Abstract
Growth and differentiation factor-15 (GDF-15) has been implicated in fibrosis, inflammation, and ventricular remodeling. The role of GDF-15 in the regulation of cardiac remodeling in idiopathic dilated cardiomyopathy (DCM) remains poorly defined. This study attempts to analyze the molecular interactions between GDF-15 and markers of fibrosis as well as its positive correlations with worsening functional capacity. The study population consisted of 24 DCM patients and 8 control subjects. All DCM patients had normal coronary angiographic studies. Plasma levels of GDF-15, matrix metalloproteinase-2 (MMP2), MMP3, MMP9, tissue inhibitor of MMP 1 (TIMP1), and soluble suppression of tumorigenicity-2 protein (sST2) were determined by enzyme-linked immunosorbent assays. Brain Natriuretic Peptide (BNP) was measured as per core laboratory protocol assay at Scott and White Memorial Hospital core laboratory. Correlation analysis was performed between GDF-15 and each of the MMPs—MMP2, MMP3, MMP9, and TIMP as well as New York Heart Association (NYHA) class and echocardiographic parameters (left ventricular ejection fraction (LVEF) and left ventricular internal dimension in diastole (LVIDd)). LVEF and LVIDd were obtained by two-dimensional echocardiography. The protocol was approved by Scott and White Memorial Hospital Institutional Review Board (S&W IRB). Correlation analysis of control versus all DCM patients showed a strong correlation of GDF-15 with TIMP1 (r = 0.83, p < 0.0001) and weaker correlation with MMP3 (r = 0.41, p = 0.011) and MMP2 (r = 0.47, p = 0.003). MMP9 showed poor correlation with GDF-15 (r = 0.3036, p = 0.046). GDF-15 correlated negatively with MMP2/TIMP1 ratio (r = −0.47, p = 0.006). sST2 correlated strongly with GDF-15 (r = 0.7, p < 0.0001). GDF-15 correlated negatively with LVEF (r = −0.49, p = 0.004) and positively with LVIDd (r = 0.58, p = 0.0006). GDF-15 showed significant positive correlation with NYHA functional class (r = 0.71, p < 0.00001) and BNP (r = 0.86, p < 0.00001). Significant associations of GDF-15 with MMPs, sST2, LVIDd, LVEF, and NYHA class reported here for the first time in nonischemic dilated hearts may open up new avenues of investigations to better understand molecular mechanisms controlling cardiac remodeling. This study is limited by its small size and needs validation in larger populations.
Keywords: sST2, GDF-15, matrix metalloproteinases, LVIDd, LVEF
Introduction
Dilated cardiomyopathy (DCM) is characterized by absence of secondary causes. Etiologies include hypertension, valvular dysfunction, coronary artery disease, myocarditis, and congenital pathophysiology. Approximately 50% of DCM patients carry an idiopathic etiology. In the absence of familial causes, idiopathic DCM (IDCM) is indistinguishable from the familial type.1,2
Growth and differentiation factor-15 (GDF-15) is a cytokine of the Transforming Growth Factor (TGF)- beta superfamily first cloned and sequenced in 1997 from an activated macrophage cell system.3 GDF-15 has been implicated in fibrosis, inflammation, and ventricular remodeling.4–6 Soluble suppression of tumorigenicity-2 protein (sST2) is the circulating counterpart of the cellular receptor Suppression of Tumorigenity Ligand (ST2L). It is usually produced by cardiomyocytes and endothelial cells along with its ligand interleukin-33 (IL-33) during cardiomyocyte injury. IL-33 binds to ST2L and promotes inhibition of myocardial hypertrophy, fibrosis, and remodeling under normal conditions. On the other hand in pathological conditions such as heart failure, there is a large increase in the circulating form (sST2) which competes with the cellular form ST2L for the same ligand IL-33. This leads to pathogenesis as the protective role of ST2L is disrupted as sST2 binding to IL-33 does not result in inhibition of myocyte hypertrophy and remodeling. sST2 has therefore been postulated in prognosis and risk stratification of chronic heart failure.7–9 Disruptions in the myocardial extracellular matrix (ECM) is the hallmark of structural pathology seen in heart failure; ECM is a dynamic unit. The synthesis and breakdown of ECM occurs via the actions of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs). It has been postulated that ECM plays an active role in myocardial remodeling and interstitial transport in addition to acting as a reservoir for growth factors and cytokines involved in remodeling making them important of therapeutic targets.10–12
The role of GDF-15 in regulation of cardiac remodeling in IDCM still remains poorly defined. This study was therefore undertaken to assess association of GDF-15 with metalloproteinases and sST2 which are molecular targets of fibrosis. The utility of these biomarkers in clinical evaluation of heart failure in IDCM will be investigated in this study from their correlation with New York heart Association (NYHA) class.
