Abstract
Background
Optimal secondary prevention is critical for the reduction of repeated cardiovascular events and the control of cardiovascular risk factors in this context is essential. Data on secondary prevention of cardiovascular disease (CVD) in sub-Saharan Africa are needed to inform intervention strategies, with a particular focus on local disparities. The aim of this study was to assess CVD management in a rural community in northeast South Africa.
Methods and Results
We recruited adults aged ≥ 40 years residing in the Agincourt sub-district of Mpumalanga province. Data collection included socioeconomic and clinical data, anthropometric measures, blood pressure, HIV status and point-of-care glucose and lipid levels. CVD was defined as self-report of myocardial infarction and stroke, or angina diagnosed by Rose Criteria. A linear regression model was built to identify variables independently associated with the number of cardiovascular risk factors controlled. Out of 5,059 subjects, 592 (11.7%) met CVD diagnostic criteria. Angina was reported in 77.0% of these subjects, stroke in 25.2% and myocardial infarction in 3.7%. Percent controlled of the five individual risk factors assessed were as follows: tobacco 92.9%; blood pressure 51.2%; BMI 33.8%; LDL 31.4%; and waist-to-hip ratio 29.7%. Only 4.4% had all five risk factors controlled and 42.4% had three or more risk factors controlled. Male sex (β-coefficient=0.44; 95%CI 0.25–0.63; p<0.001), absence of physical disability (β-coefficient=0.40; 95%CI 0.16–0.65; p=0.001) and socioeconomic status (SES) (β-coefficient=0.10; 95%CI 0.01–0.19; p=0.035) were directly associated with the number of risk factors controlled.
Conclusions
Currently, CVD is not being optimally managed in this rural area of South Africa. There are significant disparities in control of CVD risk factors by sex, SES, and level of disability. Efforts to improve secondary prevention in this population should be focused on females, subjects from lower SES, and those with physical disabilities.
Traditionally, sub-Saharan Africa (SSA) has been affected predominantly by maternal and perinatal disease, infectious communicable conditions and nutritional deficiencies. However, during recent years, the burden of non-communicable diseases (NCDs) such as cardiovascular disease (CVD) has been added to the challenges that health systems from this region face1. In 1990 CVD and other major chronic diseases accounted for approximately 28% of morbidity and 35% of mortality in SSA, but by 2020 it is projected that these will rise to 60% and 65%, respectively2.
While CVD is not yet the leading cause of death in SSA age-specific CVD mortality and morbidity are already higher in some parts of the region than in many developed countries3. Therefore, promotion of cardiovascular health and CVD prevention in an effort to mitigate the observed adverse trends in CVD is an immediate necessity4.
It is well established that optimal secondary prevention is critical for the reduction of repeated cardiovascular events and the control of cardiovascular risk factors in this context is essential5. Many studies have assessed the prevalence of cardiovascular risk factors6–11 and primary prevention strategies1, 12, but few have focused on the management of established CVD in the sub-Saharan region.
The lack of information regarding management of patients with established CVD in sub-Saharan Africa prompted us to investigate this topic in an appropriate population: the ‘Health and Aging in Africa: Longitudinal Studies of INDEPTH Communities (HAALSI)’ cohort. One of the main purposes of this cohort is to understand the reasons for changes in the prevalence, incidence, and risk factors for cardiometabolic disease in rural South Africa. To fulfill that purpose a cohort of 5059 men and women ≥40 years of age was recruited in the Agincourt Health and Demographic Surveillance System (HDSS) site in rural South Africa. The intention of this analysis is to determine CVD prevalence and its management in a rural community in northeast South Africa, focusing on identifying disparities within this population.
Methods
The HAALSI study is based in the Agincourt Health and Demographics Surveillance System (HDSS) site, a sub-district of the rural Mpumalanga province, South Africa, comprising some 115 000 people living in 21 000 households and 31 villages in an area of ~450km2. An annual census update, conducted by experienced local field staff, provides up-to-date denominator data on the full population with systematic recording of all vital events (deaths, births, in- /out-migrations).
Eligibility
All adults aged 40 years and older as of July 1, 2014 who had permanently resided in the Agincourt sub-district for at least one year prior to the 2013 census update were eligible.
Sampling and Sample Size
Participants were selected from an existing framework of the Agincourt HDSS site in Mpumalanga province. Using the full 2013 Census data, a sampling frame of adults aged 40 and older (8,974 women and 3,901 men) who met the residency criteria were identified. A target sample size, assuming an 80% response rate, was approximately 5,000 completed interviews. We selected a total of 6,281 women and men for the main household study. Among these, 391 had moved outside of the study site or were deceased. Out of the remaining 5,890 eligible individuals, 5,059 (85.9%) participated in the baseline survey13.
Ethics Approval
The study received ethical approvals from the Ethics Committees of three institutions directly involved in the project (University of the Witwatersrand Human Research Ethics Committee (ref M141159), the Harvard T.H. Chan School of Public Health, Office of Human Research Administration (ref C13-1608-02) and the Mpumalanga Provincial Research and Ethics Committee (approved on 22nd October 2014).
Recruitment and Follow Up
Prior to the survey, the HAALSI study was introduced to community members across the study villages, and discussed with a representative Community Advisory Group. This facilitated community review of study objectives and contributed to the effective response achieved.
Between November 2014 and November 2015 all identified individuals were visited at home by a supervised local field worker who briefly described the study in the local language (Shangaan), and requested permission to read and explain the relevant informed consent forms. Those who agreed to participate signed a consent form or, if not able to sign their name, were asked to have a literate witness sign and date the informed consent on their behalf. Field workers also signed and dated the informed consent form.
