Supplemental Digital Content is available in the text.
Keywords: canagliflozin, clinical trial, diabetes mellitus, primary prevention, secondary prevention
Abstract
Background:
Canagliflozin is a sodium glucose cotransporter 2 inhibitor that significantly reduces the composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke in patients with type 2 diabetes mellitus and elevated cardiovascular risk. The comparative effects among participants with and without a history of cardiovascular disease (secondary versus primary prevention) were prespecified for evaluation.
Methods:
The CANVAS Program (Canagliflozin Cardiovascular Assessment Study) randomly assigned 10 142 participants with type 2 diabetes mellitus to canagliflozin or placebo. The primary prevention cohort comprised individuals ≥50 years of age with ≥2 risk factors for cardiovascular events but with no prior cardiovascular event, and the secondary prevention cohort comprised individuals ≥30 years of age with a prior cardiovascular event. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included heart failure hospitalization and a renal composite (40% reduction in estimated glomerular filtration rate, renal replacement therapy, or renal death).
Results:
Primary prevention participants (N=3486; 34%) were younger (63 versus 64 years of age), were more often female (45% versus 31%), and had a longer duration of diabetes mellitus (14 versus 13 years) compared with secondary prevention participants (N=6656; 66%). The primary end point event rate was higher in the secondary prevention group compared with the primary prevention group (36.9 versus 15.7/1000 patient-years, P<0.001). In the total cohort, the primary end point was reduced with canagliflozin compared with placebo (26.9 versus 31.5/1000 patient-years; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75–0.97; P<0.001 for noninferiority, P=0.02 for superiority) with no statistical evidence of heterogeneity (interaction P value=0.18) between the primary (HR, 0.98; 95% CI, 0.74–1.30) and secondary prevention (HR, 0.82; 95% CI, 0.72–0.95) cohorts. Renal outcomes (HR, 0.59; 95% CI, 0.44–0.79 versus HR, 0.63; 95% CI, 0.39–1.02; interaction P value=0.73) and heart failure hospitalization (HR, 0.68; 95% CI, 0.51–0.90 versus HR, 0.64; 95% CI, 0.35–1.15; interaction P value=0.91) were similarly reduced in the secondary and primary prevention cohorts, respectively. Lower extremity amputations were similarly increased in the secondary and primary prevention cohorts (HR, 2.07; 95% CI, 1.43–3.00 versus HR, 1.52; 95% CI, 0.70–3.29; interaction P value=0.63).
Conclusions:
Patients with type 2 diabetes mellitus and prior cardiovascular events had higher rates of cardiovascular outcomes compared with the primary prevention patients. Canagliflozin reduced cardiovascular and renal outcomes with no statistical evidence of heterogeneity of the treatment effect across the primary and secondary prevention groups. Additional studies will provide further insights into the effects of canagliflozin in these patient populations.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01032629 and NCT01989754.
Editorial, see p 335
Clinical Perspective.
What Is New?
Canagliflozin reduces cardiovascular and renal outcomes in patients with type 2 diabetes mellitus.
No statistical evidence of heterogeneity was observed for the effects of canagliflozin on cardiovascular and renal outcomes in participants with prior cardiovascular events (secondary prevention) and without prior cardiovascular events but at elevated risk (primary prevention), although the power to detect differences was limited.
Lower extremity amputations were uncommon but increased with canagliflozin without statistical evidence of heterogeneity between the secondary and primary prevention cohorts.
What Are the Clinical Implications?
Patients with type 2 diabetes mellitus are at high risk for cardiovascular and renal outcomes.
Canagliflozin should be considered to manage diabetes mellitus in patients at high risk for cardiovascular events to reduce cardiovascular and renal outcomes.
Further study of canagliflozin in patients with type 2 diabetes mellitus without prior cardiac events is needed to better define the benefits on cardiovascular death, myocardial infarction, or stroke outcomes.
Caution should be used in patients at risk for amputations.
Patients with type 2 diabetes mellitus suffer substantial morbidity and mortality from cardiovascular and renal disease.1,2 Current drug therapies and lifestyle interventions are not adequate, with elevated relative and absolute risks of serious disease outcomes observed for both primary and secondary prevention cohorts. Although the largest absolute benefits of interventions for individual patients are achieved among those with established disease (secondary prevention), the large number of patients with diabetes mellitus without overt cardiovascular disease (primary prevention) makes knowledge about the effects of therapies on first events an additional priority.