Materials and methods
The study population consisted of 24 DCM patients and 8 control subjects. This is a cross-sectional single center observational study. All DCM patients had normal angiographic studies. Plasma levels of GDF-15, sST2, MMP2, MMP3, MMP9, and TIMP1 were measured by enzyme-linked immunosorbent assays (ELISA). Blood was collected from patients in EDTA tubes, and BNP was measured as per core laboratory protocol assay at Scott and White Memorial Hospital core laboratory. Blood samples (5 ml) were collected from patients in standard EthyleneDiamineTetraacetic Acid (EDTA) tubes and centrifuged and plasma obtained was aliquoted into 100-ml Eppendorf tubes (Fisher Scientific, Hampton, USA) frozen at −800°C. The commercial ELISA kits used for GDF-15, MMP3, and sST2 were obtained from Aviscera Biosciences Inc (Santa Clara, California, USA). MMP2, MMP9, and TIMP1 ELISA kits were purchased from Ray Biotech Inc( Norcross, Georgia, USA). All analytes were quantitated using commercially available ELISA kits. They were all based on Sandwich ELISA principle wherein primary antibodies against the analyte were coated on a 96-well plate. Analyte was captured from serum samples by these primary antibodies, unbound matrix was washed off, and the bound analyte quantitated using a tagged detection antibody. Calibration curves were generated using serially diluted, pre-weighed analyte. The protocol followed was that obtained with the kit for use.
Left ventricular ejection fraction (LVEF) and left ventricular internal dimension in diastole (LVIDd) were obtained by two-dimensional (2-D) echocardiography. All echocardiographic examinations were done in the S&W echo laboratory The interoperator variation for scanning and reading was estimated at around 5%. Correlation analysis was performed between sST2 and GDF-15, MMP2, MMP3, MMP9, TIMP1, NYHA class, LVEF, and LVIDd using Microsoft Excel as well as VassarStats online program. The study protocol was approved by S&W IRB. Spearman rank-order correlation analyses wereperformed between GDF-15 and each of the MMPs—MMP2, MMP3, MMP9, TIMP1, and sST2 as well as NYHA class and echocardiographic parameters (LVEF and LVIDd). LVEF and LVIDd were obtained by 2-D echocardiography. The protocol was approved by S&W IRB. All statistical analyses were performed using the VassarStats online program. Statistical significance was set at p < 0.05. Tables show both the one-tailed and two-tailed p values. Individual patient values used for correlations and the corresponding scatter plots have been provided in the Online Supplementary Material.
Results
Characteristics of study population
Table 1 summarizes the characteristics of the study population. BNP levels, LVIDd, and ejection fraction were significantly different from the DCM versus controlled subjects. Male comprised of 84% of the DCM group, while male and female were almost equally represented in the control group. This unequal gender representation in the two groups was not intentional. Table 2 shows that all the five biomarkers analyzed such as GDF-15, MMP2, MMP3, MMP9, and TIMP1 were significantly upregulated in the DCM subjects.
Table 1.
Characteristics of study population.
| Baseline characteristic | Control (n = 9) | DCM (n = 23) | p Value |
|---|---|---|---|
| Age (years) | 58.3 ± 10.3 | 50.4 ± 34 | 0.18 |
| Gender | |||
| Male | 56% | 84% | Nd |
| Female | 44% | 16% | Nd |
| BNP (pg/ml) | 50 ± 38 | 1653 ± 45 | 0.0006* |
| LVIDd (cm) | 4.5 ± 0.3 | 6.5 ± 0.5 | <0.0001* |
| LVEF (%) | 63.1 ± 7.4 | 21.6 ± 8.4 | <0.0001* |
| Creatinine (mg/dl) | 0.87 ± 0.12 | 1.6 ± 2.1 | 0.28 |
DCM: dilated cardiomyopathy; LVIDd: left ventricular internal dimension in diastole; LVEF: left ventricular ejection fraction; Nd: Not done.