Study Procedures
Interviews lasting 2–3 hours were conducted in participants’ homes. The visit included a questionnaire in two parts: household and socioeconomic data then individual interview data; followed by anthropometric, physical and cognitive functioning assessments plus blood sample collection in the form of capillary blood sample and “dried blood spots” (DBS). Data were captured on laptop computers using a Computer Assisted Personal Interview (CAPI) program.
Data collected included blood pressure (Omron M6W automated cuff; Omron, Kyoto, Japan), weight (Genesis Growth Management Electronic Scale; Johannesburg, South Africa); height using a sensor with infrared measurement; waist and hip circumferences with a flexible tape measure (SECA, Hamburg, Germany). Blood drops were used to measure glucose (Caresense© N Monitor, Seoul, Korea) and individual lipid levels (Cardiocheck© PA Silver version; Indianapolis, Indiana, USA) in point-of-care machines. The DBSs were collected via finger prick on Whatman 903 ™ paper (Whatman, Buckinghamshire, UK) and used to measure HIV-status and high sensitivity C-reactive protein (hsCRP). Three blood pressure readings (systolic and diastolic) were obtained with two minutes between each reading and the mean blood pressure was calculated using the average between the second and third reading14.
Definition of Cardiovascular Disease
Cardiovascular disease was defined by self-report of stroke and myocardial infarction, or a diagnosis of angina by Rose criteria (World Health Organization Rose questionnaire is widely used in epidemiological studies and is a validated and standardized method for defining angina pectoris)15.
Control of Cardiovascular Risk Factors
Smoking status was defined by self-report of current smoking status and the non-smokers were considered as having this risk factor controlled. LDL cholesterol was considered controlled with a value < 1.8mmol/L according to the South African Dyslipidaemia Guidelines16. Body mass index (BMI) in kg/m2 was categorized using World Health Organization cutoffs17 and values lower than 25 k/m2 were considered controlled. Waist-to-hip ratios were considered controlled if ≤ 0.90 for men and ≤ 0.85 for women18. Hypertension was considered controlled if systolic blood pressure <140 mmHg and diastolic blood pressure < 90 mmHg19
Other Covariates
Treatment of stroke, angina and myocardial infarction was assessed by self-report. Clinical determination of HIV status was made by first using the Vironostika Uniform 11 (Biomeriuex, France) screening assay. Negative results were assigned a HIV-negative status, while positive results trigged a second (confirmatory) test using the Roche Elecys (Indianapolis, Indiana, USA) assay to determine the viral load. If both screening and confirmatory tests were positive, a final status of HIV-positive was assigned. If the screening and confirmatory tests yielded opposing results, a third assay was run on the Siemens Centaur XP (Erlangen, Germany) immunoassay. This third test served as the tie-breaker to determine a final HIV-positive or negative status, in accordance with World Health Organization (WHO) guidelines20, 21. HIV-positive status was defined as a self-report of being informed of the condition by a health professional or a positive result from blood analysis. Physical disability (PD) was assessed by self-reported presence or absence of limitations in activities of daily living (ADL) (excluding difficulty in dressing as an ADL)22. Socioeconomic status (SES) was measured using the wealth asset index which is a quintile ranking of scores constructed following the Demographic Health Surveys (DHS) methodology to create a composite indicator of living standards using data on household ownership of assets. This index includes information on durables such as televisions and refrigerators, as well as housing conditions23, 24. Immigrants were defined as the subjects who were born outside South Africa. Illiteracy was assessed separately from education; respondents were asked whether they could read or write.
Analyses
All analyses were conducted using STATA ® V14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, US). Continuous variables were compared using T test (expressed in mean values and standard deviations, since they were normally distributed) and categorical variables using Chi Square test (expressed in absolute numbers and percentiles). The distribution of modifiable risk factors under control was assessed by HIV and immigration status, since these two conditions were highly prevalent in this cohort, have been shown to impact on NCD management in the region and yet need to be better studied25. A linear regression model was built to identify factors associated with the number of cardiovascular risk factors controlled. The predictors included in this model were sex, PD, age, SES, HIV status, immigrant status and illiteracy. These variables were included because they were previously reported as possible factors interfering with health management in this setting25–29. The results are presented using β-coefficients and 95% confidence intervals; level of significance was set as 5%.
Missing data
Several categories had varying levels of missing data. No imputation was performed for missing height (5%), weight (7%), waist circumference (6%), glucose (9%), total cholesterol (16%), HDL-cholesterol (16%), triglycerides (16%), and hs C-Reactive Protein (15%). Descriptive statistics in Table 1 reflect the results for all persons who had values for the respective variable. For the wealth index, 231 individuals were missing data and for these individuals the mean value for their village was imputed for this variable. In the subset of individuals with CVD, the analysis excluded subjects with missing key variables: blood pressure (3%), body mass index (7%), waist-to-hip ratio (7%), and LDL-cholesterol (16%).
Table 1.
