Abstract
The current experiments examined the impact of early-life immune activation and a subsequent mild immune challenge with lipopolysaccharide (LPS; 25μg/kg) on hippocampal-dependent learning, proinflammatory cytokine expression in the brain, and peripheral immune function in juvenile male and female rats at P24, an age when hippocampal-dependent learning and memory first emerges. Our results indicate that neonatal infection did not produce learning deficits in the hippocampal-dependent context pre-exposure facilitation effect paradigm in juvenile males and females, contrary to what has been observed in adults. Neonatal infection produced an increase in baseline IL-1b expression in the hippocampus (HP) and medial prefrontal cortex (mPFC) of juvenile rats. Furthermore, neonatally infected rats showed exaggerated IL-1β expression in the HP following LPS treatment as juveniles; and juvenile females, but not males, showed exaggerated IL-1β expression in the mPFC following LPS treatment. Neonatal infection attenuated the production of IL-6 expression following LPS treatment in both the brain and the spleen, and neonatal infection decreased the numbers of circulating white blood cells in juvenile males and females, an effect that was further exacerbated by subsequent LPS treatment. Together, our data indicate that the consequences of neonatal infection are detectable even early in juvenile development, though we found no concomitant hippocampal-dependent learning deficits at this young age. These findings underscore the need to consider age and associated on-going neurodevelopmental processes as important factors contributing to the emergence of cognitive and behavioral disorders linked to early-life immune activation.
Keywords: early life infection, learning and memory, inflammation, microglia, sex differences
INTRODUCTION
Early life exposure to infection, injury, trauma, or stress can have significant, lasting consequences for cognition and behavior in adulthood. This phenomenon is termed perinatal programming (Barker et al., 1995), and it is thought to occur via long-term changes in immune function caused by perinatal immune activation. For example, neonatal exposure to bacterial or viral agents (e.g. lipopolysaccharide [LPS] or Poly I:C) can produce permanent alterations in behavior and cognitive functions such as altered social interactions and memory (MacRae et al., 2015; Doenni et al., 2016), increased anxiety (Tenk et al., 2013), and schizophrenia-like behaviors (Meehan et al., 2016) across a number of species later in life. Bilbo et al. (2006) originally found that neonatal infection with live Escherichia coli (E. coli) during the neonatal period produces memory impairment in male rats when learning is paired with a second, low-dose immune challenge with LPS in adulthood. Furthermore, early-life immune activation produces exaggerated expression of the proinflammatory cytokine interleukin-1β (IL-1β) in the hippocampus (HP) in response to LPS challenge at the time of learning in neonatally infected adult male rats (Bilbo et al., 2006; Williamson et al., 2011). When IL-1β synthesis was prevented via a caspase-1 inhibitor, memory deficits were prevented (Bilbo et al., 2005). Importantly, if male rats are infected with E. coli on postnatal day 30 (P30), well after the neonatal period of development, no alterations in inflammatory cytokine production or behavior are observed in adulthood in response to LPS (Bilbo et al., 2006). Taken together, these data suggest that the immune system is permanently altered or programmed by early life immune activation or inflammation (Del Rey et al., 2009; Jessop et al., 2010) and that the immune system and the molecules it produces (e.g. cytokines) are key mediators of the cognitive and behavioral impairments observed in adulthood.
There is also increasing evidence linking early life immune activation with the development of behavioral, learning, and even neuropsychiatric disorders in humans (Keil et al., 2010; Leckman, 2014). A number of developmental disorders including schizophrenia, autism spectrum disorder (ASD) and Rett syndrome have all been linked to early life immune activation and subsequent dysregulation of immune function (Keil et al., 2010; Derecki et al., 2012; Atladottir et al., 2016). For example, individuals with ASD and Rett syndrome have altered peripheral cytokine levels, low immunoglobulin levels, and altered T cell activation (Derecki et al., 2010; Keil et al., 2010), consistent with the idea that early-life immune activation produces global changes in immune function in addition to microglial activation, cognitive and behavioral disorders. Furthermore, epidemiological data indicate that boys have twice the prevalence of developmental disorders such as ASD, Rett syndrome, Attention Deficit Hyperactivity Disorder and general learning disabilities than girls (Boyle et al., 2011; Schwarz and Bilbo, 2012), suggesting that sex is a critical factor that must be considered when examining the etiology of neurodevelopmental disorders linked to immune dysregulation. We recently found a sex difference in the overall number of microglia in the HP and cortex on P4 (Schwarz et al., 2011). Male rats have significantly more microglia in the HP, cortex and amygdala than females at this early age. Importantly, these brain regions are crucial for learning and memory suggesting that sex may be an important biological variable in determining the vulnerability to long-term consequences of neonatal infection on microglia function and behavior. Specifically, these data suggest that males may be more vulnerable than females to the neural and behavioral consequences of early life immune activation.
Thus, the goal of the current study was to determine the impact of early life immune activation on hippocampal-dependent learning, neuroimmune function, and peripheral immune function in juvenile male and female rats, at an age when hippocampal-dependent learning and memory first emerges. Importantly, these experiments attempted to address several gaps in the literature. First, it has never been investigated whether early life immune activation can result in learning deficits at an age when hippocampal-dependent learning first emerges (Jablonski et al., 2012), or whether deficits could be precipitated by a second immune challenge at this young juvenile age. Although it is evident that early life immune activation leads to deficits in learning in adulthood that are precipitated by a second immune challenge, it is also quite likely that the cascade of events responsible for such deficits occurs much earlier in development, and may be more severe at younger ages, when neural circuits are still being established. Second, it has never been investigated how early life immune activation alters the developing immune system as these alterations may be unique to specific periods of neural development. Last, it has never been systematically examined whether biological sex differentially affects the vulnerability to early life immune activation at these ages.
Experiments 1.1 and 1.2 were designed to determine whether early life immune activation results in learning deficits at the onset of hippocampal-dependent learning and memory (P24) in a sex-dependent manner using previously established models (Bilbo et al., 2005, 2006; Williamson et al., 2011; Jablonski et al., 2012; Westbrook et al., 2014). We predicted that males would be more vulnerable to early-life immune activation, resulting in sex-dependent deficits in hippocampal-dependent learning. Experiment 2.1 was designed to determine the impact of early life immune activation on microglial activation and proinflammatory gene expression at the age when hippocampal-dependent learning in juvenile male and female rats first emerges. We predicted that neonatal infection, in conjunction with LPS, would produce exaggerated increases in proinflammatory cytokine expression, particularly IL-1β expression, in the HP and medial prefrontal cortex (mPFC) in juvenile males, but not females. Finally, Experiment 2.2 aimed to determine the impact of early life immune activation on the developing peripheral immune system. We predicted that neonatal infection, in conjunction with LPS, would produce exaggerated increases in proinflammatory cytokine expression from the peripheral immune system and alterations in circulating immune cells in juvenile males, consistent with epidemiological data described above.
MATERIALS AND METHODS
Animals and Breeding
Adult male and female Sprague Dawley rats were ordered from Envigo Laboratories in Indianapolis, Indiana. Rats were housed in same sex pairs in clear, polyethylene cages (45 × 20.5 × 24 cm) and allowed one week of acclimation to the facility prior to breeding. The colony room was maintained at 22°C on a 12:12-h light:dark cycle (lights on at 0700 h) and all rats had ad libitum access to food and water. For breeding, male and female pairs were housed together for 5 days and the presence of sperm plugs was checked daily to determine the date of Embryonic Day 1. Two days prior to the calculated date of birth, P0, pregnant females were housed individually. Litter sizes and male to female ratios were not adjusted at the time of birth. For the behavioral experiments, only two to three rats of the same sex from any litter were assigned to an experimental condition. For gene expression analysis and whole blood analysis, at least three to four litters are represented in each experimental group. Sentinel rats were housed in the colony room and periodically examined for the presence of common rodent diseases. All tests came back negative. All experiments were approved by the University of Delaware Institutional Animal Care and Use Committee.
