Abstract
Hepatocellular carcinoma (HCC), also called malignant hepatoma, is one of the deadliest cancers due to its complexities, reoccurrence after surgical resection, metastasis and heterogeneity. Incidence and mortality of HCC are increasing in Western countries and are expected to rise as a consequence of the obesity epidemic. Multiple factors trigger the initiation and progression of HCC including chronic alcohol consumption, viral hepatitis B and C infection, metabolic disorders and age. Although Sorafenib is the only FDA approved drug for the treatment of HCC, numerous treatment modalities such as transcatheter arterial chemoembolization/transarterial chemoembolization (TACE), radiotherapy, locoregional therapy and chemotherapy have been tested in the clinics. Polymeric nanoparticles, liposomes, and micelles carrying small molecules, proteins, peptides and nucleic acids have attracted great attention for the treatment of various cancers including HCC. Herein, we discuss the pathogenesis of HCC in relation to its various recent treatment methodologies using nanodelivery of monoclonal antibodies (mAbs), small molecules, miRNAs and peptides. Synopsis of recent clinical trials of mAbs and peptide drugs has been presented with a broad overview of the pathogenesis of the disease and treatment efficacy.
Keywords: Hepatocellular carcinoma, drug delivery, miRNA, nanomedicines
1. Introduction
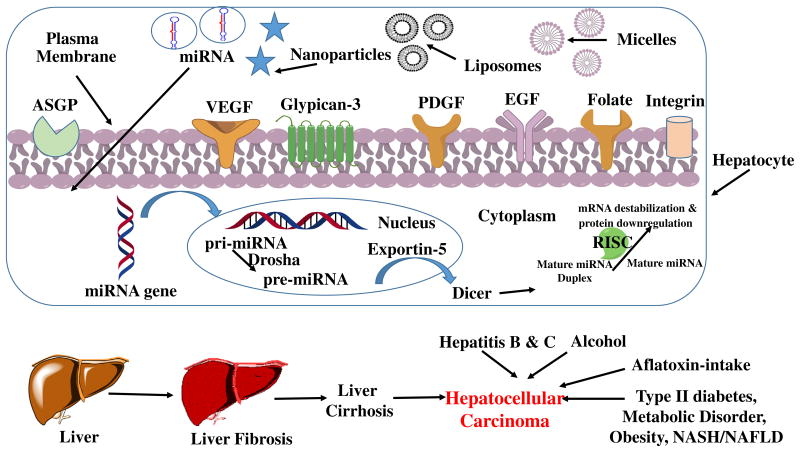
Hepatocellular carcinoma (HCC) is the sixth most common cancer and rank third in cancer-related deaths globally considering malignancies, severity, treatment challenges and 5-year survival rate (< 5 %) among cancer patients (Cabibbo, Latteri, Antonucci, & Craxi, 2009; Singh, Singh, Roberts, & Sanchez, 2014). Among various types of primary hepatic neoplasms such as cholangiocarcinoma (bile duct cancer of biliary epithelial cells), hepatoblastoma (rare early childhood malignant liver tumor), bile duct cystadenocarcinoma and epitheliod haemangioendothelima, HCC deserves special mention (Farazi & DePinho, 2006). The stimulus for HCC development in Asian and African countries arises from factors like chronic hepatitis B and C viral infection, chronic alcohol consumption, aflatoxin-contaminated food intake, other hereditary diseases (hemochromatosis due to iron overload in the body) and liver cirrhosis (Farazi & DePinho, 2006; Ge & Huang, 2015; Ghasemi, Rostami, & Meshkat, 2015). In the developed countries, the epidemiological evidence connecting the pathologies are type 2 diabetes, obesity, metabolic disorders and non-alcoholic steatohepatitis (NASH) as part of non-alcoholic fatty liver diseases (NAFLD) (Reeves, Zaki, & Day, 2016; Trojan, Zangos, & Schnitzbauer, 2016). The risk factors susceptible for the occurrence of HCC are more common in the male population compared to the females (4:1) (Yang, Ekanem, Sakyi, & Ray, 2015). Other factors such as age, tyrosinemia, galactosidemia, fructosemia, and hypothyroidism also play critical roles in the prevalence of HCC (Mazzanti, Arena, & Tassi, 2016; Zhu, Seto, Lai, & Yuen, 2016). Although surgical resection is the only curative therapy, it often causes hindrance to most patients detected at late stage of the disease. Following resection, the survival rate reaches 70 % if the tumor is < 2 cm (Varshosaz & Farzan, 2015). As a result, early diagnosis is crucial for successful treatment. Computed tomography (CT), magnetic resonance imaging (MRI), ultrasound and dynamic multiphasic multi-detector-row CT (MDCT) are the standard HCC diagnostic methods (Bertino et al., 2014; Daoudaki & Fouzas, 2014). Based on the tumor size, serum albumin, bilirubin and α-fetoprotein (AFP) levels, HCC morphology, presence of portal vein thrombosis and ascites, various classifications are used in order to categorize the stage of the disease, particularly Okuda staging system (I-III), Cancer of Liver Italian Program (CLIP) score (0-6), Barcelona Clinic Liver Cancer (BCLC) (Table 1), American Association for the Study of the Liver Disease (AASLD), National Comprehensive Cancer Network (NCCN) and European Association for the Study of the Liver (EASL) are noteworthy (Bertino et al., 2014; Trojan et al., 2016). Apart from liver transplantation, tumor local ablation, embolization, (Transcatheter arterial chemoembolization/transarterial chemoembolization or TACE) and chemotherapy are often recommended for patients with BCLC stage B-C (Table 1) (Bruix et al., 2011; Trojan et al., 2016). Advanced stage HCC leads to aggravated liver dysfunction, making systemic drug delivery ineffective. Also, development of drug resistance and untoward systemic side effects cause serious obstacles to successful treatment regimen for HCC. In practice, the usual biomarkers for the detection of HCC are serum alanine aminotransferase (ALT), aspartase aminotransferase (AST) and alpha-fetoprotein (AFP). In addition, other biomarkers such as glypican-3 (GPC-3), des-carboxyprothrombin (DCP), lens culinaris-agglutinin reactive fraction of AFP (AFP-L3), human hepatocyte growth factor (HGF), insulin-like growth factor (IGF), chromogranin A (CgA), osteopontin (OPN), alpha-1-fucosidase (AFU) and squamous cell carcinoma antigen-immunoglobulin M complexes (SSCA-IgM Cs) alone or in combinations are helpful in early detection of HCC (Bertino et al., 2014; Kondo, Kimura, & Shimosegawa, 2015; Yang et al., 2015). (See Figs. 1–3.)
Table 1.
Classification of BCLC staging of hepatocellularcarcinoma.
| Barcelona-clinic liver cancer | |
|---|---|
| Stage 0 | Single nodule; <2 cm |
| Early stage (A) | HCC patients are asymptomatic; suitable or radical therapies; single or 3 nodules; <3 cm |
| Intermediate stage (B) | Asymptomatic and multinodular tumors, not suitable for resection but for palliative systemic therapy (sorafenib) |
| Advanced stage (C) | Symptomatic tumors, vascular invasion, extrahepatic spread, preserved liver function (child-pugh turcotte classification B), not suitable for resection but for palliative systemic therapy (sorafenib) |
| Stage (D) | Poor prognosis, Okuda stage III tumors, decompensated cirrhosis (child-pugh C) |
Fig. 1.
Causes and treatment of modalities of hepatocellular carcinoma (HCC).
Fig. 3.
Pathways involved and various therapeutic agents under study for HCC treatment.
HCC is mainly the cancer of liver parenchymal cells. Although most of the risk factors of the occurrence of this solid cancers are known, the underlying mechanisms responsible for the conversion of healthy hepatic cells to neoplastic ones are still ambiguous. Various strategies have been implemented for improving the clinical benefits and better therapeutic outcome. Alcohol intake prevention, vaccination against hepatitis B virus (HBV) infection, intake of vitamin D and calcium can prevent HCC in many cases but could not obliterate the disease. The occurrence of this multi-stage carcinogenesis evolves through dysregulation of multiple signaling pathways, genetic alterations with the initiation of inflammatory responses leading to the formation of a heterogeneous solid tumorous mass.
Specific drug delivery techniques have been proven to be efficacious with respect to better targeting and minimizing the adverse toxic effects to the surrounding organs. Targeting the receptors with peptide mediated drug delivery has significantly outweighed the adversities of chemotherapy. In this review, we discuss the causes and recent treatment modalities of HCC, with an emphasis on delivery and targeting of small molecules and RNAs with different synthetic carriers.
2. Causes for Hepatocellular Carcinoma
Multiple factors are responsible for the initiation and progression of HCC include (a) virus-induced, (b) alcohol-induced, (c) fungi-induced hepatocarcinogenesis, (d) obesity and type II diabetes (Fig. 1).
2.1. Virus-induced hepatocarcinogenesis
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infect approximately 2 billion and 170 million people worldwide, respectively (Farazi & DePinho, 2006; Tamori, Enomoto, & Kawada, 2016). Almost 2.5%of the chronic HCV cases leads to HCC and the underlying virus-associated mechanisms is complex comprising both the host and viral factors where host-virus interactions lead to robust T-cell immune response elicitation (Colpitts & Baumert, 2016; Farazi & DePinho, 2006).
2.1.1. HBV
HBV belongs to the Hepadnaviridae family and apart from human hepatocytes it also infects birds and other animals (Farazi & DePinho, 2006; Levrero & Zucman-Rossi, 2016). The infectious HBV particle consists of a circular relaxed partially double-stranded DNA (covalently linked to a DNA polymerase) composed of 3200 nucleotides within the inner nucleocapsid which is in turn enveloped by a spherical lipid layer. The nucleocapsid is composed of core protein HBcAg and the membrane with 3 surface proteins HBsAg (according to sizes; preS1 (large), preS2 (middle) and preS3 (small)) along with host-derived lipids (Levrero & Zucman-Rossi, 2016). For infection the virion first attach to the cellular heparan sulfate proteoglycan (HSPGs) and subsequently bind to the hepatocyte-specific receptor irreversibly. Recently, the role of transmembrane transporter Na+-taurocholate co-transporting polypeptide (NTCP) has been elucidated to which the virus bind utilizing the preS1 domain (Zhang, Zehnder, Damrau, & Urban, 2016). Following entry within the hepatocytes via endocytosis, infection takes place in three steps, (i) viral polymerase expulsion, (ii) positive DNA strand completion and (iii) the HBV DNA conversion to covalently closed circular DNA (HBV cccDNA) and incorporation of histone and non-histone proteins to form minichromosome in the hepatocyte nucleus. Viral replication through infection of hepatocytes is manifested by cccDNA minichromosome which serves as the viral mRNA transcription template by recruiting viral proteins (HBx and HBc), transcription factors, coactivators/corepressors and other enzymes necessary for chromatin modifications. Insertional mutagenesis, genomic stability and activation of pathways related to carcinogenesis by the viral proteins such as HBx, HBc and preS (both wild-type and mutated/truncated) are the different mechanisms promoting HCC by HBV. Various targeted cancer-related genes are involved in the integration of HBV viral genome with the host DNA microdeletions. These genes are telomerase reverse transcriptase (TERT), platelet-derived growth factor receptor β (PDGFRB), mitogen-activated protein kinase 1 (MAPK1) and viral regulatory gene HBx. The expression of growth control genes (SRC tyrosine kinases, Ras, Raf, MAPK, ERK and JNK) are altered by HBx transcriptional activation which also inactivates the tumor suppressor p53 by binding to it and thus promotes cellular growth, survival and bypasses the DNA-damage checkpoints (Farazi & DePinho, 2006; Levrero & Zucman-Rossi, 2016).