The CANVAS Program (Canagliflozin Cardiovascular Assessment Study) was designed to assess the cardiovascular safety and efficacy of canagliflozin in a broad range of patients with type 2 diabetes mellitus.3–6 The main results demonstrated that canagliflozin reduced the relative risk of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke by 14% (P=0.02 for superiority) compared with placebo.6 In addition, hospitalized heart failure and serious declines in renal function were reduced by 33% and 40%, respectively.6 An unanticipated ≈2-fold increase in the risk of amputation was also observed.
By design, the CANVAS Program enrolled patients with and without prior cardiovascular disease to provide insight into the effects of canagliflozin in the primary and secondary prevention settings. In the analyses presented here, the efficacy and safety of canagliflozin are described separately for the primary and secondary prevention cohorts enrolled in the CANVAS Program.
Methods
Data from the CANVAS Program will be made available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and European Union and the study has been completed for 18 months. The trial protocols and statistical analysis plans were published along with the primary CANVAS Program article.6
The design of the CANVAS Program has been published.3–6 In brief, the CANVAS Program was a double-blind comparison of the effects of canagliflozin versus placebo made by combining data from 2 large-scale trials. The CANVAS Program was sponsored by Janssen Research & Development, LLC, and was conducted as a partnership between Janssen Research & Development, LLC, an academic Steering Committee (Appendix in the online-only Data Supplement), and an Academic Research Organization, George Clinical. The first draft of this article was written by the first author, with all coauthors contributing comments and approving the final draft for submission. The authors had access to all the data and ensured the accuracy of the analyses. All participants provided informed consent, and ethics approval was obtained for every center.
Participants
The criteria for inclusion and exclusion have been previously published.3–6 Participants were men and women with type 2 diabetes mellitus (glycohemoglobin ≥7.0% and ≤10.5%) who were either ≥30 years of age with a history of symptomatic atherosclerotic cardiovascular events defined as stroke, MI, hospitalization for unstable angina, coronary artery bypass grafting, percutaneous coronary intervention, peripheral revascularization (surgical or percutaneous), and symptomatic with documented hemodynamically significant carotid or peripheral vascular disease or amputation secondary to vascular disease (secondary prevention cohort); or ≥50 years of age with no prior cardiovascular events but with ≥2 of the following cardiovascular risk factors: duration of diabetes mellitus ≥10 years, systolic blood pressure >140 mm Hg on ≥1 antihypertensive agents, current smoker, microalbuminuria or macroalbuminuria, or high-density lipoprotein cholesterol <1 mmol/L (primary prevention cohort). The primary and secondary prevention participants were categorized based on a review of their medical histories.
Randomized Treatment
Randomization was performed through a central web-based system and used a computer-generated randomization schedule. Participants were assigned to canagliflozin or placebo, and use of other background therapy for glycemic management and other risk factor control was according to best practice instituted in line with local guidelines. By design, the secondary prevention cohort was to be ≈70% (minimum of 60%) of all patients.
Follow-Up
Follow-up after enrollment was scheduled quarterly for 1 year and then every 6 months until the end of the study. Every follow-up included inquiry about primary and secondary outcome events and serious adverse events. Serum creatinine measurement with estimated glomerular filtration rate was performed at least every 26 weeks.
Outcomes
The efficacy outcomes for these analyses were the composite of cardiovascular mortality, nonfatal MI, or nonfatal stroke; the individual components of the composite; hospitalization for heart failure; and all-cause mortality. Effects on the kidney were assessed using a composite renal outcome comprising a 40% reduction in estimated glomerular filtration rate, requirement for renal replacement therapy, or renal death. The safety events of interest were adverse events attributable to genital infection, urinary tract infection, volume depletion events, hypoglycemia, diabetic ketoacidosis, acute pancreatitis, renal adverse events, thromboembolism, cancer, fracture, and lower extremity amputation.
All major cardiovascular events, renal outcomes, and deaths as well as selected safety outcomes (diabetic ketoacidosis, acute pancreatitis, and fracture) were assessed by Endpoint Adjudication Committees (Appendix in the online-only Data Supplement) blinded to therapy. The definitions that were used for the clinical events have been published.3–6
Statistical Analysis
Evaluation of outcomes in the primary and secondary prevention participants was prespecified. Rates of cardiovascular disease, kidney disease, death outcomes, and selected adverse events were estimated for active and placebo groups combined. All analyses of the effects of canagliflozin compared with placebo on cardiovascular and renal outcomes were based on the intention-to-treat principle using all follow-up time (on or off study treatment) for all randomized participants. Safety outcomes were analyzed using an on-treatment approach (based on patient time and events accrued while on study drug or within 30 days of study drug discontinuation) except for diabetic ketoacidosis, fracture, cancer, and amputation outcomes, which were assessed using all follow-up time (on or off study treatment).
Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated for participants assigned to canagliflozin versus participants assigned to placebo separately for the primary and secondary prevention cohorts. Cardiovascular, death, and safety outcomes were analyzed using a stratified Cox proportional hazards regression model, with treatment as the exploratory variable and study as the stratification factor. Renal outcomes were analyzed using a stratified Cox proportional hazards model with treatment and the stage of baseline chronic kidney disease measured by estimated glomerular filtration rate (<60 or ≥60 mL/min/1.73 m2) as the exploratory variables and study as the stratification factor. Homogeneity of treatment effects across the primary and secondary prevention groups was examined via a test for the treatment-by-prevention interaction by adding this term and the prevention cohort as covariates to the respective Cox proportional hazards model. The risk differences were calculated by subtracting the incidence rate (per 1000 patient-years) with placebo from the incidence rate with canagliflozin and multiplying by 5 years. Similarly, the CI was estimated by multiplying the lower and upper CI values by 5 years. Analyses were undertaken using SAS version 9.2 and SAS Enterprise Guide version 7.11. Analyses were performed by statisticians at Janssen with verification by a statistician at George Clinical.
Results
Overall, 10 142 participants at 667 centers in 30 countries were enrolled in the CANVAS Program.6 Mean follow-up was 188 weeks. Discontinuation of the study drug was similar with placebo and canagliflozin in the overall population (30% versus 29%) and in the secondary prevention (29% versus 30%) and primary prevention cohorts (31% versus 28%). Vital status was available for 99.6% of patients.6
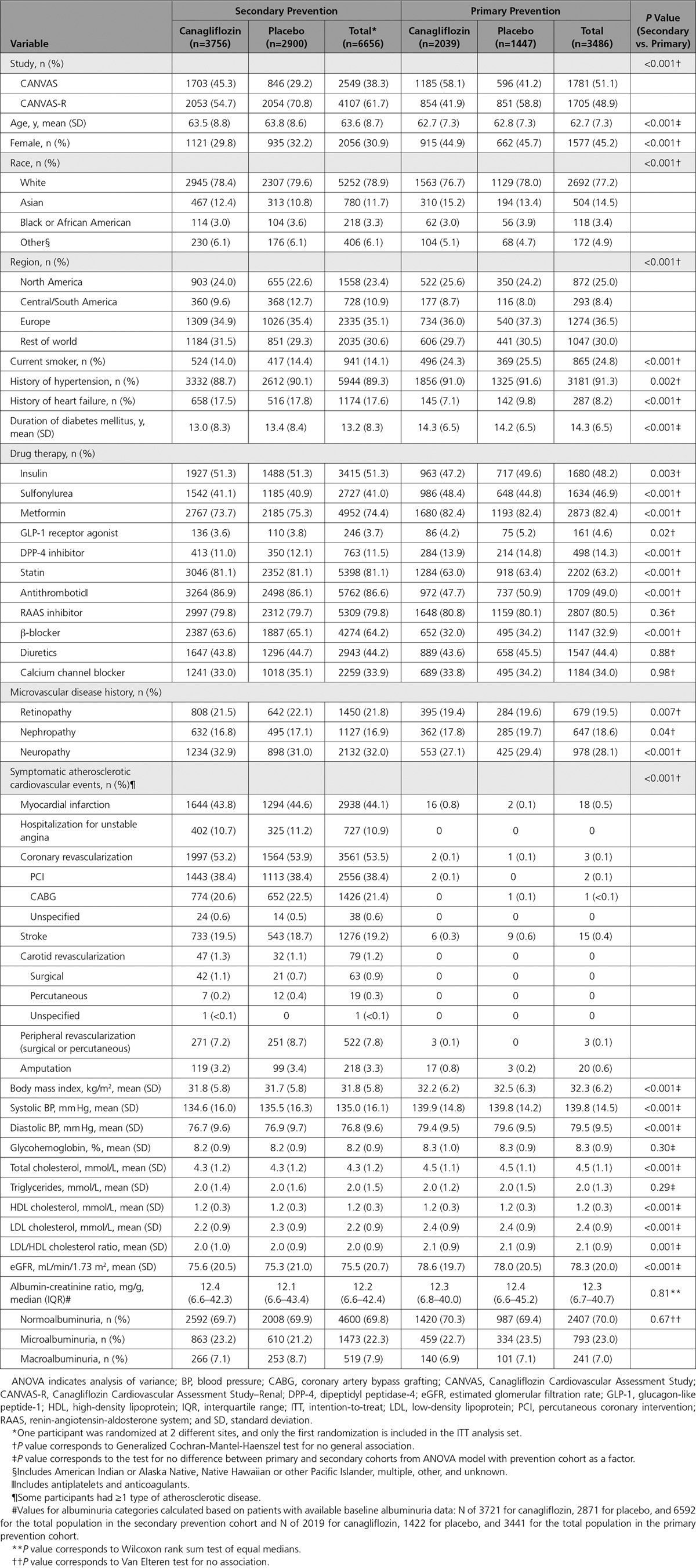
Primary prevention participants (N = 3486; 34%) were younger (63 versus 64 years), were more often female (45% versus 31%), and had longer duration of diabetes mellitus (14 versus 13 years) compared with secondary prevention participants (N = 6656; 66%). Participants in the secondary prevention group had higher use of common cardiac medications, including statins, β-blockers, and antiplatelet agents, as well as insulin, but lower use of oral antihyperglycemic agents (Table 1). Within each of the primary and secondary prevention cohorts, participant characteristics were all well balanced across canagliflozin and placebo groups (Table 1).
