To the Editor
The prevalence of food allergy continues to increase, and although there is evidence that early food introduction may be beneficial, the immunologic mechanisms predisposing an individual to develop food allergy remain elusive.1 Additionally, early atopic disease is a risk factor for the development of asthma, a progression termed the atopic march.1,2 Regulatory T cells (Tregs) play an important role in maintaining self-tolerance, and oral immunotherapy studies have shown increases in CD4+CD25+Foxp3+ cells are associated with clinical efficacy.3 We recently demonstrated that CD25+CD127loFoxp3+ cells are increased in children who outgrow their food allergy naturally,4 but it is not known if differences in Treg populations early in life, during critical times of immune development, might predispose an individual to developing food allergy and other atopic diseases.
As food allergy often begins early in life, we characterized populations of Tregs that might be important in food allergy and the development of further atopic diseases. We focused on age-related differences early in life between children with and without food allergy that could provide insight on immunologic mechanisms important for tolerance maintenance. We used low expression of IL-7Rα (CD127lo), high expression of IL-2Rα (CD25hi) and Foxp3 to more precisely define Tregs in humans.5 To study Tregs migrating to sites of inflammation (possibly gut homing) and therefore important in regulating responses to foods, we used CCR6 (chemokine receptor of CCL20), which has been shown to be important for Treg gut migration and suppression in a murine model of colitis.6
We performed a cross-sectional study on 77 patients recruited via an IRB approved study from Allergy-Immunology and General Academic Pediatric Clinics at our institution (Table S1). Food allergy was defined by oral food challenge or a history of IgE-mediated signs and symptoms within two hours of ingestion and evidence of specific IgE to the implicated food. To compare age-related differences, the cut-off of 6 years was chosen based on previous birth cohorts which demonstrate that persistent atopic phenotypes are established by this age.7 Additionally, there was a natural inflection point in Treg frequency at this age. PBMCs were isolated and stained for flow cytometry using validated methods8 (see supplemental methods; figure S1).
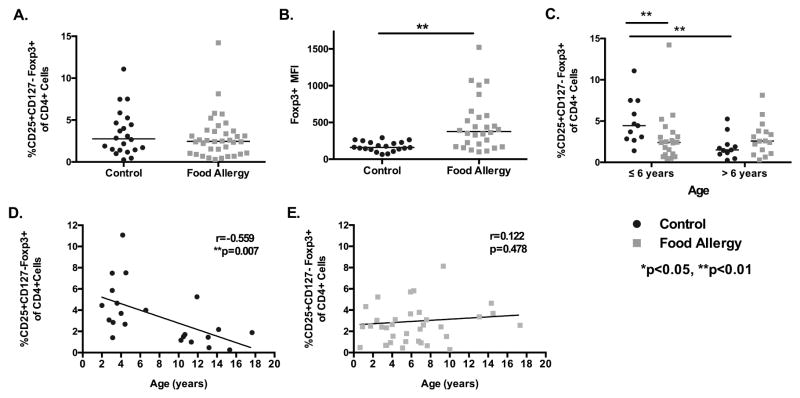
When comparing all children with and without food allergy, there was no difference in peripheral blood percentages of CD4+CD25hiCD127loFoxp3+ cells [median 2.46 vs. 2.76, p=0.471], but increased Foxp3 expression per cell [median 377.5 vs. 160 MFI, p<0.001] (Fig 1A and B) ex vivo. We hypothesize that this may be the result of ongoing immune activation in allergic individuals. When evaluating differences in Tregs associated with age, young food allergic children 0–6 years of age had significantly decreased percentages of peripheral blood CD4+CD25hiCD127loFoxp3+ Tregs compared to healthy controls of a similar age [median 2.41 vs. 4.45, p<0.05], but this difference was lost after 6 years of age (Fig 1C). Young healthy controls also had increased percentages of Tregs compared to older healthy controls [median 4.45 vs. 1.5, p=0.004] (Fig 1C), with a significant negative correlation between age and peripheral blood Tregs [r=−0.559, p=0.007] (Fig 1D). This difference was not seen among food allergic children (Fig 1E). Using a multivariable linear regression analysis, we found that age was significantly associated with Treg number in control children (p=0.001); however, this was not seen in food allergic children (p=0.467) (Table S2A and S2B). There was a combined effect of age and food allergy status (p=0.004, table S2D). These data suggest that decreased Tregs, particularly before age 6, are important in the expression of food allergy, and early life immune regulation may alter the acquisition of allergic disease.
Figure 1. Comparison of the percentage of CD25+CD127loFoxp3 Tcells in peripheral blood mononuclear cells from children with and without food allergy.
A. Comparison of CD25+CD127loFoxp3+ CD4+ T cells populations between control and food allergic children. B. Mean fluorescence intensity (MFI) of Foxp3 in CD25+CD127loFoxp3+ cells between food allergic and control children. C. CD25+CD127loFoxp3+ T-cell percentage in young children (<6 years) and older children (7–17 years). D and E. Linear correlation of age and CD25+CD127loFoxp3+ percentage in control and food allergic children. **p=0.007 for control children. *p<0.05, **p<0.01, Mann-Whitney U test. Black bars represent the median. MFI: Mean Fluorescence Intensity.