Activation of hepatic stellate cells (HSCs) due to the stimulation of various growth and survival signaling pathways leading to oxidative stress induction by viral endoplasmic reticulum (ER) interactions is another plausible mechanism for HBV-induced hepatocarcinogenesis (Farazi & DePinho, 2006).
Antiviral therapy targeting the inhibition of viral replication via reduction in cccDNA loads, blocking the polymerase and immunomodulation by IFN-α are some of the strategies applied for controlling the infection and thus the development and progression of HCC (Singh et al., 2014).
2.1.2. Hepatitis C virus (HCV)
HCV belongs to the flaviviridae family and acts as cytoplasmic-replicating virus (Farazi & DePinho, 2006). It consists of a single-stranded RNA genome (9.6 kbp) within the capsid which is layered by an outer envelope composed of host-derived lipids. The capsid contains E1, E2 glycoproteins and apolipoprotein E (ApoE), the latter acts as a mediator for interaction with the hepatocyte surface anchoring with the heparan sulfate proteoglycans (HSPGs) during infection (Colpitts & Baumert, 2016). Other cellular interactions include host cell low-density lipoprotein receptor (LDL-R) with viral ApoE, scavenger receptor class B type I (SR-BI) with virion-associated lipoproteins and E2 protein, CD81 with E2, claudin 1(CLDN1) along with involvement of other tight junction (TJ) proteins (Evans et al., 2007; Harris et al., 2010; Ploss et al., 2009). Following host internalization through Rab5-containing endosomes, viral fusion takes place, the exact mechanism of which is still not elucidated. Apart from surface receptor binding, complexation with tight junction proteins (TJ) and interaction with various cellular factors (Niemann-Pick C1-like 1 cholesterol, transferrin receptor 1, DNA fragmentation factor subunit alpha and cell-to-cell transmission as studied in Huh-7.5 hepatoma cell via EGFR signaling pathways also contributes to viral entry (Martin & Uprichard, 2013; Sainz et al., 2012; Timpe et al., 2008; Wu et al., 2014). HCV infection has been attributed to be a major concern of liver graft reinfection following transplantation. Although direct-acting antivirals (DAAs) have shown some positive therapeutic outcome, persistent viral infection still remains a challenge among HCC patients. In fact, HCV infection causes more chronic infection compared to HBV as HCV is a RNA virus which does not integrate into the host genomes unlike non-cytopathic HBV.
Fatty liver, also known as fatty liver disease (FLD) or hepatic steatosis development occurs due to the HCV core proteins by the oxidative-stress-mediated mechanism. Continuous mutation propagation and accumulation due to the hepatocyte death caused due to the immune response of the viral infection is one of the accepted theory of HCV-induced hepatocarcinogenesis. Not only HCV core proteins are responsible for the immune-evasion mechanism of cell killing involving factors such as tumor necrosis factor-α (TNF-α) receptor, IFN-α but also NS5A and NS3 non-structural proteins are also involved.
Direct-acting antiviral agents (DAAs) can act at various stages of viral infection in their life cycle, such as entry inhibitors can block the viral entry targeting the host-factors, some can target the viral replication, other can protect the naïve hepatocytes from infection. Entry inhibitors, also known as fusion inhibitors, are a class of antiretroviral drugs used in combination therapy for treating HIV infection.
2.2. Alcohol-induced hepatocarcinogenesis
Alcoholic liver diseases (ALD) cause liver injuries ranging from steatosis, steatohepatitis, fibrosis and ultimately lead to cirrhosis and consequently HCC (Farazi & DePinho, 2006). Factors contributing to ALD are increase in the hepatic iron overload through the upregulation of transferrin receptor I, thus increasing the generation of reactive oxygen species (ROS) in the hepatocytes, downregulation of hepatocyte-produced hepcidin which is a key regulator of iron entry into the circulation and thus causing increased serum iron levels (Sugimoto & Takei, 2017). This also leads to decreased activity of antioxidants, such as glutathione, hepatic tissue hypoxia due to imbalance of hypoxia-inducible factor (HIF) generation by hepatocytes or degradation by proteasomes, dysregulation of various cytokines secreted from adipocytes, inflammatory cells, Kupffer cells (adiponectin, leptin, TNF-α, IL-6), resistance to insulin and HSC activation by gut-derived lipopolysaccharides (LPS) (Chen, Tsukamoto, & Machida, 2014; Sugimoto & Takei, 2017). Amongst various causes which are responsible for alcohol-induced hepatocarcinogenesis, activation of Kupffer cells due to the activation of monocytes and elevated levels of endotoxins result in the chemokines. Release of various inflammatory cytokines limits the hepatocyte overall survival along with HSC activation, cirrhosis leading to HCC. Increased level of lipid peroxidation marker, isoprostane and oxidative stress induced oncogenic mutations along with reduced level of STAT1 phosphorylation, activation of IFNγ signaling and ultimate hepatocyte damage caused by the loss of protective effects of IFNγ add up to the development of cirrhosis and HCC (Farazi & DePinho, 2006). Higher incidence of HCC has been found in patients with HBV-infection and alcoholic cirrhosis compared to viral infection and alcoholism alone (Daoudaki & Fouzas, 2014).
2.3. Fungi–induced hepatocarcinogenesis
Mycotoxins constitute a large number of naturally occurring fungal secondary metabolites with diversified toxic effects. Among many mycotoxins, aflatoxin B1 is a food contaminant produced by the fungi. It is a known as carcinogen and is involved in p53 mutation and induction of HRAS oncogene mutation (Farazi & DePinho, 2006). Although no specific correlation exists between Aflatoxin B1 exposure and cirrhosis development, coexistence of HBV infection often leads to 5-10 folds increased risk of HCC (Farazi & DePinho, 2006; Zhu, Seto, et al., 2016).
2.4. Roles of obesity and type 2 diabetes
Obesity and type 2 diabetes mellitus (T2DM) are considered the key risk factors in the development of non-alcoholic fatty liver disease (NAFLD) and its progression to HCC (Reeves et al., 2016). It has been reported there is an annual increase of 9% in the number of NAFLD-associated HCC cases in the United States (Younossi et al., 2015). Besides the obvious contributors of obesity (due to over nutrition, sedentary life style), genetic factors also play significant role in the metabolic syndrome of the obese patients and subsequently to HCC. Various genes and their variations such as fat mass and obesity-associated protein (FTO; a nuclear protein belonging to the non-heme Fe and 2-oxoglutarate-dependent oxygenase superfamily), melanocortin 4 receptors (MC4R) are correlated to the pathogenesis of obesity, insulin resistance and T2DM (Grarup, Sandholt, Hansen, & Pedersen, 2014). Patatin-like phospholipase domain 3 (PNPLA3) expressed in the adipose tissues as well as in the liver, codes a membrane-bound triacylglycerol lipase protein responsible for energy balance. Although the exact mechanism of HCC promotion due to PNPLA3 polymorphism remains unclear, hepatic stellate cellular retinol metabolism disruption could be considered as one of the causes (Pirazzi et al., 2014). Increase in BMI creates a greater risk factor for liver cancer and obesity, impaired glucose tolerance and NAFLD singularly or as cofactor contribute to the increased risk of HCC. In obese condition, increase in free fatty acids from triacylglycerol (TG) from stressed subcutaneous adipose tissue (SAT) leads to release of TNF-α from recruited macrophages and decreased secretion of adiponectin which is an insulin sensitizer (Larter, Chitturi, Heydet, & Farrell, 2010; Zimmermann, Lass, Haemmerle, & Zechner, 2009). As a result, hepatic lipogenesis is increased due to elevated TG content leading to the development of insulin resistance and lipid storage in visceral adipose tissue (VAT) causing stimulation of the transcription factors (SREBP1, ChREBP1) which contribute substantially in the promotion of hepatocarcinogenesis. Reeves et al has reviewed elaborately the underlying cause of hyperinsulinemia and its correlation with the tumor growth through the mitogenic extracellular signal-regulated kinase (ERK) and anti-apoptotic PI3K signaling pathways in correlation with reduced insulin-like growth factor (IGF)-binding proteins production with augmented insulin secretion (Reeves et al., 2016). Not only suppression of adiponectin but also increase in leptin and their role in adipose tissue hypoxia are all related to insulin resistance and to obesity-related cancer pathologies. Even inflammatory responses (M1 and M2 phenotypes of macrophages), secretion of cytokines such as TNF-α, interleukin-6 (IL-6), IL-1β, leukotriene B4 and their roles in JNK, AMPK, MAPK pathways leads to oncogenic effects (McNelis & Olefsky, 2014). Even the potential role of TNF-α, NFΚb, IL-1, CXC chemokines from activated Kupffer cells, Toll-like receptors (TLRs) from damage-associated molecular patterns (DAMPs) of damaged hepatocytes are found to play crucial roles in the NASH-related HCC (Kubes & Mehal, 2012; Nakagawa et al., 2014). There is a good correlation between liver damage due to metabolic syndrome and the activation of hedgehog (Hh) signaling pathway. NAFLD due to metabolic syndrome, thus has indirect correlation with Hh pathway. In fact Guy et al has established this correlation in their study in NAFLD patients where metabolic syndrome, liver damage and deregulation of Hh pathway leading to advanced liver damage was found from clinical and histological results (Guy et al., 2012). In relation to steatosis and NAFLD-mediated HCC through elevated oxidative stress, autophagy, a self-destructive mechanism of disassembling dysfunctional cellular components plays critical role. Autophagy protein 5 (ATG5) is necessary for autophagy and thus its deletion inhibits autophagy and shows elevation of triglyceride and insulin sensitivity. Evidence of hepatocyte protein p62 accumulation and subsequent activation of nuclear factor erythroid 2-related factor 2 (Nrf2) in relation to “Mallory” inclusion and pathological contribution to steatohepatitis has been established in correlation to defective autophagy (Reeves et al., 2016).