Separate groups of rats were used for each experiment and each experiment had eight groups based on sex (male or female), neonatal treatment (saline or E. coli) and P24 treatment (saline or LPS). Experiment 1.1 had a total of 96 rats (48 males and 48 females, n = 12/group, four to seven litters are represented in each group). Experiment 1.2 had a total of 79 rats (40 males and 39 females, n = 9–10/group, six to eight litters are represented in each group). All behavioral testing in Experiments 1.1 and 1.2 took place between 12:00 p.m. and 5:00 p.m. and all rats were euthanized with CO2 immediately following completion of behavioral testing. Experiment 2.1, had a total of 67 rats (32 males and 35 females, n = 8–10/group). On P24, all rats in Experiment 2.1 were injected with saline or LPS between 7:00 a.m. and 9:00 a.m. and 4 h later were perfused to collect brain areas of interest (HP and mPFC) and spleen for the analysis of inflammatory cytokine expression. Experiment 2.2 had a total of 80 rats (40 males and 40 females, n = 10/group). On P24, all rats in Experiment 2.2 were injected with saline or LPS between 7:00 and 9:00 a.m. and 4 h later euthanized to collect whole blood via cardiac puncture for analysis of circulating white blood cells (WBCs). This 4-h time point for tissue and blood collection has been shown to be around the peak response of many cytokines following LPS challenge (Bilbo et al., 2005; Henry et al., 2009; Wei et al., 2015) and thus, was chosen as the appropriate time point for these experiments examining LPS-induced cytokine expression.
Bacterial Culture and Neonatal Infection
Prior to the start of the study, E. coli (ATCC 1547; American Type Culture Collection, Manassas, VA) culture vial contents were hydrated and grown overnight at 37°C in 30 mL of brain–heart infusion (BHI). Cultures were aliquoted in 1 mL stock samples, supplemented with 10% glycerol and stored at −20°C. Prior to injections, a stock culture was thawed and incubated 19–24 h in 40 mL of BHI at 37°C. The culture was removed from incubation and the number of bacteria present was determined by a microplate reader (BioTek; model ELx808) and the number of colony forming units (CFUs) was quantified by extrapolating from previously determined growth curves. Cultures were centrifuged at 300g for 15 min, supernatants were discarded, and the bacteria were re-suspended in the appropriate volume of sterile, pyrogen-free, Dulbecco’s phosphate buffered saline (DPBS) for a final concentration of 1 × 106 CFU of live bacterial E. coli. On P4, pups were removed from the dam, counted, sexed, and administered a subcutaneous injection of either 0.1 mL of 1 × 106 CFU of E. coli or 0.1 mL of sterile DPBS as a control and returned to the dam within 5 min. All neonatal injections took place between 8:00 and 11:00 a.m. or 2:00 and 5:00 p.m. All pups in a litter were injected with the same treatment to avoid the possibility of cross-contamination by E. coli treated pups. Additional concerns with E. coli treated litters may include the quality of maternal care, or perhaps that the infection could spread to the dam, thereby affecting maternal care to the pups. We quantified maternal behavior twice a day over the next three 3 days post-infection and found no significant differences in arched back nursing or licking/grooming of the pups between E. coli and saline litters (data not shown), similar to other previous reports (Bilbo et al., 2006; Spencer et al., 2006). Additionally, we found no differences in the average weight of the E. coli treated rats and control treated rats by P24, indicating no gross differences in weight gain prior to weaning. Pups were weaned into same sex groups on P21, but were otherwise allowed to mature, undisturbed, until P24 at the time of behavioral testing (Experiments 1.1 and 1.2), tissue collection (Experiment 2.1), or whole blood analysis (Experiment 2.2).
LPS Injections
All experiments used LPS derived from E. coli 0111:B4 obtained from Sigma Aldrich (Cat. No. L2630). Sterile, pyrogen-free DPBS was used to dilute the stock concentration of LPS (2,500 μg/mL) to a final concentration of 25 μg/mL for injections. On P24, rats in all experiments received an intraperitoneal (i.p.) injection of either 1 mL/kg of 25 μg/mL of LPS or the equivalent volume of DPBS as a control. This low dose of LPS was used in the current experiments to elicit an increase in cytokine production in the brain (see “Results” section) without producing overt sickness behavior that might interfere with the behavioral testing.
Euthanasia for Tissue and Whole Blood Collection
In Experiment 2.1, 4 h following saline or LPS injections on P24, rats were euthanized by administration of an overdose of Euthasol (ANADA 200–071) via i.p. injection. Once anesthetized, rats were perfused via cardiac puncture with ice-cold, 0.9% saline solution to remove blood and peripheral immune cells from the brain. Following perfusion, the whole HP, mPFC, and spleen were collected and immediately flash frozen on dry ice. Samples were stored at −80°C until further analysis. In Experiment 2.2, 4 h following saline or LPS injections on P24, rats were euthanized by administration of an overdose of Euthasol (ANADA 200–071) administered via i.p. injection. Once anesthetized, whole blood was collected via cardiac puncture into EDTA coated tubes and stored on ice until time of analysis.
Quantitative Real-Time PCR
RNA was extracted from frozen tissue samples using Isol-RNA Lysis Reagent (Cat. No. 2302700, 5 Prime). Genomic DNA was eliminated and cDNA was synthesized from extracted RNA (1,000 ng/μL) using the QuantiTect Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was quantified by real-time PCR using the RealMasterMix Fast SYBR Kit (Cat. No. 2200830, 5 Prime) in 10 μL reactions on a CFX96Touch real time PCR machine. IL-6 was analyzed using a QuantiTect Primer Assay (Cat. No. QT00182896) and diluted according to protocol. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 μM (18s, CD11b, and IL-1β; see Table 1 for a list of primer sequences). 18s was used as the housekeeping gene for all experimental groups as it did not differ significantly across any groups. Samples were numbered and blinded to treatment group and run in duplicate on real-time PCR plates. For each reaction, the average quantitative threshold amplification cycle number (Cq) value was determined from each duplicate, and the 2−ΔΔCq method was used to calculate the relative gene expression for each gene of interest relative to the housekeeping gene.
Table 1.
Rat Primers used in the Current Experiments for Quantitative Real-Time PCR
| Gene | NCBI Sequence | Primers |
|---|---|---|
| 18S | AB_970462.1 | F: ATGGTAGTCGCCGTGCCTA R: CTGCTGCCTTCCTTGGATG |
| CD11b | NM_012711.1 | F: CTGGGAGATGTGAATGGAG R: ACTGATGCTGGCTACTGATG |
| IL-1β | NM_031512.2 | F: GAAGTCAAGACCAAAGTGG R: TGAAGTCAACTATGTCCCG |
Analysis of Whole Blood Samples for WBC Counts
Whole blood samples were collected in EDTA coated tubes (Medical Supply 123, Cat. No. 07–6013) and kept on ice for a minimum of 30 min, but no more than 4 h to be analyzed on the HEMAvet machine (Drew Scientific; Model No. HV950) according to the protocol. The HEMAvet machine analyzes the counts of circulating WBCs including lymphocytes, neutrophils, monocytes, eosinophils and basophils. Prior to running samples, a species-specific, synthetic control blood (Drew Scientific; Cat. No. MULTI-1835–01) was run to ensure proper calibration of the machine.
