacy Endpoints and Assessments
The primary endpoint was ORR using criteria of the modified Response Assessment in Neuro-Oncology (modified RANO; Supplementary material) as assessed by an independent radiology facility (IRF). For implementation in this study, the primary modifications to the RANO criteria for time point responses include defining operational conventions for changes in glucocorticoid dose and removal of the clinical deterioration component to reduce subjectivity and facilitate IRF assessment. Classification of ORR required complete response (CR)/partial response (PR) confirmed on follow-up imaging at least 4 weeks from initial response. Secondary endpoints included duration of response, 6-month and median PFS, OS, and glucocorticoid use. Radiologic evaluations were performed at screening and generally every 6 to 8 weeks from enrollment until disease progression using modified RANO criteria (see Supplementary material). Tumor assessments for the primary efficacy analyses were performed by an IRF to determine response and/or progression. The minimum lesion size required for measurable disease by IRF was 10 mm × 5 mm, reflecting the implementation of RANO in this study before publication of the 10 mm × 10 mm recommended criterion for measurable disease.
Safety Assessments
Safety and tolerability assessments included monitoring AEs, performing standard clinical laboratory tests (including hematology, serum chemistry, and urinalysis) and physical examinations, and recording electrocardiograms. Severity of AEs was assessed by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Serious AEs were defined in accordance with the ICH Guidelines for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting, Topic E2A.
Statistical Analysis
Results for patients naive to antiangiogenic therapy are presented here. Efficacy and safety analyses include all such enrolled patients unless otherwise specified. ORR was defined as the proportion of patients with measurable disease at baseline per modified RANO criteria whose best overall response was confirmed CR or confirmed PR. The ORR is presented with 2-sided 95% CIs. The planned sample size to evaluate the primary endpoint was 80 patients to evaluate ORR with the following hypotheses (1-sided nominal alpha of 0.025 and power of >90%): H0: ORR = 10% and HA: ORR = 25%. The primary endpoint was to be analyzed in the last group of patients enrolled at the 100 mg/day dose; however, for simplicity, patients naive to antiangiogenic therapy at the 100 mg/day dose were retrospectively combined for the analyses presented here. For more details on the planned analyses, see Supplementary material.
PFS was calculated as the time from first cabozantinib dose to the earlier of documented disease progression per modified RANO or death from any cause. OS was calculated as the time from first cabozantinib dose to death from any cause. Efficacy analyses of secondary endpoints for duration of response, PFS, and OS used the Kaplan–Meier method. All statistical analysis was performed using SAS version 8.2 or higher.
Results
Patients
A total of 222 patients were enrolled between June 2008 and June 2012; at study entry, 152 of 222 patients (68%) were naive to prior antiangiogenic therapy and are the subject of this report. After the first 34 naive patients were enrolled and received at least 1 dose of cabozantinib at 140 mg/day, the dose was reduced to 100 mg/day and an additional 118 naive patients were treated at the 100 mg/day dose. Among patients naive to prior antiangiogenic therapy, baseline demographics and clinical characteristics were similar between the 2 cabozantinib dose groups (Table 1). The majority of patients were white males. Approximately one third of patients received >1 prior systemic therapy before study entry.
Table 1.
Baseline demographics and clinical characteristics of the patients
| Characteristic | Patients, n (%) | |
|---|---|---|
| 140 mg/day (n = 34) | 100 mg/day (n = 118) |
|
| Age, y | ||
| Median (range) | 55 (20–73) | 56.5 (21–82) |
| Sex | ||
| Male | 24 (70.6) | 72 (61.0) |
| Female | 10 (29.4) | 46 (39.0) |
| Race | ||
| White | 29 (85.3) | 104 (88.1) |
| Black | 3 (8.8) | 5 (4.2) |
| Asian | 1 (2.9) | 4 (3.4) |
| Native American | 0 | 2 (1.7) |
| Other | 1 (2.9) | 4 (3.4) |
| Karnofsky performance status | ||
| 90–100 | 22 (64.7) | 74 (62.7) |
| 70–80 | 11 (32.4) | 44 (37.3) |
| ≤60 | 1 (2.9) | 0 |
| Years since initial diagnosis | ||
| Median (range) | 0.95 (0.1–5.3) | 0.94 (0.3–16.7) |
| GBM type | ||
| Primary | 29 (85.3) | 100 (84.7) |
| Secondary | 5 (14.7) | 18 (15.3) |
| Prior radiotherapy for GBM | ||
| Yes | 30 (88.2) | 117 (99.2) |
| No | 4 (11.8) | 1 (0.8) |
| Prior lines of systemic therapy for GBM | ||
| 0 | 3 (8.8) | 0 |
| 1 | 18 (52.9) | 77 (65.3) |
| 2 | 12 (35.3) | 39 (33.1) |
| ≥3 | 1 (2.9) | 2 (1.7) |
| Steroid use at baseline* | ||
| Yes | 14 (41.2) | 56 (47.5) |
| No/unknown | 20 (58.8) | 62 (52.5) |
*Received at least 7 days of systemic steroids within 30 days before the first dose of cabozantinib.