3. Pathways involved in HCC
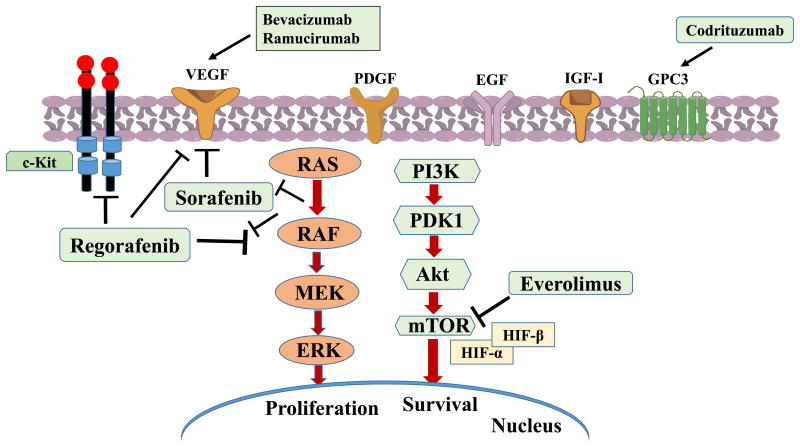
HCC is a complex multi-step process arising from combination of genetic and epigenetic alterations, somatic mutations, genomic instability and environmental factors (Bertino et al., 2014; Daoudaki & Fouzas, 2014). Genes involved in HCC are extensively reviewed by Bertino et al where c-myc, cyclin A2 & D1, PTEN, SOCS, E-cadherin, IGFR-II/M6PR, p16, Rb1, AXIN1 and their chromosomal amplification (1q, 6p, 8q, 17q, 20q) and deletions (4q, 8p, 13q, 16q, 17p) are detected (Bertino et al., 2014). Multiple molecular pathways implicated in HCC pathogenesis, involving key growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet derived growth factor (PDGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor (TGF) a and (3, insulin-like growth factors (IGFs) along with mesenchymal epithelial transition factor (c-MET) and the mammalian target of rapamycin (mTOR) pathway act as potential targets for therapeutic interventions (Bertino et al., 2014; Farazi & DePinho, 2006; Spangenberg, Thimme, & Blum, 2009). These growth factors although present during fetal development in the liver, are absent substantially in adult liver but during regeneration following injury/insult, some gets upregulated leading to dysregulation of multiple signaling pathways causing HCC (Spangenberg et al., 2009). Amongst the multiple signaling pathways that are involved in the initiation and progression of HCC, receptor tyrosine kinase plays a key role in inducing the Ras/Raf/MEK/ERK (MAPK) and phosphatidylinositol 3-kinase (PI3K/Akt/mTOR) signaling pathways. As a consequence of the activation of these pathways, Ras-Raf-ERK signaling downstream, followed by proto-oncogene c-Fos and transcription factor activator protein 1 (AP-1) activation leads to induction of transcription of cell proliferating genes (Singh et al., 2014). Also interruption in mTOR cascade occurs due to PI3K-Akt kinase signaling activation via insulin-like growth factor receptor (IGFR1). Activation of Wnt/β-catenin signaling due to β-catenin mutations, ubiquitin/proteasome degradation pathway, epigenetic DNA methylation, histone deacetylation, angiogenic pathways and telomerase also promotes liver carcinogenesis (Roberts & Gores, 2005).
4. Current treatment modalities for hepatocellular carcinoma
4.1. Chemotherapeutics
Systemic therapies using small molecule drugs to target various signaling pathways following surgical resection and liver transplantation have been applied particularly where locoregional therapy such as transcatheter arterial chemoembolization (also called transarterial chemoembolization or TACE) has failed (Cabibbo et al., 2009; Dhir et al., 2016). However, chemotherapy in HCC suffers from the drawbacks of dose-limiting toxicities, development of multidrug resistance (MDR) and unfavorable side-effects like other cancers (Sun et al., 2016; Waidmann & Trojan, 2015; Wang, Su, et al., 2016). Among the various multikinase inhibitors used for systemic treatment of HCC, sorafenib (orally active multikinase inhibitor) deserves special mention as it is the only approved drug for treatment of advanced HCC (Ribeiro de Souza, Reig, & Bruix, 2016). Monotherapy with sorafenib prolongs overall survival in patients by 3 months (from 7.9 in placebo-treated group to 10.7 months) and delays the time to progression of advanced HCC (Pascual, Herrera, & Irurzun, 2016). This tyrosine kinase inhibitor serves the dual purpose of angiogenesis and antiproliferation by targeting multiple genes, namely VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, Raf, RET and FLT-3. Sorafenib has been immensely utilized not only for adjuvant therapy after surgical resection and ablation but also in clinical trials. Both Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol (SHARP) trial and ORIENTAL study (phase III trials) with sorafenib proved that the clinical outcome for sorafenib treated group was positive among advanced/progression after surgical and virus-related HCC patients, respectively (Ge & Huang, 2015). However, diarrhea, hypertension, skin toxicity, weight loss, hypophosphatemia are the frequent symptoms amongst patients undergoing sorafenib treatment. Recently, a fluoro-derivative of Sorafenib, Regorafenib, a multikinase inhibitor (angiopoietin-Tie system, VEGFR2/3, PDGFR, c-kit, Raf kinase and c-Kit signaling) has been tested as second-line therapy in thirty-six patients with BCLC stage B/C HCC and showed promising anti-tumor activity with median overall survival of 13.8 months (Bruix etal., 2011). Various first line and second line therapies are currently under evaluation for the HCC treatment, however, potential randomized phase III trial with another multikinase drug Suni-tinib, an oral multikinase inhibitor targeting VEGF-1/2 and PDGFR-a/b as well as an inhibitor of c-kit, Fit-3 and RET has been disappointing due to the adverse effects and not superior to Sorafenib (Cheng et al., 2013). Considering the risk/benefit ratio, the efficacy/safety and toxicity assessment studies of sunitinib has been halted for its administration. Tivantinib, an orally selective MET inhibitor is a second-line treatment in advanced HCC patients where MET tumor overexpression is high. Phase III clinical trials (ARQ 197-A-U303, NCT01755767) with Tivantinib is currently undergoing with 60% of overall survival as interim analysis has been planned as the end point criteria (Porta et al., 2015). Brivanib (antiangiogenicagent) and linifanib (multikinase inhibitor; phase III clinical trial) although showed marked antitumoral effects as second-line therapy did not show improvement against placebo and was halted for further phase III trial respectively (Cainap et al., 2015; Llovet etal., 2013) Erlotinib (EGFR inhibitor) and sorafenib combination therapy was unsuccessful as first-line therapy (Zhu, Rosmorduc, et al., 2015). In view of the failures of clinical trials for most of the chemotherapeutics drugs, an elaborate and extensive information of the underlying molecular mechanisms undergoing in each patient's tumor progression is critically necessary. Other small molecule targeted therapies such as doxorubicin, gefitinib, vatalanib, cediranib, bortezomib (proteasome inhibitor), rapamycin, sirolimus, and gemcitabine as single agent or in combination with mAb have been evaluated in clinical trials targeting pathways which are activated in HCC (Spangenberg et al., 2009). Connell and colleagues have extensively reviewed the current clinical trials of HCC therapeutics which are underway along with their placebo studies in parallel (Connell, Harding, & Abou-Alfa, 2016). Other BCR/ABLand c-Kit inhibitors recently under phase II clinical trials (dasatanib, imatinib) yielded mixed results when compared as single or combination drugs.(Ohri, Kaubisch, Garg, & Guha, 2016)
4.2. Antibody therapeutics
Immunotherapy plays a critical role in HCC treatment since liver is the main “immune organ” of the lymphatic system (Hong, Li, Prasoon, & Zhang, 2015). Three different hepatic cells namely liver sinusoidal endothelial cells (LSECs), Kupffer cells and dendritic cells (DCs) are mainly involved in the immune response in the HCC patients (Pardee & Butterfield, 2012). Presence of tumor-infiltrating lymphocytes (effector T cells) in tumors of patients leading to low risk of relapse of tumor following surgical transplantation is a strong basis for the benefits of immunotherapy in HCC (Prieto, Melero, & Sangro, 2015). Combination therapy of monoclonal antibodies (mAbs) or chemotherapies along with transarterial chemoembolization (TACE) is a promising approach in increasing the overall survival of HCC patients where chances of relapse after surgery is evident (Huang et al., 2016; Li, Zhou, Ren, Zhang, & Han, 2013; Pinter et al., 2015; Wang, et al., 2017). Ma et al has shown promising efficacy and tumor targeting of the 131-Labeled-Metuximab (licartin) and TACE combination for unresectable HCC among 167 patients with stage III/IV tumors (Ma & Wang, 2015). There are various tumor-associated antigens (TAAs) which are shared antigens whose expressions are dysregulated leading to defective immune responses. CD8+ T cells are anti-TAA which have potential to reverse the tumor-suppression microenvironment. The antigenicity of the tumor-associated antigens (TAAs) arise from somatic genetic mutations leading to dysregulation of oncofetal proteins (GPC-3 and AFP) and can-cer/testis antigens (NY-ESO-1, melanoma-associated antigen-1(MAGEA), synovial sarcoma, X breakpoint 2 (SXX2) and telomerase reverse transcriptase (TERT) (Prieto et al., 2015). Various immune checkpoint mAbs blockers such as ipilimumab, tremelimumab, nivolumab, MED14736, anti-LAG-3 (BMS-986016), pembrolizumab, targeting the immune checkpoint molecules inhibit the T-cell activation like cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), ligand of PD (PD-L1) constitute for appealing therapeutic outcome (Prieto et al., 2015; Sangro et al., 2013). Anti-GPC3 mAb such as GC33 also showed encouraging result in a phase II clinical trial in advanced HCC bearing patients. Different phases of clinical trials with mAbs such as ramucirumab (VEGFR2 antagonist), bevacizumab, temsirolimus and Everolimus (mTOR antagonist) were evaluated as mono- and combination therapy where no or little improvements has been observed (Knox et al., 2015; Pinter et al., 2015; Zhu et al., 2014; Zhu, Park, et al., 2015). Recently, Codrituzumab, a humanized monoclonal antibody targeting GPC3 and interacting with CD16/FcγRIIIa has been tested in cohorts with advanced HCC (125 patients) in a randomized phase II trial with placebo (60 patients). However, the outcome of the study did not have beneficial effect and higher dose or patient selection with higher biomarkers were suggested for better therapeutic outcome (Abou-Alfa et al., 2016) (Fig. 3 and Table 2).
Table 2.