Behavioral Testing
Context Pre-Exposure Facilitation Effect Paradigm
Procedure
Hippocampal-dependent learning was assessed using a modified version of contextual fear conditioning called the Context Pre-exposure Facilitation Effect (CPFE) paradigm (Fanselow, 1990; Rudy et al., 2004; Burman et al., 2009), combined with an LPS challenge around the time of learning (Bilbo et al., 2005, 2006). All rats were numbered (i.e. blind to treatment) throughout the duration of the experiment. Rats began this task on P24, which is the earliest age at which robust and reliable learning of the CPFE task emerges (Jablonski et al., 2012). CPFE is a 3-day task where on P24 (Day 1) rats were placed in a context and allowed to freely explore and learn about the context for 5 min. Immediately following the completion of the pre-exposure, rats were administered an i.p. injection of either LPS or saline. Twenty-four hours later on P25 (Day 2), rats were put back in the same context, received an immediate shock (2, 2-s, 0.7 mA [direct current] shocks spaced 1 second apart), and then immediately removed from the context. Twenty-four hours following the immediate shock, on P26 (Day 3), rats were placed back into the context for 5 min and learning was measured by assessing the amount of time spent freezing. As a control procedure, the Immediate Shock Deficit (ISD) paradigm was administered to a separate group of juvenile male and female rats (n = 20 total; Supporting Information Fig. S1). Rats in this task do not receive the Day 1 pre-exposure to the context on P24, but do receive the immediate shock on Day 2 and test on Day 3 as described above. This control task was used to confirm that freezing on test day was a result of a hippocampal-dependent, learned-association between the context on Day 1 and the shock on Day 2, rather than a more generalized form of fear learning to the shock alone (Fanselow, 1990; Rudy et al., 2004).
Apparatus
Two identical chambers were obtained from TSE Systems (2560606 Series; Germany) and connected to the TSE Systems software program (TSE Multi Conditioning System – Fear Conditioning). Each chamber was white with two doors in the front of the chamber that locked closed and housed the conditioning boxes. Each chamber was fitted with sound attenuating foam, interior lights and a TSE Systems camera (Type No. 256060-VID-KIT/CAM) that was mounted at the top of the chamber directly above each conditioning box. All four walls of each conditioning box (44 × 37 × 44 cm) were made of black Plexiglas. Two walls had vertical, white stripes covering them (front and back walls of the boxes) constructed from painter’s tape, the left wall had a large white outline of a triangle constructed from painters tape, and the right wall had a large white outline of a square, also constructed from painters tape. The shock was delivered through a removable floor of stainless steel rods that were 0.5 cm in diameter, spaced 1cm apart, and wired to a TSE Systems generator (PROCESS CONTROL MultiConditioningSystem 256060 Series). The chambers were cleaned with Quatricide and then water before each rat was placed inside. Videos from the test session on Day 3 were exported and two observers, blind to the treatment conditions and trained to 85% inter-observer reliability, scored the freezing behavior for each rat. Freezing behavior was defined as the complete absence of movement with the exception of that required for breathing. A frequency score was generated by assessing every 5 s whether the rat was freezing or not throughout the entire 5-min testing session (60, 5-s intervals).
Novel Object Location Task
Procedure
All rats are numbered, blind to treatment, throughout the duration of the experiment. In this experiment, we tested the effect of neonatal infection and a mild immune challenge on hippocampal-dependent recognition memory using the NOL task with a 24-h delay. This paradigm is similar to previous paradigms though with slightly longer habituation trials and different objects (Ennaceur & Delacour, 1988; Westbrook et al., 2014). On P23 (Day 1), rats received two, 10-min habituation sessions spaced 5 h apart. On P24 (Day 2), 24 h following the first habituation, rats received a final habituation session followed 5 h later by a 5-min sample session in which rats could learn about the objects. Immediately following completion of the sample session, rats received an i.p. injection of either 25 μg/kg of LPS or saline. On P25 (Day 3), 24 h after the sample session, rats received a 3-min test session.
Apparatus
Four identical chambers (Stoelting Co.; 45 × 45 cm) with black, Plexiglas walls and grey plastic floors were used for the NOL task. Each chamber contained two proximal cues that are effective for this type of incidental learning in juvenile rats (Burman et al., 2009). The back wall of each chamber was marked with a cross made from white painter’s tape. The left wall of each chamber had a circle cut out from yellow poster board lined with horizontal green stripes made from painter’s tape. For the three habituation sessions, the chambers were completely devoid of any objects. For the sample session, two identical white plastic hooks were secured to the floor in one of two configurations. In Configuration 1, the hooks were placed in positions 1 and 4 and in Configuration 2 the hooks were placed in Positions 2 and 3. For the test session, the hook that changed positions was moved to one of two possible new positions. For example, in configuration 1 the hook in Position 1 could be moved to either 2 “new” or 3 “new” while the hook in Position 4 would remain in the same position it had been during the sample session (see Westbrook et al., 2014 for a diagram of the chamber configurations). Hooks were always secured such that the “hooked” side faced the left wall of the chamber and the flat side faced the right wall. All rats were placed into the middle of the chamber facing the back wall with the white cross. For all habituation sessions, rats were handled for 3 min immediately prior to being placed in the chamber. All chambers were cleaned with Quatricide prior to rats being placed in the chamber. Sample and test sessions were recorded via a video camera (Clover Electronics CM625 with Panasonic PLZ727 lens) that was mounted directly above the four chambers. Two observers blind to the treatment conditions and trained to 85% inter-observer reliability later scored each video from the testing session to record the total time spent exploring each object. Exploration of an object was defined as actively sniffing, pawing at, or whisking with its snout directed toward the object and being within < 2 cm from the object (Ennaceur, 2010). The percent of time spent exploring the novel object was calculated by dividing the total time spent exploring the novel object by the total time spent exploring both the novel and familiar object (i.e. a discrimination ratio) as described in Blaser and Hayser (2015).
Statistical Analyses
Data were analyzed using the statistical software program SPSS (IBM). Data from all experiments were analyzed using 2 × 2 × 2 ANOVAs with Sex (male vs female), Neonatal Treatment (Saline vs. E. coli), and P24 Treatment (Saline vs. LPS) as the between-subjects factors. Significant interactions were followed up with post hoc pairwise comparison tests using the Bonferroni correction to control for multiple comparisons and examine between group differences. When violations of normality were found in the data, Mann-Whitney U tests were conducted. The accepted significance level for these analyses was P < 0.05. Additionally, discrimination ratios from the NOL task in Experiment 1.2 were analyzed following convention (Akkerman et al., 2012), using one-sample t-tests to compare discrimination ratios of each group to a discrimination ratio indicating no discrimination (0.5). The accepted significance level for these analyses was P < 0.05. One male was excluded from the analyses of NOL in Experiment 1.2 because he did not spend any time investigating the objects during the test. One male was excluded from all HP gene expression analyses in Experiment 2.1 due to insufficient quantities of RNA.
RESULTS
Experiment 1.1: Impact of Neonatal Infection on Hippocampal-Dependent Learning in the CPFE Paradigm in Juvenile Males and Females Following a Mild LPS Challenge
Neonatal Infection Does Not Significantly Disrupt Learning of the CPFE at P24 Alone or in Conjunction with LPS
We tested hippocampal-dependent learning using the CPFE paradigm on P24, when learning in this task first emerges in rats (Jablonski et al., 2012). Learning was tested in control and neonatally infected male and female rats in the presence or absence of LPS given immediately after context pre-exposure on Day 1 (P24) of the paradigm. Contrary to our predictions, we found no significant interactions, Sex × Neonatal Treatment × P24 Treatment (F1,88 = 2.446, P = 0.121), or main effects of Sex (F1,88 = 0.041, P = 0.841), Neonatal Treatment (F1,88 = 0.291, P = 0.591), or P24 Treatment (F1,88 = 0.002, P = 0.967) on freezing levels in the CPFE paradigm (Fig. 1A), indicating that there are no significant learning deficits in rats on this cognitive task as a result of neonatal infection either alone or in conjunction with LPS at the time of learning at this age. Importantly, we were able to validate our parameters for the CPFE by running them in a behavioral control paradigm, the ISD task. All of our juvenile rats could learn the CPFE task relative to behavioral controls (t112 = −2.913, P = 0.004; Supporting Information Fig. S1) confirming that freezing on test day was a result of a hippocampal-dependent, learned-association between the context on Day 1 and the shock on Day 2, rather than a more generalized form of fear learning to the shock alone (Fanselow, 1990; Rudy et al., 2004).
Figure 1.