| Overall | Non CVD | CVD | p-value§ | |
|---|---|---|---|---|
| n | 5059 | 4467 | 592 | |
| Male Sex | 2345 (46.4%) | 2121 (47.5%) | 224 (37.8%) | <0.001 |
| Age (years) | 61.74 (±13.06) | 61.34 (±13.07) | 64.71 (±12.63) | <0.001 |
| Weight (kg) | 71.87 (±17.64) | 71.80 (±17.65) | 72.43 (±17.57) | 0.430 |
| Height (m) | 1.63 (±0.09) | 1.63 (±0.09) | 1.61 (±0.09) | <0.001 |
| Body Mass Index (kg/m2) | 27.25 (±6.88) | 27.16 (±6.88) | 27.98 (±6.90) | 0.009 |
| Waist Circumference (cm) | 92.39 (±15.08) | 92.18 (±14.89) | 93.95 (±16.52) | 0.009 |
| Waist-Hip Ratio | 0.91 (±0.08) | 0.91 (±0.08) | 0.91 (±0.09) | 0.070 |
| Average SBP (mmHg) | 137.99 (±23.34) | 137.88 (±23.23) | 138.79 (±24.13) | 0.380 |
| Average DBP (mmHg) | 82.14 (±12.71) | 82.15 (±12.69) | 82.04 (±12.84) | 0.840 |
| Glucose (mmol/L) | 6.67 (±3.16) | 6.65 (±3.15) | 6.81 (±3.23) | 0.270 |
| Total Cholesterol (mmol/L) | 4.24 (±1.26) | 4.22 (±1.26) | 4.38 (±1.25) | 0.006 |
| High Density Lipoprotein (mmol/L) | 1.57 (±0.55) | 1.57 (±0.53) | 1.58 (±0.63) | 0.830 |
| Triglycerides (mmol/L) | 1.76 (±1.57) | 1.77 (±1.64) | 1.67 (±0.84) | 0.170 |
| Low Density Lipoprotein (mmol/L) | 2.12 (±1.50) | 2.11 (±1.56) | 2.19 (±1.03) | 0.240 |
| hs C-Reactive Protein‡ (mg/L) | 3.26 (±3.03) | 3.24 (±3.02) | 3.42 (±3.10) | 0.210 |
| Current Smokers | 460 (9.1%) | 418 (9.4%) | 42 (7.1%) | 0.072 |
| HIV positive | 1134 (22.5%) | 1028 (23.1%) | 106 (18.0%) | 0.005 |
| Illiterate | 2108 (41.7%) | 1831 (41.0%) | 277 (46.8%) | 0.007 |
CVD – cardiovascular disease (self-report of Stroke/Myocardial Infarction or Angina by Rose criteria)
Not shown missing: height (5%), weight (7%), body mass index (7%), waist circumference (6%), waist-to-hip ratio (7%), blood pressure (3%), glucose (9%), total cholesterol (16%), HDL-cholesterol (16%), triglycerides (16%), LDL-cholesterol (16%), and C-Reactive Protein (15%).
hs - high-sensitivity c-reactive protein
p-value for comparison between Non CVD and CVD individuals.
Statistically significant at α = 0.05; Data given as mean ± SD or n (%)
Results
Out of the 5,890 eligible persons, 85.9% (5,059) agreed to be interviewed, 7.3% refused to participate, 6.0% could not be located, and 0.8% were unable to participate. The number of subjects living with self-reported CVD was 592, which was 11.7% of the overall population. In this subset of CVD patients, angina was reported in 77.0% of the subjects, stroke in 25.2% and myocardial infarction in 3.7%. The population of patients with CVD was more likely to be female, older, with higher BMI, larger waist circumference and higher total cholesterol and less likely to be smokers when compared to the non-CVD population. The overall population characteristics, as well as results of those with CVD compared to the non-CVD population are summarized in table 1.
Among the subjects with angina 6.1% (28/456) were receiving treatment. In the group with stroke 65.8% (98/149) were being treated and in the subset with previous myocardial infarction 86.4% (19/22) reported treatment of their disease. Collectively, 24% (142/592) of those with CVD were being treated.
To understand the impact of HIV on CVD in the community we compared the proportion of individuals with angina, myocardial infarction and stroke, based on their HIV status. We found that 18.6% (85/456) of those who were HIV-positive had angina compared to 22.9% (1,048/4,576) among those who were HIV-negative, and this difference was statistically significant (p=0.036). The prevalence of stroke and myocardial infarction for HIV-positive persons was 16.3% (24/147) and 9.1% (2/22), respectively, compared to 22.7% (1,110/4,894) and 22.5% (1,132/5,020) for HIV-negative persons. These differences were not statistically significant (stroke: p = 0.068; myocardial infarction: p = 0.132).
The percentage of those with CVD who attained control for each of five modifiable risk factors was assessed and the results are presented in Table 2. Higher proportions of control were attained for smoking, with 92.9% of the patients with history of CVD reported to be non-smokers. Lower levels of control were attained for adiposity, as indicated by 29.7% of patients with waist-hip ratio below the cut-off values (0.90 for males and 0.85 for women). When these rates of modifiable risk factors were compared according to HIV and immigration status, higher numbers of patients with controlled BP were found in the HIV positive patients and fewer smokers were observed in the immigrant population (see Table 2).
Table 2.
Distribution of modifiable risk factors under control among the subjects with CVD* by HIV and migration status, Agincourt sub-district, South Africa 2015.
| Overall | HIV positive | HIV negative | Immigrant | Non Immigrant | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| (%) | (%) | (%) | p-value | (%) | (%) | p-value | |
| Waist-hip ratio† | 29.7 | 34.9 | 28.7 | 0.207 | 29.1 | 30.1 | 0.809 |
| LDL Cholesterol‡ | 31.4 | 36.8 | 30.4 | 0.197 | 28.5 | 32.7 | 0.317 |
| Body mass index§ | 33.8 | 41.5 | 32.2 | 0.068 | 33.1 | 34.1 | 0.817 |
| Blood pressure|| | 51.2 | 61.3 | 48.8 | 0.019# | 48.8 | 52.0 | 0.480 |
| Not Smoking | 92.9 | 88.7 | 93.8 | 0.063 | 96.5 | 91.4 | 0.028# |
CVD – cardiovascular disease (self-report of Stroke/Myocardial Infarction or Angina by Rose criteria).
Waist-Hip Ratio: ≤ 0.90 (men) or ≤ 0.85 (women).
LDL cholesterol < 1.8mmol/L.
Body mass index < 25kg/m2
Blood pressure < 140 × 90 mmHg.
Statistically significant at α = 0.05
Figure 1 shows the cumulative number of risk factors under control in this secondary prevention population. More than 57% of the patients with CVD had only two or fewer risk factors controlled and less than 5% had all five risk factors under control. When the number of risk factors controlled was assessed by sex 55.8% of the male subjects had three or more risk factors controlled compared to 34.2% of the females (p<0.001). In contrast, 28.3% of the females had only 1 risk factor controlled compared to 13.0% of the males (p<0.001).