Monoclonal antibody (mAbs) therapeutics with/without combination therapy in various clinical trials for hepatocellular carcinoma (HCC).
| mAb | Target | Combination therapy | Clinical phase stage | References |
|---|---|---|---|---|
| Codrituzumab | GPC3 | – | Phase II | Abou-Alfa et al. (2016) |
| 131I-labeled metuximab | CD147 | TACE | Phase IV | Ma and Wang (2015) |
| Bevacizumab | VEGF-A | TACE | Phase II | Pinter et al. (2015) |
| Ramucirumab | VEGFR-2 | Sorafenib | Phase III | Zhu, Park, et al. (2015), Zhu, Rosmorduc, et al. (2015) |
| Bevacizumab | VEGF-A | Temsirolimus | Phase II | Knox et al. (2015) |
| GC33 | GPC-3 | – | Phase I | Ikeda et al. (2014) |
| Tremelimumab | CTLA-4 | – | Phase II | Sangro et al. (2013) |
| MED14736 (durvalumab) | PD-L1 | – | Phase I | |
| Nivolumab | PD-1 | – | Phase I/II, III | Melero et al. (2015) |
| PF-03446962 | ALK-1, a specific | – | Phase I | Simonelli et al. (2016) |
| TGF-β receptor |
4.3. Adjuvant therapy
Interferon (IFN)-based therapy has been reported to be effective in creating a marked decline in the HCC incidence against HCV due to the viraemia clearance with subsequent gaining of sustained virologic response. Various observational studies as well as randomized controlled trials proved that IFN-based therapy was able to achieve the reduction in HCC risk by manifolds compared to patients who failed therapy or did not receive treatment. However, IFN-α as adjuvant therapy prior to resection has been non-recommended due to conflicting data and study designs. IFN-based therapy along with combination of PEGylated interferon (PEG-IFN) and ribavirin (RBV) has been shown to be effective in patients with high viral loads. Other than IFN treatment telaprevir, boceprevir, (NS3/4A direct-acting antiviral (DAAs) protease inhibitors), adefovir, lamivudine as adjuvant therapy have also shown to increase SVR rates thus reducing the risk of HCC (Tamori et al., 2016). Although DAAs can eradicate the chronic HCV infection, it is not yet confirmed whether HCC prohibition can be achieved by this therapy (Spangenberg, Thimme, & Blum, 2008).
4.4. Targeting peptides and peptide vaccines
4.4.1. (a) SP94
SP94, a synthetic peptide has been reported to show specific activity for targeted delivery against HCC (Li et al., 2016). Lo et al reported the use of SP94 through the use of phage displayed random peptide library both in vitro and in vivo in HCC. Tumor-specific targeting peptide, SP94 (SFSIIHTPILPL) has been compared with the control peptide (FPWFPLPSPYGN) for the tumor-targeted therapeutic studies in subcutaneous model of severe combined immunodeficiency (SCID) mice. PEGylated liposomes coupled with targeting peptide SP94 along with doxorubicin (SP94-Lipo-Dox) were administered systemically into these mice. Flow cytometry analyses revealed that phage clone 94 (PC94) had best reactivity to HCC cells compared to other tested 14 clones. The authors suggested in the study that HCC cells bear an unknown target molecule which was recognized by SP94 specifically (Lo, Lin, & Wu, 2008). Recently, Medina et al have demonstrated the potential targeting activity of PEGylated particles functionalized with SP94 peptide in HepG2 cancer cells (Medina et al., 2013).
4.4.2. Glypican-3 (GPC3)
GPC3 belongs to the class of glypican family consisting of heparan sulfate proteoglycans linked through a glycosylphosphatidylinositol anchor with the outer surface of the cell membrane (Pan et al., 2013). GPC3 is a carcinoembryonic antigen which is found to be highly overexpressed in HCC and thus can act as a unique anticancer immunotherapy target. GPC3 is found in the placenta or fetal liver in normal tissues but not in other non-cancerous tissues (Haruyama & Kataoka, 2016). Based on cDNA microarray analysis of 23,040 genes, Nakatsura et al has reported GPC3 as a novel marker for human HCC. The amino acid sequence (95 % homology) and expression patterns of mouse and human of this oncofetal protein were identical (Nakatsura et al., 2004). In murine model, GPC3 was highly immunogenic. Although no autoimmunity was elicited, generation of antitumor immunity was certain. BALB/c mice were primed with GPC3-derived peptides by subcutaneous injections and were boosted for 7 days after priming. Splenocytes were then collected and harvested for CD8+ cytotoxic T lymphocytes (CTLs). Komori et al identified human leukocyte antigens, HLA-A2 or HLA-A24 restricted CTL epitopes which could be useful for GPC-3 specific immunotherapy of HCC (Komori et al., 2006). Mouse GPC3298-306 (EYILSLEEL) which is an H-2Kd–restricted antigenic peptide was observed to be recognized by mouse CD8+ CTLs. It was further investigated by the group that GPC3 peptide could be utilized for HLA-A24+ HCC treatment as an immunotherapeutic platform since the structural motifs of HLA-A24+ and mouse H-2Kd were identical. Depending on the relative gene frequency of HLA-A24 (A* 2402) and HLA-A2 (A*0201), GPC3-derived CTL epitopes were examined from HCC patients' peripheral blood mononuclear cells (PBMCs) to check for HLA-A2 or HLA-A24- restricted epitope peptides could actually induce GPC3- reactive CTLs using HLA-A2.1 transgenic mice (Tgm). In another study, Zhu et al selected a 12-mer peptide, TJ12P1 (DHLASLWWGTEL) through phage display library showing GPC3 binding affinity via conjugation with near-infrared fluorescent dye (Cy5.5) not only in vitro but also in vivo xenografts of GPC3-expressing HepG2 cell line (Zhu, Qin, et al., 2016). Nobuoka et al has described the potential application of GPC3 peptide vaccine following Phase I and Phase II trials where the authors suggested combination or specific immunotherapies along with peptide vaccine could lead to superior results for advanced HCC treatment (Nobuoka, Yoshikawa, Sawada, Fujiwara, & Nakatsura, 2013). Recently, Iwama and colleagues synthesized liposome-coupled GPC3-derived epitope peptide (pGPC3-liposome) and showed its vaccination stimulated CTLs and was able to inhibit GPC3-expressing tumor growth in mice (Iwama et al., 2016).
4.4.3. (c) Multidrug resistance-associated protein 3 (MRP 3)
Among the ABC transporters, multidrug resistance protein3 (MRP3) is highly overexpressed in various cancers including HCC tissues. MRP3 is an organic anion transporter able to carry anticancer drugs opposite to the concentration gradient with the help of ATP-dependent mechanisms (Borst & Elferink, 2002). Mizukoshi et al suggested MRP3 as a target HCC immunotherapeutic antigen as they reported the induction of MRP3-specific CTLs against MRP3 overexpressed HCC cells irrespective of the HCC stage, AFP level or hepatic functions. MRP3-derived peptide vaccine has been proven to be tolerated and effective among glioblastoma and prostate cancer patients as revealed from clinical trials and the safety and effectiveness in HCC patients undergone hepatic arterial infusion chemotherapy (HAIC) as potential immunological vaccine. Twelve HLA-A24–positive patients were first treated with PEGylated IFN-α-2b/5-fluorouracil+cisplatin through HAIC (Mizukoshi et al., 2015). Yutani et al reported the safety and immune response efficacy in a Phase II study of personalized peptide vaccine (PPV) for treating HCV–positive advanced HCC patients. From 15 tumor-associated antigens (TAAs), 31 pooled peptides (for human leukocyte antigens (HLA) A2, A24,A3 (subtypes A3, A11, A31 or A33) and A26 12, 16, 9 and 4 peptides) were derived from which 4 HLA matching peptides were selected based on the patients' preexisting immunity for the study. A C-35 peptide (YLLPRRGPRL) applicable to all mentioned HLA types and derived from HCV core protein was used for vaccination along with31 peptides. 2-4 peptides were selected based on the IgG titers specific to 32 candidates and HLA typing and host previous immunity profile and were sub-cutaneously administered with incomplete Freund's adjuvant (IFA) once weekly for 8 consecutive weeks. IgG levels for peptide specificity were measured for humoral immune response using Luminex system and IFN-γ were evaluated for CTL-response specific to peptide vaccines. The outcome of the study revealed that the peptide-specific IgG1 response to be a potential prognostic marker where the patients' vaccine for both C-35 and TAA-derived peptides has a higher survival rate than with single or no peptide treated groups. The study strongly recommended the fact that PPV along with HCV-derived CTL epitope and TAA derived peptides could be a safe and immunity inducing factor among HCC patients who are HCV-positive and at the advanced stage in the disease (Yutani et al., 2015).
4.5. miRNA-based Therapies
Apart from small molecule chemotherapeutics, nucleic acid-based drugs such as small interfering RNA (siRNA) and microRNAs (miRNAs) also have promising therapeutic potential for the HCC treatment. miRNAs are highly conserved small non-coding RNA molecules consisting of 22 nucleotides which play a major role in RNA silencing and post-translational regulation of gene expression (Hayes & Chayama, 2016). These can act as oncogenic or tumor suppressor in diseased conditions. While miRNA is a single-stranded RNA natural molecule, siRNA is a double stranded RNA, which can be either a natural or synthetic RNA. The basic difference between miRNA and siRNA lies in the binding of the antisense strand to the mRNA after being incorporated into the RNA-induced silencing complex (RISC). Whereas miRNA binds imperfectly at several sites, siRNA binds at a single site and forms a perfect match with its target (Farra, Grassi, Grassi, & Dapas, 2015). However, two drawbacks notable for miRNA and siRNA-based therapeutics are off-target effect and ineffective delivery to the site of interest. Off-target effect leads to silencing of genes other than the target gene, in many cases the healthy genes. Therefore, efficient chemical modification, proper sequence and optimal concentration are of utmost importance. Amongst the delivery obstacles, extracellular nucleases-mediated degradation and elimination/renal filtration leads to inefficient delivery to the target site (Farra et al., 2015; Huang et al., 2011; Jackson & Linsley, 2010; Layzer et al., 2004). Notably, these nucleic acid based drugs cause immunogenicity in many instances. Necessary targeting approaches are required for the maximum cellular uptake which otherwise is reduced due to the repulsion of the negatively-charged plasma membrane to these hydrophilic negatively charged moieties and also to by-pass the endosomal uptake.