Table 1.
Baseline Characteristics of Primary and Secondary Prevention Cohorts in the CANVAS Program
Risks of Cardiovascular, Renal, Death, and Safety Outcomes in the Primary and Secondary Prevention Cohorts
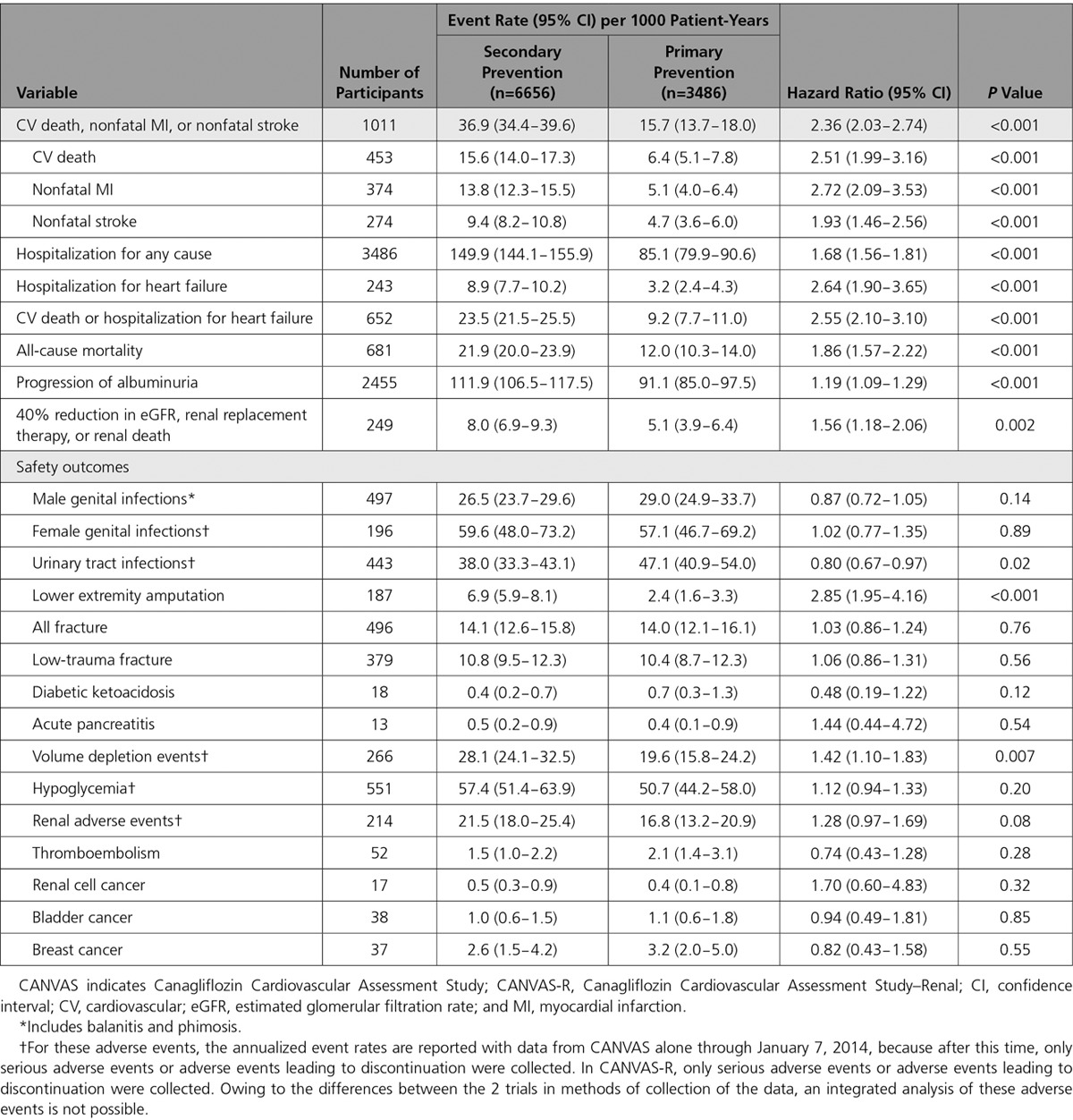
Secondary prevention participants had higher rates of the primary cardiovascular composite outcome compared with the primary prevention participants (HR, 2.36; 95% CI, 2.03-2.74; P<0.001) (Table 2). There were also more hospitalizations for heart failure (HR, 2.64; 95% CI, 1.90-3.65), more deaths (HR, 1.86; 95% CI, 1.57-2.22), and more of the composite renal outcome (HR, 1.56; 95% CI, 1.18-2.06) in the secondary prevention compared with the primary prevention group. Rates of safety outcomes were not different except for lower extremity amputation (HR, 2.85; 95% CI, 1.95-4.16) and volume depletion events (HR, 1.42; 95% CI, 1.10-1.83), which were more frequent among the secondary prevention participants, and urinary tract infection, which was less common in the secondary prevention participants (HR, 0.81; 95% CI, 0.67-0.97).
