Abstract
Dental pulp represents a promising and easily accessible source of mesenchymal stem cells for clinical applications. Many studies have investigated the use of human dental pulp stem cells and stem cells isolated from the dental pulp of human exfoliated deciduous teeth for bone tissue engineering in vivo. However, the type of scaffold used to support the proliferation and differentiation of dental stem cells, the animal model, the type of bone defect created, and the methods for evaluation of results were extremely heterogeneous among these studies conducted. With this issue in mind, the main objective of this study is to present and summarize, through a systematic review of the literature, in vivo studies in which the efficacy of human dental pulp stem cells and stem cells from human exfoliated deciduous teeth (SHED) for bone regeneration was evaluated. The article search was conducted in PubMed/MEDLINE and Web of Science databases. Original research articles assessing potential of human dental pulp stem cells and SHED for in vivo bone tissue engineering, published from 1984 to November 2017, were selected and evaluated in this review according to the following eligibility criteria: published in English, assessing dental stem cells of human origin and evaluating in vivo bone tissue formation in animal models or in humans. From the initial 1576 potentially relevant articles identified, 128 were excluded due to the fact that they were duplicates and 1392 were considered ineligible as they did not meet the inclusion criteria. As a result, 56 articles remained and were fully analyzed in this systematic review. The results obtained in this systematic review open new avenues to perform bone tissue engineering for patients with bone defects and emphasize the importance of using human dental pulp stem cells and SHED to repair actual bone defects in an appropriate animal model.
Keywords: Mesenchymal stem cells, dental pulp, bone regeneration, dental pulp stem cells, stem cells from human exfoliated deciduous teeth
Introduction
Tissue engineering is a multidisciplinary field based on combination of cells and/or proteins with biomaterials to generate a new tissue. Some factors are very important to succeed in tissue engineering: the choice of stem cell source, the strategies used to isolate and expand the specific cells, the choice of the biomaterial to be used as a scaffold, and the correct association between them.1 To bone regeneration, it is necessary that the chosen biomaterial allows the cells to migrate, proliferate, and differentiate into bone cells in order to regenerate the bone tissue defect, but also it is necessary that local angiogenesis occurs to provide the necessary nutrients and environment factors for the correct development of the bone tissue.2
Reconstruction of maxillofacial critical bone defects represents a major challenge in the areas of dentistry and medicine and they are the main objective pursued by several research groups. With this aim in mind, mesenchymal stem cells (MSCs) are being investigated as an appropriate type of stem cells to be used in bone tissue engineering due to their ability to differentiate in both osteoblasts and endothelial cells supporting hematopoiesis.3 This type of stem cell, despite being classically isolated from bone marrow4 (bone marrow stromal cells (BMSCs)), can also be obtained from several neonatal and adult tissues, including dental pulp,5 orbicularis oris muscle,6 and fat.7 Many studies are focusing on the isolation of MSCs from more accessible sources aiming for a safer clinical application of these cells for tissue engineering purposes.8–10 In this context, the dental pulp is currently being proposed as one of the most promising source of MSCs for tissue engineering. The advantages that the use of MSCs isolated from dental pulp tissue (dental pulp stem cells (DPSCs)) represent are related to their accessibility and great proliferative and multilineage differentiation potential.5,11,12
Human dental pulp stem cells (hDPSCs) were first isolated and characterized by Gronthos et al.13 They are extremely proliferative and capable of osteogenic,11 dentinogenic,13 adipogenic,12 chondrogenic,12 and neurogenic12 differentiation. A number of studies were conducted in which hDPSCs were used in combination or not with several types of scaffolds and transplanted to repair a number of bone defects in animal models and in human clinical trials.14–16 In particular, a study conducted by Miura et al.17 was the first study to successfully isolate stem cells from the dental pulp of human exfoliated deciduous teeth (SHED) and to use them associated with a scaffold for bone tissue engineering purposes in vivo. With regard to the method of isolation of both stem cells from human exfoliated deciduous teeth (SHED) and hDPSCs, the main difference between SHED and hDPSC is related to the fact that hDPSCs are isolated from the dental pulp of permanent teeth or isolated from deciduous teeth without exfoliation while SHED are obtained from the dental pulp of exfoliated deciduous teeth.13,17 As a result, SHED represent a more immature than permanent teeth DPSC subpopulation. SHED have higher proliferation rate and differentiation capability in comparison with permanent DPSCs and, considering the ease to obtain deciduous teeth exfoliated of children, the clinical use of SHED may be particularly advantageous.17 The main objective of this research is, therefore, to present and summarize, through a systematic review of the literature, in vivo studies in which the efficacy of hDPSCs and SHED for bone regeneration was evaluated. Furthermore, this study aimed to assess the influence of host-related factors (animal model and defect type) and the scaffold used on the efficiency of hDPSCs for bone regeneration.
Methodology
An electronic customized search of scientific articles published between 1984 and November 2017 using PubMed/MEDLINE and Web of Science databases was conducted. The following key words were used separately and in combination: stem cells, dental pulp, isolation, human DPSCs, SHED, MSCs, mesenchymal stromal cells, deciduous tooth, deciduous teeth, tooth exfoliation, inflamed pulp, in vivo differentiation, stem cell transplantation, MSC transplantation, cell transplantation, cell differentiation, regeneration, tissue engineering, tissue regeneration, bone tissue engineering, bone transplantation, tissue-engineered bone, bone regeneration, osteogenesis, osteoblast, osteoporosis, scaffold, tissue scaffolds, mandible.
The application of the inclusion and exclusion criteria for each article was conducted by two independent researchers (A.L.J. and C.C.G.P.) through the screening of titles and abstracts. Disagreements were resolved by discussion by a third reviewer (D.F.B.). Duplicate articles were excluded from the analysis. Furthermore, articles written in languages other than English, in vitro studies, review manuscripts, studies that evaluated the regeneration of tissues other than bone, studies that used only MSCs isolated from other human tissues, studies that used only non-human MSCs, and studies in which stem cells were not used were also identified and removed through the application of the exclusion criteria. In order to select the manuscripts that were included in this systematic review, an inclusion criteria were applied and only the manuscripts that described in vivo studies in which hDPSCs and SHED were used for bone tissue engineering purposes were elected for complete evaluation. After this, the selected articles were reviewed and classified according to the type of scaffold used, the in vivo experimental model chosen, the type of defect created, the method of evaluating bone regeneration, the time elapsed of evaluation after transplantation, and the results of bone tissue engineering obtained.
Results
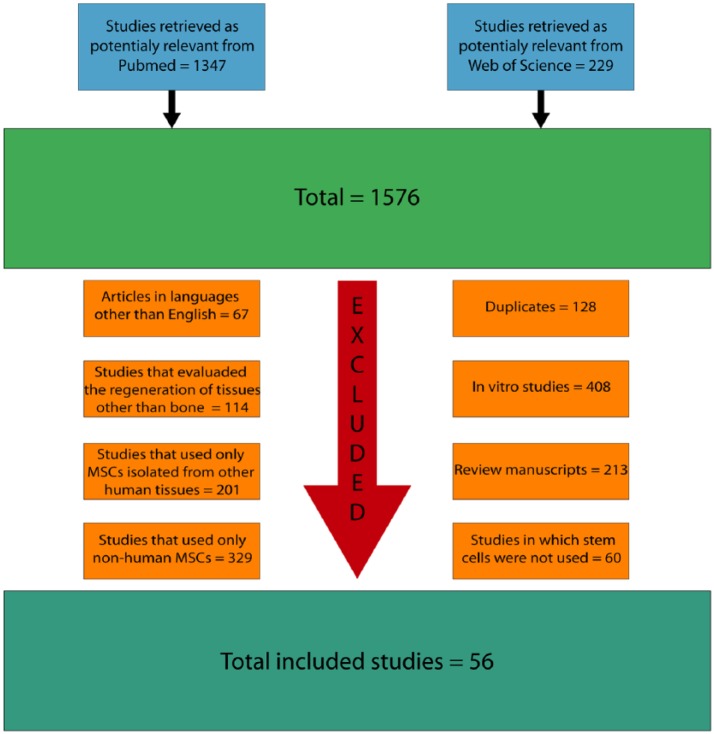
The initial search resulted in 1576 articles. Among them, 128 articles were excluded because they were duplicates, 67 articles written in languages other than English, 408 in vitro studies, 213 review manuscripts, 114 studies that evaluated the regeneration of tissues other than bone, 201 studies that used only MSCs isolated from other human tissues, 329 studies that used only non-human MSCs, and 60 studies in which stem cells were not used were also removed from the analysis (Figure 1).
Figure 1.
Flow diagram presenting the results of the literature search and the strategy used to select manuscripts that performed in vivo studies on the use of hDPSCs for bone tissue engineering.
After application of both exclusion and inclusion criteria, a total of 56 studies17–72 were selected and formed the basis of this systematic review. A list of the experimental model, type of scaffold, bone defect created, and the time and the methodology employed by the articles selected are presented in Table 1.
Table 1.