Dysregulation of miRNA at different stages of HCC is noticed through miRNA profiling of HCC patients and healthy subjects and are detected not only on liver tumor tissues but also as circulating miRNA in serum and urine (Table 3). Noticeably, miRNAs are both upregulated (miR-21, miR-221, miR-222, miR-224, miR-17-92) and downregulated (miR-29, miR-122, miR-200, miR-123, miR-199a, miR-199b, let-7) in association with HCC and have been extensively reviewed by Yang et al. (2015). As a result, two kinds of approaches are used for the management of miRNA level in HCC, namely, miRNA mimic and anti-miRNA (or antimiR). Additionally, circulating miRNAs (miR-939, miR-595, miR-519d) have been found to be notable biomarkers in cirrhotic HCC patients (Fornari et al., 2015). Herein, we discuss the recent reports of the most frequently dysregulated miRNAs affecting HCC etiology and malignancy (Hayes & Chayama, 2016).
Table 3. Below are the recent reported circulating and serum miRNA found to be at elevated or decreased levels in HCC patients.
| Circulating and serum miRNA | References |
|---|---|
| miR-25, miR-375, miR-206, miR-223, miR-92a, miR-222, miR-1, let-7f, miR-21 | Mirzaei et al. (2016) |
| miR-939, miR-595, miR-519d, miR-494 | Fornari et al. (2015) |
| miR-18a, miR-221, miR-222 and miR-224 (higher) and miR-101, miR-106b, miR-122 and miR-195 (lower) | Sohn et al. (2015) |
| miR-122, miR-99a, miR-331, miR-125b, miR-23b, miR-92a, and miR-26a (higher risk) and miR-652, miR-23a, miR-27a, miR-34a, miR-145, miR-10a, miR-150, and let-7f (lower risk) | Wang, Hann, et al. (2016) |
4.5.1. miRNA-21
miRNA-21 or miR-21is an oncogenic miRNA and it is overexpressed in many human cancers including HCC, particularly plasma miR-21 proved to a novel circulating biomarker for HCC (Kamel et al., 2016; Mirzaei et al., 2016). Tomimaru et al. reported circulating miR-21 as a novel biomarker for HCC where HCC patients' plasma were measured for miR-21 by qRT-PCR after pre and post-surgical resection. Plasma levelofmiR-21 was significantly higher in another groupof126 HCC patients compared to healthy cohorts (Tomimaru et al., 2012).
4.5.2. miRNA-221
Zhang et al reported the delivery of miR-221 antisense oligonucleotide (anti-miR-221) using negatively charged liposomes with transfer-rin (Tf) as a targeting ligand in in vitro and also in HepG2 tumor-bearing xenograft mice (Zhang, Peng, et al., 2015).
4.5.3. miRNA-29
miRNA-29 or miR-29 family members (a/b/c) are significantly downregulated in HCC tissues and targets T cell leukemia/lymphoma 1 (TCL1), myeloid cell leukemia sequence 1 (Mcl-1), cell division cycle 42 (CDC42) and phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) which are characteristics of this family. Xiong et al demonstrated the potential role of miR-29 on apoptosis, tumorigenicity and prognosis of HCC. They also demonstrated Bcl-2 and Mcl-1 asdirect targets of miR-29-regulated apoptosis through a mitochondrial pathway (Xiong et al., 2010). In another study by Dong and colleagues, lower miR-29c expression was found in tumor tissues from HCC patients when compared with adjacent noncancerous tissues (Dong, Wang, Du, Ding, & Hu, 2016).
4.5.4. miRNA-122
miR-122 is expressed profusely constituting of 70% of the hepatic miRNAs in normal hepatocytes, but is downregulated in HCC. The validated targets of miR-122 are ADAM10 (a disintegrin and metalloprotease family 10), serum response factor (SRF) and insulin-like growth factor 1 receptor (IGF1R). Bai et al demonstrated the loss of miR-122 results in an increase of cell migration and invasion and its restoration can inhibit tumorigenicity of HCC and sensitization to cancer cells (Bai et al., 2009; Hayes & Chayama, 2016; Thakral & Ghoshal, 2015; Wang, Wang, Shen, et al., 2016).
4.5.5. miRNA-7
Regulation of EGFR expression and its downstream P13/AKT/mTOR pathway are correlated to miR-7 overexpressed in HCC tumors. miR-7 has been demonstrated by Fang et al in inhibiting tumor growth and reversing metastasis by targeting the phosphoinositide 3-kinase/Akt/mTOR pathway in HCC by targeting phosphoinositide 3-kinase catalytic subunit delta (PIK3CD), p70S6K and mammalian target of rapamycin (mTOR) (Fang, Xue, Shen, Chen, & Tian, 2012).
4.5.6. miRNA-34a
miRNA-34a is a potent tumor suppressor and has been found to act through various signaling pathways related to cancer, such as p53 and Wnt/β-catenin pathway. Restoration of miR-34a leads to inhibition of tumor growth and progression. miR-34a mimics, plasmid expression vectors, nanoparticle-based non-viral vehicles, liposomes, are the general strategies for restoration of miR-34a level. A natural compound which is a chalcone derivative, termed Rubone has been reported to up-regulate miR-34a expression in HCC cells. Deng et al reported recently the use of carboxymethyl dextran-stabilized polyethylenimine-poly (epsilon-caprolactone) nanoparticles mediated modulation of miR-34a expression with the help of Rubone for HCC therapy (Deng et al., 2016).
4.5.7. miRNA-224
Okajima et al recently reported the identification of plasma miR-224 as an important and novel biomarker in HCC where the findings lead to the conclusion that detection of tumors smaller than 18 mm is possible with this biomarker preoperatively (Okajima et al., 2016).
5. Nanomedicines and drug delivery for treating hepatocellular carcinoma
Nanotechnology is a powerful tool for the delivery and targeting of therapeutics in hepatocarcinogenesis. Since hepatocytes undergo genetic and phenotypic changes compared to other hepatic cells, targeting of hepatocytes is an obvious avenue for treatment of HCC. Various receptors have been explored for targeting nanoparticles (NPs), micelles, liposomes both passively and receptor-mediated active targeting (Figs. 1 and 2). Unfavorable side-effects of systemic administration of chemo-therapeutics can be overcome by improving the pharmacokinetics and biodistribution, accumulating cytotoxic agents in tumor site and elevating the effectiveness of treatment through the use of novel drug delivery carrier systems. Nanometer (nm) size range helps in reaching the tumor cells through the leaky vasculature and enhance site-specific enhanced delivery. Herein we discussed some of the recent findings in the field of nano-based medicines for passive and receptor-specific active targeting for the treatment of hepatocellularcarcinogenesis.
Fig. 2.
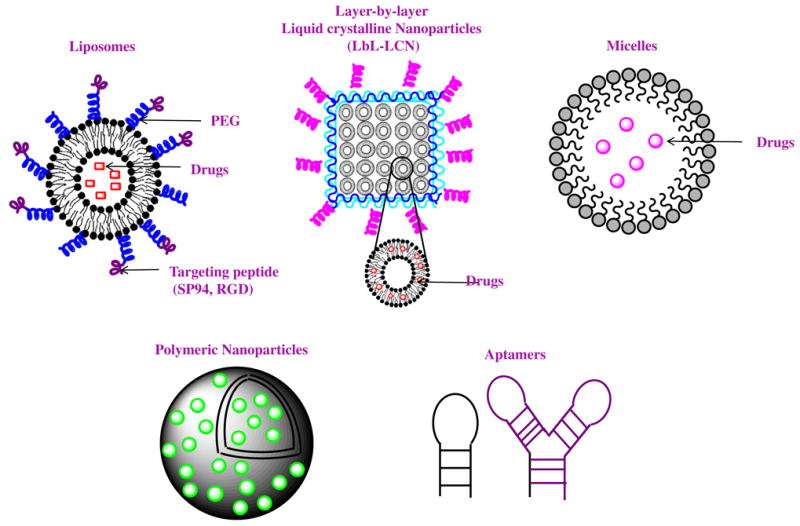
Recent nanomedicines for the application of HCC treatment.
5.1. Passive Targeting
In case of passive targeting, nanomedicines can reach tumor via the leaky vasculature of the tumors by the enhanced permeability and retention (EPR) effect (Varshosaz & Farzan, 2015). Various stimuli including pH, enzymatic, redox, light and heat have been used for passive targeting of nanoparticles carrying drugs in HCC. Recently, D-α-tocopheryl PEG1000-poly-(β-amino ester) block copolymer containing disulfide linkages (TPSS) were synthesized for developing docetaxel (DTX)-carrying TPSS nanoparticles for pH–triggered charge reversal (from -47.6 ± 2.5 mV to 22.5 ± 3.2 mV) release of DTX due to decrease in pH from 7.4 to 6.5 at extracellular tumor environment (Chen, Zhang, Wang, Wang, & Chen, 2015). Apart from liposomes and conventional nanoparticles, liquid crystalline nanoparticles (LCN) have gained immense focus as drug delivery vehicles. In presence of suitable surfactants, these LCN structures self assembles when exposed to polar lipids forming non-lamellar structures providing rigid and stable assembly with higher payload and sustained release of cargo. Kim and colleagues loaded Sorafenib (SF) in monoolein-based LCNs formed by layer-by-layer polymer assembly (LBL-LCN/SF) and showed in vitro high cellular uptake and enhanced apoptosis in HepG2 cells (Thapa et al., 2015).
5.2. Site-specific delivery and targeting
Although liposomal system has gained tremendous success for delivering chemotherapeutic agents, their rapid clearance by the reticulo-endothelial system (RES) after systemic administration restricts them from their delivery to hepatocytes which are the main site for HCC origination (Dewhirst, Landon, Hofmann,& Stauffer, 2013; Ohri et al., 2016). As a result, site specific or targeted drug delivery approaches amenable to distinct hepatic cells and their receptors are being studied extensively by researchers (Ashley et al., 2011).
5.2.1. Asialoglycoprotein receptor (ASGPR)
Asialoglycoprotein receptor (ASGPR) is a commonly found lectin receptor which are profoundly expressed in mammalian liver cells. Use of natural ligands such as asialofeutin as well as synthetic ligands (galactosylated cholesterol, glycolipids, arabinogalactan (AG), lactosylated/galactosylated polymers) has achieved specific liver ASGPR targeting. Among the various physiological roles such as specific binding of galactose (Gal) or N-acetylgalactosamine (GalNAc) and their internalization deserves special mention. These high-capacity C-type lectins mainly on human hepatocytes consist of two homologous poly-peptides,H1 and H2 (Hu, Liu, Yang, Lu, & Yin, 2014). Liposomes incorporating various lactosylated lipids have been evaluated for targeting efficacy to hepatic tumor cells. Zhou et al. synthesized lactosylated liposomes encapsulating calcein/doxorubicin (Lac-L-calcein/DOX) and found significantly stronger HCC tumor inhibitory activity compared to non-targeted doxorubicin encapsulated liposomes (L-DOX) and free DOX, along with a higher drug accumulation in HepG2 xenograft tumors in nude mice (Zhou et al., 2012). Lactoferrin (Lf) which is a mammalian cationic iron-binding glycoprotein has been reported to be potential ligand for ASGPR for HCC. Wei et al and colleagues successfully demonstrated the significant antitumor efficacy of DOX-loaded, Lf-modified, PEGylated liposomes (Lf-PLS) (DOX-loaded Lf-PLS) in male BALB/c nude mice bearing HepG2 xenografts compared with PLS (Pb0.05) and free DOX (Wei et al., 2015). Shah et al have investigated the chemically modified palmitoylated arabinogalactan (PAG) for DOX delivery in in vitro ASGPR+ HepG2 cells as well in immunocompromised mice. Compared to conventional liposomes PAG liposomes targeting ASGPR resulted in higher plasma AUC, liver accumulation and enhanced tumor suppression (Shah et al., 2014). In another study, cholesterol arabinogalactan anchored liposomes (CHOL-Al-AG) carrying DOX targeting ASGPR receptors on mammalian hepatocytes in HCC were studied where improved delivery of DOX was reported in terms of stability, loading efficiency, tumor regression both in in vitro and in vivo compared to unmodified liposomes (Pathak et al., 2016).
