Short abstract
Introduction
Macrophages play an important role in HIV, where they are a cellular reservoir. Macrophages are polarized into two phenotypes: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages, which may have altered expression of drug efflux transporters, including BCRP and MRP1. These differences may result in subtherapeutic concentrations of antiretrovirals inside of macrophages and viral replication.
Methods
U937 and U1 cells were polarized to the M1 or M2 phenotype via IFN-γ and LPS, or IL-4, IL-13, and LPS. Transporter expression was assessed via PCR and Western blotting, and transporter function was assessed via fluorescent dye assays. Transporter function was blocked with the inhibitors MK571 or KO143. Protein expression was confirmed in monocyte-derived macrophages. p24 production was assessed in U1 cells via enzyme-linked immunosorbent assay.
Results
mRNA and protein analysis demonstrated higher expression of MRP1 in M1 macrophages, while BCRP expression was downregulated in M1 macrophages. Treatment with inhibitors of transporter function decreased the difference in intracellular fluorescence between polarized macrophages. Differences in protein expression, which were observed with U937 cells, were confirmed in monocyte-derived macrophages. M1, but not M2 cells treated with MK571, showed decreased p24 production, consistent with reported MRP1 transporter expression.
Conclusions
These results support our hypothesis that there is differential expression of MRP1 and BCRP on M1 and M2 polarized macrophages and suggests that these differences may result in altered intracellular concentrations of antiretrovirals in macrophages and alter viral production in these cells. Targeting these differences may be a strategy to decrease viral replication in HIV-infected individuals.
Keywords: AIDS, drug resistance, HIV, molecular biology
Introduction
Macrophages are key effectors of innate immunity and are significantly involved in the immune response to pathogens and inflammatory process.1 Macrophages are dynamic cells characterized by great diversity and plasticity that undergo polarization in the presence of acute or chronic infection.1 Polarized macrophages have been broadly categorized as pro-inflammatory classically activated (M1) macrophages and anti-inflammatory alternatively activated (M2) macrophages; macrophage activation is plastic, fully reversible, and dependent on their microenvironment.2 These subsets of macrophages are phenotypically dissimilar, differing in terms of receptors, cytokine stimuli, and chemokine expression.1–3
Macrophages serve as a long-lived reservoir site for HIV and are a significant contributing factor of the progression of HIV to AIDS.4,5 Although the role of polarized macrophages in HIV is largely uncertain, M1 polarization occurs preferentially during acute HIV infection, while a shift towards M2 polarization takes place at later stages of disease progression.6 Differentiated subsets of macrophages are additionally involved in acute and chronic infection and play differing roles in the pathophysiology of numerous disease states. M1 polarization initiates and sustains inflammation; M2 polarized states are prevalent in chronic inflammation, parasite infections, allergy, and various cancers.1 The progression of many diseases is significantly impacted by macrophage polarization.7
Due to macrophage involvement in a variety of diseases, macrophages serve as major cellular targets for drugs, including antibiotics and antiviral agents.8 To date, very little is known on transporter expression on polarized macrophages; we hypothesized that altered transporter expression between M1 and M2 macrophages could serve as a key component for combating macrophage-mediated diseases.
Cellular drug efflux transporters have the potential to substantially impact intracellular concentrations of various medications at their target site within macrophages, decreasing cellular drug accumulation and potentially resulting in multidrug resistance.9 P-glycoprotein (PGP), multidrug-resistant protein 1 (MRP1), and breast cancer resistance protein (BCRP) are ATP-binding cassette (ABC) transporters expressed throughout the body, including macrophages.9 These transporters are associated with decreased steady-state cellular accumulation of drugs.10 Activation of macrophages with lipopolysaccharide (LPS) has previously been shown to increase the expression of MRP1.11
We have previously shown that PGP expression is higher in M2 macrophages as compared to M1 macrophages.12 Based on these previous findings, we hypothesized that additional efflux transporters would have differential expression between macrophage subsets. With specific macrophage-targeted therapies under development,1 understanding how expression of MRP1 and BCRP is uniquely expressed on differentiated polarized macrophage subsets may provide potential strategies for overcoming subtherapeutic antiretroviral concentrations within macrophages.
Methods
Cell culture
The U937 cell line was purchased from ATCC (CRL-1593.2). U1 cells were obtained from the NIH AIDS Reagent Program. Cells were cultured in RPMI, 1640 (Thermofisher, Waltham, MA), medium supplemented with 2 mM glutamine, 10 mM HEPES, 4.5 g/l glucose and 10% fetal calf serum. Low passage cells were used for all experiments.