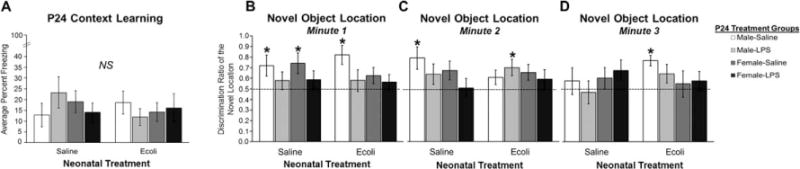
Impact of neonatal infection on hippocampal-dependent learning and memory in juvenile male and female rats following a mild immune challenge of LPS (25 μg/kg). (A) The average percent freezing on Day 3 of the CPFE paradigm. (B) The average discrimination ratio of time spent exploring the novel location during the first minute of the NOL task. (C) The average discrimination ratio of time spent exploring the novel location during the second minute of the NOL task. (D) The average discrimination ratio of time spent exploring the novel location during the third minute of the NOL task. Error bars represent the ±SEM. *P < 0.05 indicates discrimination ratios significantly greater than chance value (50%). NS indicates no statistically significant differences.
Experiment 1.2: Impact of Neonatal Infection on Hippocampal-Dependent Learning in the NOL Task in Juvenile Males and Females Following a Mild LPS Challenge
LPS Challenge Produces Learning Deficits in the NOL Task in Juvenile Males and Females
Next, we examined hippocampal-dependent learning and memory using the NOL task, on P24 with a 24-h retention period between the sample and test sessions. We found distinct effects throughout the 3-min NOL test, indicative of rapid recognition memory during early phases of the test, thus we analyzed each phase of the 3-min test separately. Analysis of the data collected during the first minute of the NOL test using one-sample t-tests against chance revealed that LPS produced significant learning deficits in control (t8 = 0.906, P = 0.388) and neonatally infected males (t9 = 0.775, P = 0.458) and control (t9 = 0.989, P = 0.349) and neonatally-infected females (t9 = 0.846, P = 0.419; Figure 1B), as none of the LPS-treated rats were able to express significantly above chance discrimination for the novel object location. Neonatal infection alone was also sufficient to produce learning deficits, but only in females (t8 = 1.570, P = 0.155). A 2 × 2 × 2 ANOVA for the data collected in the first minute supports these overall results, revealing a significant main effect of P24 Treatment (F1,70 = 7.356, P = 0.008). Analysis of the data collected during the second minute of the NOL test using one sample t-tests revealed that the LPS-induced learning deficit was still evident in all groups with the exception of neonatally infected males treated with LPS (t9 = 2.525, P = 0.032; Fig. 1C). A 2 × 2 × 2 ANOVA for the data collected in the second minute, however, revealed no significant main effects or interactions for Sex, Neonatal Treatment, or P24 Treatment. Finally, analysis of the data collected during the third minute of the NOL test revealed that only neonatally infected control males continued to show above chance levels of discrimination (t9 = 5.466, P < 0.001) while no other groups were showing discrimination ratios above chance value (Fig. 1D). A 2 × 2 × 2 ANOVA revealed no significant main effects or interactions of Sex, Neonatal Treatment, or P24 Treatment for Minute 3. Taken together, these data indicate that at P24 rats are capable of recognizing the novel object location within the first and second minute of the NOL task, but that treatment with LPS significantly interferes with this recognition memory. Moreover, in females neonatal infection alone or in conjunction with LPS produced significant learning deficits such that these females never showed a discrimination ratio for the novel location above chance value during any minute of the task.
Experiment 2.1: Impact of Neonatal Infection on the Neuroimmune Response of Juvenile Males and Females Following a Mild LPS Challenge
Neonatal Infection Produces Increased Baseline IL-1β Expression and, in Conjunction with LPS, Produces Exaggerated IL-1β Expression and Suppressed IL-6 Expression in the Juvenile HP
In this experiment, control and neonatally infected rats were administered LPS or saline on P24 and inflammatory gene expression was examined in the brain 4 h later. In the HP, we found a main effect of Neonatal Treatment on IL-1β expression (F1,58 = 4.251, P = 0.044). Specifically, neonatally infected males and females showed increased expression of IL-1β as juveniles, even in the absence of LPS exposure (Fig. 2A). We also found a main effect of P24 Treatment which revealed that LPS-treated juvenile rats had increased IL-1β expression relative to saline-treated juvenile rats (F1,58 = 27.962, P < 0.001; Fig. 2A). Interestingly, post hoc tests indicated that both treatments were additive, as neonatally infected males and females treated with LPS as juveniles had significantly greater IL-1β expression relative to their uninfected controls treated with LPS as juveniles (P’s < 0.0001; Fig. 2A). We also found a main effect of Neonatal Treatment on IL-6 expression in the HP, with neonatally infected rats having lower IL-6 expression compared to uninfected controls (F1,58 = 5.991, P = 0.017; Fig. 2B). No significant main effects or interactions were found for the expression of CD11b, a marker of microglial activation, in the HP (Fig. 2C).
Figure 2.

Impact of neonatal infection on proinflammatory gene expression in the HP of juvenile male and female rats following a mild immune challenge of LPS (25 μg/kg). (A) Relative gene expression of the proinflammatory cytokine IL-1β in the HP. (B) Relative gene expression of the proinflammatory cytokine IL-6 in the HP. (C) Relative gene expression of a marker of microglia activation, CD11b, in the HP. Error bars represent ± SEM. *P < 0.05 indicates the main effect of neonatal infection causing 1) increased baseline IL-1β expression and 2) attenuated IL-6 expression in juvenile male and female rats. **P < 0.01 indicates the main effect of LPS causing increased IL-1β expression in juvenile male and female rats regardless of previous neonatal infection. †P < 0.0001 indicates post hoc tests showing the additive effect of neonatal infection and LPS causing exaggerated IL-1β expression in juvenile male and female rats. NS indicates no statistically significant differences.
Neonatal Infection Produces Exaggerated IL-1β Expression in Females and Suppressed IL-6 Expression in Males in Response to LPS in the Juvenile mPFC
In the mPFC, a brain region that is also important for the expression of spatial learning (Asok et al., 2013), statistical analysis revealed a significant Sex × Neonatal Treatment × P24 Treatment interaction for IL-1β expression (F1,59 = 6.421, P = 0.014) and post hoc tests revealed that while all rats showed increased IL-1β expression following LPS (P’s < 0.05) compared with saline treated rats, only neonatally-infected females, not males, showed exaggerated expression following LPS challenge as juveniles (P = 0.002; Fig. 3A). Statistical analysis also revealed a significant Sex × Neonatal Treatment × P24 Treatment interaction for IL-6 expression in the mPFC (F1,59 = 6.707, P = 0.012). Post hoc tests indicated that neonatally infected males, but not females, had attenuated expression of IL-6 in response to LPS as juveniles relative to neonatal control males treated with LPS (P < 0.001; Fig. 3B). This three-way interaction is explained by the finding that control females express low levels of IL-6 in response to LPS and neonatal infection does not affect this response. A main effect of P24 Treatment was found for CD11b expression in the mPFC (F1,59 = 6.345, P = 0.015) indicating that LPS treatment increases the expression of CD11b, a microglial activation marker, in the mPFC regardless of neonatal treatment in both juvenile males and females (Fig. 3C).
Figure 3.

Impact of neonatal infection on proinflammatory gene expression in the mPFC of juvenile male and female rats following a mild immune challenge of LPS (25 μg/kg). (A) Relative gene expression of the proinflammatory cytokine IL-1β in the mPFC. (B) Relative gene expression of the proinflammatory cytokine IL-6 in the mPFC. (C) Relative gene expression of a marker of microglia activation, CD11b, in the mPFC. Error bars represent ±SEM. *P < 0.05 indicates the main effect of neonatal infection causing juvenile male and female rats to have increased baseline IL-1β expression; **P < 0.05 indicates the effect of LPS causing increased IL-1β and CD11b expression in juvenile male and female rats regardless of neonatal infection status. †P < 0.005 indicates post hoc tests following significant Sex × Neonatal Treatment × P24 Treatment interactions showing 1) the additive effect of neonatal infection and LPS causing exaggerated IL-1β expression in juvenile females, but not males and 2) the suppression of IL-6 expression in neonatally infected juvenile males, but not females following LPS.