5.2.2. Integrin receptors
Integrins are transmembrane receptors which are responsible for cell-cell and cell-extracellular matrix interactions. Hepatic stellate cells expresses integrins in fibrotic liver. As a result, the peptide sequence RGD (Arginine-Glycine-Aspartate) acts as a targeting ligand for collagen VI and RGD-coupled liposomes has been extensively applied for targeting integrin receptors in HCC. To deliver the poorly water soluble drug paclitaxel (PTX) effectively in HCC, integrin-αvβ3 receptor targeted liposomal PTX was used by Chen et al. where RGD-motif was successfully conjugated to 1,2-distearoyl-phosphatidyl Ethanolamine-Methyl-Polyethyleneglycol (DSPE-mPEG) to prepare RGD-modified liposomes (RGD-LP). Both in vitro and in vivo studies demonstrated RGD-LP-PTX had enhanced anti-proliferative and tumor growth inhibitory effects activity against HepG2 cells and HepG2–bearing mice tumor respectively than RGD-LP or free PTX (Chen, Liu, Wang, & Liu, 2015). Combination of DOX and sorafenib (SOR) in iRGD decorated lipid-polymer hybrid core-shell structured nanoparticles (DOX+SOR/iRGD NPS) demonstrated enhanced antitumor efficacy in HCC xenograft mouse models with improved bioavailability and longer circulation time (Zhang, Hu, et al., 2016). Recently, another combination therapy of So-rafenib and quercetin (QT), an active flavonoid has been tested in PLGA nanoparticle formulations with RGD functionalization (particle size = 136.5 ± 3.2) by Wang et al for targeted delivery in HepG2 xenograft HCC tumor models. However, in the targeted formulation percentage drug loading of both Sorafenib and quercetin (SRF=1.9±0.4;QT= 2.4 ± 0.6) was reduced compared to the non-targeted combination (SRF=6.5 ± 0.9; QT= 8.4 ± 0.7) and individual mono-formulation (SRF=7.9 ± 0.8; QT= 9.1 ± 0.5) (Wang, Su, et al., 2016). Vandetanib (vanib), an oral inhibitor of VEGFR, EGFR, RET-tyrosine kinase and which has been approved for medullary thyroid cancer has been explored for HCC as encapsulated nanomedicine in PEG-PLA with iRGD targeting moiety with enhanced antitumor efficacy (Wang, Wang, Li, et al., 2016).
5.2.3. Transferrin receptors (TfR)
HCC cells overexpressed transferrin (Tf) receptors which have become promising targeting avenues for potential treatment (Martin & Uprichard, 2013). Co-delivery of DOX and cisplatin loaded in Tf-modified PLGA nanoparticles displayed higher cytotoxicity in vitro and active targeting in human HepG2 cells compared to non-targeted and single drug loaded nanoparticles (Zhang, Li, & Yan, 2016). In an interesting study by Szwed et al it was proved that DOX-transferrin conjugates leads to increased cytotoxicity in HepG2 and a non-HCC cell line (A549) due to induction of oxidative stress as observed by decreased glutathione level and increased hydroperoxide level (Szwed, Wrona, Kania, Koceva-Chyla, & Marczak, 2016).
5.2.4. Epidermal growth factor receptor (EGFR)
CL4 RNA aptamer specific to EGFR and CD133 targeted aptamers (A15 RNA aptamer) conjugated to PLGA nanoparticles was studied for salinomycin delivery to cancer stem cells (CD133+ and CD133-) in HCC treatment. The dual targeted nanoparticles (CESN) were internalized readily with subsequent cytoplasmic delivery of salinomycin thus resulting in better targeting efficacy to HCC cells (Jiang et al., 2015).
5.2.5. Folate receptors (FR)
Folate receptors (FR) are 38 kDa glycosylphosphatidylinositol membrane-anchored glycoproteins which are overexpressed in tumor tissues compared to normal tissues. Like other cancers, FR are significantly overexpressed in HCC and to target these receptors, its natural ligand, folic acid (FA) has been explored for nanoparticle drug delivery to the cancer cells. Liu et al studied the antitumor potency of the folate receptor-targeted liposomes (FA-PEG) carrying di-acid metabolite of norcantharidin (DM-NCTD) in H22 HCC both in vitro and in vivo models (Liu, Liu, et al., 2016)
5.2.6. Vascular endothelial growth factor receptor (VEGFR)
VEGF receptor are found to be overexpressed in HCC due to the receptor activation via multiple signaling pathways. Recently, the role of miR-195 has been demonstrated in inhibiting HCC which is otherwise downregulated in the tumor setup. Liu et al studied the combination effect of miR-195 and Rho-kinase blocker Fasudil in encapsulated nano-particles for the suppression of the vasculogenic mimicry (VM). The authors utilized a cell penetrating peptide (CPP)-modified aptamer (ST21) for specific delivery of Fasudil and miR-195. ST21-H3R5-PEGmiR195 is their delivery vehicle composed of the cationic peptide of arginine and histidine (H3R5) along with the Y-shaped ST21 (SFSIIHTPILPL-TATSFSIIHTPILPL) targeting probe made up of SP94 pep-tide. Active targeting by aptamer-mediated delivery showed significant anti-tumor efficacy when tested both in vitro and in vivo (Liu, Wu, et al., 2016).
5.2.7. Glycyrrhetinic acid (GA) receptor
GA receptor is overexpressed on the hepatocyte surface and has been extensively studied for drug targeting. Cai et al reviewed the roles of this receptor and its natural ligand 18β-Glycyrrhetinic acid (GA) for GA-mediated drug delivery in HCC (Cai et al., 2016). GA-modified liposomal formulations of oxaliplatin, calcein, docetaxel, wogonin and also pDNA were discussed for effective targeted therapy for HCC. Zhang and colleagues studied poly (L-Histidine) (PHIS) mediated polymeric system (GA-PEG-PHIS-PLGA) with GA for targeted delivery of the anti-cancer agent andrographolide (Zhang, Zhang, et al., 2015).
6. Miscellaneous
Recently, the use of exosomes (30-100nm in diameter; extracellular membrane enclosed vesicles) which are released by various cells in the extracellular matrix and biological fluids by exocytosis has been suggested as carriers for heat shock proteins (HSP) for the immunotherapy of HCC. Exosomes from resistant HepG2 cells have been reported to improve tumor immunogenicity with the induction of HSP-specific natural killer (NK) cell responses (Lv et al., 2012).
7. Challenges and Future Opportunities
With the rising incidence of HCC and high morbidity and mortality rates associated with this cancer, there is an urgent need to develop effective treatment and prevention strategies. We have reviewed the various pathways and recent treatment modalities of HCC with emphasis on small molecule therapeutics, vaccines, monoclonal antibody and nanotechnology development in the field of hepatocarcinogenesis. Since HCC is a complex multi-step process influenced by many factors for its initiation and progression, therapeutic interventions are challenging for this disease. Multiple factors are correlated including inter-related pathways and genetic and epigenetic alterations, which requires thorough understanding of prognosis for successful treatment. While surgical resection and liver transplantation are the only strategies available for treating advanced stage patients, nanomedicines and immunotherapy are promising approach for successful treatment of HCC. Not only safety, toxicity, preclinical efficacy studies are required thoroughly before any clinical application of these delivery strategies are further explored.
Acknowledgments
We acknowledge the financial support from the National Institutes of Health (1R01EB017853 and R01GM113166) and the Faculty Startup fund from the University of Nebraska Medical Center.