Human monocyte isolation by RosetteSep
Buffy coats from deidentified donors were obtained from Interstate Blood Bank (Memphis, TN, USA). Monocytes were isolated by RosetteSep Human Monocyte enrichment cocktail (Stemcell Technologies, Vancouver, CA). The RosetteSep cocktail was incubated with the buffy coats for 20 min at room temperature. Samples were diluted with phosphate buffered saline (PBS) containing 2% fetal bovine serum (FBS) and 1 mM ethylenediaminetetraacetic acid, layered on the top of a Ficoll density gradient medium, and centrifuged for 20 min at 1200 × g at room temperature with the brake off. The interface-enriched cells were harvested and washed three times, and then resuspended in RPMI 1640 for later use.
Macrophage polarization
Cells were polarized to the M1 phenotype via treatment with LPS (100 ng/mL, E. Coli origin, Sigma Aldrich, St. Louis, MO, USA) and interferon-γ (IFN-γ) (20 ng/mL, Life Technologies, Carlsbad, CA, USA) or polarized to the M2 phenotype via treatment with LPS (100 ng/mL) + Interleukin (IL)4 (10 ng/mL, CST, Danvers, MA, USA)+ IL13 (10 ng/mL, CST, Danvers, MA, USA). Cells (0.5 ×106/mL) were treated with cytokines for 48 h. Unstimulated cells were utilized as a control. Cell viability was unaltered in all three groups.
RNA isolation and qRTPCR
RNeasy® Mini Kit (Qiagen, Valencia, CA, USA) was used to harvest RNA from U937 cells. The final RNA concentrations were determined by Nano drop. RNA (100 ng) from each sample was reverse transcribed into cDNA using the high-capacity RNA to cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The generated cDNA was used to perform qRTPCR following the supplier’s instruction (TaqMan Gene Expression Kit, Applied Biosystems) using a StepOnePlus real-time PCR system (Thermofisher, Waltham, MA). The primers utilized were ABCC1, Hs01561502_m1; ABCG2 primer HS 01053790_m1; endogenous control GAPDH, HS03929097_g1. Relative gene expression was calculated for each gene by the 2−ΔΔCt method.
Western blotting
Cells were lysed in cold RIPA lysis buffer with protease/phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA) for whole cell lysates. A cell fractionation kit (CST, Danvers, USA) was used to isolate cell membrane protein. Cell membrane protein was collected for MRP1 detection. Protein (5–50 μg) was loaded in a mini gel (4% stacking, 8% separating SDS-PAGE). After separation, gels were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Membranes were blocked using 5% non-fat milk in TBS buffer, then incubated at 4°C with the respective primary antibody overnight; anti-BCRP primary antibody (Abcam, Cambridge, UK, 1:2000), or anti-MRP1 antibody (Abcam, Cambridge, UK, 1:25). Whole cellular protein was normalized using β-Actin (Cell Signaling Danvers, MA, USA, 1:2000). Membrane protein was normalized using a Na-K-ATPase antibody (Abcam, Cambridge, UK, 1:2000). The secondary antibody (IRDye® 800CW goat anti-rabbit or IRDye® 680RD Goat anti-Mouse (1:15,000)) was incubated in the dark at room temperature for 45 min. Dual-channel infrared scan and quantitation of immunoblots were conducted using the Odyssey Sa infrared imaging system with Image Studio (Ver. 3.1.4) (LI-COR, Lincoln, NE, USA).
BCRP and MRP1 function
Calcein AM (ThermoFisher, NY, USA) cellular accumulation assays were used for MRP1 function, while BCRP function was assessed with Hoechst 33342 (ThermoFisher, NY, USA). MK571 (Tocris, Bristol, UK) and KO143 (Tocris, Bristol, UK), specific MRP1 and BCRP inhibitors, respectively, were used in the functional assay. M1, M2, and unstimulated U937 cells were washed and resuspended in serum-free RPMI, and then seeded in 96-well Black Clear-Bottom Plates (Costar, Washington, DC, USA). Plates were incubated at 37°C with or without inhibitor (MK571, 10 min incubation; KO143, 2 h incubation). After incubation, 10 μM Calcein AM or 10 μM Hoechst 33342 was added to the plate. Plates were immediately placed in an FLx800 Fluorescence Reader (BioTek, Winooski, VT, USA) for 60 min, and read at 485/528 (ex/em). Cell viability was determined via trypan blue staining.