Experiment 2.2: Impact of Neonatal Infection on Peripheral Immune Function in Juvenile Males and Females Following a Mild LPS Challenge
Neonatal infection Attenuates the Induction of IL-6 Expression in the Spleen of Juvenile Males and Females in Response to LPS
In the spleen, analysis of IL-1β expression revealed a main effect of P24 treatment (F1,58 = 87.679, P < 0.001), as all rats showed increased expression in response to LPS as juveniles (Fig. 4A). We found a significant Sex × Neonatal Treatment × P24 Treatment interaction for IL-6 expression in the spleen (F1,58 = 6.716, P = 0.012; Fig. 4B). Post hoc tests revealed that while LPS increased IL-6 expression in the spleen of neonatal control males and females (P < 0.001 and P = 0.010, respectively), neonatally infected males, but not females, had significantly attenuated expression of IL-6 (P = 0.0001) relative to neonatal control males treated with LPS as juveniles (Fig. 4B).
Figure 4.

Impact of neonatal infection on proinflammatory gene expression in the spleen, a peripheral immune organ, of juvenile male and female rats following a mild immune challenge of LPS (25 μg/kg). (A) Relative gene expression of the proinflammatory cytokine IL-1β from the spleen. (B) Relative gene expression of the proinflammatory cytokine IL-6 from the spleen. Error bars represent ± SEM. *P < 0.05 indicates the main effect of LPS causing increased IL-1β and increased IL-6 expression in male and female rats regardless of neonatal infection status. †P < 0.0001 indicates post hoc tests following significant Sex × Neonatal Treatment × P24 Treatment interaction showing the effect of neonatal infection further attenuating IL-6 expression following LPS in juvenile males.
Neonatal Infection Suppresses Levels of Circulating WBCs, Including Monocytes, in Juvenile Males and Females, and Suppresses Levels of Circulating Neutrophils in Juvenile Males, but Not Females in Response to LPS
We found a significant Neonatal Treatment × P24 Treatment interaction on the levels of circulating WBCs in juvenile rats (F1,72 = 6.081, P = 0.016; Table 2). Post hoc tests showed that neonatal infection significantly reduced the number of circulating WBCs in response to LPS in juvenile males (P = 0.005) and females showed a trend towards significance (P = 0.054) compared with all other groups (Table 2). We found a main effect of P24 Treatment on the levels of circulating lymphocytes (F1,72 = 4.117, P = 0.046); Table 2) such that LPS-treated rats showed decreased levels of circulating lymphocytes relative to their control counterparts (Table 2). Main effects of both Neonatal Treatment and P24 Treatment were observed for the number of circulating monocytes (F1,72 = 5.882, P = 0.018 and F1,72 = 4.810, P = 0.032, respectively; Table 2) such that neonatally infected rats and LPS-treated rats showed decreased levels of circulating monocytes relative to their control counterparts. A significant Sex × Neonatal Treatment interaction (F1,72 = 6.201, P = 0.015) and a significant Neonatal Treatment × P24 Treatment interaction (F1,72 = 5.599, P = 0.021) was found for the number of circulating neutrophils (Table 2). Post hoc tests revealed that neonatal infection significantly reduced the number of circulating neutrophils in juvenile males compared with females at baseline and in response to LPS (P = 0.019 and 0.042, respectively; Table 2). No significant main effects or interactions for Sex, Neonatal Treatment, or P24 Treatment were observed for the number of circulating eosinophils and basophils (Table 2).
Table 2.
Impact of Neonatal Infection on Circulating Peripheral WBCs, Including Lymphocytes, Neutrophils, Monocytes, Eosinophils, and Basophils in Juvenile Male and Female Rats Following a Mild Immune Challenge of LPS (25 μg/kg)
| Cell Type | Male
|
Female
|
Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline
|
E. coli
|
Saline
|
E. coli
|
||||||
| Saline | LPS | Saline | LPS | Saline | LPS | Saline | LPS | ||
| Total WBC (k/μL) | 2.97 ±0.21 | 3.2 ±0.35 | 2.45 ± 0.37 | 1.72 ±0.22† | 2.61 ± 0.24 | 3.41 ± 0.48 | 3.18 ±0.46 | 2.41 ± 0.45† |
Neonatal Trmt × P24 Trmt: F1,72 = 6.081; P = 0.016 |
| Lymphocytes (k/μL) | 1.62 ±0.13 | 1.46 ± 0.20# | 1.42 ±0.27 | 0.92 ±0.14† | 1.48 ±0.17 | 1.43 ±0.18# | 1.3 ±0.09 | 1.06±0.28† |
P24 Trmt: F1,72 = 4.117; P = 0.046 |
| Neutrophils (k/μL) | 1.16 ±0.12 | 1.61 ± 0.24 | 0.84 ± 0.13† | 0.75 ± 0.08† | 0.97 ±0.10 | 1.8 ±0.29 | 1.41 ± 0.23 | 1.26 ±0.20 |
Sex × Neonatal Trmt: F1,72 = 6.201; P = 0.015 Neonatal Trmt × P24 Trmt: F1,72 = 5.599; P = 0.021 |
| Monocytes (k/μL) | 0.16 ±0.04 | 0.12 ± 0.03# | 0.10 ± 0.02* | 0.06 ± 0.01† | 0.15 ± 0.03 | 0.15 ± 0.04# | 0.15 ± 0.04* | 0.07 ± 0.02*# |
Neonatal Trmt: F1,72 = 5.882; p = 0.018 P24 Trmt: F1,72 = 4.810; p = 0.032 |
| Eosinophils (k/μL) | 0.008 ± 0.002 | 0.013 ± 0.0021 | 0.013 ± 0.004 | 0.013 ±0.004 | 0.007 ± 0.003 | 0.023 ± 0.005 | 0.019 ± 0.008 | 0.015 ± 0.004 | NS |
| Basophils (k/μL) | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.025 ± 0.024 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.006 ± 0.003 | 0.016 ±0.010 | 0.002 ± 0.001 | NS |
| % Lymphocytes | 55.36 ± 2.93 | 45.71 ±3.57 | 58.59 ± 4.06 | 50.67 ± 2.4 | 56.37 ± 3.24 | 43.44 ± 1.95 | 50.48 ± 3.99 | 41.11 ± 3.81 | |
| % Neutrophils | 38.56 ± 2.17 | 50.36 ± 3.37 | 32.52 ± 2.05 | 45.09 ± 2.3 | 37.63 ± 3.01 | 51.7 ± 1.76 | 43.93 ±4.08 | 55.33 ± 3.93 | |
| % Monocytes | 5.73 ± 1.11 | 3.31 ± 0.59 | 4.16 ± 0.72 | 3.13 ±0.24 | 4.85 ± 0.66 | 3.84 ± 0.68 | 4.27 ± 0.56 | 2.79 ± 0.47 | |
| % Eosinophils | 0.295 ± 0.073 | 0.429 ± 0.085 | 0.461 ± 0.115 | 0.637 ±0.128 | 0.304 ± 0.087 | 0.740 ±0.183 | 0.656 ± 0.235 | 0.673 ± 0.160 | |
| % Basophils | 0.034 ± 0.012 | 0.077 ± 0.032 | 0.078 ± 0.030 | 0.115 ± 0.030 | 0.048 ± 0.031 | 0.280 ± 0.115 | 0.207 ± 0.102 | 0.084 ± 0.024 | |
Data are presented as the mean ± SEM for each cell type.
P < 0.05 indicates a main effect of Neonatal Treatment;
P < 0.05 indicates a main effect of P24 Treatment;
P < 0.05 indicates significant post hoc tests. The percentage of WBCs that individual cell types represent is listed for reference.