*p < 0.05 was considered significant.
Table 2.
Biomarker levels in control and DCM populations.
| GDF-15 (pg/ml) | MMP2 (ng/ml) | MMP9 (ng/ml) | TIMP1 (ng/ml) | MMP3 (ng/ml) | |
|---|---|---|---|---|---|
| Control (n = 8) | 516 ± 146 | 626 ± 100 | 592 ± 210 | 280 ± 23 | 5.5 ± 2 |
| DCM (n = 24) | 3203 ± 1354 | 848 ± 167 | 976 ± 238 | 565 ± 173 | 9.7 ± 2.8 |
| One-tailed pvalue | 0.01 | 0.01 | 0.006 | 0.001 | 0.006 |
| Two-tailed p value | 0.02 | 0.02 | 0.013 | 0.002 | 0.013 |
GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; TIMP1: tissue inhibitor of MMP 1; DCM: dilated cardiomyopathy; sST2: soluble suppression of tumorigenicity-2 protein.
Correlation of GDF-15 with MMPs
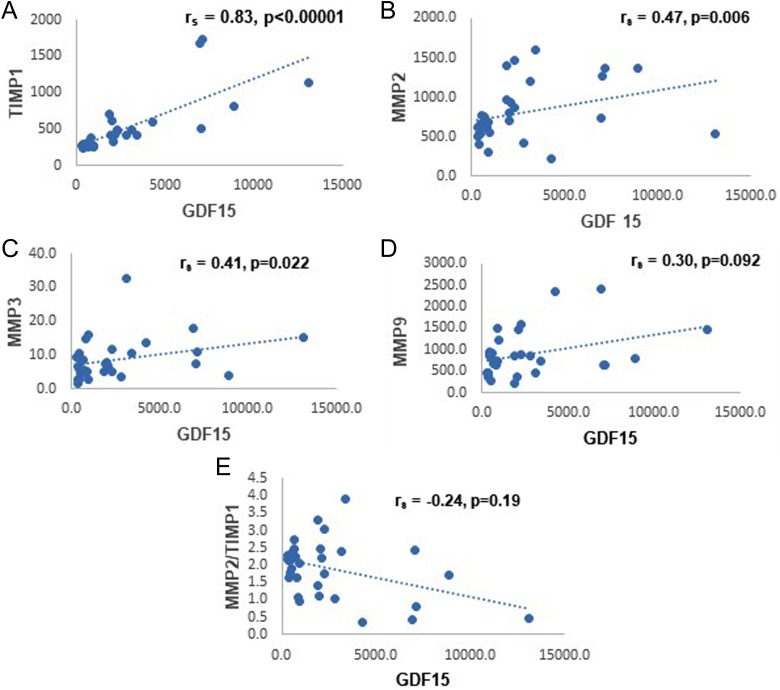
Correlation of GDF-15 with MMPs is shown in Table 3. Correlation analysis of control versus DCM patients showed a strong correlation of GDF-15 with TIMP1 (r = 0.83, p < 0.0001) and weaker correlation with MMP3 (r = 0.41, p = 0.011) and MMP2 (r = 0.47, p = 0.003). Correlation with MMP9 was only borderline significant (r = 0.3036, p = 0.046). GDF-15 correlated negatively with MMP2/TIMP1 ratio (r = −0.24, p = 0.09) but was not statistically significant at p < 0.05. Figure 1(a) to (e) shows the scatter plots for the correlations between GDF-15 and TIMP1, MMP2, MMP3, MMP9, and MMP2/TIMP1 ratio, respectively.
Table 3.
Correlation of GDF-15 with MMPs.
| Correlation coefficient (rs) | p Value (one-tailed) | p Value (two-tailed) | |
|---|---|---|---|
| TIMP1 | 0.83 | p < 0.00001 | p < 0.00001 |
| MMP2 | 0.47 | p = 0.003 | p = 0.006 |
| MMP3 | 0.41 | p = 0.011 | p = 0.022 |
| MMP9 | 0.304 | p = 0.046 | p = 0.092 |
| MMP2/TIMP1 | −0.24 | p = 0.09 | p = 0.19 |
GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; TIMP1: tissue inhibitor of MMP 1.