p24 ELISA
U1 cells, a constitutively HIV-1-infected subclone of the U937 cell line, were polarized as previously described. After polarization, cells were treated with the MRP1 inhibitor MK571 and either 10 or 27 nM LPV, a substrate for both MRP1 and PGP for 24 h. After treatment, cell supernatants were collected, and p24 production was assessed via p24 ELISA (Zeptometrix, Buffalo, NY). p24 production was normalized to the total amount of p24 production in the untreated cells for each phenotype.
Statistical analysis
Statistical analysis was performed with Graphpad Prism (GraphPad Software, La Jolla, CA, USA). All data shown are mean ± SD and obtained from at least three independent experiments. Significance was determined by utilizing ANOVA with Tukey’s multiple comparison test.
Results
MRP1 and BCRP expression is altered between M1 and M2 macrophages
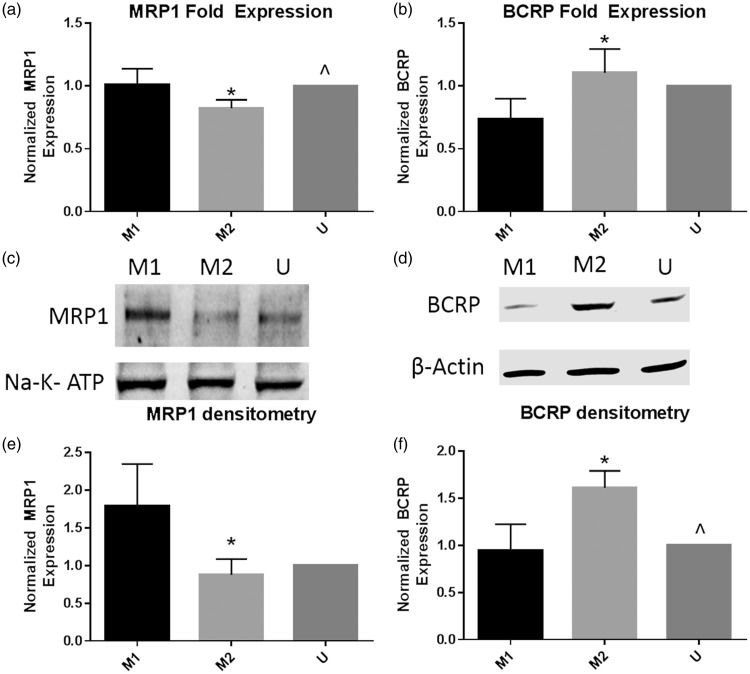
We first assessed the expression of both MRP1 and BCRP mRNA in M1, M2, and unstimulated U937 macrophages via qRTPCR. We observed a small but significant increase in MRP1 expression in M1 macrophages as compared to M2 cells (Figure 1(a)). Contrariwise, BCRP mRNA expression was significantly upregulated in M2 cells as compared to M1 cells (Figure 1(b)). To complement the mRNA data, we next assessed protein expression of MRP1 and BCRP in polarized U937 macrophages. Similar to the mRNA data, we observed more MRP1 expression in the M1 cells (Figure 1(c)), confirmed via averaged densitometry (Figure 1(e)), with an over two-fold increase in MRP1 expression in the M1 cells as compared to the M2 and unstimulated cells. BCRP protein expression was increased in the M2 cells as compared to the M1 and unstimulated macrophages (Figure 1(d)). The densitometry data showed that M2 macrophages significantly upregulated BCRP expression as compared to the M1 and unstimulated macrophages (Figure 1(f)).
Figure 1.
MRP1 and BCRP mRNA and protein expression is altered between M1 and M2 U937 macrophages. U937 cells were polarized to the M1 or M2 phenotype, or left unstimulated for 48 h. After 48 h, cells were collected, and mRNA was isolated for real-time PCR, while cell lysates were collected for Western blotting. Real-time PCR and Western blotting were performed for MRP1 and BCRP. (a) and (b) show real-time PCR results for three replicates of the experiments, with results from each replicate normalized to the unstimulated condition. (c) and (d) show representative Western blots from three replicates, while (e) and (f) show average densitometry normalized to the unstimulated condition in each blot. Groups were compared via one-way ANOVA with Tukey’s post-test, and *p value < 0.05 vs. M1 cells, ^p value < 0.05 vs. M2 cells.