Table 2.
Rates of Events for Cardiovascular Disease, Kidney Disease, Fatal Outcomes, and Safety Events for the Primary and Secondary Prevention Cohorts in the CANVAS Program in the Active and Control Groups Combined
Effects of Canagliflozin on Cardiovascular and Renal Outcomes in Primary and Secondary Prevention Cohorts
The primary end point was reduced with canagliflozin compared with placebo (26.9 versus 31.5/1000 patient-years; HR, 0.86; 95% CI, 0.75-0.97; P<0.001 for noninferiority, P=0.02 for superiority) in the total cohort, with no statistical evidence of heterogeneity (P=0.18) between the primary (HR, 0.98; 95% CI, 0.74-1.30) and secondary (HR, 0.82; 95% CI, 0.72-0.95) prevention groups (Figure 1). Likewise, no statistical evidence of heterogeneity was found between the primary and secondary prevention cohorts for hospitalization for heart failure, all-cause mortality, and the composite renal outcome (all P values for homogeneity ≥0.10) (Figure 1). Kaplan-Meier curves for the composite cardiovascular outcome, cardiovascular death, hospitalization for heart failure, all-cause mortality, and the composite renal outcome are shown in Figure 2.
Figure 1.
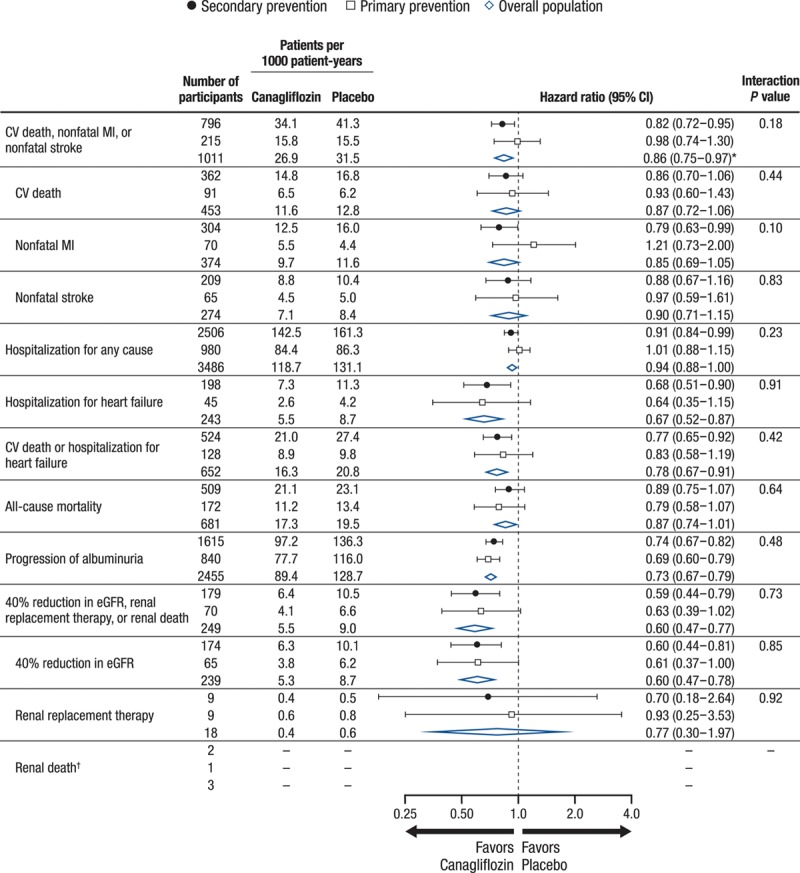
Comparative effects of canagliflozin and placebo on cardiovascular, kidney, and mortality outcomes in the total population and the primary and secondary prevention cohorts in the CANVAS Program. Hazard ratios and 95% CIs were estimated using Cox regression models, with stratification by trial for all canagliflozin groups combined vs. placebo. CANVAS indicates Canagliflozin Cardiovascular Assessment Study; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; and MI, myocardial infarction. *P<0.001 for noninferiority and P=0.02 for superiority for the primary outcome of CV death, nonfatal MI, or nonfatal stroke in the overall population. †Incidence rates and HRs not calculated because of the small number of events.