List of in vivo studies in which the potential of hDPSCs for bone tissue engineering was evaluated and the experimental model, type of scaffold, bone defect created, and the time and methodology employed by each of them.
| Author | Experimental model | Type of scaffold | Defect | Method of evaluation | Time of evaluation |
|---|---|---|---|---|---|
| Miura et al.17 | Immunocompromised mice | HA/TCP ceramic scaffold | Cranial defect | Histology immunohistochemistry, in situ hybridization, and RT-PCR | 8 weeks |
| Laino et al.18 | Immunocompromised rats | Woven bone obtained by hDPSCs | Subcutaneous implantation | Histology | 4 weeks |
| Papaccio et al.19 | Immunocompromised rats | Woven bone obtained by hDPSCs | Subcutaneous implantation | Histology and immunofluorescence | 4 weeks |
| Laino et al.20 | Immunocompromised rats | Woven bone obtained by hDPSCs | Subcutaneous implantation | Histology | 4 weeks |
| Graziano et al.21 | Immunocompromised rats | PLGA membrane | Subcutaneous implantation | Histology, immunohistochemistry and immunofluorescence | 30, 45, and 60 days |
| d’Aquino et al.22 | Immunocompromised rats | Woven bone obtained by hDPSCs in vitro and PGA-TMC scaffold | Subcutaneous implantation | Histology and immunohistochemistry | 4, 6, and 8 weeks |
| Otaki et al.23 | Immunocompromised mice | HA/TCP powder | Subcutaneous implantation | Histology | 7 and 15 weeks |
| Graziano et al.24 | Immunocompromised rats | PLGA membrane | Subcutaneous implantation | Histology, immunohistochemistry, and X-ray diffraction | 4 and 8 weeks |
| De Mendonça Costa et al.25 | Rats | Collagen membrane | Cranial defects | Histology | 1, 3, 4, and 8 weeks |
| Abe et al.26 | Immunocompromised rats | HA scaffold | Subcutaneous implantation | Histology, immunohistochemistry, and immunofluorescence | 12 weeks |
| Zhang et al.27 | Immunocompromised mice | HA/TCP ceramic scaffold | Subcutaneous implantation | Histology and real-time PCR | 5 and 10 weeks |
| Morito et al.28 | Immunocompromised mice | CaP/PLGA scaffold | Subcutaneous implantation | Histology, immunohistochemistry, and in situ hybridization | 5 and 10 weeks |
| d’Aquino et al.29 | Humans | Collagen sponge | Post third molar extraction defect | Histology, X-ray, immunofluorescence, and clinical evaluation | 1, 2, and 3 months |
| Kraft et al.30 | Immunocompromised mice | HA/TCP granules | Subcutaneous implantation | Histology, immunohistochemistry, and histomorphometry | 8 weeks |
| Feitosa et al.31 | Sheep | None | Osteonecrosis of the femoral head | Histology | 4 weeks |
| Chan et al.32 | Immunocompromised mice | Self-assembling peptide nano-fibre material | Subcutaneous implantation | Histology, X-ray, and immunohistochemistry | 4 weeks |
| Ikeda et al.33 | Immunocompromised mice | HA granules | Subcutaneous implantation | Histology and histomorphometry | 8 weeks |
| Li et al.34 | Immunocompromised mice | 3D gelatin scaffold | Subcutaneous implantation | Histology, X-ray, and immunohistochemistry | 4 weeks |
| Pisciotta et al.35 | Rats | Collagen scaffold | Cranial defect | Histoloy, immunohistochemistry, and histomorphometry | 6 weeks |
| Abe et al.36 | Immunocompromised mice | HA scaffold | Subcutaneous implantation | Histology and immunofluorescence | 12 weeks |
| Chen et al.37 | Immunocompromised mice | HA/TCP scaffold | Subcutaneous implantation | Histology, histomorphometry, and clinical evaluation | 12 weeks |
| Kawanabe et al.38 | Immunocompromised mice | β-TCP scaffold | Subcutaneous implantation | Histoloy and immunohistochemistry | 8 weeks |
| Wang et al.39 | Immunocompromised mice | Ceramic bovine bone | Subcutaneous implantation | Histology and clinical evaluation | 8 weeks |
| Bressan et al.40 | Immunocompromised rats | HA scaffold | Calvarial defect | Histology and real-time PCR | 3 weeks |
| Riccio et al.41 | Rats | Fibroin scaffold | Cranial defect | Histology, X-ray, and immunohistochemistry | 4 weeks |
| Annibali et al.42 | Immunocompromised mice | β-TCP, GDPB, and Aga/nHA | Calvarial defect | Histology and histomorphometry | 1, 2, 4, and 8 weeks |
| El-Gendy et al.43 | Immunocompromised mice | 3D Bioglass(R) scaffold | Intraperitonial implantation | Histoloy and immunohistochemistry | 8 weeks |
| Maraldi et al.44 | Immunocompromised rats | Collagen scaffold | Cranial defect | Histoloy, X-ray, immunohistochemistry, and histomorphometry | 4 and 8 weeks |
| Alkaisi et al.45 | Rabbits | None | Mandibular bone defect | Histoloy histomorphometry and radiological and clinical evaluation | 2, 4, and 6 weeks |
| Giuliani et al.46 | Humans | Collagen sponge | Post third molar extraction defect | Histoloy, histomorphometry, synchrotron X-ray phase-contrast microtomography, holotomography, and radiological and clinical evaluation | 6 months, 1 and 3 years |
| Niu et al.47 | Immunocompromised mice | NSC and ISCS | Subcutaneous implantation | Histology and immunohistochemistry | 8 weeks |
| Acasigua et al.48 | Rats | PLGA scaffold | Calvarial defect | Histology and histomorphometry | 60 days |
| Annibali et al.49 | Immunocompromised rats | GDPB and β-TCP scaffold | Calvarial defect | Micro-computed tomography and positron emission tomography analysis | 2, 4, 8, and 12 weeks |
| Kim et al.50 | Immunocompromised mice | MBCP scaffold | Subcutaneous implantation | Histology, immunohistochemistry, real-time PCR, and ELISA | 8 weeks |
| Asutay et al.51 | Rats | HA/TCP paste | Calvarial defect | Histology, histomorphometry, and micro-computed tomography | 8 weeks |
| Cao et al.52 | Mini pigs | HA/TCP scaffold | Periodontal bone defect | Histology, histomorphometry, and radiological and clinical evaluation | 12 weeks |
| Kuo et al.53 | Mini pigs | CSD, α-CSH/ACP, and β-TCP scaffold | Mandibular bone defect | Histology and histomorphometry | 8 weeks |
| Qian et al.54 | Immunocompromised mice | HA/TCP scaffold | Subcutaneous implantation | Histology and histomorphometry | 2 and 3 months |
| Petridis et al.55 | Rats | HA-based hydrogel scaffold | Calvarial defect | Histology and histomorphometry | 8 weeks |
| Kwon et al.56 | Rats | Computer-designed scaffold | Cranial defect | Histology and micro-computed tomography | 4, 8, and 12 weeks |
| Jang et al.57 | Rats | In vivo-forming hydrogel | Subcutaneous implantation | Histology, reverse transcription PCR, and micro-computed tomography | 2, 4, and 6 weeks |
| Jahanbin et al.58 | Rats | Collagen matrix | Maxillary alveolar bone defect | Histology and histomorphometry | 1 and 2 months |
| Yasui et al.59 | Immunocompromised mice | Matrigel matrix scaffold | Calvarial defect | Immunohistochemistry and micro-computed tomography | 4 weeks |
| Monti et al.60 | Humans | Collagen sponge | Post third molar extraction defect | Histology and radiological evaluation | 60 days |
| Wongsupa et al.61 | Rabbits | PCL-BCP scaffold | Calvarial defect | Histology, histomorphometry, micro-computed tomography, and clinical evaluation | 2, 4, and 8 weeks |
| Paino et al.62 | Immunocompromised rats | Woven bone obtained by hDPSCs | Mandibular bone defect | Histology, immunofluorescence, synchrotron X-ray phase-contrast microtomography, and holotomography | 30 and 40 days |
| Ma et al.63 | Immunocompromised Mice | None and HA/TCP scaffold | Calvarial defect, subcutaneous implantation, and intravenous administration | Histology, ELISA, and immunofluorescence | 4 and 8 weeks |
| Liu et al.64 | Immunocompromised mice | None | Intravenous administration | Histology, histomorphometry ELISA, and micro-computed tomography | 8 weeks |
| Behnia et al.65 | Dogs | Collagen scaffold | Mandibular bone defect | Histology, clinical evaluation, and image segmentation | 12 weeks |
| Jeon et al.66 | Immunocompromised mice | MBCP scaffold | Subcutaneous implantation | Histology, immunohistochemistry, qPCR, and quantitative assay of the alkaline phosphatase levels | 9 weeks |
| Ma et al.67 | Mice | None | Intravenous administration | Histology, ELISA, real-time RT-PCR, and micro-computed tomography | 4 weeks |
| Feng et al.68 | Rabbits | None | Tibial bone defect | Histology, micro-computed tomography, radiography, dual-energy X-ray absorptiometry, and mechanical evaluation | 8 weeks |
| Li et al.69 | Humans | β-TCP scaffold | Periodontal bone defect | Clinical and radiological evaluation | 1, 3, and 9 months |
| Hilkens et al.70 | Immunocompromised mice | 3D-printed HA scaffold | Subcutaneous implantation | Histology and scanning electron microscopy | 12 weeks |
| Kang et al.71 | Immunocompromised mice | HA/TCP and DDM granule | Subcutaneous implantation | Histology, immunohistochemistry, qRT-PCR, and micro-computed tomography | 1 and 8 weeks |
| Seo et al.72 | Immunocompromised mice | HA/TCP scaffold | Calvarial defect | Histology, immunohistochemistry, in situ hybridization, and RT-PCR | 6 and 8 weeks or 6 months |
ELISA: enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; RT-PCR: Real Time Polymerase Chain Reaction; qPCR : quantitative polymerase chain reaction; qRT-PCR :quantitative Real Time Polymerase Chain Reaction GDPB: granular deproteinized bovine bone; PLGA: poly(lactide-co-glycolide); CaP/PLGA: calcium phosphate/poly(lactide-co-glycolide); PGA-TMC: polyglycolic acid–trimethylene carbonate; HA: hydroxyapatite; HA/TCP: hydroxyapatite/tri-calcium phosphate; β-TCP: beta-tri-calcium phosphate; Aga/nHA: agarose/nanohydroxyapatite; CSD: calcium sulfate dehydrate; NCS: nonsilicified collagen scaffolds; ISCS: intrafibrillar-silicified collagen scaffolds; MBCP: macroporous biphasic calcium phosphate; α-CSH/ACP: alpha-calcium sulfate hemihydrate/amorphous calcium phosphate; CSD/β-TCP: calcium sulfate dehydrate/beta-tri-calcium phosphate; PCL-BCP: poly-ε-caprolactone–biphasic calcium phosphate; DDM: demineralized dentin matrix; hDPSC: human dental pulp stem cell.
In vivo model
Fifty-two17–28,30–45,47–59,61–68,70–72 out of 56 studies17–72 using hDPSCs and SHED for bone tissue engineering were performed on animal models and only four of these 56 selected manuscripts evaluated the potential of hDPSCs for bone tissue engineering in humans.29,46,60,69 Among the studies that used animal models, 20 manuscripts used rats,18–22,24–26,35,40,41,44,48,49,51,55–58,62 24 manuscripts used mice,17,23,27,28,30,32–34,36–39,42,43,47,50,54,59,63,64,66,67,70–72 3 manuscripts used rabbits,45,61,68 2 manuscripts used mini pigs,52,53 one manuscript used sheep,31 and one manuscript used dogs.65 The use of these different experimental models for bone tissue engineering in the articles reviewed is graphically represented in Figure 2.