Response to Treatment
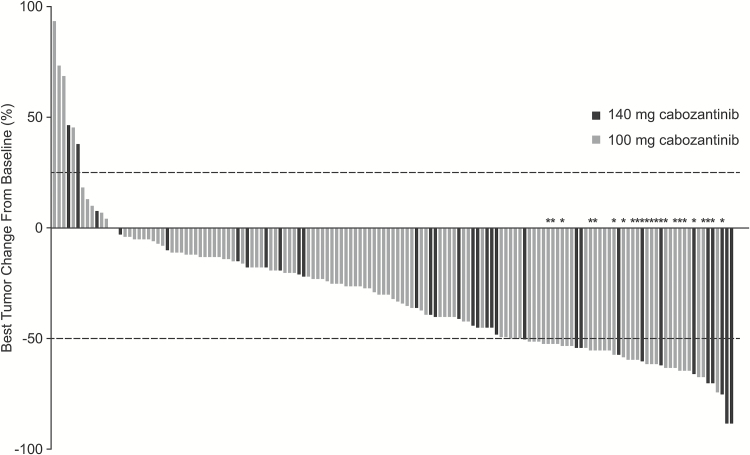
Among the 152 patients naive to prior antiangiogenic therapy, 151 had measurable disease at baseline by the IRF and were included in the response assessment (Table 2). Subgroup analysis by cabozantinib starting dose demonstrated an ORR of 17.6% (95% CI, 6.8%–34.5%) in patients who received 140 mg/day and 14.5% (95% CI, 8.7%–22.2%) in those who received 100 mg/day. The results for ORR did not meet the predefined statistical target for success. The median duration of ORR was 5.9 months (range, 1.9–12.8 mo) in the 140 mg/day group and 8.5 months (range, 1.0–9.3 mo) in the 100 mg/day group. Overall, 90% of patients with measurable disease at baseline and ≥1 evaluable postbaseline assessment had a detectable decrease in tumor volume (Fig. 1). The median follow-up for scans was 3.6 months (range, 0.03–25.0) in the 140 mg/day group and 3.6 months (range, 0.03–17.5) in the 100 mg/day group.
Table 2.
Best overall response to treatment by modified RANO criteria (per IRF)
| Patients, n (%) | ||
|---|---|---|
| 140 mg/day (n = 34) | 100 mg/day (n = 117)* | |
| Objective response rate | 6 (17.6) | 17 (14.5) |
| Best overall response | ||
| Confirmed partial response | 6 (17.6) | 17 (14.5) |
| Stable disease | 20 (58.8) | 79 (67.5) |
| Progressive disease | 4 (11.8) | 14 (12.0) |
| Unevaluable or missing** | 4 (11.8) | 7 (6.0) |
| Duration of objective response, mo | ||
| Median (range) | 5.9 (1.9–12.8) | 8.5 (1.0–9.3) |
*One patient lacked measurable disease at baseline and is not included.
**Unevaluable by modified RANO or no postbaseline tumor assessments.
Fig. 1 .
Best tumor size change from baseline in target lesion per IRF using modified RANO criteria in patients who had measurable disease at baseline and ≥1 evaluable postbaseline radiographic scan. Lines indicate the threshold for response and progression per RANO criteria, ≥50% decrease and ≥25% increase, respectively. Partial responses were confirmed in 6 patients in the 140 mg/day group and 17 in the 100 mg/day group. *Confirmed partial response.