DISCUSSION
The goal of the current experiments was to increase our understanding of how neonatal immune activation may impact hippocampal-dependent learning and the function of the immune system during juvenile development. Thus, we examined the effect of neonatal infection on hippocampal-dependent learning and memory, cytokine production in the HP and mPFC, peripheral immune function in the spleen, and levels of circulating WBCs in juvenile male and female rats at an age when hippocampal-dependent learning first emerges (Jablonski et al., 2012). Contrary to our initial predictions, we found no effect of neonatal infection or LPS treatment on learning in the CPFE paradigm in juvenile male and female rats. Given these surprising findings, we sought to determine whether neonatal infection may result in deficits in a separate hippocampal-dependent task, the NOL task. Again, contrary to expectations, we found that LPS treatment, administered immediately after the sample phase (i.e. learning) of the NOL task, produced significant learning deficits during the task in both males and females, regardless of previous neonatal infection.
We found that neonatal infection in conjunction with LPS produced exaggerated IL-1β expression in the HP of juvenile males and females and even increased expression at baseline (in the absence of subsequent LPS) relative to uninfected controls. IL-6 expression in response to LPS was attenuated in neonatally infected juvenile males and females, and we observed no changes in CD11b, the marker of microglial activation in the HP. In the mPFC, neonatal infection in conjunction with LPS produced exaggerated IL-1β expression in juvenile females, but not males and suppressed IL-6 expression in juvenile males, but not females, suggesting sex-specific programming effects of neonatal infection on cytokine expression within the juvenile mPFC. We saw no changes in CD11b expression in the mPFC as a result of neonatal infection; however, LPS produced increased CD11b expression in both juvenile males and females. In the spleen, LPS increased IL-1β expression in males and females, regardless of neonatal infection. LPS increased IL-6 expression in neonatally infected and control rats, but neonatal infection significantly attenuated this response in males only in the spleen. Overall, neonatal infection suppressed circulating WBCs (with the exception of eosinophils and basophils), an effect that was further exacerbated in response to LPS challenge in juvenile rats. Neonatal infection produced sex-specific suppression of neutrophils such that males showed a significantly greater suppression than females in response to LPS.
Neonatal Infection and Hippocampal-Dependent Learning in Juveniles
We predicted that neonatal infection in conjunction with a second immune challenge at the time of learning would result in learning deficits in males in the CPFE paradigm. Contrary to our predictions, all rats were able to learn the CPFE task and neither neonatal infection nor LPS produced learning deficits in male or female rats. Importantly, all rats exhibited freezing levels around 20–25% which is typical of juveniles at this age and with these task parameters (Chang et al., 2009; Schiffino et al., 2011; Jablonski et al., 2012; Dokovna et al., 2013). In adult male rats, neonatal infection results in learning deficits following a second immune challenge at the time of learning (Bilbo et al., 2005). These adult rats exhibit exaggerated cytokine production (e.g. IL-1β) in response to LPS in the HP, and administration of a caspase-1 inhibitor to block IL-1β synthesis prevents these learning deficits providing strong evidence that exaggerated IL-1β expression in the HP produces these cognitive deficits (Bilbo et al., 2005). These data support the notion that immune activation during the perinatal period leads to programming of inflammatory responses making male rats more vulnerable to the effects of later-life immune challenges in adulthood. Despite the fact that we found no effect of our “two-hit” model of immune activation on learning in the CPFE paradigm in juvenile rats, we did find exaggerated IL-1β expression in the HP. From these data, we can conclude that exaggerated IL-1β expression had no effect on learning in the CPFE paradigm, an effect that may be unique to this young age.
Similarly, we can also conclude that age itself is a critical factor for determining whether, and possibly how, neonatal infection produces cognitive vulnerabilities that are precipitated by immune dysregulation. Indeed, if we consider findings from aged rodents, this is not a surprising notion. Aged rodents are far more vulnerable to the consequences of immune activation compared with younger animals (Bilbo, 2010; Liu et al., 2012; Norden et al., 2016; for a review see Niraula et al., 2016) and, in humans cognitive impairments observed in the aged population following infection or illness are prolonged and more severe relative to a younger population (Bodles and Barger, 2004; Perry, 2004). Much like aging, it is likely that earlier periods of neural development (e.g. juvenile and adolescence) also provide a unique stage of life within which the impact of neonatal or prenatal infection on the brain, cognition, and behavior, either alone or in conjunction with a second immune challenge, may be different relative to other periods of development (Zuckerman et al., 2003; Meyer et al., 2006; MacRae et al., 2015). These data suggest that this age may confer some level of resilience to this type of immune activation for hippocampal-dependent learning, at least in a short-term capacity.
In contrast to the CPFE, we found that LPS produced learning deficits in the hippocampal-dependent NOL task, regardless of neonatal infection or sex, an effect that was evident in both males and females in the first minute of the task, suggesting a delay in the ability to recall the previous location or identify the novel location of the object in rats that received the low-dose immune challenge immediately after learning (Fig. 1B). These LPS-induced deficits persisted into the second minute for all groups except the neonatally infected males suggesting that these rats are able to learn the novel location after a delay whereas LPS challenge prevents the other groups from ever learning the task, at least within the 3 min examined here. These data also indicate that control males and females are capable of learning the NOL task at this age as they show a preference for the novel location (i.e. discrimination ratio significantly above 0.5) immediately during the first and/or second minutes of the task. Interestingly, neonatally infected males that only received saline on P24 showed a preference for the novel location in Minutes 1 and 3, but not Minute 2. These males spent more time exploring the object in the familiar location in Minute 2 (7.40 s in Minute 2 compared with 3.00 and 3.71 s in Minutes 1 and 3, respectively) indicating that, while these males ultimately learn the task, they show a unique pattern of exploration for both object locations that is only observable when the task is analyzed minute-by-minute. Additionally, our data indicate that females may be more vulnerable to these immune challenges that disrupt memory in the NOL task. This finding is supported by the minute-by-minute analysis of the test as females that received LPS, neonatal infection alone, or neonatal infection in conjunction with LPS never showed learning above chance levels during any minute of the task. Importantly, the overall amount of time spent exploring the objects did not differ between females and males in any group, except that females consistently spent a greater amount of time exploring the object in the familiar location than males in a given minute or during the overall 3-min test. Indeed, there is some evidence to suggest that brain regions, including the HP, develop at different rates in males and females (Duarte-Guterman et al., 2015) and that males and females process spatial information differently (Daniel, 2006; Pleil & Williams, 2010), and thus may exhibit differential vulnerability to immune activation at this age. Importantly, these deficits in the NOL task cannot be explained simply by a decrease in the time spent exploring the objects as all rats spent the same amount of time, on average, exploring the objects during each minute of the test (data not shown). Deficits in the NOL task could be a result of increased IL-1β in the HP, as we predicted to find in the CPFE, or they may be a result of the increased IL-1β expression and CD11b expression in the mPFC. This is the first time the NOL task has been used in this “two-hit” model of immune activation; therefore, a causal link between changes in cytokine production and microglial activation on cognitive performance on the NOL remain to be determined. Notably, while both the CPFE and the NOL task are considered hippocampal-dependent, incidental learning tasks, there is evidence that the parahippocampal-hippocampal system that supports conjunctive representations (Rudy, 2009) is critical for spatial and context learning, but not incidental object recognition learning (Mumby et al., 2002; Barker and Warburton, 2011) indicating that these two tasks rely on somewhat different hippocampal circuits. Given that we found no impact of neonatal infection or LPS on learning in the CPFE, but found significant learning deficits in the NOL task as a result of LPS, it is likely that the different hippocampal neural circuits underlying the emergence and maintenance of these tasks develop at slightly different ages and are impacted differently by our model of immune activation during early juvenile development. Indeed, others have found that the ability to learn in the NOL task emerges several days prior to that of the CPFE (Krüger et al., 2012; Westbrook et al., 2014).
Collectively, our findings highlight the important role of age in determining the impact of perinatal programming by inflammation and subsequent immune activation on learning and cognitive deficits. In adult male rats, neonatal infection produces robust deficits in hippocampal-dependent learning that can be assessed using the CPFE paradigm; however, it is completely unknown at what age these deficits first become evident in development as a result of neonatal infection and/or in response to a subsequent immune challenge. By characterizing the ontogeny of these learning deficits in relation to immunological or microglial development we may better understand the etiology of these deficits, informing our understanding of neurodevelopmental disorders associated with early-life inflammation and immune dysregulation.