Figure 2.
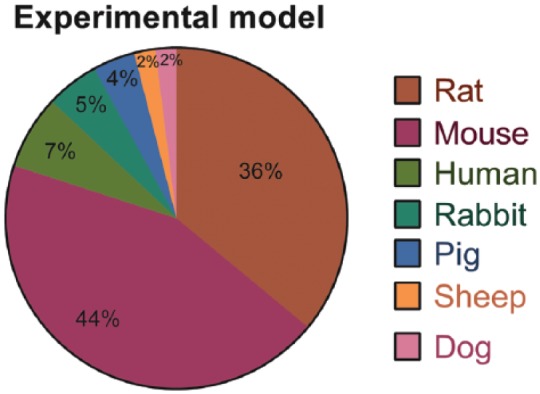
Representative graph of the different experimental models employed to assess the bone tissue engineering potential of hDPSCs and SHED in the articles reviewed. It is important to note the high prevalence of studies in which the rat and mouse was chosen as the experimental model, in contrast with the low amount of studies in which an animal model with a greater similarity to human bone (as the pig, sheep, and dog model) was chosen. The prevalence of studies conducted in humans is also low among the articles reviewed.
The experimental designs
Among the 52 manuscripts that used animal models,17–28,30–45,47–59,61–68,70–72 the authors tested the ectopic bone formation using hDPSC in 27 studies,18–24,26–28,30,32–34,36–39,43,47,50,54,57,63,66,70,71 in 26 studies they created local bone defects to test the efficacy of the use of hDPSC and SHED for bone tissue engineering,17,25,29,31,35,40–42,44–46,48,49,51–53,55,56,58–62,63,65,68,69,72 and in three studies63,64,67 SHED were intravenously administered.
In order to assess ectopic bone formation, the authors implanted hDPSCs and SHED associated with scaffolds subcutaneously in 26 studies18–24,26–28,30,32–34,36–39,47,50,54,57,63,66,70,71 and in one manuscript, the authors implanted hDPSCs intraperitoneally associated with a scaffold.43 All 27 manuscri-pts18–24,26–28,30,32–34,36–39,43,47,50,54,57,63,66,70,71 showed the ectopic bone formation when hDPSC or SHED were used for bone tissue engineering.
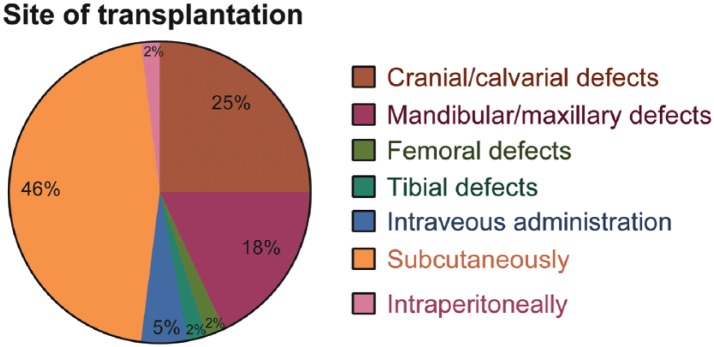
To study the local bone formation using hDPSCs and SHED, cranial defects were created in rats on 11 manuscri-pts,25,35,40,41,44,48,49,51,55,56 in mice on 4 manuscripts,17,42,59,72 and in only one manuscript they were made on rabbits.61 Furthermore, 10 studies created maxillary and mandibular bone defects29,45,46,52,53,58,60,62,65,69 to assess the potential of hDPSCs for bone tissue engineering. In 3 out of these 10 studies, the researchers used the alveolar bone to test the tissue engineering strategies after extraction of third molars of maxillary60 and mandibular29,46 regions in humans. In addition, one study transplanted hDPSCs to periodontal defect areas in patients undertaking periodontal treatment.69 Six studies were conducted in different animal models. Among these studies, one used rats and created the bone defect in the anterior part of the maxilla,58 one used rat and created the defect in the left side of mandible,62 one used rabbits and created the defect between the first premolar and the mental foramen,45 one used mini pigs to create the defects in the mesial region of the maxilla and mandibular first molars,52 one used mini pigs and created the defect in front of the mandibular angle,53 and one used dogs and created the defect on each side of the inferior mandibular border.65 Furthermore, one study evaluated the potential of hDPSCs for bone tissue engineering in rabbits tibia submitted to distraction osteogenesis68 and one study investigated the osteogenic potential of hDPSCs in an ovine model of induced osteonecrosis of femoral head.31 Finally, the therapeutic potential of SHED intravenously administered for the treatment of osteoporosis was assessed in three studies conducted in a mice model of the disease.63,64,67 The use of different sites of hDPSCs and SHED transplantation for bone regeneration purposes in the articles reviewed is graphically represented in Figure 3.
Figure 3.
Representative graph of the different sites of transplantation used to assess the bone tissue engineering potential of hDPSCs and SHED in the articles reviewed. It is imperative to note the high prevalence of studies in which dental stem cells were subcutaneously and intraperitoneally transplanted to assess ectopic bone formation. The amount of studies in which dental stem cells were used to repair actual bone defects were only around half of the articles reviewed.
It is important to note that while most studies evaluated the potential of hDPSCs to produce ectopic bone when implanted subcutaneously or intraperitoneally,18–24,26–28,30,32–34,36–39,43,47,50,54,57,63,66,70,71 the majority of the recent in vivo studies published applied hDPSCs to repair local bone defects.17,25,29,31,35,40–42,44–46,48,49,51–53,55,56,58–62,63,65,68,69,72 In Figure 4, the prevalence of articles in which dental stem cells were employed to assess ectopic bone formation per year is graphically represented and summarizes the change observed in the literature.
Figure 4.
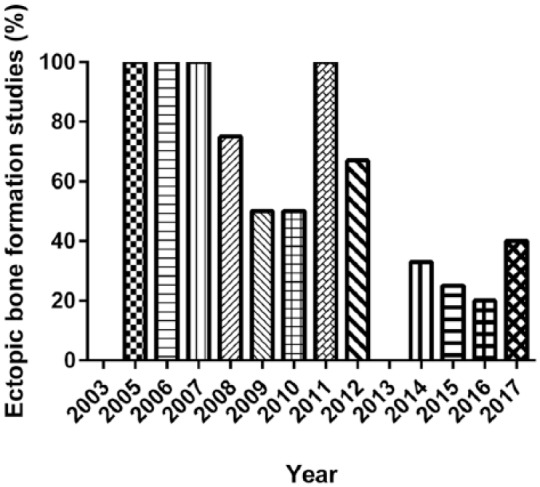
The prevalence of studies in which the potential of hDPSCs and SHED to produce ectopic bone when implanted subcutaneously or intraperitoneally was evaluated per year. It is important to note that in the majority of the studies conducted recently, dental stem cells were used to repair actual bone defects, in contrast with the high prevalence of studies that employed dental stem cells to produce ectopic bone observed in previous years.
The scaffold
The scaffolds were used to seed the hDPSCs for bone tissue engineering in the bone defect sites. Different types of scaffolds were used in these studies that were summarized in this systematic review. The most common scaffolds used were collagen sponge or collagen membrane (observed in 8 studies),25,29,35,44,46,58,60,65 and hydroxyapatite/tri-calcium phosphate (HA/TCP) granules or HA/TCP ceramic (observed in 11 studies).17,23,27,30,37,51,52,54,63,71,72 Furthermore, six studies used scaffolds composed only by HA,26,33,36,40,55,70 five studies used scaffolds composed by beta-tri-calcium phosphate (β-TCP),38,42,49,53,69 three studies used PLGA membranes,21,24,48 two studies used granular deproteinized bovine bone (GDPB) scaffolds,42,49 and two studies used macroporous biphasic calcium phosphate (MBCP) scaffolds.50,66 The other types of scaffolds were used only once and are listed in Table 1.
In 11 manuscripts, scaffolds were not used to deliver hDPSC to the defect site18–20,22,31,45,62–64,67,68 for bone tissue engineering applications. Among them, five studies transplanted the woven bone produced by hDPSCs in rats18–20,22,62 (subcutaneously18–20,22 and to bone defects sites62) and, in three studies, undifferentiated hDPSCs were directly injected to the defect site in sheep31 and rabbits.45,68 Finally, in three studies, SHED were intravenously injected in mice for the treatment of osteoporosis.63,64,67 The use of these different types of scaffolds for bone tissue engineering purposes in the articles reviewed is graphically represented in Figure 5.
Figure 5.
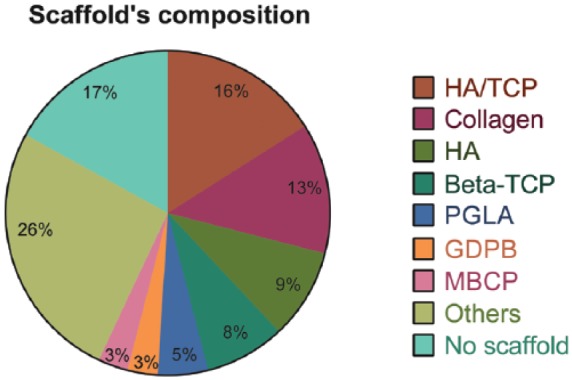
Representative graph of the different types of scaffolds employed in combination with hDPSCs or SHED in the articles reviewed. It is important to note that despite the fact that HA/TCP scaffolds were used by the majority of the articles reviewed, the number of papers in which collagen, HA, and β-TCP scaffolds were used is also significative. In 26% of the studies reviewed, however, other types of scaffolds were used for bone tissue engineering purposes, showing a great heterogeneity among the studies in this regard. In addition, a considerable amount of articles did not use a scaffold to deliver dental stem cells to the site of transplantation.