Figure 1.
The scatter plots depicting the correlations between GDF-15 and TIMP1, MMP2, MMP3, MMP9, and MMP2/TIMP1 ratio. GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; TIMP1: tissue inhibitor of MMP 1.
Correlation of GDF-15 with worsening function and echocardiographic parameters
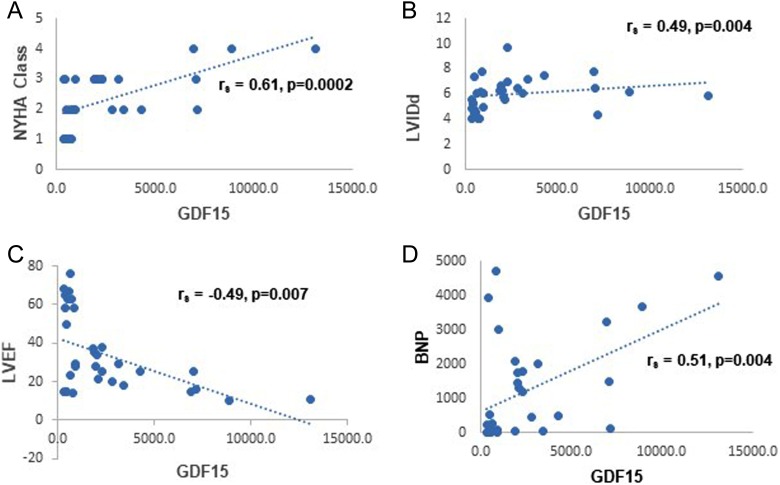
Table 4 shows GDF-15 correlated negatively with LVEF (r = −0.49, p = 0.003) and positively with LVIDd (r = 0.5, p = 0.002). GDF-15 showed significant positive correlation with NYHA functional class (r = 0.71, p < 0.00001) and BNP (r = 0.51, p = 0.002). Figure 2(a) to (d) shows the scatter plots for the correlations between GDF-15 and NYHA class, LVIDd, LVEF, and BNP, respectively.
Table 4.
Correlation of GDF-15 with NYHA class, LVIDd, LVEF, and BNP.
| Correlation coefficient (rs) | p Value (one-tailed) | p Value (two-tailed) | |
|---|---|---|---|
| NYHA class | 0.7 | p = 0.0001 | p = 0.0002 |
| LVIDd | 0.5 | p = 0.002 | p = 0.004 |
| LVEF | −0.49 | p = 0.003 | p = 0.007 |
| BNP | 0.51 | p = 0.002 | p = 0.004 |
GDF: growth and differentiation factor-15; NYHA: New York Heart Association; LVIDd: left ventricular internal dimension in diastole; LVEF: left ventricular ejection fraction.
Figure 2.
The scatter plots depicting the correlations between GDF-15 and NYHA class, LVIDd, LVEF, and BNP. GDF: growth and differentiation factor-15; NYHA: New York Heart Association; LVIDd: left ventricular internal dimension in diastole; LVEF: left ventricular ejection fraction.