Key Secondary Efficacy Endpoints
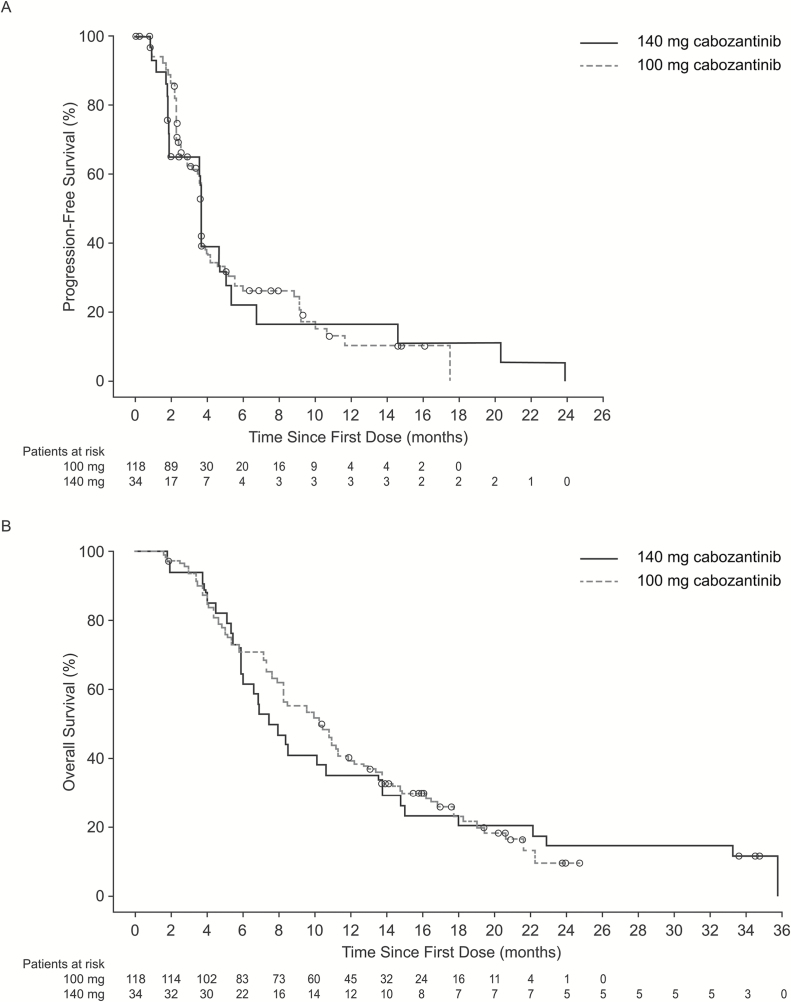
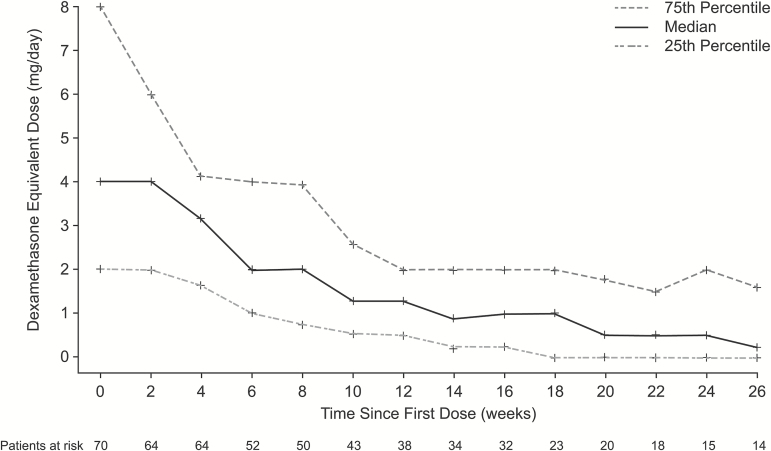
The median PFS was 3.7 months in both the 140 mg/day and 100 mg/day dose groups (Fig. 2A). The Kaplan–Meier estimates of the proportion of patients alive and progression free at 6 months were 22.3% in the 140 mg/day group and 27.8% in the 100 mg/day group. The estimated median OS was 7.7 months in the 140 mg/day group and 10.4 months in the 100 mg/day group (Fig. 2B). Overall, 100 of 152 patients (66%) subsequently received salvage therapy with bevacizumab. Among patients who reported glucocorticoid use at baseline (n = 70), there was a trend for them to receive stable or decreasing glucocorticoid doses over time (Fig. 3).
Fig. 2 .