MRP1 and BCRP function are different between M1 and M2 macrophages
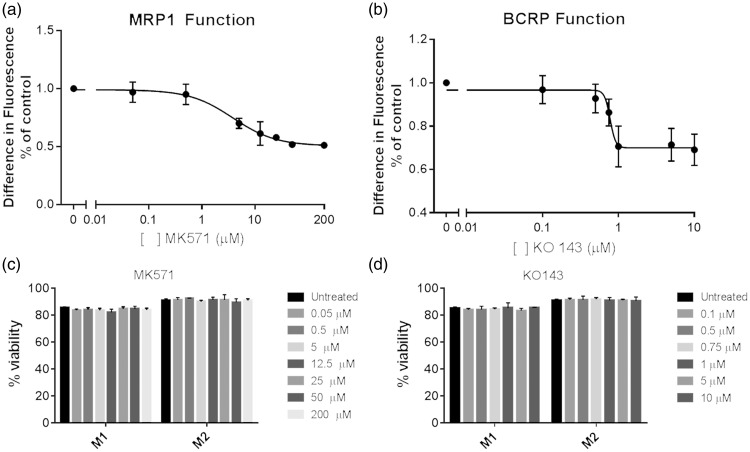
Next, we assessed the function of MRP1 and BCRP in the polarized U937 macrophage subsets. Calcein AM was used as a dye substrate for MRP1 function, while BCRP function was assessed with Hoechst 33342. Specific small molecule inhibitors were used for both transporters: MK571 for MRP113 and KO143 for BCRP.14 We assessed the differences in intracellular fluorescence between the M1 and M2 U937 macrophages in the presence of increasing concentrations of the inhibitors (Figure 2). We observed an approximately 50% decline in the difference in intracellular fluorescence between the M1 and M2 macrophages in cells that were treated with the MRP1 inhibitor MK571 (Figure 2(a)). We additionally noted an approximately 30% decrease in the difference in intracellular fluorescence in cells pre-treated with the BCRP inhibitor KO143 (Figure 2(b)). Additionally, we assessed cell viability in the cells via trypan blue staining, and observed that increasing concentrations of both MK571 and KO143 had no effect on cell viability in the cells (Figure 2(c) and (d)).
Figure 2.
Macrophage subsets have different MRP1 and BCRP function. U937 cells were polarized to the M1 or M2 phenotype for 48 h. Cells were treated with increasing concentrations of either (a) the MRP1 inhibitor MK571 or (b) the BCRP inhibitor KO 143, and the difference in fluorescence was assessed at 60 min. Data are representative of three replicates. Cell viability was also assessed at the same time points and with the same concentrations of small molecule inhibitors (c, d). Lines of best fit were calculated via a four-parameter variable slope model.
MRP1 and BCRP protein expression is differentially expressed in polarized monocyte-derived macrophages
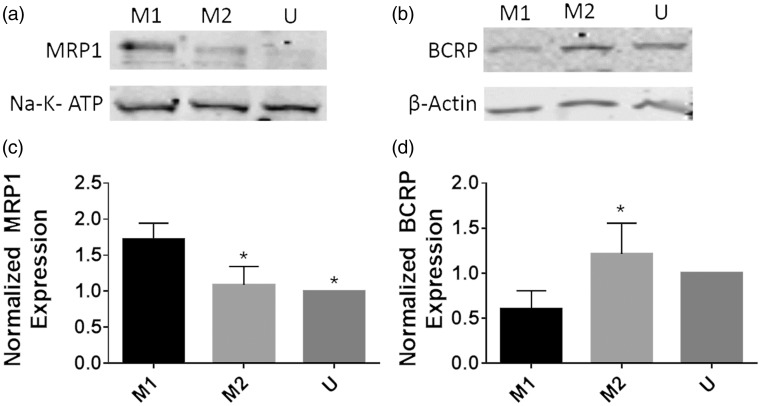
Next, we confirmed our findings in U937 cells with primary monocyte-derived macrophages from healthy volunteers (Figure 3). While there are differences between U937 cells and primary monocyte-derived macrophages, the U937 cell line correlates very strongly to monocyte-derived macrophages (MDM).4,15–17 Monocytes were enriched via Rosettesep, a technique which we have previously used to provide an initial monocyte population that is 90% pure.12 After differentiation and polarization, polarized macrophages were lysed and Western blots were performed.