Neonatal Infection and Cytokine Expression in the Brain
Importantly, this is the first time that perinatal programming of proinflammatory cytokine expression has been shown in juvenile rats, in both males and females. Though we predicted that males may be more vulnerable to the effects of perinatal programming by early life immune activation, it is important to note that we found this effect to be consistent across both sexes. Notably, our finding of exaggerated IL-1β expression in the HP corroborates what has been previously reported in adult male rats suggesting that neonatal infection can cause permanent alterations in the immune system. Our results extend these findings by highlighting that they are evident quite early in development and also present in females. The cognitive deficits associated with exaggerated IL-1β in the HP previously reported in adult male rats, however, were not observed in our juvenile male and female rats. Our results suggest that there may be something unique about the juvenile HP in order to allow intact learning and memory despite neonatal infection and the subsequent immune challenge, making them less likely to exhibit learning deficits that are seen in adult rats using the same paradigm. Alternatively, the overall levels of cytokine expression (in particular, IL-1β in our model) produced in the brain at this immature stage of development (P24) may be relatively low compared with the levels produced by the same dose of LPS in adult rats (P60). There is some evidence for this notion, particularly in aged rats (Bossu et al., 2012; Fu et al., 2014), though how changes in cytokine expression produced by neonatal infection and subsequent LPS challenge may differ between juveniles and adults remain to be determined. It is likely that the neuroimmune system is still developing and therefore, cytokine production in the brain at P24 may not be as robust as it is at later ages, thus it is less likely to disrupt learning at P24.
We also found increased IL-1β expression, even in the absence of LPS, in juvenile males and females in the HP and mPFC. These findings suggest that neonatal infection programs a new baseline level of IL-1β expression, at least throughout this juvenile period of development, in two brain regions important for learning and memory. It is also possible that this increased baseline expression of IL-1β is the result of prolonged cytokine expression following the infection itself, rather than perinatal programming caused by the infection. We believe this is unlikely given previous data showing that, 1) IL-1β expression resolves itself within the neonatal brain 48-h post-infection, and 2) the presence of E. coli protein is cleared from the brain of neonates within 48 h of infection (Bilbo et al, 2005). Additionally, we found no significant effect of neonatal infection on baseline IL-1β expression in the spleen of juveniles, suggesting that this increase in baseline IL-1β in the brain is not the lingering result of infection, but is perinatal programming of cytokine expression that is specific to IL-1β in the brain. Importantly, however, previous data in adult male rats did not find an increase in baseline IL-1β expression in the HP (Bilbo et al., 2005) indicating that increased baseline IL-1β expression in the HP and mPFC of neonatally infected juveniles is specific to this period of neural development and possibly the result of interactions with on-going neural or immunological developmental processes.
In the HP, neonatal infection decreased the expression of IL-6, regardless of a second immune challenge in both male and female juvenile rats. In the mPFC, IL-6 expression was only decreased in neonatally infected males following LPS, suggesting that the LPS-induced inflammatory response in neonatally infected rats may be more sexually dimorphic in the mPFC compared with the HP during this period of neural development. Finally, we did not find any significant changes in our marker of microglial activation, CD11b, in the HP as a result of neonatal infection or LPS. This is not entirely surprising as we only administered a 25 μg/kg dose which often does not elicit overt increases in microglial activation (Williamson et al., 2011) and others have shown increases in proinflammatory cytokine expression in the absence of overt increases in markers of microglia activation (Giovanoli et al., 2016). Despite the lack of increase in CD11b expression observed in the HP, previous data confirm that microglia (i.e. CD11b+ cells) are almost exclusively the sole source of IL-1β in the HP (Williamson et al., 2011); therefore, we are confident in our conclusion that the changes in cytokine expression as a result of early life immune activation are reflective of changes in neuroimmune function (e.g. microglia function) even if increases in CD11b expression are not observed. LPS did increase microglial activation in the mPFC in both males and females regardless of neonatal infection, suggesting that the consequences of immune activation with the same stimulus can impact microglia differently depending on their location in the brain (Gautier et al., 2012; Grabert et al., 2016). These data provide evidence that some brain regions (or networks) are more vulnerable than others to immune activation during juvenile development. Regardless, it will be important to investigate other potential markers of microglial activation and different doses of LPS to elucidate the impact of neonatal infection and subsequent immune activation on microglia function and cytokine expression in brain regions important for learning and cognition.
Neonatal Infection and Peripheral Immune Function
In the spleen, we found that neonatal infection did not increase baseline expression of IL-1β, nor did it produce exaggerated IL-1β expression following subsequent immune challenge (Figure 4A). These data are consistent with what has been previously observed in neonatally infected adult male rats (Bilbo et al., 2006), suggesting that neonatal programming of IL-1β is possibly restricted to the brain. Although it has been shown that peripheral cytokines can influence cytokine production in the brain (Quan and Banks, 2007), a number of studies have also shown that the peripheral and central cytokines do not necessarily respond in the same fashion (Bilbo et al., 2005, 2006; Henry et al., 2009; Roque et al., 2016). In contrast, neonatal infection attenuated IL-6 expression in the spleen of males (Fig. 4B) in response to LPS, and this attenuation was mirrored in the HP of males and females and in the mPFC of males. There have been no previous reports of perinatal programming of IL-6 expression in the adult brain or periphery by neonatal infection, suggesting that the suppression of IL-6 in the spleen may be specific to the juvenile period of development.
Neonatal infection also decreased the levels of circulating WBCs at this age, including monocytes and neutrophils (though only in males; see Table 2) and LPS decreased the production of circulating lymphocytes. To our knowledge, this is the first time the effects of neonatal infection and a subsequent immune challenge on circulating WBCs have ever been examined. Our data confirm that neonatal infection can significantly impact the production of circulating peripheral immune cells or perhaps, their production and infiltration into specific tissues, although this was not explicitly examined here. In monocytes and neutrophils, LPS effects were only observed in neonatally infected males, suggesting sex-specific perinatal programming of long-term peripheral immune function for at least some cell types. These data are interesting in the context of epidemiological data indicating that individuals with certain neurodevelopmental disorders also display altered function of innate and adaptive immunity within the periphery (Derecki et al., 2010). It is unclear how these changes in peripheral immune function may directly influence neural function or behavior, but our goal is to one day, identify bio-markers that manifest with or contribute to learning or cognitive deficits and thus, gain a more comprehensive understanding of peripheral immune function and its impact on neural development and behavior. Future experiments will aim to understand how these changes in peripheral immune cells may influence neural function and behavior at this particular young age.
In conclusion, the goal of these experiments was to further our understanding of how perinatal programming may influence immune and cognitive function during juvenile brain development, a period of development that is relatively unexplored. Our results reveal that immune activation can produce alterations in cognitive function that are distinct across ages (Henry et al., 2009; Liu et al., 2012). Neuroscientists are only beginning to understand how the maturation of certain structures in the brain allows for the emergence of specific behaviors throughout development (Albani et al., 2014). Similarly, immunologists understand very little about the developing peripheral and central immune systems (Sharma et al., 2012). Thus, researchers understand perhaps even less about how the development of these two systems (neural and immune) may be perturbed by early life inflammation, resulting in the emergence of behavioral or cognitive disorders later in life. Future experiments in this area will continue to explore the impact of early life immune activation and perinatal programming on the development of immune and cognitive function with the goal of gaining a better understanding of the factors that contribute to the emergence or etiology of neurodevelopmental disorders linked to early life immune activation. Advancing our understanding of when and how learning deficits emerge as a result of early life immune activation is critical to refining current treatments and developing new therapies or preventative measures for human neurodevelopmental disorders.
Supplementary Material
Acknowledgments
We would like to acknowledge Arun Asok and Mark Stanton for their help in establishing the parameters necessary for juvenile learning in the CPFE and Hollie Sanders for her help in establishing the parameters necessary for juvenile learning in the NOL task. We would also like to thank Caitlin Posillico, Laurne Terasaki, Taylor Medina, Sarah Beamish, and Hallye Rosenbloom for their assistance in collecting the data presented here.