Kaplan–Meier estimates of (A) progression-free survival and (B) overall survival by dose group.
Fig. 3.
Average daily glucocorticoid dose up to last treatment date among patients who reported any glucocorticoid use at baseline.
Safety and Tolerability
The median duration of treatment was 14.8 weeks (range, 0.3–108.4 wk) in the 140 mg/day group and 15.1 weeks (range, 0.7–79.0 wk) in the 100 mg/day group. All patients received ≥1 dose of cabozantinib and reported ≥1 treatment-emergent adverse event (TEAE); those occurring in ≥15% of patients (all grades) are summarized in Table 3. The most common TEAEs included fatigue (76.3%), diarrhea (62.5%), decreased appetite (47.4%), palmar-plantar erythrodysesthesia syndrome (39.5%), nausea (38.8%), headache (36.8%), constipation (34.2%), hypertension (32.2%), weight decrease (31.6%), and dysphonia (30.9%).
Table 3.
Treatment-emergent adverse events (TEAEs) reported in ≥15% of patients
| Adverse Event* | Patients, n (%) | |||
|---|---|---|---|---|
| 140 mg/day (n = 34) | 100 mg/day (n = 118) | |||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Any TEAE | 34 (100) | 27 (79.4) | 118 (100) | 100 (84.7) |
| Fatigue | 27 (79.4) | 13 (38.2) | 89 (75.4) | 22 (18.6) |
| Diarrhea | 23 (67.6) | 2 (5.9) | 72 (61.0) | 8 (6.8) |
| Decreased appetite | 18 (52.9) | 1 (2.9) | 54 (45.8) | 5 (4.2) |
| PPES | 14 (41.2) | 3 (8.8) | 46 (39.0) | 11 (9.3) |
| Nausea | 12 (35.3) | 2 (5.9) | 47 (39.8) | 3 (2.5) |
| Headache | 16 (47.1) | 5 (14.7) | 40 (33.9) | 7 (5.9) |
| Constipation | 17 (50.0) | 0 | 35 (29.7) | 0 |
| Hypertension | 12 (35.3) | 1 (2.9) | 37 (31.4) | 9 (7.6) |
| Weight decreased | 9 (26.5) | 0 | 39 (33.1) | 7 (5.9) |
| Dysphonia | 13 (38.2) | 0 | 34 (28.8) | 0 |
| AST increased | 11 (32.4) | 1 (2.9) | 35 (29.7) | 3 (2.5) |
| ALT increased | 11 (32.4) | 3 (8.8) | 33 (28.0) | 10 (8.5) |
| Convulsion | 12 (35.3) | 3 (8.8) | 29 (24.6) | 12 (10.2) |
| LDH increased | 8 (23.5) | 0 | 33 (28.0) | 3 (2.5) |
| Hypophosphatemia | 6 (17.6) | 3 (8.8) | 35 (29.7) | 17 (14.4) |
| Confusional state | 8 (23.5) | 4 (11.8) | 31 (26.3) | 2 (1.7) |
| Stomatitis | 13 (38.2) | 1 (2.9) | 26 (22.0) | 3 (2.5) |
| Vomiting | 10 (29.4) | 1 (2.9) | 25 (21.2) | 4 (3.4) |
| Abdominal pain | 10 (29.4) | 1 (2.9) | 24 (20.3) | 3 (2.5) |
| Thrombocytopenia | 1 (2.9) | 0 | 33 (28.0) | 8 (6.8) |
| Pain in extremity | 8 (23.5) | 0 | 25 (21.2) | 2 (1.7) |
| Insomnia | 11 (32.4) | 0 | 21 (17.8) | 0 |
| Gait disturbance | 9 (26.5) | 2 (5.9) | 23 (19.5) | 7 (5.9) |
| Hair color changes | 8 (23.5) | 0 | 23 (19.5) | 0 |
| Leukopenia | 1 (2.9) | 1 (2.9) | 30 (25.4) | 11 (9.3) |
| Lipase increased | 4 (11.8) | 3 (8.8) | 26 (22.0) | 12 (10.2) |
| Cough | 7 (20.6) | 0 | 21 (17.8) | 0 |
| Dysgeusia | 6 (17.6) | 0 | 22 (18.6) | 0 |
| Anxiety | 6 (17.6) | 0 | 21 (17.8) | 0 |
| Oral pain | 4 (11.8) | 2 (5.9) | 23 (19.5) | 3 (2.5) |
| Depression | 4 (11.8) | 0 | 22 (18.6) | 1 (0.8) |