Figure 2.
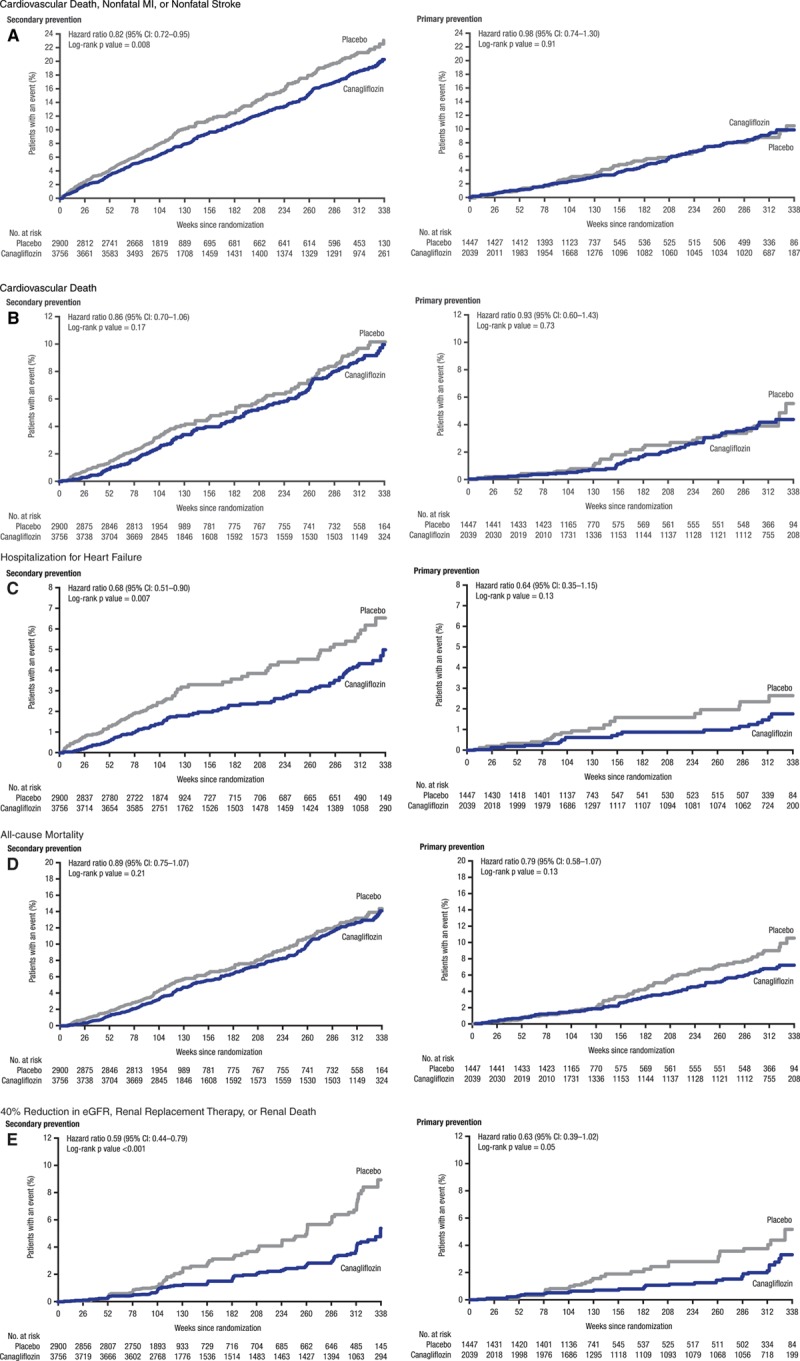
Effects of canagliflozin and placebo on cardiovascular and renal outcomes by primary and secondary prevention cohorts in the CANVAS Program. CANVAS indicates Canagliflozin Cardiovascular Assessment Study; CI, confidence interval; MI, myocardial infarction; and eGFR, estimated glomerular filtration rate.