Figure 3.
MRP1 protein expression is altered between M1 and M2 monocyte-derived macrophages. Buffy coats from three healthy deidentified subjects were collected, monocytes isolated via Rosettesep, and differentiated to macrophages for six days with m-CSF. After differentiation, macrophages were polarized to the M1 or M2 phenotype for 48 h, and lysates were collected for Western blotting for MRP1 or BCRP. (a) and (b) show representative Western blots for the replicates, while (c) and (d) show average densitometry normalized to the unstimulated condition in each blot. Groups were compared via one-way ANOVA with Tukey’s post-test, and *p values < 0.05 vs. the M1 group.
MRP1 inhibition decreases viral replication in U1 cells in a phenotype-specific manner
Finally, we assessed the effect of MRP1 inhibition on viral production in U1 cells treated with lopinavir (LPV). Lopinavir is a protease inhibitor which is a substrate of both MRP1 and PGP.18 Previously, we have shown that intracellular concentrations of LPV are different between M1 and M2 macrophages in a matter consistent with the transporter differences that we have reported between M1 and M2 macrophages.12 We treated polarized M1 and M2 cells of the U1 cell line, a constitutively infected U937 subclone with either 10 or 27 nM LPV (the range of reported in vitro IC50) for 24 h.19,20 In addition, some cells were treated with MK571 concurrently with LPV (Figure 4). Treatment with both LPV and MK571 significantly decreased viral production in M1 cells as compared to untreated, LPV treated, and MK571-treated cells at both 10 and 27 nM concentrations of LPV. In M2 cells, however, treatment with LPV and MK571 had no additional effect compared to treating cells with only LPV.
Figure 4.
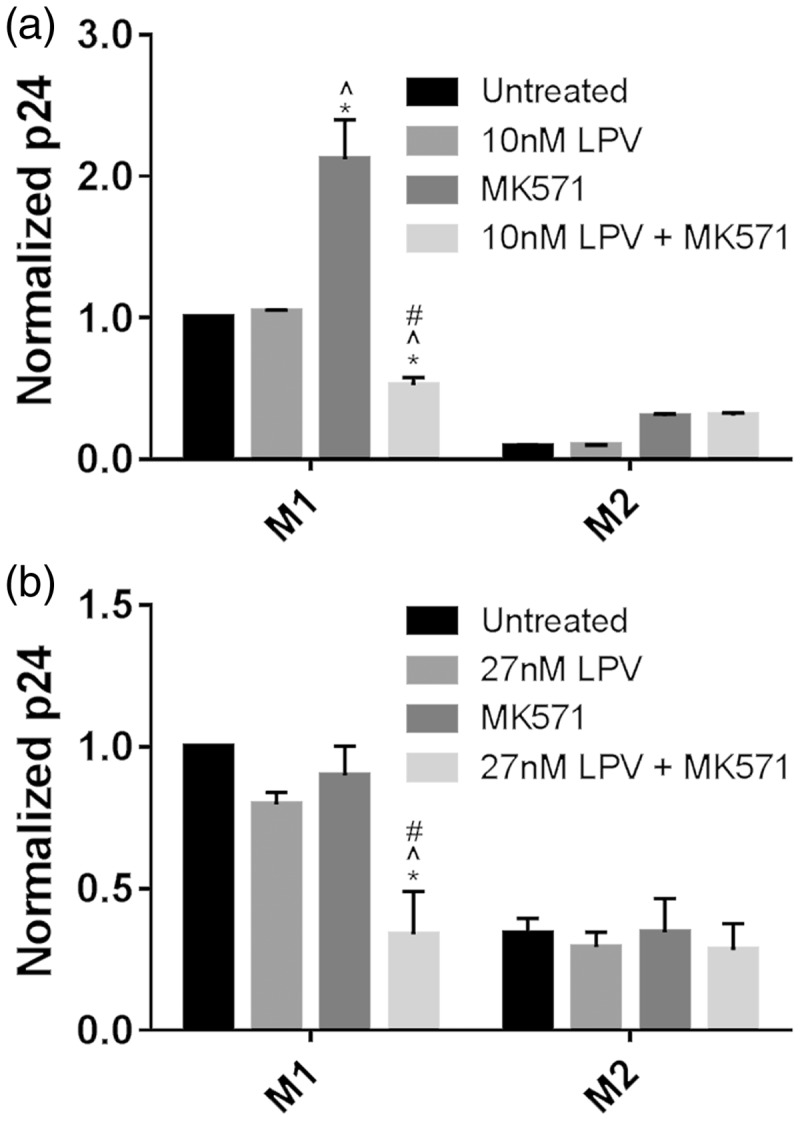
Inhibition of MRP1 function decreases viral replication in lopinavir-treated U1 cells in a phenotype-specific manner. U1 cells were polarized to the M1 or M2 phenotype with cytokines for 48 h. After polarization, cells were treated with either 10 or 27 LPV (a, b), the MRP1 inhibitor MK571, or a combination of the two. Supernatants were collected, and p24 production was analyzed via ELISA. P24 production was normalized by the untreated condition for each phenotype. Groups were compared via two-way ANOVA with Tukey’s post-test, and significance calculated with a p value < 0.05 vs. *untreated cells, ^LPV-treated cells, # MK571-treated cells.