| Dry skin | 12 (35.3) | 0 | 14 (11.9) | 0 |
| Hemiparesis | 3 (8.8) | 0 | 23 (19.5) | 10 (8.5) |
| Dyspepsia | 5 (14.7) | 0 | 20 (16.9) | 0 |
| Edema peripheral | 4 (11.8) | 0 | 21 (17.8) | 0 |
| Oropharyngeal pain | 9 (26.5) | 0 | 16 (13.6) | 0 |
| Rash | 6 (17.6) | 0 | 19 (16.1) | 1 (0.8) |
| Hypokalemia | 5 (14.7) | 1 (2.9) | 19 (16.1) | 7 (5.9) |
| Neutropenia | 5 (14.7) | 2 (5.9) | 19 (16.1) | 5 (4.2) |
| Dyspnea | 4 (11.8) | 0 | 18 (15.3) | 1 (0.8) |
| Dizziness | 10 (29.4) | 1 (2.9) | 11 (9.3) | 0 |
| Cognitive disorder | 7 (20.6) | 1 (2.9) | 13 (11.0) | 1 (0.8) |
| Lymphopenia | 7 (20.6) | 3 (8.8) | 13 (11.0) | 4 (3.4) |
| Proteinuria | 8 (23.5) | 0 | 12 (10.2) | 1 (0.8) |
| Aphasia | 6 (17.6) | 1 (2.9) | 9 (7.6) | 2 (1.7) |
| Vision blurred | 7 (20.6) | 0 | 7 (5.9) | 0 |
| Bilirubin increased | 6 (17.6) | 2 (5.9) | 7 (5.9) | 1 (0.8) |
| Epistaxis | 6 (17.6) | 0 | 6 (5.1) | 0 |
| Skin discoloration | 6 (17.6) | 0 | 1 (0.8) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; LDH, lactate dehydrogenase; MedDRA, Medical Dictionary for Regulatory Activities; PPES, palmar-plantar erythrodysesthesia syndrome.
*MedDRA v. 15 Preferred Terms (converted to US spelling), CTCAE v. 3.0 grading.
Adverse events were generally manageable with dose reductions or dose interruptions. Dose reductions due to an AE were required in 21 (61.8%) patients in the 140 mg/day group and 85 (72.0%) in the 100 mg/day group. Adverse events were reported as the primary reason for a dose interruption in 20 (58.8%) patients in the 140 mg/day group and 73 (61.9%) patients in the 100 mg/day group. The median average daily dose was 111.1 mg/day (range, 28.4–140 mg/day) in the 140 mg/day group and 71.5 mg/day (range, 25.1–100 mg/day) in the 100 mg/day group. Adverse events resulted in treatment discontinuation in 6 (17.6%) patients in the 140 mg/day group and 22 (18.6%) patients in the 100 mg/day group.
Serious AEs were reported in 18 (52.9%) patients in the 140 mg/day group and 61 (51.7%) patients in the 100 mg/day group and were similar in the 2 dose groups. In the overall population, serious AEs that occurred at a frequency >2% were convulsion (9.9%), deep vein thrombosis (5.3%), hemiparesis (4.6%), dehydration (3.9%), intracranial hemorrhage (2.6%), confusional state (2.6%), and vomiting (2.6%). One hundred twenty-two deaths were reported: 31 (91.2%) in the 140 mg/day group and 91 (77.1%) in the 100 mg/day group. Grade 5 AEs were reported in 8 (6.8%) patients in the 100 mg/day group and no patients in the 140 mg/day group. Grade 5 AEs consisted of pulmonary embolism (in 2 patients) and intracranial hemorrhage, acute respiratory failure, cardiac arrest, death, disease progression, and gastrointestinal ulcer hemorrhage (1 patient each). Four of these grade 5 AEs (2 pulmonary embolisms, 1 gastrointestinal ulcer hemorrhage, and 1 death) were assessed as treatment related.