Correlation of sST2 with GDF-15, MMPs, worsening function, and echocardiographic parameters
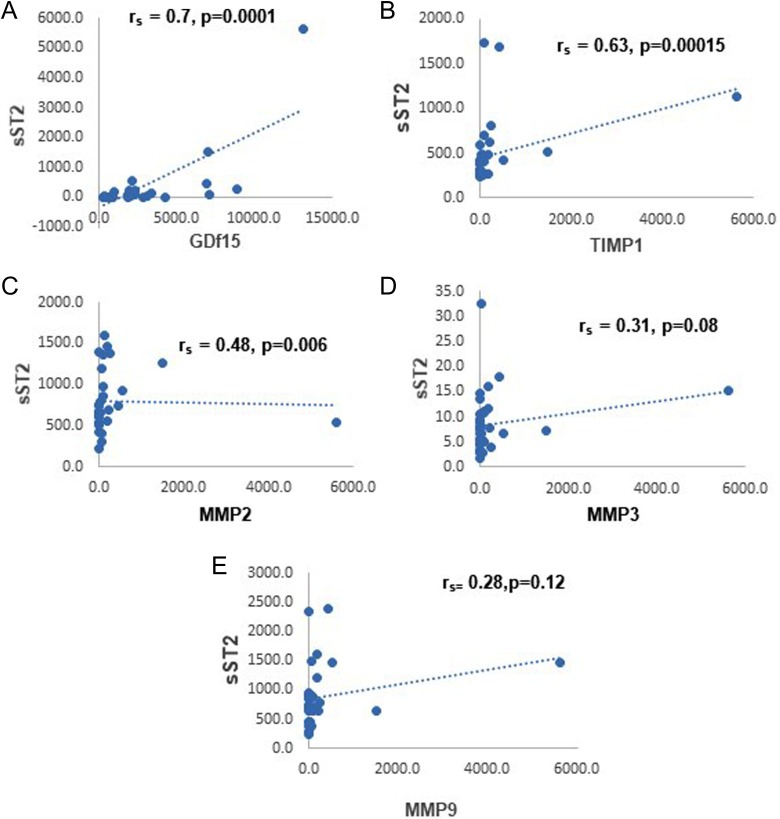
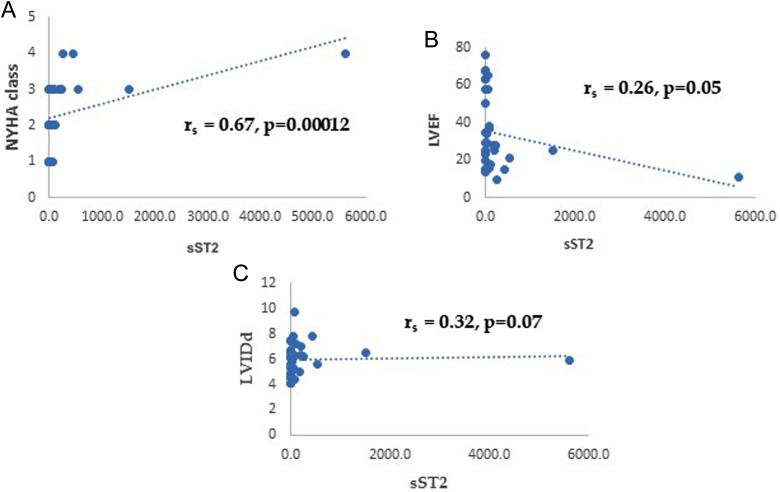
Table 5 shows correlation of sST2 with GDF-15 and MMPs. Plasma sST2 correlated strongly with GDF-15 (r = 0.7, p = 0.000005) and TIMP1 (r = 0.68, p = 0.00007). Moderate correlation was noted with MMP2 (r = 0.48, p = 0.003) and weaker correlation with MMP3 (r = 0.31, p = 0.04). MMP9 showed poor correlation with no essential statistical significance (r = 0.31, p = 0.06). Figure 3(a) to (e) shows the scatter plots for the correlations between sST2 and GDF-15, TIMP1, MMP2, MMP3, and MMP9, respectively. Interestingly, sST2 correlated significantly with NYHA class (r = 0.31, p = 0.000012) but not with LVEF or LVIDd as shown in Table 6. Figure 4(a) to (c) shows the scatter plots for the correlations between sST2 and NYHA class, LVEF, and LVIDd, respectively.
Table 5.
Correlation of sST2 with GDF-15 and MMPs.
| Correlation coefficient (rs) | p Value (one-tailed) | p Value (two-tailed) | |
|---|---|---|---|
| GDF-15 | 0.7 | p = 0.000005 | p = 0.00001 |
| TIMP1 | 0.63 | p = 0.00007 | p = 0.00015 |
| MMP2 | 0.48 | p = 0.003 | p = 0.006 |
| MMP3 | 0.31 | p = 0.04 | p = 0.08 |
| MMP9 | 0.31 | p = 0.06 | p = 0.12 |
GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; TIMP1: tissue inhibitor of MMP 1; sST2: soluble suppression of tumorigenicity-2 protein.
Figure 3.
The scatter plots depicting the correlations between sST2 and GDF-15, TIMP1, MMP2, MMP3, and MMP9. sST2: soluble suppression of tumorigenicity-2 protein; GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; TIMP1: tissue inhibitor of MMP 1.
Table 6.