Figure 1. Number of risk factors* controlled among subjects with CVD† stratified by sex, Agincourt sub-district, South Africa 2015 (n=592).
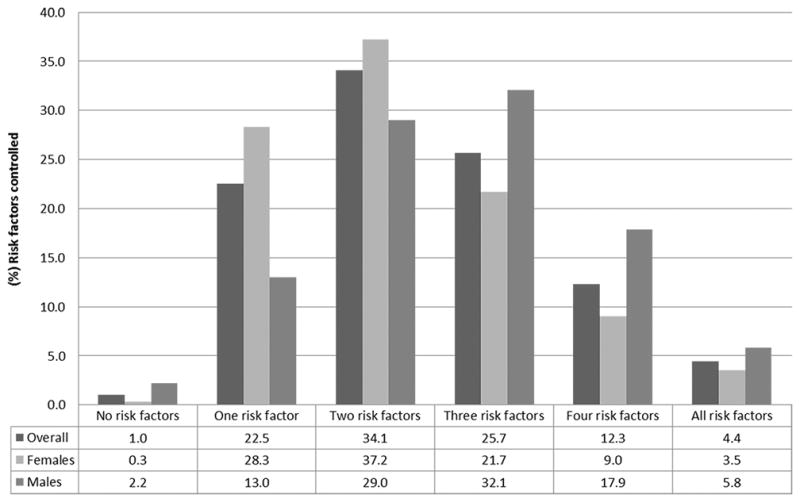
*LDL cholesterol < 1.8mmol/L; blood pressure < 140 × 90 mmHg; not smoking; waist-hip ratio: ≤ 0.90 (men) or ≤ 0.85 (women); body mass index < 25 kg/m2. †CVD – cardiovascular disease (self-report of Stroke/Myocardial Infarction or Angina by Rose criteria).
An additional analysis was conducted to address the individual impact of each of the five risk factors on angina, myocardial infarction and stroke. The only statistically significant difference we found was a higher percentage of waist-to-hip ratio in the ideal range among those with angina when compared to those with stroke (31.4% versus 22.8%; p=0.045). All other comparisons for the remaining risk factors were not statistically significantly different (see Figure 2).
Figure 2. Cardiovascular risk factors controlled* stratified by angina, myocardial infarction and stroke, Agincourt sub-district, South Africa 2015.
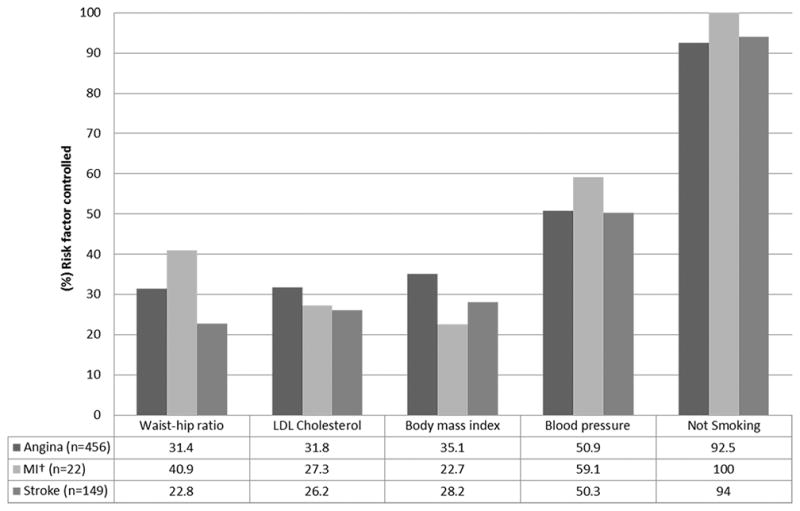
*LDL cholesterol < 1.8mmol/L; blood pressure < 140 × 90 mmHg; not smoking; waist-hip ratio: ≤ 0.90 (men) or ≤ 0.85 (women); body mass index < 25 kg/m2. †MI – myocardial infarction.
A multivariable linear regression model was developed to identify factors independently associated with the number of cardiovascular risk factors controlled, as shown in Table 3. Better management of CVD in this context was associated with male sex, absence of physical disability and higher SES.
Table 3.
Linear regression coefficients for the number of risk factors controlled among subjects with CVD*, Agincourt sub-district, South Africa 2015
| Variables | β-coefficient | [95% CI] | p-value |
|---|---|---|---|
| Male sex | 0.44 | 0.25 – 0.63 | <0.001§ |
| Absence of PD† | 0.40 | 0.16 – 0.65 | 0.001§ |
| SES‡ | 0.10 | 0.01 – 0.19 | 0.035§ |
| Age | 0.00 | −0.01 – 0.00 | 0.687 |
| HIV positive | 0.20 | −0.04 – 0.44 | 0.106 |
| Immigrant | −0.05 | −0.26 – 0.15 | 0.582 |
| Illiterate | −0.04 | −0.24 – 0.16 | 0.675 |
CVD – cardiovascular disease (self-report of Stroke/Myocardial Infarction or Angina by Rose criteria).
Physical disability - self-reported presence or absence of limitations in activities of daily living
SES – socioeconomic status (measured using the wealth asset index)
Statistically significant at α = 0.05
Discussion
Many different aspects of CVD have been studied in sub-Saharan Africa, particularly the prevalence of cardiovascular risk factors and primary prevention strategies1, 6, 10–12, 30–32. However, to our knowledge, no publications have assessed the management of patients with established CVD focusing on individuals’ disparities related to cardiovascular risk factors control. Even though CVD prevalence is increasing in sub-Saharan Africa, our results suggest that the secondary prevention of the disease is currently not being optimally managed, especially considering the need to have all cardiovascular risk factors controlled. Less than 5% of our study population had all five risk factors that were assessed under control, and the majority of the group had two or fewer risk factors controlled. In addition to that, gender, SES and physical disability were identified as associated to CVD management in this setting.