Discussion
Patients with recurrent GBM have limited treatment options. Despite not meeting the predefined statistical target for success, results from this phase II trial suggest that cabozantinib has clinical activity in patients who have not received prior antiangiogenic therapy. Comparison of ORR with cabozantinib (15.2%) across both dosing cohorts with bevacizumab monotherapy is difficult as the studies evaluating these agents employed different response criteria (Macdonald vs modified RANO). The trial did not have a comparator arm, and interpretations based on cross-trial comparisons are difficult due to differences in patient populations, assessments, and study design. Nonetheless, the results for PFS and OS with cabozantinib compare well with those reported for other therapies. The median PFS of 3.7 months in both cabozantinib groups is similar to that of monotherapy with bevacizumab (4.2 mo),7 cediranib (3.1 mo),26 and axitinib (3.0 mo)27 in patients with recurrent GBM. The median OS of 10.4 months in the cabozantinib 100 mg/day group is also similar to results observed with bevacizumab (median OS, 9.2 mo). Although the effect of salvage bevacizumab therapy on survival in the current study is unknown, it may not be inconsequential.7 In addition, most patients receiving cabozantinib did not experience a change in the pattern of tumor progression (most had a local pattern of progression at baseline) and experienced a decrease in glucocorticoid usage over time.
This was the first study of cabozantinib in patients with GBM, and changes were made to the study design, endpoints, and assessments during the trial. The decision to analyze patients by prior treatment with antiangiogenic therapy was based on initial data that showed efficacy differences between these populations. Assessment of response using modified RANO was introduced later in the study; however, all groups were analyzed using the same modified RANO criteria. In addition, groups were retrospectively combined for determination of ORR for simplicity of the data presentation, increasing the size of the analysis group used for ORR beyond the planned size. Some differences in entry criteria and the schedule of tumor assessments also existed between these groups. Nonetheless, this study provides an initial assessment of the activity of cabozantinib in patients with GBM.
The patient population in the cabozantinib trial is typical of patients with recurrent disease in that most had received standard first-line therapy for GBM (ie, surgery and radiotherapy plus temozolomide). In addition, many of the patients received high doses of corticosteroids for prolonged periods of time. A high incidence of comorbidities that may be attributable to either advanced GBM or prior therapy was observed. Overall, the reported AEs were consistent with the known safety profile of cabozantinib23,28 and are consistent with AEs typically associated with this class of agents. The overall incidence of AEs was not lower at the 100 mg/day dose compared with the 140 mg/day dose, and dose reductions or interruptions were frequently used to manage AEs in both dose groups. Efficacy results were similar in the 2 dose groups, and additional studies might determine if efficacy could be maintained and tolerability improved with a lower starting dose. A lower dose of 60 mg/day has been approved for the treatment of advanced renal cell carcinoma, whereas the 140 mg/day dose has been approved for treatment of medullary thyroid cancer, based on results from the pivotal phase III trials.23,28
Although the predefined statistical target for success was not met, cabozantinib showed evidence of clinical activity in patients with refractory or recurrent GBM who had not received prior antiangiogenic therapy. Further assessment of activity and tolerability at a lower starting dose would be necessary to better evaluate cabozantinib in this patient population.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
Research funding and financial support for medical editorial assistance were provided by Exelixis, Inc (South San Francisco, California).
Conflict of interest statement. The conflict of interest statement follows:
Employment: J. Holland: Exelixis; J. Ping: Exelixis; R. Weitzman: Exelixis
Leadership Position: None
Stock or Other Ownership: A. Desjardins: Istari Oncology; J. Drappatz: Exelixis; J. Holland: Exelixis; J. Ping: Exelixis; R. Weitzman: Exelixis
Honoraria: J. de Groot: Merck; J. Drappatz: Angiochem, Geron; D. Reardon: Roche/Genentech, Merck, Amgen, Stemline, Novartis; D. Schiff: Merck
Consultant and/or Advisory Role: P. Wen: Agios, Genentech/Roche, Cavion, Cortice Biosciences, Foundation Medicine, Insys, Monteris, Novartis, Vascular Biogenics, VBI Vaccines; J. de Groot: Celldex, Deciphera Pharmaceuticals, Foundation Medicine, Inc., AstraZeneca, Novartis, Genentech; M.D. Prados: Novartis; D. Reardon: Roche/Genentech, Merck, Amgen, Stemline, Novartis; D. Schiff: DMSCs for Celldex, Vascular Biogenics; consultant/advisory board for Tau Pharmaceutical, Genentech, Sigma Tau, Midatech, Heron; T. Mikkelsen: Celgene, Roche/Genentech; A. Desjardins: Genentech USA, Inc, PTC Therapeutics; T. Cloughesy: Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, MedQIA; M Chamberlain: Eli Lilly, Genentech/Roche, Nativis, NeurOnc, Pharmacokinesis.