Correlation of sST2 with NYHA class, LVEF, and LVIDd.
| Correlation coefficient (rs) | p Value (one-tailed) | p Value (two-tailed) | |
|---|---|---|---|
| NYHA class | 0.67 | p = 0.000012 | p = 0.000024 |
| LVEF | 0.26 | p = 0.05 | p = 0.1 |
| LVIDd | 0.32 | p = 0.03 | p = −0.07 |
sST2: soluble suppression of tumorigenicity-2 protein; NYHA: New York Heart Association; LVIDd: left ventricular internal dimension in diastole; LVEF: left ventricular ejection fraction.
Figure 4.
The scatter plots depicting the correlations between sST2 and NYHA class, LVEF, and LVIDd. sST2: soluble suppression of tumorigenicity-2 protein; NYHA: New York Heart Association; LVIDd: left ventricular internal dimension in diastole; LVEF: left ventricular ejection fraction.
Discussion
GDF-15 has been implicated in fibrosis, inflammation, and ventricular remodeling. The role of GDF-15 in regulation of cardiac remodeling in IDCM is not well-defined. This study has attempted to address the association of GDF-15 with sST2 and metalloproteinases, which are possible molecular targets of regulation in pathogenesis of DCM. Pathophysiology of IDCM at the molecular and cellular levels remains poorly understood. The correlations of sST2 with GDF-15 and MMPs show significant associations between these molecules.
In this study of IDCM patients, GDF-15 appears to have significant associations with markers of fibrosis and remodeling such as soluble ST2 and MMPs. Additionally, in our study, GDF-15 appears to significantly correlate with structural (echocardiographic) and functional (NYHA class) parameters of cardiac function. Increases in GDF-15 in heart failure and its negative correlations with LVEF as well as positive correlations with LVIDd suggest that GDF-15 possibly influences structure/functional interactions affecting ventricular remodeling in these patients and that such influences could influence mortality. However, most of the existing studies on GDF-15 upregulation are in heart failure patient populations with heterogeneous etiologies.13,14
A strong correlation between improved ejection fraction and decreased mortality has been demonstrated in a recent meta-analysis.15 In another study of an acute heart failure population of mixed etiologies, increases in GDF-15 were associated with a greater risk of 60-day heart failure rehospitalizations/cardiovascular death as well as cardiac death at 180 days.16 In a meta-analysis of a heterogeneous heart failure population, elevated GDF-15 levels were associated with increased risk of mortality.17 Further studies are needed for better characterization of GDF-15 use in prognostication in heart failure populations of specific etiologies.
This study focuses on a single group of well-characterized IDCM patients at a single center. Patients with DCM in this study had no coronary artery disease as evidenced by coronary angiography. We present here some findings for the first time in IDCM population which will possibly open up pathways of investigation to further knowledge on role of GDF-15 and markers of fibrosis such as soluble ST2 and the MMPs on ventricular remodeling in this population. GDF-15 correlates significantly and positively with NYHA class and worsening functionality. GDF-15 negatively correlates with MMP2/TIMP1. This finding sheds light on effect of GDF-15 on metalloproteinase metabolism, therefore suggesting a role in ventricular fibrosis and remodeling. It is also noteworthy that GDF-15 strongly correlates with BNP suggesting that GDF-15 is released analogous to the natriuretic peptides in response to cardiac injury signaling a possible protective response.
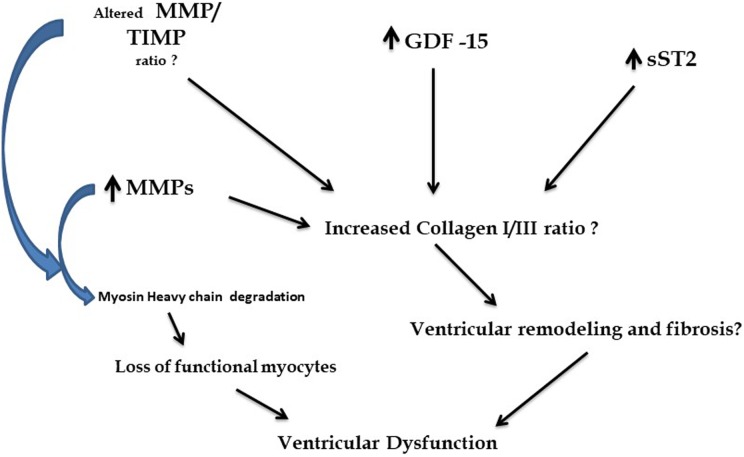
Figure 5 shows a possible effect of interactions of GDF-15, sST2, and MMPs on alterations in collagen I/III ratios, MMP/TIMP ratios, and increased myosin heavy chain degradation; all of which could contribute to loss of myocytes/ventricular fibrosis/remodeling.18 GDF-15 has also been implicated in anti-apoptosis as well as induction of hypertrophy in ventricular myocytes19 further contributing to ventricular remodeling.