The method of evaluation
The most common methods used to evaluate the bone tissue engineering potential of hDPSCs were histology, immunohistochemistry, and histomorphometry. Histology was applied to obtain results regarding bone regeneration in 53 out of the 56 articles analyzed,17–48,50–58,60–68,70–72 while immunohistochemistry was used in 20 studies17,21,22,24,26,28,30,32,34,35,38,41,43,44,47,50,59,66,71,72 and histomorphometry was applied in 16 articles.30,35,37,42,44–46,48,51–55,58,61,64 Other methods included immunofluorescence (7 studies),19,21,26,29,36,62,63 micro-computed tomography (10 studies),49,51,56,57,59,61,65,68,69,71 real-time polymerase chain reaction (9 studies)17,27,40,50,57,66,67,71,72 and radiological (10 studies)29,32,34,41,45,46,52,60,68,69 and clinical (10 studies)29,37,39,45,46,52,61,65,69 evaluation. The other methods of evaluation that were used in the articles selected are shown in Table 1.
Outcomes
The results obtained and the methods employed by the articles reviewed were analyzed individually and divided according to the site of transplantation of dental stem cells for in vivo bone tissue engineering purposes. Table 2 summarizes the results obtained in the studies selected in this systematic review.
Table 2.
List of in vivo studies in which the potential of hDPSCs for bone tissue engineering was evaluated, the site of transplantation and the results obtained.
| Author | Site of transplantation | Results |
|---|---|---|
| El-Gendy et al.43 | Intraperitonial implantation | Formation of condensed tissue with polarized cuboidal/columnar cells in a parallel orientation adjacent to the scaffold surface expressing collagen type I and osteocalcin and woven bone-like spicules positive for alizarin red staining |
| Laino et al.18 | Generation of a well-developed lamellar bone with osteocytes entrapped within the lamellae | |
| Papaccio et al.19 | Generation of a well-developed lamellar bone of human origin (as confirmed by the HLA-1 positivity of the transplanted bone) with osteocytes entrapped within the lamellae | |
| Laino et al.20 | Formation of a well-developed lamellar bone with osteocytes entrapped within the lamellae and osteoblasts surrounding the tissue | |
| Graziano et al.21 | Formation of lamellar bone of human origin containing osteocytes entrapped within the lamellae expressing HLA-1, collagen I, bone alkaline phosphatase, bone sialoprotein, osteocalcin, and osteonectin | |
| d’Aquino et al.22 | Generation of an adult bone-like tissue positive for alizarin red staining and expressing von Willebrand factor 1 and 2, PECAM-1, bone alkaline phosphatase, bone sialoprotein, osteocalcin, and osteonectin in both groups | |
| Otaki et al.23 | Formation of lamellar bone-like tissue composed of regular parallel lamellae containing osteoblasts on the bone surface and osteocytes trapped in osseous lacunae | |
| Graziano et al.24 | Presence of bone nodules of human origin composed of crystals of human bone expressing osteonectin, fibronectin, bone sialoprotein, bone alkaline phosphatase, osteocalcin, PECAM-1, von Willebrand factor, and HLA-1 | |
| Abe et al.26 | Formation of a bone-like tissue of human origin (positive for human-specific mitochondria proteins) with osteocyte-like cells embedded within the calcified matrix and cells resembling osteoblasts on the bone surface | |
| Zhang et al.27 | No specific new bone-like tissue formation was observed; however, a fibril-like extracellular matrix and sporadic mineral deposits within the collagen-like tissues were formed. No upregulation of the Runx2 and osteocalcin genes was detected | |
| Morito et al.28 | Subcutaneous implantation |
Formation of a bone matrix containing osteoclasts, osteocytes, and a cuboidal-shaped active osteoblast lining on the matrix surface expressing aggrecan, FABP-4, Runx2, and osteocalcin |
| Kraft et al.30 | Formation of a lamellar bone-like tissue with alkaline phosphatase-positive cells surrounding and within the newly formed bone and TRAP-positive osteoclasts. 1.8% of the area of the scaffold was occupied by the bone-like tissue produced | |
| Chan et al.32 | Presence of multiple radio-opaque foci of mineralization occupying 78% area of the scaffold. Generation of sparse extracellular matrix lobules expressing parathyroid hormone receptor, osteopontin, osteocalcin, osteonectin, and type I collagen | |
| Ikeda et al.33 | Generation of a hard tissue containing osteocyte-like cell inclusions inside the pores of the HA scaffold. Furthermore, the area occupied by the bone-like tissue was 56% of the total area | |
| Li et al.34 | Generation of a bone-like tissue on the 3D gelatin scaffold expressing bone sialoprotein, osteocalcin, and osteopontin. X-ray images results also revealed the presence of a high-density osteoid | |
| Abe et al.36 | The existence of an ectopic bone tissue on the border of the porous HA scaffold expressing osteocalcin was detected | |
| Chen et al.37 | Formation of a lamellar bone with osteoblasts on the surface of the biomaterial (HA-TCP). The total area of the newly mineralized tissue was significantly bigger when DPSCs were associated with the biomaterial compared with the control group | |
| Kawanabe et al.38 | Generation of a bone-like structure of human origin expressing collagen type I and osteocalcin | |
| Wang et al.39 | Generation of bone-like structures on the surface of the scaffold and a connective tissue with small amounts of mineralized tissues when SHED and DPSCs were transplanted, respectively | |
| Niu et al.47 | Formation of a bone-like tissue containing many osteocyte-like cells and capillaries within the newly formed bone trabeculae. Positivity for alizarin red staining and expression of osteocalcin was also observed | |
| Kim et al.50 | Formation of a typical lamellar bone containing osteocytes in lacunae and osteoblast-like cells within the matrix expressing the collagen I, Runx2, bone sialoprotein, and osteocalcin mRNAs and the alkaline phosphatase and osteopontin proteins | |
| Qian et al.54 | The formation of a bone-like tissue was observed. Furthermore, histometric measurements showed the presence of higher amounts of bone-like tissues in the groups treated with DPSCs when compared to the control group | |
| Jang et al.57 | The transplantation of the hydrogel scaffold containing hDPSCs induced the formation of a tissue-engineered bone-like tissue expressing the osteocalcin, osteopontin, and osteonectin mRNAs | |
| Jeon et al.66 | Transplantation of SHED resulted in the production of a bone-like tissue with a compact lamellar matrix with cells embedded within it at the periphery of the MBCP scaffold at 9 weeks after the subcutaneous implantation. The immunohistochemical analysis revealed the expression of osteocalcin in the bone-like tissue produced while the quantitative PCR results have shown the expression of Runx2, osteocalcin, and osteopontin mRNAs. High levels of alkaline phosphatase were also observed through a quantitative assay | |
| Hilkens et al.70 | Subcutaneous implantation |
Strongly organized and concentric layers of collagen and mineralized tissue were found in constructs containing DPSCs. Ultrastructural analysis demonstrated the presence of a mineralized tissue with occasional cellular protrusions in the group transplanted with DPSCs. Deposits of collagen, hydroxyapatite, and mineralized tissue were also present in the samples containing DPSCs |
| Kang et al.71 | The transplantation of DPSCs with both HA-TCP and DDM granule showed great ectopic bone formation efficacy in vivo when compared with the control group (scaffold alone). However, the mineral volumes in transplants of HA-TCP/hDPSCs and DDM/hDPSCs were increased by 15.3% and by 28.7%, respectively, in comparison with those of control without cells. In addition, more osteoids were formed in transplant of HA-TCP/hDPSCs than those in DDM/hDPSCs, and lacuna structure and the immature bone formed in both transplants were similar to each other. Furthermore, quantitative PCR analysis demonstrated that the expression of late osteogenic markers such as osteonectin and osteocalcin in transplants of DDM/hDPSCs was higher than those in transplants of HA-TCP/hDPSCs while the expression levels of the early osteogenic markers osteopontin and bone sialophosphoprotein in transplants of HA-TCP/hDPSCs were higher than those in transplants of DDM/hDPSCs. Finally, the osteoconduction potential of the group transplanted with DPSCs in combination with HA-TCP scaffold was as good as that of DDM, and there was no significant difference in calcium deposition or osteogenesis after 8 weeks of transplantation between the two groups | |
| Ma et al.63 | Eight weeks after subcutaneous implantation, bone-like structures of human origin were formed around the surface of the HA/TCP scaffold in the group transplanted with SHED but not in the control group | |
| Miura et al.17 | In situ hybridization results demonstrated that SHED, despite not been able to differentiate directly into osteoblasts, were able to induce recipient murine cells to differentiate into osteoblasts and osteocytes. SHED transplantation was capable of inducing significant bone formation in 40% of the mice while in 60% of them the amount of bone tissue formed was limited | |
| De Mendonça Costa et al.25 | Cranial/calvarial defects |
The formation of a more mature bone of human origin was observed after 20 days and 1 month when compared to collagen scaffold only controls. After 60 days, the cranial defect was apparently healed in all groups |
| Pisciotta et al.35 | Formation of a bone like-tissue with an osteoblast layer surrounding the islets of the bone expressing osteonectin and von Willebrand factor. The area of occupied by the bone-like tissue was significantly higher when DPSCs were used | |
| Riccio et al.41 | The formation of a bone-like tissue of human origin and an increased radiopacity of defect area in cell-containing groups was detected | |
| Maraldi et al.44 | Formation of a mineralized tissue in DPSC-seeded groups composed of cells of human origin. Complete bridging of the bone defects by 8 weeks in the DPSC-seeded group | |
| Kwon et al.56 | The use of hDPSCs seeded on the scaffold resulted in extensive bone-like ingrowths when compared to the control group. Furthermore, bone regeneration increased from 0% to 35%, 46%, and 53% at 4, 8, and 12 weeks, respectively | |
| Bressan et al.40 | Presence of osteoblast-like cells capable of producing a extracellular matrix consisting of collagen type I expressing osteopontin, RUNX, vWF VEGF, osteonectin, osteocalcin, CD31, and vascular endothelial growth factor mRNAs | |