Discussion
Here, we report that there are previously unreported differences in expression and function of the drug efflux transporters MRP1 and BCRP between M1 and M2 macrophages and show that for a substrate of one of these transporters, inhibition of the relevant transporter results in decreased p24 production in the macrophage subset, where expression of the transporter was higher.
We first examined the mRNA and protein expression of MRP1 and BCRP in both M1 and M2 macrophages. While the difference in MRP1 mRNA between M1 and M2 macrophages was small, when we examined protein expression via Western blotting, the differences were significantly more pronounced. These differences, in addition to the differences in PGP expression that we previously observed, support the hypothesis that macrophage subsets differentially express efflux transporters.12 Additionally, the findings with MRP1 expression are consistent with the findings of others in RAW 264.7 cells, showing that treatment with LPS increases the expression of MRP1.11 Here, we have extended these findings to cells of human origin, as well as assessed cells polarized to the M2 phenotype.
When we treated the polarized macrophages with small molecule inhibitors of either MRP1 or BCRP, as well as fluorescent dyes which were substrates for these transporters, we observed that increasing concentrations of the inhibitors decreased the difference in fluorescence between M1 and M2 macrophages, consistent with the two transporters functioning different between the two cell subtypes.
The differences in fluorescence between the two subsets of macrophages were not entirely eliminated by treating cells with the inhibitors of transporter function, most likely be due to off target affinity of the dyes for other transporters.13,21 This functional data, coupled with the mRNA and Western blot data, strongly support the hypothesis that these transporters are differentially expressed in M1 and M2 macrophages.
Similar to our results in U937 cells, we observed upregulated MRP1 in the M1 polarized MDM, while BCRP expression was upregulated in M2 polarized MDM (Figure 3(a) to (d)). We observed similar findings on protein expression in these cells as we did with the U937 cells, strongly suggesting that the changes that we have observed are true in both U937 cells and primary macrophages and support the relevance of further investigating this interaction in HIV-infected individuals. Finally, our findings showing that MRP1 inhibition decreases viral production in LPV-treated M1, but not M2 macrophages further supports the importance of investigating strategies that target both subsets of macrophages to increase intracellular antiretroviral concentrations, rather than investigating macrophages as a singular cellular target for HIV-1.
There are, however, limitations associated with our research. BCRP, MRP1, and similar transporters share affinity for many compounds.18 We did, however, use inhibitors that have high affinity for one, but not both transporters. With MRP1 having higher expression on M1 macrophages and BCRP having higher expression on M2 macrophages, there is the possibility that the differences in fluorescence that we see in the presence of the inhibitors are at least partially negated by other transporters. This potentially explains why the difference in fluorescence in the presence of transporter blockage only decreased by 50% in the presence of the MRP1 transporter, and approximately 30% for the BCRP transporter. While we were unable to fully block function of our transporters of interest, the data still support our hypothesis that there are differences in transporter expression between macrophage subsets.
These differences in drug efflux transporters may result in altered intracellular concentrations of a variety of drugs, including other commonly used antiretrovirals. Many antiretrovirals are substrates for a variety of drug efflux transporters, and it is important to understand how they are differentially effluxed by macrophages.18,22–24 If these transporters are upregulated in certain subsets of macrophages, this may result in subtherapeutic concentrations of drugs in these cells, leading to an inadequate response. Contrariwise, excessive downregulation of efflux transporters in macrophage subsets may result in toxicity in these cells. A better understanding of how these transporters are modulated in polarized macrophages may lead to improved outcomes for individuals with HIV or other diseases.