Figure 5.
A summary of possible interactions of GDF-15 with soluble ST2 and MMPs in DCM. GDF: growth and differentiation factor-15; MMP: matrix metalloproteinase; DCM: dilated cardiomyopathy.
A delicate balance between MMPs and TIMPs has always been postulated in preventing adverse remodeling, but circulating levels of TIMP1 have been shown in human DCM to be elevated.20 The levels of circulating biomarkers appear to be different in their concentrations and trends as compared to all the literature on tissue samples where MMPs and TIMPs seem to hold an inverse relationship.20,21 Many factors such as cytokines, hormones and growth factors and stage of DCM as well as extent of upregulation of the neurohormonal system may operate synergistically to regulate the levels of MMPs and TIMPs in the myocardial tissue versus in circulation. It is unclear if the extent of inflammation also influences the level balance between MMPs and TIMPs in the remodeling processes.20,21 Hence, differences noted in this small study will need to be further investigated in different stages of DCM progression to determine whether changes in MMPS/TIMPs would change with disease severity.
BNP is known to counteract the hemodynamic stress by inducing vasodilation, natriuresis, and diuresis. The cardioprotective properties of ST2 and IL-33 are also well established. The protective effects in the heart of GDF-15 occur through inhibition of apoptosis, hypertrophy, and adverse remodeling in the injured heart. Correlations between GDF-15, BNP, and sST2 serve to address the complicated interactions that may exist in myocardial stress.22–25 It is however important to note that protective roles of these molecules may vary in efficiency as the disease progresses; hence, further studies are warranted in this domain.
Limitations
The study is limited by its small population size and the fact that GDF-15 and all the biomarkers described here were only assayed in circulation. Site of production of GDF-15 in heart failure of different etiologies still remains ambiguous.25,26
Conclusions
This study shows for the first time that the strongest relationships were between GDF-15 and NYHA class as well as between GDF15 and TIMP, sST2 with GDF-15, and with TIMP in IDCM patients. This investigation opens the pathway for newer investigations on interactions between these molecular markers of fibrosis in the process of ventricular remodeling and dysfunction. Further studies are required to precisely define the molecular interactions between GDF-15, sST2, and MMPs in ventricular remodeling as well as influencing worsening functional capacity in DCM.
Future directions
Precise studies to elucidate the extent of changes in MMPs, TIMPs, and biomarkers of heart failure such as GDF-15 and soluble standard deviation in relation to BNP need to be undertaken. This is especially needed in the context of disease progression in order to help with prognostication and development of therapeutics. Protective roles of BNP, GDF-15, and ST2 should be studied in the context of worsening heart failure and disease progression. Interactions between GDF-15, BNP, and soluble ST2 need to be further investigated in order to define molecular mechanisms that work in synergy when subjected to myocardial strain and remodeling. Another area of importance is to investigate the use of these biomarkers and heart failure to monitor therapy and progression of disease. Further studies would help in developing a panel of biomarkers which can be used to risk stratify as well as for the development of therapeutics as interactions between biomarkers define the convergence divergence of complex biochemical pathways. This is especially important as heart failure is a complex syndrome where interactions between inflammation, myocardial strain, and remodeling contribute to the pathophysiology and progression of the disease.
Supplementary Material
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Division of Cardiology at Scott and White Memorial Hospital, Temple, Texas, USA, under an internal grant.
Supplemental material: Supplementary material for this article is available online.