This work was supported by the National Institutes of Health [grant numbers R21MH101663, P20GM103653, and R01MH106553]
References
- Akkerman S, Prickaerts J, Steinbusch H, Blokland A. Object recognition testing: statistical considerations. Behav Brain Res. 2012;232:317–322. doi: 10.1016/j.bbr.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Albani S, McHall D, Dumas T. Developmental studies of the hippocampus and hippocampal-dependent behaviors: Insights from interdisciplinary studies and tips for new investigators. Neurosci Biobehav Rev. 2014;43:183–190. doi: 10.1016/j.neubiorev.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Schreiber W, Jablonski S, Rosen J, Stanton M. Egr-1 increases in the prefrontal cortex following training in the context pre-exposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 2013;106:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir H, Schendel D, Henriksen T, Hjort L, Parner E. Gestational age and autism spectrum disorder: trends in risk over time. Autism Res. 2016;9:224–231. doi: 10.1002/aur.1525. [DOI] [PubMed] [Google Scholar]
- Barker D, Gluckman P, Robinson J. Conference Report: fetal origins of adult diseases – report of the first international study group, Sydney, 29–30 October 1994. Placenta. 1994;16:317–320. doi: 10.1016/0143-4004(95)90118-3. [DOI] [PubMed] [Google Scholar]
- Bilbo S. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;64:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S, Biedenkapp J, Der-Avakian A, Watkins L, Rudy J, Maier S. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neuro. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S, Rudy J, Watkins L, Maier S. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Barker G, Warburton E. When is the hippocampus involved in recognition memory? J Neuro. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R, Hayser C. Spontaneous object recognition: A promising approach to the comparative study of memory. Front Behav Neurosci. 2015;9:1–12. doi: 10.3389/fnbeh.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodles A, Barger S. Cytokines and the aging brain – what we don’t know might help us. Trends Neurosci. 2004;27:621–626. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bossu P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J Neuroinflammation. 2012;29:101–113. doi: 10.1186/1742-2094-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle C, Boulet S, Schieve L, Cohen R, Blumberg S, Yeargin-Allsopp M, Kogan M. Trends in the prevalence in developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- Burman M, Murawski N, Schiffino F, Rosen J, Stanton M. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav Neurosci. 2009;123:1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Knapska E, Orsini C, Rabinak C, Zimmerman J, Maren S. Fear extinction in rodents. Curr Protoc Neurosci. 2009;8 doi: 10.1002/0471142301.ns0823s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. Effects of oestrogen on cognition: What have we learned from basic research? Neuroendocrinology. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Randolf A, Wildmann J, Besedovsky H, Jessop D. Re-exposure to endotoxin induces differential cytokine gene expression in the rat hypothalamus and spleen. Brain Behav Immun. 2009;23:776–783. doi: 10.1016/j.bbi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki N, Cron J, Lu Z, Xu E, Abbott S. Wild type microglia arrest pathology in a mouse model of rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki N, Privman E, Kipnis J. Rett syndrome and other autism spectrum disorders–brain diseases of immune malfunction? Mol Psychiatry. 2010;15:355–363. doi: 10.1038/mp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenni V, Gray J, Song C, Patel S, Hill M, Pittman Q. Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain Behav Immun. 2016;1591:346–400. doi: 10.1016/j.bbi.2016.07.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokovna L, Jablonski S, Stanton M. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav Brain Res. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea L. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1″ behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fanselow M. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Fu H, Yang T, Xiao W, Fan L, Wu Y, Terrando N, Wang T. Prolonged neuroinflammation after lipopolysac-charide exposure in aged animals. PLoS. 2014;9 doi: 10.1371/journal.pone.0106331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier E, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek K, Gordonov S, Mazloom A, Ma’ayan A, Chua W, Hansen T, Turley S, Merad M, Randolph G, Immunological Genome Consortium Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglial anomalies. Brain Behav Immun. 2016;55:25–38. doi: 10.1016/j.bbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoe T, Karavolos M, Clohisey S, Baillie J, Stevens M, Freeman T, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Huang Y, Wynne A, Godbout J. Peripheral lipopolysaccharide challenge promotes microglial activity in aged mice that is associated with exaggerated induction of both proinflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S, Schiffino L, Stanton M. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context pre-exposure facilitation effect. Dev Psychobiol. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop D, Besedovsky H, del Rey A. New insights into cytokine gene expression in the rat hypothalamus following endotoxin challenge. Neurochem Res. 2010;35:909–911. doi: 10.1007/s11064-009-0071-0. [DOI] [PubMed] [Google Scholar]
- Keil A, Daniels J, Forssen U, Hultman C, Cnattingquis S, Soderberg K, Feychting M, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiol. 2010;21:805–808. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger H, Brockmann M, Salamon J, Ittrich H, Hanganu-Opatz I. Neonatal hippocampal lesion alters the functional maturation of the prefrontal cortex and the early cognitive development of pre-juvenile rats. Neurobiol Learn Mem. 2012;97:470–481. doi: 10.1016/j.nlm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Leckman J. Commentary: what does immunology have to do with brain development and psychopathology? J Child Psychol Psychiatry. 2014;55:632–634. doi: 10.1111/jcpp.12259. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neurosci. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- MacRae M, Macrina T, Khoury A, Migliore M, Kentner A. Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neurosci. 2015;298:455–466. doi: 10.1016/j.neuroscience.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Meehan C, Harms L, Frost J, Barreto R, Todd J, Schall U, Shannon-Weickert C, et al. Effects of immune activation during early or late gestation on schizophrenia-related behavior in adult rat offspring. Brain Behav Immun. 2016;1591:30338–30350. doi: 10.1016/j.bbi.2016.07.144. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee B. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- Mumby D, Gaskin S, Glenn M, Schramek T, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niralula A, Sheridan J, Godbout J. Microglia priming with aging and stress. Neuropsychopharmacology. 2016;42:318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden D, Trojanowski P, Walker F, Godbout J. Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol Aging. 2016;44:22–41. doi: 10.1016/j.neurobiolaging.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pleil K, Williams C. The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm Behav. 2010;57:360–367. doi: 10.1016/j.yhbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks W. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Roque A, Ochoa-Zarzosa A, Torner L. Maternal separation activates microglial cells and induces and inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav Immun. 2016;55:39–48. doi: 10.1016/j.bbi.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Rudy J. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J, Huff N, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schiffino F, Murawski N, Rosen J, Stanton M. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J, Bilbo S. Sex, glia, and development: Interactions in health and disease. Horm Behav. 2012;62:243–253. doi: 10.1016/j.yhbeh.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J, Sholar P, Bilbo S. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2011;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Jen R, Butler A, Lavoie P. The developing human preterm neonatal immune system: A case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Martin S, Mouihate A, Pittman Q. Early-life immune challenge: Defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006;31:1910–1918. doi: 10.1038/sj.npp.1301004. [DOI] [PubMed] [Google Scholar]
- Tenk C, Kavaliers M, Ossenkapp K. Neonatal treatment with lipopolysaccharide differentially affects adult anxiety responses in the light-dark test and taste neophobia test in male and female rats. Int J Dev Neurosci. 2013;31:171–180. doi: 10.1016/j.ijdevneu.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Wei P, Liu Q, Li D, Zheng Q, Zhou J, Li J. Acute nicotine treatment attenuates lipopolysaccharide-induced cognitive dysfunction by increasing BDNF expression and inhibiting neuroinflammation in the rat hippocampus. Neurosci Lett. 2015;14:161–166. doi: 10.1016/j.neulet.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Westbrook S, Brennan L, Stanton M. Ontogeny of object versus location recognition in the rat: Acquisition and retention effects. Dev Psychobiol. 2014;56:1492–1506. doi: 10.1002/dev.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L, Sholar P, Mistry R, Smith S, Bilbo S. Microglia and memory: Modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