| Annibali et al.42 | Increased bone regeneration was detected in all groups. However, no significant difference was observed between scaffolds seeded with DPSCs and scaffolds alone | |
| Acasigua et al.48 | Greater bone neoformation in the hDPSC-seeded PLGA scaffold group maintained 13 days in osteogenic medium. The neoformation occurred in the peripheric region or as “islands” in the center of the defect area | |
| Annibali et al.49 | The addition of DPSCs to the grafts induced a small increase in bone mineral density and standardized uptake values compared to the scaffolds alone group | |
| Asutay et al.51 | The area of bone neoformation in the defect zone was significantly increased in the group treated with DPSCs. Furthermore, the bone mineral density values in the group treated with DPSCs were significantly higher than the control | |
| Petridis et al.55 | Formation of a lamellar bone in the edges of the defect area and a dense connective tissue bridging the defect area with lacunae and osteocytes in it. The percentage of new bone formation detected was 32.78% in the cell–scaffold treated group | |
| Yasui et al.59 | Cranial/calvarial defects |
Transplanted DPSCs promoted central and peripheral wound healing and new bone formation with osteoblasts expressing osteocalcin |
| Wongsupa et al.61 | Formation of a mature bone at the periphery and in the middle of the defect areas. The bone regeneration of the hDPSCs transplanted group in combination with PCL-BCP scaffolds was significantly higher at 4 and 8 weeks when compared to the control | |
| Ma et al.63 | Transplantation of SHED associated with HA/TCP scaffolds was able to regenerate the calvarial defects of mice with a large amount of bone-like structures and bone-marrow-like components of human origin compared to the implantation with only the scaffold | |
| Seo et al.72 | The transplantation of SHED associated with HA/TCP scaffolds to the calvarial bone defect site of immunocompromised mice resulted in robust generation of mineralized tissues to repair the defects. The group that was transplanted with the scaffold alone, however, lacked mineralized tissue. After 6 months posttransplantation, SHED was able to maintain the bone the continuity and complete the calvarial repair. The dental stem cells transplanted were able to both induce recipient cells to differentiate into osteogenic cells to form bone and to actively contribute to bone formation, as demonstrated by the presence of human mitochondria-positive cells and by the expression human-specific osteogenic cell markers, including bone sialoprotein and osteocalcin. Furthermore, immunohistochemical analysis demonstrated that the bone tissue regenerated expressed alkaline phosphatase, bone sialoprotein, and collagen I | |
| d’Aquino et al.29 | Formation of an organized bone with a lamellar architecture surrounding the Haversian canals expressing BMP-2, VEGF, osteopontin, osteocalcin, and osteonectin. Greater bone neoformation was observed in the site transplanted with hDPSCs | |
| Alkaisi et al.45 | Formation of highly vascular bony trabeculae with thick cortices and marrow cavity in the SHED transplantation group. Greater radiodensity in the distraction gap and clear corticalization were also observed | |
| Giuliani et al.46 | Presence of a compact bone–like architecture with Haversian canals surrounded by several lamellae. Furthermore, the DPSC-treat group presented a larger volume of bone and a better vertical bone height than the control group | |
| Cao et al.52 | A higher amount of mineralized tissue combined with collagen fiber was generated in the HGF-hDPSC group. Furthermore, the newly regenerated bone was significantly higher and larger in all treatment groups compared with the control group | |
| Kuo et al.53 | Mandibular/maxillary defects |
The sites treated with hDPSCs seeded on α-CSH/ACP scaffolds presented less unhealed cavities compared to the CSD and CSD/β-TCP groups. The ratio of new bone formation was also lower in the CSD/β-TCP + hDPSCs group |
| Jahanbin et al.58 | There were no significant differences for bone neoformation between iliac bone graft and DPSCs plus collagen scaffold groups at 1 or 2 months after transplantation. Maximum fetal bone formation was reached in the iliac bone graft group | |
| Monti et al.60 | The generation of a well-differentiated bone with structures resembling Haversian canals could be observed. The bone regeneration and the radio-opacity level of the site transplanted with hDPSCs were also higher when compared to control | |
| Paino et al.62 | Woven bone, after transplantation, was remodeled to a bone tissue with small clusters of mineralized bone. The bone tissue also integrated with the surrounding tissue, giving rise to a lamellar bone tissue with Haversian canals and osteocytes visible | |
| Behnia et al.65 | Mandibular/maxillary defects |
For both control and SHED-seeded groups, the formation of a new compact bone was observed in both lingual and floor parts of the defect. In the middle part of the defect, a lamellar and woven bone with limited connective was produced and in the lateral cortex of mandible, the defect site was restored with connective tissue. However, no difference between the control group and de SHED-seed group could be observed |
| Li et al.69 | Transplantation of DPSCs associated with β-TCP scaffolds greatly improved the clinical symptoms of periodontitis and exerted a repair effect on periodontal hard tissue defects caused by the condition | |
| Ma et al.63 | Intravenous administration |
Intravenous administration of SHED increased the bone mineral density and recovered the trabecular bone structures in the long bones in a mice model of osteoporosis |
| Liu et al.64 | SHED systemic intravenous administration resulted in a marked increase in bone volume, trabecular thickness, trabecular number, bone mineral density, connectivity density and in the trabecular bone area, along with decreased trabecular space and structure model index in a mice model of ovariectomy-induced osteoporosis. SHED transplantation also resulted in an increase in the cortical bone parameters, including total cross-sectional area, cortical bone area, cortical bone fraction, and cortical thickness. Furthermore, histological analysis revealed that the trabecular bone volume in the SHED-treated group was markedly elevated compared with the control group. Finally, ELISA results demonstrated that transplantation of SHED down-regulated the serum levels of receptor activator of nuclear factor kappa-B ligand (RANKL) and up-regulated the level of osteoprotegerin (OPG) | |
| Ma et al.67 | The systemic transplantation of SHED recovered the bone mineral density and trabecular bone structures in a mice model of osteoporosis. The bone formed expressed higher levels of the osteoblast-specific genes Runx2, alkaline phosphatase, and osteocalcin in the group transplanted with SHED when compared to the control group. Furthermore, ELISA results demonstrated that SHED systemic transplantation markedly reduced the serum concentration of the bone resorption markers RANKL and C-terminal telopeptides of type I collagen (CTX) compared with the control group | |
| Feng et al.68 | Tibial bone defects | Formation of new trabeculae was seen in all groups. In the control group treated only with saline, bone formation was sparse and disordered and focal defects were seen in the regenerated region while in the group treated with DPSCs, the partial emergence of both sclerotic zones could be observed and the distraction gap was partially bridged with incompact and tiny trabecular bones. In the group treated with DPSCs infected with adenovirus-Runx2-GFP, however, a complete bony continuity of the distraction gap and a more mature, regular trabecular bone with the highest bone mineral density, bone volume-to-total volume ratio, trabecular thickness and trabecular number, and with the lowest trabecular separation among the three groups was observed |
| Feitosa et al.31 | Femoral defects | Formation of an organized trabecular bone containing live bone marrow in the bone trabeculae |
BMP-2: bone morphogenetic protein-2; vWF: von Willebrand factor; VEGF: vascular endothelial growth factor; TRAP: tartrate-resistant acid phosphatase; FABP-4: fatty acid–binding protein 4; PECAM-1: platelet endothelial cell adhesion molecule 1; HLA-1: human leukocyte antigen 1; Runx2: runt-related transcription factor 2; GFP: green fluorescent protein; HA: hydroxyapatite; HA-TCP: hydroxyapatite–tri-calcium phosphate; DPSC: dental pulp stem cell; hDPSC: human dental pulp stem cell; SHED: stem cells from human exfoliated deciduous teeth; MBCP: macroporous biphasic calcium phosphate; PCR: polymerase chain reaction; DDM: demineralized dentin matrix; PLGA: poly(lactide-co-glycolide); PCL-BCP: poly-ε-caprolactone–biphasic calcium phosphate; α-CSH: alpha-calcium sulfate hemihydrate; ACP: amorphous calcium phosphate; CSD: calcium sulfate dehydrate; β-TCP: beta-tri-calcium phosphate; ELISA: enzyme-linked immunosorbent assay.
Subcutaneous and intraperitonial implantation of hDPSCs
Among the 27 articles that assessed ectopic bone formation,18–24,26–28,30,32–34,36–39,43,47,50,54,57,63,66,70,71 no evidence of bone tissue formation could be found in only one study which used HA/TCP ceramic scaffolds to deliver hDPSCs to a mouse model.27 Most common histological findings included the positivity for alizarin red staining22,43,47 and the observation of a lamellar bone formation with osteocytes entrapped within the lacunae and lamellae and osteoblasts on the bone surface.18–21,23,30,37,50,66 In addition, the expression of human leukocyte antigen 1 (HLA-1),19,21,24 collagen-I,21,32,38,43,72 bone alkaline phosphatase,21,22,24,30,50,66,72 bone sialoprotein,21,22,24,34,72 osteocalcin,21,22,24,28,32,34,36,38,43,47,66 osteonectin,21,22,24,32 osteopontin,32,34,50 platelet endothelial cell adhesion molecule 1 (PECAM-1),22,24 Runx2,28 and von Willebrand factor22,24 in the newly formed bone were commonly observed by immunohistochemistry and immunofluorescence staining. Gene expression analysis by RT-PCR has also shown upregulation of the collagen-I,50 bone sialoprotein,50,71,72 Runx2,27,50,66,67 osteocalcin,27,50,57,66,67,71,72 osteopontin,56,66,71 osteonectin,56,71 and alkaline phosphatase67 mRNA levels. In most studies, the implants were retrieved at 4, 8, or 12 weeks after transplantation.