Abbreviations
- AASLD
American Association for the Study of the Liver Disease
- AFP
α-fetoprotein
- ALT
alanine aminotransferase
- AST
aspartase aminotransferase
- AFP-L3
agglutinin reactive fraction of AFP
- cccDNA
covalently closed circular DNA
- CTL
cytotoxic T lymphocyte
- DAA
direct-acting antivirals
- DOX
Doxorubicin
- ERK
Extracellular signal-regulated kinase
- GPC3
Glypican-3
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HCC
Hepatocellular carcinoma
- HLA
human leukocyte antigen
- JNK
Jun amino-terminal kinases
- LCN
liquid crystalline nanoparticles
- LP
liposomes
- mTOR
mammalian target of rapamycin
- MAPK
mitogen-activated protein kinase
- mAbs
monoclonal antibodies
- NASH
non-alcoholic steatohepatitis
- NAFLD
non-alcoholic fatty liver diseases
- PDGF
platelet derived growth factor
- PEG
polyethylene glycol
- ROS
reactive oxygen species
- TACE
Transarterial chemoembolization
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- T2DM
type 2 diabetes mellitus
- VEGF
vascular endothelial growth factor
Footnotes
Associate editor: B. Teicher
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
References
- Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Yen CJ. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. Journal of hepatology. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Materials. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, Ghoshal K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertino G, Demma S, Ardiri A, Proiti M, Gruttadauria S, Toro A, Di Carlo I. Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future ther-apies. 2014;2014:203693. doi: 10.1155/2014/203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annual Review of Biochemistry. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md) 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabibbo G, Latteri F, Antonucci M, Craxi A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nature Clinical Practice. Gastroenterology & Hepatology. 2009;6:159–169. doi: 10.1038/ncpgasthep1357. [DOI] [PubMed] [Google Scholar]
- Cai Y, Xu Y, Chan HF, Fang X, He C, Chen M. Glycyrrhetinic Acid Mediated Drug Delivery Carriers for Hepatocellular Carcinoma Therapy. 2016;13:699–709. doi: 10.1021/acs.molpharmaceut.5b00677. [DOI] [PubMed] [Google Scholar]
- Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, El-Nowiem S. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Tsukamoto H, Machida K. Oncogenic signaling pathways and origins of tumor-initiating stem-like cells of hepatocellular carcinomas induced by hepatitis C virus, alcohol and/or obesity. 2014;8:330–338. doi: 10.1007/s12072-014-9545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhang J, Wang L, Wang Y, Chen M. Tumor pH(e)-triggered charge-reversal and redox-responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale. 2015a;7:15763–15779. doi: 10.1039/c5nr04612b. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu Y, Wang W, Liu K. Effect of integrin receptor-targeted liposomal paclitaxel for hepatocellular carcinoma targeting and therapy. 2015b;10:77–84. doi: 10.3892/ol.2015.3242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Raymond E. Su-nitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- Colpitts CC, Baumert TF. Hepatitis C virus cell entry: a target for novel antiviral strategies to address limitations of direct acting antivirals. Hepatology International. 2016;10(5):741–748. doi: 10.1007/s12072-016-9724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell LC, Harding JJ, Abou-Alfa GK. Advanced Hepatocellular Cancer: the Current State of Future Research. Current Treatment Options in Oncology. 2016;17:43-016, 0415-3. doi: 10.1007/s11864-016-0415-3. [DOI] [PubMed] [Google Scholar]
- Daoudaki M, Fouzas I. Hepatocellular carcinoma. Wiener Medizinische Wochenschrift (1946) 2014;164:450–455. doi: 10.1007/s10354-014-0296-7. [DOI] [PubMed] [Google Scholar]
- Deng X, Yin Z, Zhou Z, Wang Y, Zhang F, Hu Q, Zeng Y. Carboxymethyl Dextran-Stabilized Polyethylenimine-Poly(epsilon-caprolactone) Nanoparticles-Me-diated Modulation of MicroRNA-34a Expression via Small-Molecule Modulator for Hepatocellular Carcinoma Therapy. 2016;8:17068–17079. doi: 10.1021/acsami.6b03122. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW, Landon CD, Hofmann CL, Stauffer PR. Novel approaches to treatment of hepatocellular carcinoma and hepatic metastases using thermal ablation and thermosensitive liposomes. 2013;22:545–561. doi: 10.1016/j.soc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Annals of Surgery. 2016;263:1112–1125. doi: 10.1097/SLA.0000000000001556. [DOI] [PubMed] [Google Scholar]
- Dong CW, Wang YX, Du FT, Ding W, Hu SY. Low miR-29c expression is a prognostic marker in hepatocellular carcinoma. Genetics and Molecular Research: GMR. 2016;15 doi: 10.4238/gmr.15037316. http://dx.doi.org/10.4238/gmr.15037316. [DOI] [PubMed] [Google Scholar]
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2012;55:1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nature Reviews Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Farra R, Grassi M, Grassi G, Dapas B. Therapeutic potential of small interfering RNAs/micro interfering RNA in hepatocellular carcinoma. 2015;21:8994–9001. doi: 10.3748/wjg.v21.i30.8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari F, Ferracin M, Trere D, Milazzo M, Marinelli S, Galassi M, Gramantieri L. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. 2015;10:e0141448. doi: 10.1371/journal.pone.0141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Huang D. Systemic therapies for hepatocellular carcinoma. Drug Discoveries & Therapeutics. 2015;9:352–362. doi: 10.5582/ddt.2015.01047. [DOI] [PubMed] [Google Scholar]
- Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. 2015;21:11984–12002. doi: 10.3748/wjg.v21.i42.11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grarup N, Sandholt CH, Hansen T, Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia. 2014;57:1528–1541. doi: 10.1007/s00125-014-3270-4. [DOI] [PubMed] [Google Scholar]
- Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, NASHCRN Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HJ, Davis C, Mullins JG, Hu K, Goodall M, Farquhar MJ, McKeating JA. Claudin association with CD81 defines hepatitis C virus entry. 2010;285:21092–21102. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immuno-therapeutic target in hepatocellular carcinoma. 2016;22:275–283. doi: 10.3748/wjg.v22.i1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CN, Chayama K. MicroRNAs as Biomarkers for Liver Disease and Hepa-tocellular Carcinoma. 2016;17:280. doi: 10.3390/ijms17030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YP, Li ZD, Prasoon P, Zhang Q. Immunotherapy for hepatocellular carcinoma: From basic research to clinical use. 2015;7:980–992. doi: 10.4254/wjh.v7.i7.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Liu J, Yang D, Lu M, Yin J. Physiological roles of asialoglycoprotein receptors (ASGPRs) variants and recent advances in hepatic-targeted delivery of therapeutic molecules via ASGPRs. Protein and Peptide Letters. 2014;21:1025–1030. doi: 10.2174/0929866521666140626102429. [DOI] [PubMed] [Google Scholar]
- Huang W, You L, Yang S, Liu D, Liu M, Wang H, Fan X. Metronomic S-1 chemotherapy plus transcatheter arterial chemoembolization (TACE): a promising treatment of hepatocellular carcinoma refractory to TACE. Journal of B U ON : Official Journal of the Balkan Union of Oncology. 2016;21:909–916. [PubMed] [Google Scholar]
- Huang Y, Hong J, Zheng S, Ding Y, Guo S, Zhang H, Liang Z. Elimination pathways of systemically delivered siRNA. Molecular Therapy: The Journal of the American Society of Gene Therapy. 2011;19:381–385. doi: 10.1038/mt.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C, Suzuki I, Yamamoto S, Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Science. 2014;4:455–462. doi: 10.1111/cas.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama T, Uchida T, Sawada Y, Tsuchiya N, Sugai S, Fujinami N, Nakatsura T. Vaccination with liposome-coupled glypican-3-derived epitope peptide stimulates cytotoxic T lymphocytes and inhibits GPC3-expressing tumor growth in mice. Biochemical and Biophysical Research Communications. 2016;496:138–143. doi: 10.1016/j.bbrc.2015.11.084. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature Reviews Drug Discovery. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chen H, Yu C, Zhang Y, Chen M, Tian S, Sun C. The promotion of salinomycin delivery to hepatocellular carcinoma cells through EGFR and CD133 aptamers conjugation by PLGA nanoparticles. Nanomedicine (London, England) 2015;10:1863–1879. doi: 10.2217/nnm.15.43. [DOI] [PubMed] [Google Scholar]
- Kamel RR, Amr KS, Afify M, Elhosary YA, Hegazy AE, Fahim HH, Ezzat WM. Relation between microRNAs and Apoptosis in Hepatocellular Carcinoma. Open Access Macedonian Journal of Medical Sciences. 2016;4:31–37. doi: 10.3889/oamjms.2016.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JJ, Qin R, Strosberg JR, Tan B, Kaubisch A, El-Khoueiry AB, Erlichman C. A phase II trial of bevacizumab plus temsirolimus in patients with advanced hepatocellular carcinoma. 2015;33:241–246. doi: 10.1007/s10637-014-0169-3. [DOI] [PubMed] [Google Scholar]
- Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Nishimura Y. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kimura O, Shimosegawa T. Significant biomarkers for the management of hepatocellular carcinoma. 2015;8:109–115. doi: 10.1007/s12328-015-0568-9. [DOI] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogen-esis. Part 1: the metabolic movers. 2010;25:672–690. doi: 10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA (New York, N Y) 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carci-noma. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu Y, Xiao J, Liu G, Li X, Zhao Y, Cheng D. Investigation of SP94 Pep-tide as a Specific Probe for Hepatocellular Carcinoma Imaging and Therapy. 2016;6:33511. doi: 10.1038/srep33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhou JX, Ren JZ, Zhang WJ, Han XW. Clinical value of iodine [131I] metuximab infusion combined with TACE for treatment of patients with post-intervention relapse of mid or advanced stage hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi = Zhonghua Ganzangbing Zazhi = Chinese Journal of Hepatology. 2013;21:728–733. doi: 10.3760/cma.j.issn.1007-3418.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Liu MC, Liu L, Wang XR, Shuai WP, Hu Y, Han M, Gao JQ. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. International Journal of Nanomedicine. 2016a;11:1395–1412. doi: 10.2147/IJN.S96862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu X, Gao Y, Zhang J, Zhang D, Gu S, Li X. Aptamer-functionalized peptide H3CR5C as a novel nanovehicle for codelivery of fasudil and miRNA-195 targeting hepatocellular carcinoma. 2016b;11:3891–3905. doi: 10.2147/IJN.S108128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Park JW. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- Lo A, Lin CT, Wu HC. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. 2008;7:579–589. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang JH. 131I-Labeled-Metuximab Plus Transarterial Chemoembolization in Combination Therapy for Unresectable Hepatocellular Carcinoma: Results from a Multicenter Phase IV Clinical Study. 2015;16:7441–7447. doi: 10.7314/apjcp.2015.16.17.7441. [DOI] [PubMed] [Google Scholar]
- Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10777–10782. doi: 10.1073/pnas.1301764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World Journal of Experimental Medicine. 2016;6:21–36. doi: 10.5493/wjem.v6.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Medina SH, Tiruchinapally G, Chevliakov MV, Durmaz YY, Stender RN, Ensminger WD, Elsayed ME. Targeting hepatic cancer cells with pegylated dendrimers displaying N-acetylgalactosamine and SP94 peptide ligands. 2013;2:1337–1350. doi: 10.1002/adhm.201200406. [DOI] [PubMed] [Google Scholar]
- Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, Chopra A, Grosso J, Lang L, Anderson J, Dela Cruz CM, Sangro B. (Jun 20 2015 Phase I/II safety and antitu-mor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. Journal of Clinical Oncology 33(18_suppl) LBA101. http://dx.doi.org/10.1200/jco.2015.33.18_suppl.lba101.