The prevalence of CVD in our population was 13% and is similar to the 10% for combined angina, stroke, and MI reported in the adult US population from the 2010 American Heart Association (AHA) estimates33. According to SANHANES, the South African National Health Survey from 2013, the self-reported prevalence of heart disease (2.2%) and stroke (1.8%) were lower than was found in our cohort (10.7% and 3%, respectively)34. However, it should be noted that in both the AHA and SANHANES studies the CVD prevalence was estimated for the adult population (>18 years), whilst for HAALSI, only subjects older than forty years were included so we would anticipate higher prevalence rates in our older population.
The overall prevalence of self-reported angina in our study was 2.35%, similar to previous findings reported for the same Agincourt population (4.02%)35, while the prevalence of angina as determined using the Rose criteria is 9.04%. It is likely that relying only on self-report underestimates the true prevalence of angina.
It is well stablished that human immunodeficiency virus-infected individuals are at increased risk for CVD, due to, among other factors, the high prevalence of cardiovascular risk factors in HIV-infected individuals36. Despite the fact that prevalence of HIV positivity was higher in the non-CVD population (23.1%) when compared to the CVD population (18%) of our study we stratified our analysis of angina, myocardial infarction and stroke by HIV-status. This approach addresses the important interaction between stroke and HIV37. Our results showed similar HIV infection numbers in individuals with and without myocardial infarction and stroke, and an even lower percentage of HIV infected subjects with angina (18.6%) when compared to those without angina (22.9%). These findings might be explained by previous findings from the same cohort that showed that HIV-positive patients are more likely to receive healthcare services for diabetes and hypertension38, which ultimately can lead to fewer cardiovascular events.
In the CLARIFY registry39 that included 33,283 outpatients (77% male, mean age: 64 years) with proven stable CAD from 45 countries, 12% of patients smoked, 78% were overweight, 71% of patients had hypertension and the control rates ranged from 47 to 66%. Dyslipidemia was reported in 75% of patients and LDL cholesterol control was between 35 and 76%. The HAALSI cohort is notably different from this study that assessed patients with CVD in its lower smoking rate (7.1%). Since we only investigated currently smoking this lower smoking rates in the CVD group may be attributable to smoking cessation after a cardiovascular event40.
Within the current study, only 4.4% of the population had all risk factors controlled and the majority of the subjects (57.6%) had ≤ 2 risk factors under control. A similar analysis from the National Health and Nutrition Examination Survey (NHANES) showed that 10.6% of the US population (with no significant differences noted across subgroups defined by race-ethnicity) on secondary prevention had all risk factors controlled, as well as an increasing temporal trend in this result41. Considering that having all risk factors under control is the most effective action in secondary prevention of CVD5, 42, we can conclude that the management of CVD in the region is far from optimal. Even more concerning is the poor level of CVD control found in women especially considering the greater effects of cardiovascular risk factors on women when compared to men43, 44.
Since each risk factor assessed in this population has a different impact as a secondary prevention strategy on angina, myocardial infarction and stroke45, 46, we assessed the proportion of control for each the five risk factors individually. Once again, this stratification is of particular interest when dealing with stroke, since it is already known that hypertension is the most dominant risk factor for cerebrovascular events45, and additionally a focus on blood pressure and excess weight control would yield best preventive results for stroke in this populaion46. Our results show that across all 3 CVD groups blood pressure is better controlled than lipids and this is consistent for what has been observed with stroke prevention in the Agincourt population46. However, still only 50% of stroke sufferers have controlled blood pressure and this need to be improved.
Using the HAALSI data, we developed a multivariable linear regression model to identify the factors associated with the number of CVD risk factors controlled. Included in this model were variables previously reported as possible factors interfering with health management in this setting25–29, including illiteracy, immigration status, physical disability and SES. Male sex, absence of physical disability and SES were positively associated with the number of risk factors controlled. Higher SES has been consistently associated with better risk factor control47 in secondary prevention of CVD and even within the HAALSI cohort, hypertension was better managed in those individuals from higher SES25. A possible explanation for the male sex and absence of physical disability results might be related to a better adherence to treatment in these two groups, when compared to females and individuals with co-morbidities48, 49.
A potential limitation of this particular study is the fact that the sub-district from which the cohort was recruited has been part of a HDSS since 1992. This may create a specific environment of improved health care surveillance that might increase the level of risk factor control among patients with CVD, when compared to subjects with CVD living in other parts of South Africa, as we previously showed in a study addressing hypertension management25. The low control rates of CVD risk factors, observed in this particular area under surveillance suggests that levels of risk factor’s control may be even worse at sites not covered by a HDSS.
Another limitation is the absence of data about specific drugs/strategies for secondary prevention. Information about antiplatelet drugs, beta-blockers, blockers of the renin–angiotensin system, and statins was not obtained, limiting these specific comparisons with other epidemiological studies50. However, we were able to make comparisons with another study for the number of individuals with established CVD not taking any appropriate medication. In this scenario, our results (24% not being treated) are better than the mean level observed in South Africa and other upper middle-income countries (Argentina, Brazil, Chile, Malaysia, Poland, and Turkey) (48.4%)51, reinforcing the hypothesis of better healthcare associated to HDSS coverage25.