Long term, it is of key importance to have a greater understanding of how transporter expression is modulated in polarized macrophages. This information, coupled with an understanding of how this results in altered drug concentrations, may result in the need to adopt novel strategies to increase intracellular concentrations of antiretrovirals in macrophages. These strategies may include changing therapeutic modalities, concurrently treating individuals with drugs that inhibit these efflux transporters, or developing new drugs that have lower affinity for these efflux transporters.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the UTHSC College of Pharmacy and by the National Institutes of Health (R01 AA022063).
References
- 1.Sica A andMantovani A.. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit M Desnues B andMege JL.. Macrophage polarization in bacterial infections. J Immunol 2008; 181: 3733–3739. [DOI] [PubMed] [Google Scholar]
- 3.Shirey KA, Pletneva LM, Puche AC, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol 2010; 3: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassol E, Alfano M, Biswas P, et al. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J Leukoc Biol 2006; 80: 1018–1030. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JH, Hearps AC, Martin GE, et al. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 2014; 28: 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlaepfer E, Rochat MA, Duo L, et al. Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J Virol 2014; 88: 9769–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beceiro S, Radin JN, Chatuvedi R, et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol 2017; 10: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau A, Le Vee M, Jouan E, et al. Drug transporter expression in human macrophages. Fundam Clin Pharmacol 2011; 25: 743–752. [DOI] [PubMed] [Google Scholar]
- 9.Leslie EM Deeley RG andCole SP.. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 2005; 204: 216–237. [DOI] [PubMed] [Google Scholar]
- 10.Hipfner DR Deeley RG andCole SP.. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta 1999; 1461: 359–376. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein PS, Audus KL, Qureshi N, et al. Lipopolysaccharide increases the expression of multidrug resistance-associated protein 1 (MRP1) in RAW 264.7 macrophages. J Neuroimmune Pharmacol 2010; 5: 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cory TJ, He H, Winchester LC, et al. Alterations in P-glycoprotein expression and function between macrophage subsets. Pharm Res 2016; 33: 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogan AL, Legrand O, Faussat AM, et al. Evaluation and comparison of MRP1 activity with three fluorescent dyes and three modulators in leukemic cell lines. Leuk Res 2004; 28: 619–622. [DOI] [PubMed] [Google Scholar]
- 14.Allen JD, van Loevezijn A, Lakhai JM, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 2002; 1: 417–425. [PubMed] [Google Scholar]
- 15.Baek YS, Haas S, Hackstein H, et al. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol 2009; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood 1998; 91: 258–265. [PubMed] [Google Scholar]
- 17.Yang Y, Tikhonov I, Ruckwardt TJ, et al. Monocytes treated with human immunodeficiency virus Tat kill uninfected CD4(+) cells by a tumor necrosis factor-related apoptosis-induced ligand-mediated mechanism. J Virol 2003; 77: 6700–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kis O, Robillard K, Chan GNY, et al. The complexities of antiretroviral drug–drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci 2010; 31: 22–35. [DOI] [PubMed] [Google Scholar]
- 19.Kaletra (R) [Package insert] Abbvie Inc., North Chicago, IL; 2015.
- 20.Folks TM, Justement J, Kinter A, et al. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 1987; 238: 800–802. [DOI] [PubMed] [Google Scholar]
- 21.Colabufo NA, Pagliarulo V, Berardi F, et al. Bicalutamide failure in prostate cancer treatment: involvement of Multi Drug Resistance proteins. Eur J Pharmacol 2008; 601: 38–42. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet L, Pruvost A, Didier N, et al. Emtricitabine: inhibitor and substrate of multidrug resistance associated protein. Eur J Pharm Sci 2008; 35: 247–256. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet L, Roucairol C, Hembury A, et al. Comparison of ABC transporter modulation by atazanavir in lymphocytes and human brain endothelial cells: ABC transporters are involved in the atazanavir-limited passage across an in vitro human model of the blood-brain barrier. AIDS Res Hum Retroviruses 2008; 24: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 24.Weiss J, Theile D, Ketabi-Kiyanvash N, et al. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab Dispos 2007; 35: 340–344. [DOI] [PubMed] [Google Scholar]