Effects of Canagliflozin on Safety Outcomes in Primary and Secondary Prevention Cohorts
The rates of adverse events, including genital infections, urinary tract infections, fractures, diabetic ketoacidosis, and acute pancreatitis, were not statistically different between treatment groups in the primary and secondary prevention participants (Figure 3). The adverse event profile for canagliflozin compared with placebo was consistent in the primary and secondary prevention participants (all interaction P values ≥0.07).
Figure 3.
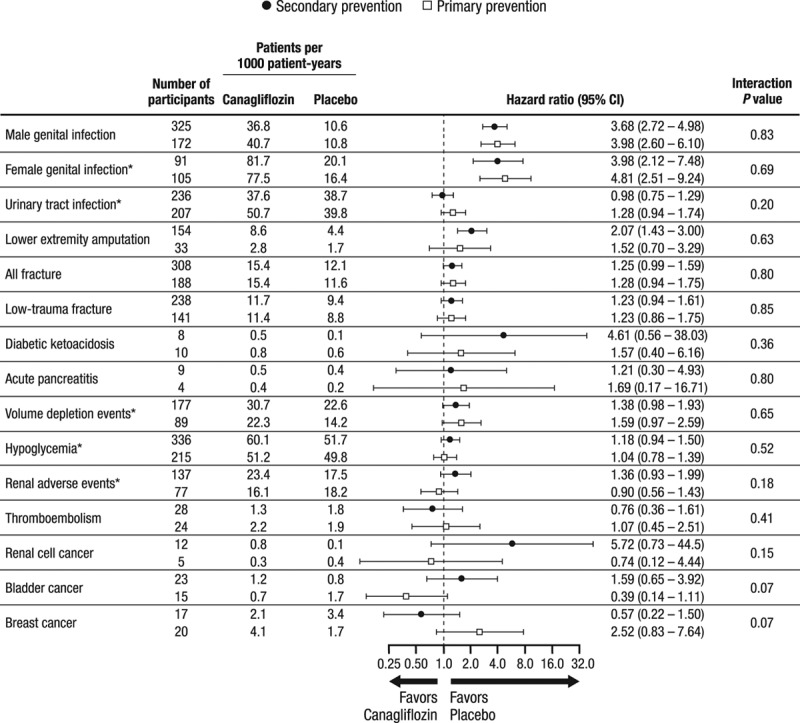
Summary of adverse events in the primary and secondary prevention cohorts in the CANVAS Program. CANVAS indicates Canagliflozin Cardiovascular Assessment Study; CANVAS-R, Canagliflozin Cardiovascular Assessment Study–Renal; and CI, confidence interval. *For these adverse events, the annualized event rates are reported with data from CANVAS alone through January 7, 2014, because after this time, only serious adverse events or adverse events leading to discontinuation were collected. In CANVAS-R, only serious adverse events or adverse events leading to discontinuation were collected. Owing to the differences between the 2 trials in methods of collection of the data, an integrated analysis of these adverse events is not possible.
Risk Differences for Cardiovascular, Renal, and Amputation Outcomes in Primary and Secondary Prevention Participants
Figure 4 shows the event rates and risk differences for the primary composite (cardiovascular death, nonfatal MI, or nonfatal stroke), hospitalization for heart failure, renal composite outcome, and amputation for canagliflozin compared with placebo in the overall study, the secondary prevention participants, and the primary prevention participants.
Figure 4.
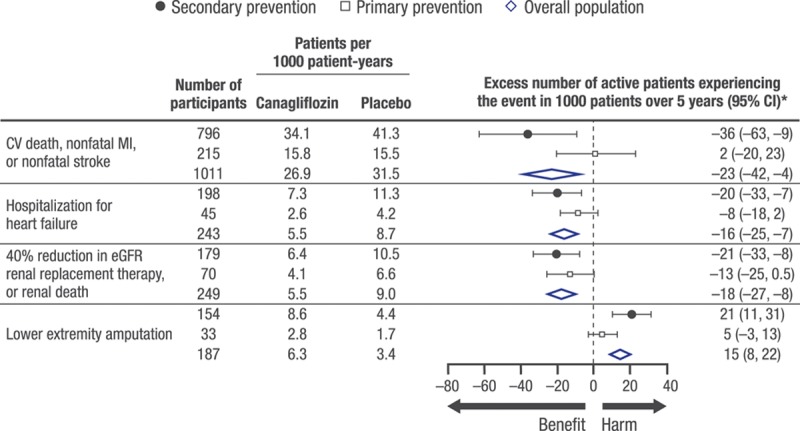
Benefits and risks per 1000 patients over 5 years with canagliflozin vs. placebo in the overall population, secondary prevention cohort, and primary prevention cohort. CI indicates confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; and MI, myocardial infarction. *Excess number is relative to the placebo group. If the number is negative, then fewer subjects in the canagliflozin group experienced the event compared with the placebo group.