Cranial bone defect
The first study to use SHED for bone tissue engineering purposes was conducted by Miura et al.17 This study was able to demonstrate that SHED, despite not been able to differentiate directly into osteoblasts, were able to induce recipient murine cells to differentiate into osteoblasts and osteocytes when transplanted to the brain of immunocompromised mice associated with HA/TCP scaffolds.17 A more recent study conducted by Ma et al.63 demonstrated that the transplantation of SHED associated with HA/TCP scaffold was able to regenerate the calvarial bone defects of mice. The tissue regenerated, however, contained a large amount of bone-like structures and bone-marrow-like components of human origin as demonstrated by the presence of human mitochondria-positive cells arranged on the mineralized matrix.63 Other studies have shown that the transplantation of hDPSCs in combination with collagen and fibroin scaffolds resulted in significant bone regeneration after 4–8 months in rat cranial defects.25,35,41 The use of hDPSCs seeded on hydroxyapatite-based hydrogel scaffolds also resulted in superior healing compared to both untreated and scaffold-treated defects in rat calvarial bone defects, although complete healing could not be observed in any group of animals.55 Furthermore, when hDPSCs were seeded on poly(lactide-co-glycolide) (PLGA) scaffolds, a greater bone neoformation was observed in the hDPSC-seeded scaffold group maintained 13 days in osteogenic medium before transplantation in rat calvarial bone defects.48 In addition, the transplantation of both hDPSCs and SHED associated with HA/TCP scaffolds resulted in a greater regeneration of calvarial bone defects in rats51 and mice72 when compared to the control group (scaffold alone). The bone mineral density values in the group treated with hDPSCs associated with the HA/TCP scaffolds were also significantly higher than in the untreated control group.51 A study conducted by Yasui et al.59 demonstrated that LNGFRLow + THY-1High + hDPSCs associated with matrigel matrix scaffolds promoted greater central and peripheral would healing and new bone formation compared to the group transplanted with the scaffold alone when transplanted into immunocompromised mice to treat calvarial bone defects.59 Transplantation of hDPSCs seeded on circular computer-designed Poly L-lactide-co-glycolide-co-epsilon-caprolactone (PLGC) scaffolds resulted in extensive bone-like ingrowths when compared to the control group in which computer-designed PLGC scaffolds were transplanted without hDPSCs.56 Furthermore, no significant increases in the bone regeneration volumes were detected in the group treated with the scaffold alone. However, in the group treated with hDPSCs associated with the computer-designed PLGC scaffold, bone regeneration increased from 0% to 35%, 46%, and 53% at 4, 8, and 12 weeks, respectively.56 Finally, a study by Wongsupa et al.61 has shown that transplantation of hDPSCs seeded on poly-ε-caprolactone–biphasic calcium phosphate (PCL-BCP) scaffolds in combination with the modified melt stretching and multilayer deposition technique resulted in higher bone regeneration in the middle of the defects at 4 and 8 weeks when compared to the control group (scaffold alone) in a rabbit model of calvarial bone defects. However, bone regeneration in the group that received autogenous bone transplantation was superior, at all time points, to the groups of scaffolds seeded with hDPSCs and scaffold alone. When hDPSCs were transplanted in combination with GDPB, β-TCP, or Aga/nHA scaffold, however, little to no difference was observed between the group transplanted with the scaffold alone and the group treated with the scaffold seeded with hDPSCs.42
Mandibular and maxillary bone defect
In a total of 10 studies, dental stem cells were transplanted for mandibular and maxillary bone defect reconstruction.29,45,46,52,53,58,60,62,65,69 In three of the four human studies selected for this systematic review, hDPSCs were used in combination with collagen scaffolds to repair bone defects created at the site of extracted maxillary and mandibular third molars.29,46,60 In one study, however, hDPSCs were transplanted in combination with a β-TCP scaffold to repair a periodontal bone defect in patients undertaking periodontal treatment.69 In general, results from these studies demonstrated that a greater bone neoformation is achieved when hDPSCs are transplanted to the bone defect site associated with collagen or β-TCP scaffolds compared to control (scaffold alone).29,46,60,69
A study conducted by d’Aquino et al.,29 for instance, demonstrated that the sites that received the transplantation of hDPSCs seeded on collagen scaffolds presented a high rate of mineralization 1 month after transplantation. Furthermore, it was observed vertical bone regeneration in the site transplanted with hDPSCs seeded on collagen scaffolds but not in the site transplanted with the collagen scaffold alone at 2 months after transplantation. Three months after transplantation, it was confirmed that the bone defect in the site that received the combination of collagen scaffold and hDPSCs was completely regenerated and the cortical bone level was much higher in this site than in the site that was transplanted with the collagen scaffold alone.29 An increase of clinical attachment was further observed in the site transplanted with hDPSCs seeded on collagen scaffolds compared to the site transplanted with the scaffold alone. Histological analysis also revealed that the tissue regenerated in the site that received the combination of hDPSCs and collagen scaffolds consisted of a well organized and well vascularized bone with a lamellar architecture surrounding the Haversian canals. In the site that received only the collagen scaffold, however, a more immature bone was formed, with fibrous bone entrapped among new lamellae, incomplete and large Haversian canals and evidence of bone reabsorption. Immunofluorescence analysis also revealed that the site that was transplanted with hDPSCs seeded on collagen scaffolds expressed higher levels of BMP-2 and VEGF when compared to the site transplanted with the scaffold alone. The results of the study conducted by d’Aquino et al.29 were further investigated by Giuliani et al.,46 in a secondary report of the same experiment containing the results of a 3-year follow up. Results from this study demonstrated that the bone tissue regenerated in the sites that received hDPSCs seeded on collagen scaffolds was uniformly vascularized and compact. Furthermore, the bone tissue regenerated in these sites was larger in volume and had a better vertical bone height compared to the sites transplanted with the scaffold alone. In addition, a study conducted by Monti et al.60 showed that 6 days after transplantation, the sites that were transplanted with hDPSCs seeded on collagen scaffolds presented stronger radio-opacity when compared to the sites transplanted with the scaffold alone. It was also observed a greater regeneration of a well-differentiated bone tissue with Haversian system formation in the sites that received the transplantation of hDPSCs associated with collagen scaffolds. Finally, a study conducted by Li et al.69 demonstrated that the transplantation of hDPSCs associated with β-TCP scaffolds greatly improved the clinical symptoms of periodontitis on patients and exerted a repair effect on periodontal hard tissue defects caused by the condition.
The other six studies selected for this systematic review in which hDPSCs were used to repair maxillary and mandibular bone defects were conducted by Alkaisi et al.,45 Behnia et al.,65 Cao et al.,52 Kuo et al.,53 Jahanbin et al.,58 and Paino et al.62 Alkaisi et al.45 created a bone defect between the first premolar and the mental foramen in a rabbit model and investigated the potential for bone neoformation of SHED. No scaffold was used associated with SHED in this study. Radiological evaluation showed that at 2 weeks postoperatively, in the group that received the SHED transplantation, radiopacity had partially bridged the gap between the distracted bones and a greater bony union was observed at the center of the surgical area when compared to the groups treated with the scaffold alone. Furthermore, the tissue regenerated in the group that was transplanted with SHED has shown bony continuity with greater radiodensity when compared to the group treated with the scaffold alone, 4 weeks after transplantation. At 6 weeks postoperatively, greater radiodensity in the distraction gap and clear corticalization with a marrow cavity was observed in the group that received SHED transplantation. Histomorphometric findings also revealed that the total amount of bone regenerated in the groups that received SHED transplantation was significantly greater when compared to the groups treated with the scaffold alone across all measurement points and the percentage of remaining fibrous tissue was significantly lower in the group that received SHED transplantation. In a study conducted by Behnia et al.,65 dogs had bone defects created on each side of their inferior mandibular border. In this study, no difference could be noted between the site that received the transplantation of SHED associated with a collagen scaffold and the site that was only transplanted with the collagen scaffold alone. The bone tissue regenerated in both sites was compact in both lingual and floor parts of the defect, in the middle part of the defect a lamellar and woven bone with limited connective was produced, and in the lateral cortex of mandible the defect site was restored with connective tissue.65 Cao et al.52 created a bone defect in the mesial region of the maxilla and mandibular first molars in a miniature swine model in order to assess the bone tissue engineering potential of hDPSCs disassociated or seeded on HA-TCP scaffolds. Results from this study indicated that at 12 weeks postoperatively, the transplantation of hDPSCs disassociated or associated with HA-TCP scaffolds promoted the regeneration of a bone tissue higher and larger in volume when compared to the control group (scaffold alone). Kuo et al.53 created bone defects in the front of the mandibular corner in a miniature swine model in order to evaluate the bone tissue engineering potential of hDPSCs seeded on various resorbable calcium sulfate/calcium phosphate scaffolds. This study has shown that at 8 weeks postoperatively, the sites treated with hDPSCs seeded on alpha-calcium sulfate hemihydrates (α-CSH)/ACP scaffolds presented less unhealed cavities when compared to the sites transplanted with hDPSCs seeded on calcium sulfate dehydrate (CSD) and CSD/β-TCP scaffolds. In contrast, some new bone but many unhealed cavities were observed in the empty control group. Furthermore, the ratio of new bone formation was significantly lower in the group treated with hDPSCs seeded on CSD/β-TCP scaffolds when compared to the sites treated with hDPSCs seeded on α-CSH/ACP and CSD scaffolds. Jahanbin et al.58 created bone defects in the anterior part of the maxilla in a rat model to evaluate the potential for bone tissue regeneration of hDPSCs seeded on collagen scaffolds. This study demonstrated that maximum fetal bone neoformation was achieved in the group that received iliac bone graft 2 months after transplantation. However, there were no significant differences for bone neoformation between iliac bone graft and stem cell plus collagen scaffold groups at 1 or 2 months after transplantation. Finally, Paino et al.62 created bone defects in the left mandible of rats in order to investigate whether a woven bone obtained by hDPSCs in vitro can be efficiently grafted to repair bone defects in a rat model. The results obtained demonstrated that woven bone, after transplantation, was remodeled to a bone tissue highly vascularized containing small clusters of mineralized bone. The bone tissue transplanted also integrated with the surrounding tissue, giving rise to a lamellar bone tissue with Haversian canals and osteocytes visible.
Intravenous administration
In three of the studies analyzed, SHED were intravenously administered for the treatment of osteoporosis in a mice model of the disease.63,64,67 In a study by Ma et al.,63 SHED, when intravenously administered, were able to increase the bone mineral density and to recover the trabecular bone structures in the long bones in a mice model of osteoporosis. Furthermore, a study conducted by Liu et al.64 demonstrated that the systematic transplantation of SHED was able to increase the bone volume, the trabecular thickness, the trabecular number, the bone mineral density, the connectivity density, and the trabecular bone area and to decrease the trabecular space and structure model index in a mice model of ovariectomy-induced osteoporosis. SHED transplantation also resulted in an increase in the cortical bone parameters, including total cross-sectional area, cortical bone area, cortical bone fraction, and cortical thickness. Furthermore, histological analysis revealed that the trabecular bone volume in the SHED-treated group was markedly elevated compared with the control group.64 Finally, Ma et al.67 showed that the intravenous administration of SHED successfully recovered the bone mineral density and trabecular bone structures in a mice model of osteoporosis. The bone regenerated also expressed higher levels of the osteoblast-specific genes Runx2, alkaline phosphatase, and osteocalcin in the group transplanted with SHED when compared to the control group. Enzyme-linked immunosorbent assay (ELISA) results from the studies conducted by both Liu et al.64 and Ma et al.67 demonstrated that SHED systemic transplantation markedly reduced the serum concentration of the bone resorption markers receptor activator of nuclear factor kappa-B ligand (RANKL) and C-terminal telopeptides of type I collagen (CTX) and up-regulated the level of osteoprotegerin (OPG) compared with the control group. It is believed, however, that the reduction in bone loss observed after SHED intravenous transplantation is mainly due to their immunomodulatory properties.