The strengths of this study include the investigation of variables such as immigration and HIV status, which have not previously been assessed for their effects on CVD risk factor management. In these analyses, fewer immigrants with CVD were found to be smokers when compared to non-immigrants. This suppression of smoking in the immigrant population, was previously shown in the literature, although not in individuals with known CVD, and may reflect more general social sanctions against personal consumption among immigrants who have taken on the responsibility of leaving home to earn money and build savings for their households52. The better blood pressure control in HIV positive subjects is aligned with the results of a recent meta-analysis that assessed the association between HIV and cardiometabolic diseases in SSA53. Their results suggested that HIV positive patients have, on average, lower blood pressure than their HIV negative counterparts. An additional strength is the fact that this study provides relevant information on disparities in CVD management, which ultimately can be used to develop more equitable health models in a setting with limited resources.
Mortality from CVD has increased globally, particularly in the developing world from 1990 to 2013. Overall mortality from these conditions in sub-Saharan Africa is low (ischemic heart disease 4.7%, stroke 5.0%) but South Africa’s are slightly higher (7.6% and 6.3%, respectively)54, 55. Our data shows that one of the reasons for these high levels of mortality from cardiovascular events may be due to the poor control of CVD risk factors. This suggests that mortality may be decreased through improvement in treatment of these risk factors coupled with greater community education on the importance of lifestyle modification. Initiatives to achieve these goals, like population-wide screening for hypertension and diabetes, engagement of community resources and governance structures, geographic decentralization of care services, and group medical visits alone or integrated into microfinance groups, have been previously proposed in SSA56 and some of these interventions are currently under investigation57.
In conclusion, this analysis of the HAALSI study demonstrates that CVD is currently not being optimally managed in this rural area of South Africa and that there are significant opportunities to improve secondary prevention in this population. A particular focus should be placed on females, subjects from lower SES and those with physical disabilities.
What is Known
The burden of non-communicable diseases (NCDs) such as cardiovascular disease (CVD) has become an increasing challenge for health systems in sub-Saharan Africa.
For CVD in this region, optimal secondary prevention is critical for the reduction of repeated cardiovascular events and the control of cardiovascular risk factors.
To date, few studies have focused on the management of established CVD in sub-Saharan Africa.
What the Study Adds
Despite the growing prevalence of CVD in sub-Saharan Africa, we found that secondary prevention is currently suboptimal, especially for the outcome of control of all cardiovascular risk factors.
Fewer than 5% of our study population had all five cardiovascular risk factors under control, and the majority of the group had two or fewer risk factors controlled.
In addition, there were differences in the rates of optimal risk factor management by gender, socioeconomic status, and physical disability, suggesting potential disparities in care.
Acknowledgments
Sincere thanks to the Agincourt Unit field staff, quality checkers and data team who toiled long and hard to ensure the quality of data on which this paper is based. Thanks also to the Unit research management, community engagement and administration teams; your efforts are essential. A further thank you to Mark Collinson and Carren Ginsburg for advice on how to conceptualize the health effects of migration. The Division of Clinical Pharmacology Laboratory at the University of Cape Town, South Africa is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701, U01 AI068632, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632].
Source of funding: The HAALSI study, funded by the National Institute on Aging (P01 AG041710), is nested within the Agincourt Health and Demographic Surveillance System site, funded by the University of the Witwatersrand and Medical Research Council, South Africa, and the Wellcome Trust, UK (058893/A/99A; 069683/Z/02/Z; 085477/Z08/Z). The HAALSI study has been carried out through a collaboration between the Harvard Center for Population and Development Studies from the Harvard T.H. Chan School of Public Health, the MRC/Wits Rural Public Health and Health Transitions Research Unit from the School of Public Health at the University of the Witwatersrand in South Africa, and the INDEPTH Network in Accra, Ghana. AW is supported by the Fogarty International Center of the National Institutes of Health under Award Number K43TW010698. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: None.
References
- 1.Sampson UKA, Amuyunzu-Nyamongo M, Mensah GA. Health Promotion and Cardiovascular Disease Prevention in Sub-Saharan Africa. Progress in Cardiovascular Diseases. 2013;56:344–355. doi: 10.1016/j.pcad.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Madu EC, Richardson KD, Ozigbo OH, Baugh DS. Improving cardiovascular disease prevention and management in Africa: issues to consider for the 21st century. Ethn Dis. 2003;13:S71–6. [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT-A, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S, Khoo J-P, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Memish ZA, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwan A. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update. Circulation. 2011;124:2458. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 6.Kandala NB, Tigbe W, Manda SO, Stranges S. Geographic variation of hypertension in sub-saharan Africa: a case study of South Africa. Am J Hypertens. 2013;26:382–91. doi: 10.1093/ajh/hps063. [DOI] [PubMed] [Google Scholar]