References
- 1. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 2. Mestroni L, Brun F, Spezzacatene A, et al. Genetic causes of dilated cardiomyopathy. Prog Pediatr Cardiol 2014; 37: 13–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 1997; 94: 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jankovic-Tomasevic R, Pavlovic SU, Jevtovic-Stoimenov T, et al. Prognostic utility of biomarker growth differentiation factor-15 in patients with acute decompensated heart failure. Acta Cardiol 2016; 71: 587–595. [DOI] [PubMed] [Google Scholar]
- 5. Boulogne M, Sadoune M, Launay JM, et al. Inflammation versus mechanical stretch biomarkers over time in acutely decompensated heart failure with reduced ejection fraction. Int J Cardiol 2017; 226: 53–59. [DOI] [PubMed] [Google Scholar]
- 6. George M, Jena A, Srivatsan V, et al. GDF 15: a novel biomarker in the offing for heart failure. Curr Cardiol Rev 2016; 12: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinning C, Kempf T, Schwarzl M, et al. Biomarkers for characterization of heart failure—distinction of heart failure with preserved and reduced ejection fraction. Int J Cardiol 2017; 227: 272–277. [DOI] [PubMed] [Google Scholar]
- 8. Aimo A, Vergaro G, Passino C, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail 2017; 5: 287–296. [DOI] [PubMed] [Google Scholar]
- 9. Xanthakis V, Larson MG, Wollert KC, et al. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. J Am Heart Assoc 2013; 2: e000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takawale A, Sakamuri SS,, Kassiri Z. Extracellular matrix communication and turnover in cardiac physiology and pathology. Compr Physiol 2015; 5: 687–919. [DOI] [PubMed] [Google Scholar]
- 11. Müller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 2012; 17: 395–409. [DOI] [PubMed] [Google Scholar]
- 12. Müller AL, Freed D, Hryshko L, et al. Implications of protease activation in cardiac dysfunction and development of genetic cardiomyopathy in hamsters. Can J Physiol Pharmacol 2012; 90: 995–1004. [DOI] [PubMed] [Google Scholar]
- 13. Santhanakrishnan R, Chong JP, Ng TP, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2012; 14: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 14. Chan MM, Santhanakrishnan R, Chong JP, et al. Growth differentiation factor 15 in heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2016; 18: 81–88. [DOI] [PubMed] [Google Scholar]
- 15. Kramer DG, Trikalinos TA, Kent DM, et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010; 56: 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cotter G, Voors AA, Prescott MF, et al. Growth differentiation factor 15 (GDF-15) in patients admitted for acute heart failure: results from the RELAX-AHF study. Eur J Heart Fail 2015; 17: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 17. Zeng X, Li L, Wen H, et al. Growth-differentiation factor 15 as a predictor of mortality in patients with heart failure: a meta-analysis. J Cardiovasc Med (Hagerstown) 2017; 18: 53–59. [DOI] [PubMed] [Google Scholar]
- 18. Rouet-Benzineb P, Buhler JM, Dreyfus P, et al. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation. Eur J Heart Fail 1999; 1: 337–352. [DOI] [PubMed] [Google Scholar]
- 19. Heger J, Schiegnitz E, von Waldthausen D, et al. Growth differentiation factor 15 acts anti-apoptotic and pro-hypertrophic in adult cardiomyocytes. J Cell Physiol 2010; 224: 120–126. [DOI] [PubMed] [Google Scholar]
- 20. Schwartzkopff B, Fassbach M, Pelzer B, et al. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur J Heart Fail 2002; 4: 439–444. [DOI] [PubMed] [Google Scholar]
- 21. Picard F, Brehm M, Fassbach M, et al. Increased cardiac mRNA expression of matrix metalloproteinase-1 (MMP-1) and its inhibitor (TIMP-1) in DCM patients. Clin Res Cardiol 2006. May; 95: 261–269. [DOI] [PubMed] [Google Scholar]
- 22. Nakao K, Ogawa Y, Suga S, et al. Molecular biology and biochemistry of the natriuretic peptide system. II: natriuretic peptide receptors. J Hypertens 1992; 10: 1111–1114. [DOI] [PubMed] [Google Scholar]
- 23. Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117: 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iqbal N, Wentworth B, Choudhary R, et al. Cardiac biomarkers: new tools for heart failure management. Cardiovasc Diagn Ther 2012; 2: 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006; 98: 351–360. [DOI] [PubMed] [Google Scholar]
- 26. Lok SI, Winkens B, Goldschmeding R, et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail 2012; 14: 1249–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.