Speakers’ Bureau: P. Wen: Merck; D. Reardon: Roche/Genentech, Merck; M Chamberlain: Genentech
Research Funding: P. Wen: Acerta, Agios, AstraZeneca, Ely Lilly, Exelixis, Genentech/Roche, Karyopharm, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics; J. de Groot: Eli Lilly, Sanofi-Aventis, EMD-Serono, AstraZeneca, Novartis, Deciphera Pharmaceuticals; D. Schiff: Novocure, Angiochem; J. Drappatz: Angiochem, AbbVie, Celldex, Genentech; M.D. Prados: Genentech, Novartis; A. Desjardins: Genentech USA, PTC Therapeutics, Celldex
Patents, Royalties, Other Intellectual Property: A. Desjardins: PVSRIPO (modified poliovirus—clinical)
Expert Testimony: T. Cloughesy: Roche
Travel, Accommodations, Expenses: J. de Groot: Genentech, Deciphera, Celldex; T. Mikkelsen: Celgene, Roche/Genentech; J. Ping: Exelixis; M Chamberlain: Genentech; J. Drappatz: Angiochem, AbbVie, Geron, Genentech
Other Relationship: J. de Groot: DSMB for VBL Therapeutics and Novella
Prior Publication: Previous analysis of data presented in part at 2010 ASCO Annual Meeting (oral presentation); abstract published as Wen et al. J Clin Oncol. 2010;28:15s (suppl; abstr 2006).
Supplementary Material
Acknowledgments
We thank Michael Hobert, PhD, Scientific Strategy Partners, Inc, for medical editorial assistance with this manuscript. The authors are fully responsible for all content and editorial decisions for this manuscript.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. . CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology—Central Nervous System Cancers v1 2016. Available at: http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed April 23, 2017.
- 4. Reardon DA, Turner S, Peters KB et al. . A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9(4):414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AVASTIN (bevacizumab) solution for intravenous infusion [prescribing information]. South San Francisco, CA: Genentech; 2015. Available at: http://www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed September 15, 2016. [Google Scholar]
- 6. Kreisl TN, Kim L, Moore K et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman HS, Prados MD, Wen PY et al. . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 8. Taal W, Oosterkamp HM, Walenkamp AM et al. . Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 9. Wick W, Brandes AA, Gorlia T et al. . EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016;(suppl; abstr 2001):34. [Google Scholar]
- 10. Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7(4): 436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arrieta O, Garcia E, Guevara P et al. . Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94(12):3210–3218. [DOI] [PubMed] [Google Scholar]
- 12. Kong DS, Song SY, Kim DH et al. . Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115(1):140–148. [DOI] [PubMed] [Google Scholar]
- 13. Lu KV, Chang JP, Parachoniak CA et al. . VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jahangiri A, De Lay M, Miller LM et al. . Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of anti-angiogenic therapy resistance. Clin Cancer Res. 2013;19(7):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hutterer M, Knyazev P, Abate A et al. . Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(1):130–138. [DOI] [PubMed] [Google Scholar]
- 16. Keating AK, Kim GK, Jones AE et al. . Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onken J, Torka R, Korsing S et al. . Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget. 2016;7(9):9876–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vajkoczy P, Knyazev P, Kunkel A et al. . Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103(15):5799–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn GP, Rinne ML, Wykosky J et al. . Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yakes FM, Chen J, Tan J et al. . Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. [DOI] [PubMed] [Google Scholar]
- 21. You WK, Sennino B, Williamson CW et al. . VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71(14):4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navis AC, Bourgonje A, Wesseling P et al. . Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PLoS One. 2013;8(3):e58262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elisei R, Schlumberger MJ, Müller SP et al. . Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurzrock R, Sherman SI, Ball DW et al. . Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith DC, Smith MR, Sweeney C et al. . Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Batchelor TT, Mulholland P, Neyns B et al. . Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duerinck J, Du Four S, Van Binst A, Everaert H, D’Haens J, Neyns B. Axitinib for the treatment of recurrent glioblastoma—early results from a randomized phase II trial [poster]. Eur J Cancer. 2013;49(suppl 2):S791. [Google Scholar]
- 28. Choueiri TK, Escudier B, Powles T et al. ; METEOR Investigators Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.