- 7.Oladapo OO, Falase AO, Salako L, Sodiq O, Shoyinka K, Adedapo K. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: a population-based survey. Cardiovascular Journal of Africa. 2010;21:26–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Ngoungou EB, Aboyans V, Kouna P, Makandja R, Ecke Nzengue JE, Allogho CN, Laskar M, Preux P-M, Lacroix P. Prevalence of cardiovascular disease in Gabon: A population study. Archives of Cardiovascular Diseases. 2012;105:77–83. doi: 10.1016/j.acvd.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Solet JL, Baroux N, Pochet M, Benoit-Cattin T, De Montera AM, Sissoko D, Favier F, Fagot-Campagna A. Prevalence of type 2 diabetes and other cardiovascular risk factors in Mayotte in 2008: The MAYDIA study. Diabetes & Metabolism. 2011;37:201–207. doi: 10.1016/j.diabet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Peltzer K, Phaswana-Mafuya N. Hypertension and associated factors in older adults in South Africa. Cardiovasc J Afr. 2013;24:67–71. doi: 10.5830/CVJA-2013-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karwalajtys T, Kaczorowski J. An integrated approach to preventing cardiovascular disease: community-based approaches, health system initiatives, and public health policy. Risk Management and Healthcare Policy. 2010;3:39–48. doi: 10.2147/RMHP.S7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne CF, Gómez-Olivé FX, Kahn K, Berkman L. Physical Function in an Aging Population in Rural South Africa: Findings From HAALSI and Cross-National Comparisons With HRS Sister Studies. The Journals of Gerontology: Series B. 2017;72:665–679. doi: 10.1093/geronb/gbx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Contro and Preventionl (CDC) National Health and Nutrition Examination Survey: 1999 – 2000 Data Documentation, Codebook, and Frequencies. 2002;2015 [Google Scholar]
- 15.Achterberg S, Soedamah-Muthu SS, Cramer MJ, Kappelle LJ, van der Graaf Y, Algra A. Prognostic value of the Rose questionnaire: a validation with future coronary events in the SMART study. Eur J Prev Cardiol. 2012;19:5–14. doi: 10.1177/1741826710391117. [DOI] [PubMed] [Google Scholar]
- 16.Klug EQ, Raal F, Marais A, Taskinen M, Dalby A, Schamroth C, Rapeport N, Jankelow D, Blom D, Catsicas R. South African Dyslipidaemia Guideline Consensus StatementA joint statement from the South African Heart Association (SA Heart) and the Lipid and Atherosclerosis Society of Southern Africa (LASSA): guidelines. South African Family Practice. 2015;57:22, 24–31. [Google Scholar]
- 17.World Health Organization Technical Report Series. Vol. 894. World Health Organization; Geneva, Switzerland: 2000. Obesity: preventing and managing the global epidemic. Report of a WHO consultation; pp. i–xii.pp. 1–253. [PubMed] [Google Scholar]
- 18.Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; Geneva. 8–11 December 2008; Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JLJ, Jones DW, Materson BJ, Oparil S, Wright JTJ, Roccella EJ National Heart L, Blood Institute Joint National Committee, on., Prevention D, Evaluation, and Treatment of High Blood, Pressure and National High Blood Pressure Education Program Coordinating C. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the Jnc 7 Report.[Comment][Erratum Appears in Jama. 2003;290:197] JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 21.World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. World Health Organization; 2014. [PubMed] [Google Scholar]
- 22.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–85. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutstein SO, Johnson K MEASURE OM. The DHS wealth index: ORC Macro. MEASURE DHS; 2004. [Google Scholar]
- 24.Rutstein SO. The Demographic and Health Surveys (DHS) Program. Rockville, MD: US Agency for International Development (USAID); [Accessed on March 18, 2016]. 2014. Steps to constructing the new DHS Wealth Index. (Wealth Index Construction). Available at: http://dhsprogram.com/programming/wealth%20index/Steps_to_constructing_the_new_DHS_Wealth_Index.pdf. [Google Scholar]
- 25.Jardim TV, Reiger S, Abrahams-Gessel S, Gomez-Olive FX, Wagner RG, Wade A, Barnighausen TW, Salomon J, Tollman S, Gaziano TA. Hypertension management in a population of older adults in rural South Africa. J Hypertens. 2017;35:1283–1289. doi: 10.1097/HJH.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginsburg C, Bocquier P, Beguy D, Afolabi S, Augusto O, Derra K, Herbst K, Lankoande B, Odhiambo F, Otiende M, Soura A, Wamukoya M, Zabre P, White MJ, Collinson MA. Healthy or unhealthy migrants? Identifying internal migration effects on mortality in Africa using health and demographic surveillance systems of the INDEPTH network. Soc Sci Med. 2016;164:59–73. doi: 10.1016/j.socscimed.2016.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, Nojilana B, Joubert JD, Matzopoulos R, Prinsloo M, Nannan N, Gwebushe N, Vos T, Somdyala N, Sithole N, Neethling I, Nicol E, Rossouw A, Bradshaw D. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health. 2016;4:e642–53. doi: 10.1016/S2214-109X(16)30113-9. [DOI] [PubMed] [Google Scholar]
- 28.Pisa PT, Behanan R, Vorster HH, Kruger A. Social drift of cardiovascular disease risk factors in Africans from the North West Province of South Africa: the PURE study. Cardiovasc J Afr. 2012;23:371–8. e379–88. doi: 10.5830/CVJA-2012-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howitt SC, Jones MP, Jusabani A, Gray WK, Aris E, Mugusi F, Swai M, Walker RW. A cross-sectional study of quality of life in incident stroke survivors in rural northern Tanzania. Journal of neurology. 2011;258:1422–30. doi: 10.1007/s00415-011-5948-6. [DOI] [PubMed] [Google Scholar]
- 30.Oladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: a population-based survey. Cardiovasc J Afr. 2010;21:26–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Shisana O, Labadarios DRT, Simbayi L, Zuma K, Dhansay A, Reddy P, Parker WHE, Naidoo P, Hongoro C, Mchiza Z, Steyn NP, Dwane N, Makoae M, Maluleke T, Ramlagan SZN, Evans MG, Jacobs L, Faber M SANHANES-1 Team. The South African National Health and Nutrition Examination Survey (SANHANES-1) Cape Town: HSRC Press; 2013. [Google Scholar]