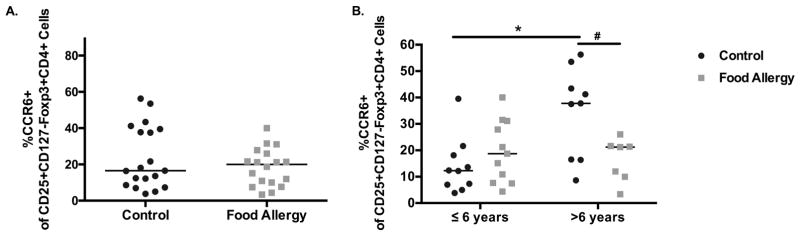
When evaluating subpopulations of CD4+CD25hiCD127loFoxp3+ Tregs we found that older subjects had an increased expression of CD45RO (memory) compared to younger subjects in both food allergic children [median 41.8 vs. 15.7, p=0.039] and healthy controls [median 17.3 vs. 7.83, p=0.011] (Fig S2 and Table S4). Expression of CCR6 was increased significantly with age in healthy controls [median 12.3 to 37.8, p=0.011] but not in food allergic children [median 18.7 to 21.2, p=0.717] (Fig 2). A stratified multivariable linear regression analysis confirmed that Treg expression of CCR6 was significantly and positively associated with age in control children only (p=0.005, Table S3A). As the immune system matures and develops in early childhood,9 we hypothesize that the acquisition of CCR6 on Tregs among healthy children may be important in tolerance maintenance in the gut and other peripheral sites of inflammation. Interestingly, older healthy controls (>6 years) also had a higher expression of CCR6 among CD4+CD25+CD45RO+ T effector cells10 [median 38.3 to 27.05, p=0.0433], and this difference was not seen in food allergic children (Figure S3), further suggesting that the T cell compartment in control children changes with age. Lastly, there was no difference in IL-10 expressing Tregs between food allergic and healthy control children [median 2.17 vs. 2.42, p=0.745] (Fig S4). These data provide evidence that decreased Treg quantity, particularly before age 6, are important in the expression of food allergy and further atopic disease development, providing support that early life immune regulation can alter disease expression.
Figure 2. Phenotypic Characterization of tTregs (CD25+CD127loFoxp3+CD4+ T cells.
A The percentage of peripheral blood tTregs (CD25+CD127loFoxp3+T cells) expressing CCR6 in control and food allergic children. B. The percentage of peripheral blood tTregs (CD25+CD127loFoxp3+T cells) in control and food allergic children in young (≤ 6 years) and older (> 6 years) control and food allergic children that express the putative gut homing marker, CCR6. *p=0.01; #p<0.1. Black bars represent the median.
While interpreting our results, we acknowledge certain limitations. While we utilized a cross-sectional study design, a longitudinal study following individual patients over time would be valuable in testing causality. Food allergic patients had an increased prevalence of atopic dermatitis, allergic rhinitis, and asthma as would be expected, and differences in Tregs may be important in the atopic march process and not solely food allergy. Therefore, some of our findings could have broader implications for the general predisposition of atopy.
In summary, we found that young, atopic food allergic children have lower percentages of strictly defined Tregs compared to healthy controls of similar age. Moreover, age-related increases in Treg expression of CCR6 were observed in healthy controls but not food allergic children, which may be important for Treg migration to peripheral sites of inflammation in the maintenance of tolerance. Our study supports the concept that Treg frequency is associated with the maintenance of tolerance in allergy.
Sincerely,
Supplementary Material
Clinical Implications.
Age-related changes in Treg percentages and phenotype may be important factors in the development and maintenance of tolerance.
Acknowledgments
Project Support: Northwestern University Feinberg School of Medicine, Thrasher Research Fund, Bunning Food Allergy Initiative, National Institutes of Health NIAID K23 A1-100995, Glen and Wendy Miller Family Foundation, Melchiorre Family Foundation; R37HL068546, R01HL078860 and P01AI106683 from the NIH, the Ernest S. Bazley Foundation
Abbreviations
- tTreg
Thymically derived regulatory T cells
- Treg
regulatory T cells
- PHA
phytohemaggluttinin
- PBMC
peripheral blood mononuclear cells
Footnotes
Conflicts of Interest/Corporate Sponsors: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toit Du G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5 doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed A, Garcia MA, Lyu S-C, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–11. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ, et al. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy. 2015;45:1663–72. doi: 10.1111/cea.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura K, Farber JM, Kelsall BL. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. The Journal of Immunology. 2010;185:3295–304. doi: 10.4049/jimmunol.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemanske RF. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 8.Hulse KE, Reefer AJ, Engelhard VH, Satinover SM, Patrie JT, Chapman MD, et al. Targeting Fel d 1 to FcgammaRI induces a novel variation of the T(H)2 response in subjects with cat allergy. J Allergy Clin Immunol. 2008;121:756–762.e4. doi: 10.1016/j.jaci.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Goenka A, Kollmann TR. Development of immunity in early life. J Infect. 2015;71(Suppl 1):S112–20. doi: 10.1016/j.jinf.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers (Basel) 2016;8 doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.