- Mirzaei HR, Sahebkar A, Yazdi F, Salehi H, Jafari MH, Namdar A, Mirzaei H. Circulating microRNAs in hepatocellular carcinoma: potential diagnostic and prognostic biomarkers. Current Pharmaceutical Design. 2016;22:5257–5269. doi: 10.2174/1381612822666160303110838. [DOI] [PubMed] [Google Scholar]
- Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, Kaneko S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. 2015;369:242–249. doi: 10.1016/j.canlet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Karin M. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Nishimura Y. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2004;10:8630–8640. doi: 10.1158/1078-0432.CCR-04-1177. [DOI] [PubMed] [Google Scholar]
- Nobuoka D, Yoshikawa T, Sawada Y, Fujiwara T, Nakatsura T. Peptide vaccines for hepatocellular carcinoma. Human Vaccines & Immunotherapeutics. 2013;9:210–212. doi: 10.4161/hv.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohri N, Kaubisch A, Garg M, Guha C. Targeted Therapy for Hepatocellular Carcinoma. Seminars in Radiation Oncology. 2016;26:338–343. doi: 10.1016/j.semradonc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Okajima W, Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Hirajima S, Otsuji E. Circulating microRNA profiles in plasma: identification of miR-224 as a novel diagnostic biomarker in hepatocellular carcinoma independent of hepatic function. Oncotarget. 2016;7:53820–53836. doi: 10.18632/oncotarget.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Chen C, Long H, Lei C, Tang G, Li L, Chen F. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. 2013;7:969–974. doi: 10.3892/mmr.2013.1279. [DOI] [PubMed] [Google Scholar]
- Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology. 2012;1:48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma. World Journal of Hepatology. 2016;8:421–438. doi: 10.4254/wjh.v8.i9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Dhawan V, Magarkar A, Danne R, Govindarajan S, Ghosh S, Nagarsenker M. Design of cholesterol arabinogalactan anchored liposomes for asialoglycoprotein receptor mediated targeting to hepatocellular carcinoma: In silico modeling, in vitro and in vivo evaluation. 2016;509:149–158. doi: 10.1016/j.ijpharm.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Pinter M, Ulbrich G, Sieghart W, Kolblinger C, Reiberger T, Li S, Peck-Radosavljevic M. Hepatocellular Carcinoma: A Phase II Randomized Controlled Double-Blind Trial of Transarterial Chemoembolization in Combination with Biweekly Intravenous administration of bevacizumab or a Placebo. radiology. 2015;277:903–912. doi: 10.1148/radiol.2015142140. [DOI] [PubMed] [Google Scholar]
- Pirazzi C, Valenti L, Motta BM, Pingitore P, Hedfalk K, Mancina RM, Romeo S. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. 2014;23:4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Giglione P, Ferrari A, Reversi F, Liguigli W, Imarisio I, Ganini C. Tivantinib (ARQ197) in hepatocellular carcinoma. Expert Review of Anticancer Therapy. 2015;15:615–622. doi: 10.1586/14737140.2015.1050383. [DOI] [PubMed] [Google Scholar]
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nature Reviews. Gastroenterology & Hepatology. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. 2016;61:1234–1245. doi: 10.1007/s10620-016-4085-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro de Souza A, Reig M, Bruix J. Systemic treatment for advanced hepato-cellular carcinoma: the search of new agents to join sorafenib in the effective therapeutic armamentarium. 2016;17:1923–1936. doi: 10.1080/14656566.2016.1225722. [DOI] [PubMed] [Google Scholar]
- Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. 2005;25:212–225. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- Sainz B, Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Uprichard SL. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. 2012;18:281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C59. 2013:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Shah SM, Goel PN, Jain AS, Pathak PO, Padhye SG, Govindarajan S, Nagarsenker MS. Liposomes for targeting hepatocellular carcinoma: use of conjugated arabinogalactan as targeting ligand. 2014;477:128–139. doi: 10.1016/j.ijpharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Simonelli M, Zucali P, Santoro A, Thomas MB, de Braud FG, Borghaei H, Berlin J, Denlinger CS, Noberasco C, Rimassa L, Kim TY, English PA, Abbattista A, Gallo Stampino C, Carpentieri M, Williams JA. Phase I study of PF-03446962, a fully human monoclonal antibody against activinreceptor-like kinase-1, in patients with hepatocellular carcinoma. Ann Oncol. 2016;9:1782–1787. doi: 10.1093/annonc/mdw240. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nature Reviews. Gastroenterology & Hepatology. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, Shim SG, Paik YH. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg HC, Thimme R, Blum HE. Targeted therapy for hepatocellular carcinoma. Nature Reviews. Gastroenterology & Hepatology. 2009;6:423–432. doi: 10.1038/nrgastro.2009.86. [DOI] [PubMed] [Google Scholar]
- Spangenberg HC, Thimme R, Blum HE. Evolving therapies in the treatment of hepatocellular carcinoma. Biologics: Targets & Therapy. 2008;2:453–462. doi: 10.2147/btt.s3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Takei Y. Pathogenesis of alcoholic liver disease. Hepatology Research: The Official Journal of the Japan Society of Hepatology. 2017;46:70–79. doi: 10.1111/hepr.12736. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N, Zhang N. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. 2016;10:85–91. doi: 10.5582/bst.2015.01128. [DOI] [PubMed] [Google Scholar]
- Szwed M, Wrona D, Kania KD, Koceva-Chyla A, Marczak A. Doxorubicin-transferrin conjugate triggers pro-oxidative disorders in solid tumor cells. Toxicology in Vitro: An International Journal Published in Association with BIBRA. 2016;31:60–71. doi: 10.1016/j.tiv.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Tamori A, Enomoto M, Kawada N. Recent Advances in Antiviral Therapy for Chronic Hepatitis C2016. 2016:6841628. doi: 10.1155/2016/6841628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral S, Ghoshal K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Current Gene Therapy. 2015;15:142–150. doi: 10.2174/1566523214666141224095610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa RK, Choi JY, Poudel BK, Hiep TT, Pathak S, Gupta B, Kim JO. Multilayer-Coated Liquid Crystalline Nanoparticles for Effective Sorafenib Delivery to Hepatocellular Carcinoma. ACS Applied Materials & Interfaces. 2015;7:20360–20368. doi: 10.1021/acsami.5b06203. [DOI] [PubMed] [Google Scholar]
- Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, McKeating JA. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology (Baltimore, Md) 2008;47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Mori M. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. Journal of hepatology. 2012;56:167–175. doi: 10.1016/j.jhep.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visceral Medicine. 2016;32:116–120. doi: 10.1159/000445730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshosaz J, Farzan M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World Journal of Gastroenterology. 2015;21:12022–12041. doi: 10.3748/wjg.v21.i42.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidmann O, Trojan J. Novel drugs in clinical development for hepatocellular carcinoma. Expert Opinion on Investigational Drugs. 2015;24:1075–1082. doi: 10.1517/13543784.2015.1058776. [DOI] [PubMed] [Google Scholar]
- Wang C, Hann HW, Ye Z, Hann RS, Wan S, Ye X, Block PD, Li B, Myers RE, Wang X, Juon HS, Civan J, Chang M, Bae HS, Xing J, Yang H. Prospective evidence of a circulating microRNA signature as a non-invasive marker of hepatocellularcarcinoma in HBV patients. Oncotarget. 2016 May 24; http://dx.doi.org/10.18632/oncotarget.9429. [Epub ahead of print]
- Wang C, Su L, Wu C, Wu J, Zhu C, Yuan G. RGD peptide targeted lipid-coated nanoparticles for combinatorial delivery of sorafenib and quercetin against hepatocellular carcinoma. Drug Development and Industrial Pharmacy. 2016:1–7. doi: 10.1080/03639045.2016.1185435. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang H, Li J, Liu Z, Xie H, Wei X, Zheng S. iRGD-Decorated Polymeric Nanoparticles for the Efficient Delivery of vandetanib to Hepatocellular Carcinoma: Preparation and in Vitro and in Vivo Evaluation. ACS Applied Materials & Interfaces. 2016;8:19228–19237. doi: 10.1021/acsami.6b03166. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang Q, Shen D, Sun X, Cao X, Wu D. Downregulation of microRNA-122 promotes proliferation, migration, and invasion of human hepatocellular carcinoma cells by activating epithelial-mesenchymal transition. OncoTargets and Therapy. 2016;9:2035–2047. doi: 10.2147/OTT.S92378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bai W, Wang E, Zhao Y, Liu L, Yang M, Han G. mRECIST response combined with sorafenib-related adverse events is superior to either criterion alone in predicting survival in HCC patients treated with TACE plus sorafenib. International Journal of Cancer. 2017;140:390–399. doi: 10.1002/ijc.30451. [DOI] [PubMed] [Google Scholar]
- Wei M, Guo X, Tu L, Zou Q, Li Q, Tang C, Wu C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. 2015;10:5123–5137. doi: 10.2147/IJN.S87011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lee EM, Hammack C, Robotham JM, Basu M, Lang J, Tang H. Cell death-inducing DFFA-like effector b is required for hepatitis C virus entry into hepa-tocytes. Journal of Virology. 2014;88:8433–8444. doi: 10.1128/JVI.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology (Baltimore, Md) 2010;51:836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- Yang N, Ekanem NR, Sakyi CA, Ray SD. Hepatocellular carcinoma and microRNA: new perspectives on therapeutics and diagnostics. Advanced Drug Delivery Reviews. 2015;81:62–74. doi: 10.1016/j.addr.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology (Baltimore, Md) 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- Yutani S, Ueshima K, Abe K, Ishiguro A, Eguchi J, Matsueda S, Noguchi M. Phase II Study of Personalized Peptide Vaccination with Both a Hepatitis C Virus-Derived Peptide and Peptides from Tumor-Associated Antigens for the Treatment of HCV-Positive Advanced Hepatocellular Carcinoma Patients. 2015;2015:473909. doi: 10.1155/2015/473909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hu J, Chan HF, Skibba M, Liang G, Chen M. iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomedicine: Nanotechnology, Biology and Medicine. 2016a;12:1303–1311. doi: 10.1016/j.nano.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang M, Ji J, Fang X, Pan X, Wang Y, Chen M. Glycyrrhetinic Acid-Mediated Polymeric Drug Delivery Targeting the Acidic Microenvironment of Hepatocellular Carcinoma. 2015a;32:3376–3390. doi: 10.1007/s11095-015-1714-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Peng F, Zhou T, Huang Y, Zhang L, Ye P, Xiang G. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. 2015b;10:4825–4836. doi: 10.2147/IJN.S79598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li J, Yan M. Targeted hepatocellular carcinoma therapy: Transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Development and Industrial Pharmacy. 2016a;42:1590–1599. doi: 10.3109/03639045.2016.1160103. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zehnder B, Damrau C, Urban S. Visualization of hepatitis B virus entry — Novel tools and approaches to directly follow virus entry into hepatocytes. FEBS Letters. 2016b;590:1915–1926. doi: 10.1002/1873-3468.12202. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang M, Yung B, Li H, Zhou C, Lee LJ, Lee RJ. Lactosylated lipo-somes for targeted delivery of doxorubicin to hepatocellular carcinoma. International Journal of Nanomedicine. 2012;7:5465–5474. doi: 10.2147/IJN.S33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Chen LT. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of soraf-enib: The EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, … REACHTrialInvestigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with so-rafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. The Lancet Oncology. 2015b;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Kang YK. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2015a;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- Zhu D, Qin Y, Wang J, Zhang L, Zou S, Zhu X, Zhu L. Novel glypican-3-binding peptide for in vivo hepatocellular carcinoma fluorescent imaging. Bioconjugate Chemistry. 2016a;27:831–839. doi: 10.1021/acs.bioconjchem.6b00030. [DOI] [PubMed] [Google Scholar]
- Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific Region. Gut and Liver. 2016b;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: The role of adipose triglyceride lipase in lipolysis. Biochimica et Biophysica Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]