- 32.Solet JL, Baroux N, Pochet M, Benoit-Cattin T, De Montera AM, Sissoko D. Prevalence of type 2 diabetes and other cardiovascular risk factors in Mayotte in 2008: the MAYDIA study. Diabetes Metab. 2011;37:201–217. doi: 10.1016/j.diabet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 34.Shisana OLD, Rehle T, Simbayi L, Zuma K, Dhansay A, Reddy P, Parker W, Hoosain E, Naidoo P, Hongoro C, Mchiza Z, Steyn NP, Dwane N, Makoae M, Maluleke T, Ramlagan S, Zungu N, Evans MG, Jacobs L, Faber M SANHANES-1 Team. South African National Health and Nutrition Examination Survey (SANHANES-1) 2013 [Google Scholar]
- 35.Gomez-Olive FX, Thorogood M, Clark B, Kahn K, Tollman S. Self-reported health and health care use in an ageing population in the Agincourt sub-district of rural South Africa. Global health action. 2013;6:19305. doi: 10.3402/gha.v6i0.19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373–81. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- 37.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:1320–1324. doi: 10.1136/jnnp.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manne-Goehler J, Montana L, Gómez-Olivé FX, Rohr J, Harling G, Wagner RG, Wade A, Kabudula CW, Geldsetzer P, Kahn K, Tollman S, Berkman LF, Bärnighausen TW, Gaziano TA. The ART advantage: healthcare utilization for diabetes and hypertension in rural South Africa. Journal of acquired immune deficiency syndromes (1999) 2017;75:561–567. doi: 10.1097/QAI.0000000000001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari R, Ford I, Greenlaw N, Tardif JC, Tendera M, Abergel H, Fox K, Hu D, Shalnova S, Steg PG. Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: Data from the contemporary CLARIFY registry. Eur J Prev Cardiol. 2015;22:1056–65. doi: 10.1177/2047487314547652. [DOI] [PubMed] [Google Scholar]
- 40.Snaterse M, Scholte op Reimer WJM, Dobber J, Minneboo M, ter Riet G, Jorstad HT, Boekholdt SM, Peters RJG. Smoking cessation after an acute coronary syndrome: immediate quitters are successful quitters. Netherlands Heart Journal. 2015;23:600–607. doi: 10.1007/s12471-015-0755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muntner P, DeSalvo KB, Wildman RP, Raggi P, He J, Whelton PK. Trends in the Prevalence, Awareness, Treatment, and Control of Cardiovascular Disease Risk Factors among Noninstitutionalized Patients with a History of Myocardial Infarction and Stroke. American Journal of Epidemiology. 2006;163:913–920. doi: 10.1093/aje/kwj124. [DOI] [PubMed] [Google Scholar]
- 42.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal. 2016;37:2315. [Google Scholar]
- 43.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, Age, Cardiovascular Risk Factors, and Coronary Heart Disease. Circulation. 1999;99:1165. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 44.Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE. Risk factors for coronary heart disease: implications of gender. Cardiovascular Research. 2002;53:538. doi: 10.1016/s0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- 45.Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. The INTERSTROKE Study: hypertension is by far the most important modifiable risk factor for stroke. Blood Pressure. 2017;26:131–132. doi: 10.1080/08037051.2017.1292456. [DOI] [PubMed] [Google Scholar]
- 46.Maredza M, Bertram MY, Gomez-Olive XF, Tollman SM. Burden of stroke attributable to selected lifestyle risk factors in rural South Africa. BMC Public Health. 2016;16:143. doi: 10.1186/s12889-016-2805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips JE, Klein WMP. Socioeconomic Status and Coronary Heart Disease Risk: The Role of Social Cognitive Factors. Social and personality psychology compass. 2010;4:704–727. doi: 10.1111/j.1751-9004.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in Myocardial Infarction Secondary Prevention: The National Health and Nutrition Examination Surveys (NHANES), 1999–2012. Journal of the American Heart Association. 2015;4:e001709. doi: 10.1161/JAHA.114.001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desai NR, Choudhry NK. Impediments to adherence to post myocardial infarction medications. Current cardiology reports. 2013;15:322. doi: 10.1007/s11886-012-0322-6. [DOI] [PubMed] [Google Scholar]
- 50.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. The New England journal of medicine. 2014;371:818–27. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Lisheng L, Wei L, Lopez-Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. The Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 52.Tong E, Saito N, Tancredi DJ, Borges G, Kravitz RL, Hinton L, Aguilar-Gaxiola S, Medina-Mora ME, Breslau J. A Transnational Study of Migration and Smoking Behavior in the Mexican-Origin Population. American journal of public health. 2012;102:2116–2122. doi: 10.2105/AJPH.2012.300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, Levitt NS, Crowther NJ, Nyirenda M, Njelekela M, Ramaiya K, Nyan O, Adewole OO, Anastos K, Azzoni L, Boom WH, Compostella C, Dave JA, Dawood H, Erikstrup C, Fourie CM, Friis H, Kruger A, Idoko JA, Longenecker CT, Mbondi S, Mukaya JE, Mutimura E, Ndhlovu CE, Praygod G, Pefura Yone EW, Pujades-Rodriguez M, Range N, Sani MU, Schutte AE, Sliwa K, Tien PC, Vorster EH, Walsh C, Zinyama R, Mashili F, Sobngwi E, Adebamowo C, Kamali A, Seeley J, Young EH, Smeeth L, Motala AA, Kaleebu P, Sandhu MS. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2013;42:1754–71. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepard D, VanderZanden A, Moran A, Naghavi M, Murray C, Roth G. Ischemic Heart Disease Worldwide, 1990 to 2013: Estimates From the Global Burden of Disease Study 2013. Circulation Cardiovascular quality and outcomes. 2015;8:455–6. doi: 10.1161/CIRCOUTCOMES.115.002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vedanthan R, Kamano JH, Bloomfield GS, Manji I, Pastakia S, Kimaiyo SN. Engaging the Entire Care Cascade in Western Kenya: A Model to Achieve the Cardiovascular Disease Secondary Prevention Roadmap Goals. Global heart. 2015;10:313–7. doi: 10.1016/j.gheart.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vedanthan R, Kamano JH, Lee H, Andama B, Bloomfield GS, DeLong AK, Edelman D, Finkelstein EA, Hogan JW, Horowitz CR, Manyara S, Menya D, Naanyu V, Pastakia SD, Valente TW, Wanyonyi CC, Fuster V. Bridging Income Generation with Group Integrated Care for cardiovascular risk reduction: Rationale and design of the BIGPIC study. American heart journal. 2017;188:175–185. doi: 10.1016/j.ahj.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