Discussion
Currently, the gold standard for alveolar bone repair is to perform an autologous bone transplantation, in which the bone is removed from the patient and transplanted in order to fill the bone defect.73 However, there are some problems related with this method that should be taken into consideration. For example, in cleft lip and palate patients, the amount of bone material removed from the donor site may not be enough to fill the alveolar cleft in case of wide fissures.74 Furthermore, bone absorption, pain in the donor site area, infection and secretory disorders may also occur.75–77 Due to these complications, bone tissue bioengineering has been pointed out as a promising strategy for the reconstruction of critical orofacial defects, and the MSCs are currently being considered the main candidates for bone tissue engineering applications.74 Bone marrow has been considered as one of the main sources of MSCs. However, the process of obtaining BMSCs requires a painful surgical incision.4
Taking all these problems into consideration, several groups are trying to isolate of MSCs from more accessible sources and to use them in bone tissue engineering applications.8–10 One of the most promising source of MSCs for reconstruction of bone defects currently investigated is the dental pulp.9 DPSCs can be easily isolated from deciduous and permanent teeth, expanded in vitro and used, in combination or not with a scaffold, for the repair of bone defects.14–16 The use of hDPSCs and SHED is particularly advantageous for bone reconstruction in cleft lip and palate patients. Due to the great benefits that the use of DPSCs can generate for the treatment of bone injuries and bone congenital malformations several in vitro studies have been conducted to investigate the potential use of dental pulp stem cells for bone tissue engineering applications.78–80 However, there is a limited number of clinical trials in humans in which the potential of hDPSCs and SHED for bone tissue engineering is evaluated. Furthermore, the methodologies applied for the evaluation of the potential of hDPSCs for bone regeneration vary considerably between distinct studies.
A variety of animal models were used in the studies analyzed in this systematic review, including mice,17,23,27,28,30,32–34,36–39,42,43,47,50,54,59,63,64,66,67,70–72 rats,18–22,24–26,35,40,41,44,48,49,51,55–58,62 rabbits,45,61,68 sheep31 and mini pigs.52,53 Most studies evaluated in this systematic review were conducted in mice or rats. However, it is known that the swine and the ovine model represents the models with the highest similarity to human.81 Future studies should put their focus on the use of animal models that have a greater similarity to human. In only four studies29,46,60,69 out of fifty-six analyzed,17–72 hDPSCs were used to treat bone defects in humans. It is imperative to conduct more human studies to reach a safer and more efficient use of hDPSCs for bone tissue engineering.
The type of defect created is also a factor of influence on bone regeneration. While the great majority of studies evaluated ectopic bone formation, an increasing number of studies are focusing on the use of hDPSCs to repair the site of bone defects in locus as evidenced in Figure 4. This change is extremely positive because the bone formation process occurs in a distinct manner when hDPSCs are implanted subcutaneously in comparison with the bone formation process in a bone defect site. As a consequence of the lack of all mechanical and chemical influences that the bone usually receives in an area of bone regeneration, subcutaneous implantation of hDPSCs hardly simulates clinical conditions.82 Furthermore, in one of the studies analyzed in this systematic review, the process of ectopic bone formation completely failed.27 Studies in which hDPSCs were transplanted to actual cranial, maxillary, and mandibular bone detects had better outcomes in general. However, in a study conducted by Annibali et al.42 using a mice model in which calvarial bone defects were created, it was not possible to observe any difference in bone regeneration between the animals transplanted with the scaffold alone and the animals transplanted with the combination of the scaffold and hDPSCs. In addition, Behnia et al.,65 conducted a study in which dogs had bone defects created on each side of their inferior mandibular border. One side was transplanted with a combination of SHED and a collagen scaffold and the other side was transplanted with the collagen scaffold alone. No difference could be noted between the side that received the transplantation of SHED associated with a collagen scaffold and the side that was transplanted with the collagen scaffold alone and, as a result, the ability of SHED to contribute to the regeneration of mandibular bone defects in vivo in this dog experimental model could not be demonstrated.65
Finally, the type of scaffold used to support the proliferation and differentiation of dental stem cells can also play a crucial role in the process of bone tissue regeneration. For instance, Zhang et al.27 did not observe ectopic bone formation in vivo when hDPSCs were seeded on HA/TCP scaffolds. A study by Kuo et al.,53 however, was able to demonstrate that the use of α-CSH/ACP scaffolds in combination with hDPSCs promoted a more efficient bone regeneration when compared to hDPSCs seeded on CSD and CSD/β-TCP scaffolds. This study also demonstrated that the ratio of new bone formation was significantly lower in the group treated with hDPSCs seeded on CSD/β-TCP scaffolds when compared to the sites treated with hDPSCs seeded on α-CSH/ACP and CSD scaffolds. Furthermore, a study conducted by Acasigua et al.48 has shown that a superior bone regeneration is reached when hDPSCs seeded on PLGA scaffolds are maintained in osteogenic induction medium for 13 days before transplantation. Finally, a study conducted by Kang et al.71 demonstrated that both HA-TCP and demineralized dentin matrix (DDM) scaffolds had similar effects on the ability of hDPSCs to produce ectopic bone in a mice experimental model. hDPSCs, when used in combination with both scaffolds, showed great ectopic bone formation efficacy in vivo when compared with the control group (scaffold alone).71 Three of the four human studies evaluated in this systematic review used collagen scaffolds as cell carries and reported successful bone formation for the repair of the bone defect produced after the process of extraction of third molars.29,46,60
Final considerations
Most of the studies analyzed in this systematic review reported positive results when they used hDPSC for bone tissue engineering. While most studies evaluated the potential of hDPSCs to produce ectopic bone when transplanted subcutaneously other evaluated their bone tissue engineering potential in actual bone defects using, in most cases, rats and mice as animal models. This emphasizes, therefore, the importance of a change of attitude regarding the use of hDPSCs to repair actual bone defects in an animal model with a greater similarity to human.
Acknowledgments
Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Programa de Desenvolvimento Institucional do Sistema Único de Saúde (PROADI – SUS). To Instituto de Ensino e Pesquisa - Hospital Sírio Libanês, for provide us all support to develop stem cells and tissue engineering researches.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alessander Leyendecker Junior  https://orcid.org/0000-0003-3973-424X
https://orcid.org/0000-0003-3973-424X
References
- 1. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926. [DOI] [PubMed] [Google Scholar]
- 2. Kaigler D, Pagni G, Park CH, et al. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A 2010; 16(9): 2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi S, Bartold PM, Miura M, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 2005; 8(3): 191–199. [DOI] [PubMed] [Google Scholar]
- 4. Caplan AI. Mesenchymal stem cells. J Orth Res 1991; 9(5): 641–650. [DOI] [PubMed] [Google Scholar]
- 5. Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81(8): 531–535. [DOI] [PubMed] [Google Scholar]
- 6. Bueno DF, Kerkis I, Costa AM, et al. New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng Part A 2008; 15(2): 427–435. [DOI] [PubMed] [Google Scholar]
- 7. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13(12): 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morsczeck C, Schmalz G, Reichert TE, et al. Somatic stem cells for regenerative dentistry. Clin Oral Investig 2008; 12(2): 113–118. [DOI] [PubMed] [Google Scholar]
- 9. Guilak F, Awad HA, Fermor B, et al. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004; 41(3–4): 389–399. [PubMed] [Google Scholar]
- 10. Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008; 34(8): 962–969. [DOI] [PubMed] [Google Scholar]
- 11. d’Aquino R, Papaccio G, Laino G, et al. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev 2008; 4(1): 21–26. [DOI] [PubMed] [Google Scholar]
- 12. Casagrande L, Cordeiro MM, Nör SA, et al. Dental pulp stem cells in regenerative dentistry. Odontology 2011; 99(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci 2000; 97(25): 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang GJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 2009; 88(9): 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang W, Walboomers XF, Van Kuppevelt TH, et al. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials 2006; 27(33): 5658–5668. [DOI] [PubMed] [Google Scholar]
- 16. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213(2): 341–347. [DOI] [PubMed] [Google Scholar]
- 17. Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci 2003; 100(10): 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laino G, d’Aquino R, Graziano A, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res 2005; 20(8): 1394–1402. [DOI] [PubMed] [Google Scholar]
- 19. Papaccio G, Graziano A, d’Aquino R, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol 2006; 208(2): 319–325. [DOI] [PubMed] [Google Scholar]
- 20. Laino G, Graziano A, d’Aquino R, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol 2006; 206(3): 693–701. [DOI] [PubMed] [Google Scholar]
- 21. Graziano A, d’Aquino R, Angelis MG, et al. Concave pit-containing scaffold surfaces improve stem cell-derived osteoblast performance and lead to significant bone tissue formation. PLoS ONE 2007; 2(6): e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. d’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 2007; 14(6): 1162–1171. [DOI] [PubMed] [Google Scholar]
- 23. Otaki S, Ueshima S, Shiraishi K, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int 2007; 31(10): 1191–1197. [DOI] [PubMed] [Google Scholar]
- 24. Graziano A, d’Aquino R, Laino G, et al. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif 2008; 41(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Mendonça Costa A, Bueno DF, Martins MT, et al. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg 2008; 19(1): 204–210. [DOI] [PubMed] [Google Scholar]
- 26. Abe S, Yamaguchi S, Watanabe A, et al. Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun 2008; 371(1): 90–93. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Walboomers XF, Van Osch GJ, et al. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A 2008; 14(2): 285–294. [DOI] [PubMed] [Google Scholar]
- 28. Morito A, Kida Y, Suzuki K, et al. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol 2009; 72(1): 51–64. [DOI] [PubMed] [Google Scholar]
- 29. d’Aquino R, De Rosa A, Lanza V, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 2009; 18(7): 75–83. [DOI] [PubMed] [Google Scholar]
- 30. Kraft DC, Bindslev DA, Melsen B, et al. Mechanosensitivity of dental pulp stem cells is related to their osteogenic maturity. Eur J Oral Sci 2010; 118(1): 29–38. [DOI] [PubMed] [Google Scholar]
- 31. Feitosa MLT, Fadel L, Beltrão-Braga PCB, et al. Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head: preliminary results. Acta Cir Bras 2010; 25(5): 416–422. [DOI] [PubMed] [Google Scholar]
- 32. Chan B, Wong RWK, Rabie B. In vivo production of mineralised tissue pieces for clinical use: a qualitative pilot study using human dental pulp cell. Int J Oral Maxillofac Surg 2011; 40(6): 612–620. [DOI] [PubMed] [Google Scholar]
- 33. Ikeda H, Sumita Y, Ikeda M, et al. Engineering bone formation from human dental pulp-and periodontal ligament-derived cells. Ann Biomed Eng 2011; 39(1): 26–34. [DOI] [PubMed] [Google Scholar]
- 34. Li JH, Liu DY, Zhang FM, et al. Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin Med J 124(23): 4022–4028. [PubMed] [Google Scholar]
- 35. Pisciotta A, Riccio M, Carnevale G, et al. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS ONE 2012; 7(11): e50542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abe S, Hamada K, Miura M, et al. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int 2012; 36(10): 927–936. [DOI] [PubMed] [Google Scholar]
- 37. Chen B, Sun HH, Wang HG, et al. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials 2012; 33(20): 5023–5035. [DOI] [PubMed] [Google Scholar]
- 38. Kawanabe N, Murata S, Fukushima H, et al. Stage-specific embryonic antigen-4 identifies human dental pulp stem cells. Exp Cell Res 2012; 318(5): 453–463. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Sha XJ, Li GH, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol 2012; 57(9): 1231–1240. [DOI] [PubMed] [Google Scholar]
- 40. Bressan E, Ferroni L, Gardin C, et al. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS ONE 2012; 7(11): e49146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riccio M, Maraldi T, Pisciotta A, et al. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A 2012; 18(9–10): 1006–1013. [DOI] [PubMed] [Google Scholar]
- 42. Annibali S, Cicconetti A, Cristalli MP, et al. A comparative morphometric analysis of biodegradable scaffolds as carriers for dental pulp and periosteal stem cells in a model of bone regeneration. J Craniofac Surg 2013; 24(3): 866–871. [DOI] [PubMed] [Google Scholar]
- 43. El-Gendy R, Yang XB, Newby PJ, et al. Osteogenic differentiation of human dental pulp stromal cells on 45S5 Bioglass® based scaffolds in vitro and in vivo. Tissue Eng Part A 2012; 19(5–6): 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maraldi T, Riccio M, Pisciotta A, et al. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther 2013; 4(3): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alkaisi A, Ismail AR, Mutum SS, et al. Transplantation of human dental pulp stem cells: enhance bone consolidation in mandibular distraction osteogenesis. J Oral Maxillofac Surg 2013; 71(10): 1758.e1–13. [DOI] [PubMed] [Google Scholar]
- 46. Giuliani A, Manescu A, Langer M, et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cell Transl Med 2013; 2(4): 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Niu LN, Sun JQ, Li QH, et al. Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells. J Dent 2014; 42(7): 839–849. [DOI] [PubMed] [Google Scholar]
- 48. Acasigua GAX, Bernardi L, Braghirolli DI, et al. Nanofiber scaffolds support bone regeneration associated with pulp stem cells. Curr Stem Cell Res Ther 2014; 9(4): 330–337. [DOI] [PubMed] [Google Scholar]
- 49. Annibali S, Bellavia D, Ottolenghi L, et al. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: preliminary data. J Biomed Mater Res B Appl Biomater 2014; 102(4): 815–825. [DOI] [PubMed] [Google Scholar]
- 50. Kim S, Song JS, Jeon M, et al. Ectopic hard tissue formation by odonto/osteogenically in vitro differentiated human deciduous teeth pulp stem cells. Calcif Tissue Int 2015; 97(1): 80–89. [DOI] [PubMed] [Google Scholar]
- 51. Asutay F, Polat S, Gül M, et al. The effects of dental pulp stem cells on bone regeneration in rat calvarial defect model: micro-computed tomography and histomorphometric analysis. Arch Oral Biol 2015; 60(12): 1729–1735. [DOI] [PubMed] [Google Scholar]
- 52. Cao Y, Liu Z, Xie Y, et al. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther 2015; 6(1): 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuo TF, Lee SY, Wu HD, et al. An in vivo swine study for xeno-grafts of calcium sulfate-based bone grafts with human dental pulp stem cells (hDPSCs). Mater Sci Eng C Mater Biol Appl 2015; 50: 19–23. [DOI] [PubMed] [Google Scholar]
- 54. Qian J, Jiayuan W, Wenkai J, et al. Basic fibroblastic growth factor affects the osteogenic differentiation of dental pulp stem cells in a treatment-dependent manner. Int Endod J 2015; 48(7): 690–700. [DOI] [PubMed] [Google Scholar]
- 55. Petridis X, Diamanti E, Trigas GC, et al. Bone regeneration in critical-size calvarial defects using human dental pulp cells in an extracellular matrix-based scaffold. J Craniomaxillofac Surg 2015; 43(4): 483–490. [DOI] [PubMed] [Google Scholar]
- 56. Kwon DY, Kwon JS, Park SH, et al. A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci Rep 2015; 5: 12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jang JY, Park SH, Park JH, et al. In vivo osteogenic differentiation of human dental pulp stem cells embedded in an injectable in vivo-forming hydrogel. Macromol Biosci 2016; 16(8): 1158–1169. [DOI] [PubMed] [Google Scholar]
- 58. Jahanbin A, Rashed R, Alamdari DH, et al. Success of maxillary alveolar defect repair in rats using osteoblast-differentiated human deciduous dental pulp stem cells. J Oral Maxillofac Surg 2016; 74(4): 829.e1–9. [DOI] [PubMed] [Google Scholar]
- 59. Yasui T, Mabuchi Y, Toriumi H, et al. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res 2016; 95(2): 206–214. [DOI] [PubMed] [Google Scholar]
- 60. Monti M, Graziano A, Rizzo S, et al. In vitro and in vivo differentiation of progenitor stem cells obtained after mechanical digestion of human dental pulp. J Cell Physiol 2017; 232(3): 548–555. [DOI] [PubMed] [Google Scholar]
- 61. Wongsupa N, Nuntanaranont T, Kamolmattayakul S, et al. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(ε-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J Mater Sci Mater Med 2017; 28(5): 77. [DOI] [PubMed] [Google Scholar]
- 62. Paino F, La Noce M, Giuliani A, et al. Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering. Clin Sci 2017; 131(8): 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma L, Makino Y, Yamaza H, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE 2012; 7(12): e51777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu Y, Wang L, Liu S, et al. Transplantation of SHED prevents bone loss in the early phase of ovariectomy-induced osteoporosis. J Dent Res 2014; 93(11): 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Behnia A, Haghighat A, Talebi A, et al. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J Stem Cell 2014; 6(4): 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jeon M, Song JS, Choi BJ, et al. In vitro and in vivo characteristics of stem cells from human exfoliated deciduous teeth obtained by enzymatic disaggregation and outgrowth. Arch Oral Biol 2014; 59(10): 1013–1023. [DOI] [PubMed] [Google Scholar]
- 67. Ma L, Aijima R, Hoshino Y, et al. Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice. Stem Cell Res Ther 2015; 6(1): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Feng G, Zhang J, Feng X, et al. Runx2 modified dental pulp stem cells (DPSCs) enhance new bone formation during rapid distraction osteogenesis (DO). Differentiation 2016; 92(4): 195–203. [DOI] [PubMed] [Google Scholar]
- 69. Li Y, Zhao S, Nan X, et al. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther 2016; 7(1): 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hilkens P, Bronckaers A, Ratajczak J, et al. The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cell Int 2017; 2017: 2582080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kang KJ, Lee MS, Moon CW, et al. In vitro and in vivo dentinogenic efficacy of human dental pulp-derived cells induced by demineralized dentin matrix and HA-TCP. Stem Cell Int 2017; 2017: 2416254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seo BM, Sonoyama W, Yamaza T, et al. SHED repair critical-size calvarial defects in mice. Oral Dis 2008; 14(5): 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raposo-Amaral CE, Bueno DF, Almeida AB, et al. Is bone transplantation the gold standard for repair of alveolar bone defects? J Tissue Eng 2014; 5: 2041731413519352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gimbel M, Ashley RK, Sisodia M, et al. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg 2007; 18(4): 895–901. [DOI] [PubMed] [Google Scholar]
- 75. Heary RF, Schlenk RP, Sacchieri TA, et al. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery 2002; 50(3): 510–517. [DOI] [PubMed] [Google Scholar]
- 76. Priya VK, Reddy JS, Ramakrishna Y, et al. Post-surgical dentofacial deformities and dental treatment needs in cleft-lip-palate children: a clinical study. J Indian Soc Pedod Prev Dent 2011; 29(3): 229. [DOI] [PubMed] [Google Scholar]
- 77. Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration: current concepts and future directions. BMC Med 2011; 9(1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pinheiro CCG, de Pinho MC, Aranha ACC, et al. Low laser therapy: a strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from cleft lip and palate patients. Tissue Eng Part A. Epub ahead of print 2017. DOI: 10.1089/ten.TEA.2017.0115. [DOI] [PubMed] [Google Scholar]
- 79. Bakopoulou A, Leyhausen G, Volk J, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 2011; 56(7): 709–721. [DOI] [PubMed] [Google Scholar]
- 80. Laino G, Carinci F, Graziano A, et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg 2006; 17(3): 511–515. [DOI] [PubMed] [Google Scholar]
- 81. Pearce AI, Richards RG, Milz S, et al. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater 2007; 13(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 82. Scott MA, Levi B, Askarinam A, et al. Brief review of models of ectopic bone formation. Stem Cell Dev 2011; 21(5): 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]