Discussion
The CANVAS Program included patients with established cardiovascular disease and those at risk for cardiovascular disease. Overall, 34% of participants were included in the primary prevention group. Secondary prevention participants had higher rates of cardiovascular and renal outcomes compared with the primary prevention participants. Canagliflozin reduced the composite risk of cardiovascular death, nonfatal MI, or nonfatal stroke compared with placebo, and there was no statistical evidence of heterogeneity in the proportional treatment effect in the primary prevention and secondary prevention participants. Canagliflozin was also associated with better hospitalization for heart failure and renal outcomes, with a similar proportional reduction achieved for the primary and secondary prevention participants.
Some large cardiovascular outcome clinical trials in patients with type 2 diabetes mellitus have included primary and secondary prevention cohorts by design using various inclusion and exclusion criteria.7–11 However, others did not include a primary prevention cohort.12,13 For the CANVAS Program, the primary prevention cohort included participants ≥50 years of age, whereas other programs typically used 40 or 50 years of age to define the entry criteria. Compared with trials with primary prevention participants,7–11 the CANVAS Program included a higher proportion in the primary prevention group (≈35% versus ≈15% to 25%). Similar to other programs, cardiovascular event rates were lower in the primary prevention participants, but there was no evidence of heterogeneity in relative treatment effects in the primary and secondary prevention groups by statistical testing. The design and results from the CANVAS Program suggest that a broader group of patients has been studied with canagliflozin compared with other drugs, including an SGLT2 inhibitor.12
The absolute reductions in cardiovascular events with canagliflozin were numerically greater in patients in the secondary prevention cohort compared with the primary prevention cohort. The relative reductions in cardiovascular events, however, showed no statistical evidence of heterogeneity between the 2 prevention groups. There appeared to be consistent reductions in hospitalization for heart failure and renal outcomes in the primary and secondary prevention participants, as well as increases in amputations in both groups that were numerically less frequent than the reductions in cardiovascular and renal outcomes. The composite outcome (cardiovascular death, nonfatal MI, nonfatal stroke) was also clearly reduced in the secondary prevention population. Although formal statistical testing did not find evidence of heterogeneity in the results for this outcome in the primary prevention population, more data are required because the interaction testing has limited power based on the size of the subpopulation. The ongoing CREDENCE study (Canagliflozin and Renal Endpoints in Diabetes With Established Nephropathy Clinical Evaluation; ClinicalTrials.org; NCT02065791) will provide more evidence on the effects of canagliflozin on clinical renal outcomes, including end-stage kidney disease and renal and cardiovascular death, whereas the DECLARE (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events; ClinicalTrials.org; NCT01730534) will provide additional data regarding the effects of SGLT2 inhibition in primary prevention.
The general safety profile of SGLT2 inhibitors has been well described.6,14 The rates of common adverse events in the CANVAS Program were generally similar in participants in the primary and secondary prevention groups. Bone fractures have been reported previously with canagliflozin,6,15 and consistent findings were observed in the primary and secondary prevention participants in the CANVAS Program. The rate of lower extremity amputation was ≈3-fold higher in the secondary prevention group compared with the primary prevention group. A statistically significant 2-fold increase in lower extremity amputation with canagliflozin versus placebo was observed in the secondary prevention group, with a statistically similar result between canagliflozin and placebo in the primary prevention group, although only 33 events were reported in that group. Additional analyses of these findings are ongoing to understand the potential mechanism for amputations with canagliflozin. Until further information is available, caution should be used in patients at risk for amputations.
The balance of cardiovascular and renal benefits compared with the major safety event of amputations was evaluated by calculating the number of patients with events prevented or caused over 5 years for 1000 treated patients. A favorable profile was observed for the overall study population, with 23 fewer cardiovascular death, nonfatal MI, or nonfatal stroke events; 16 fewer hospitalizations for heart failure; and 18 fewer renal outcomes (40% reduction in estimated glomerular filtration rate, requirement for renal replacement therapy, or renal death) occurring in canagliflozin-treated patients compared with placebo, with an excess of 15 lower extremity amputations (10 toe or metatarsal, 5 above the ankle). As expected, numerically more events were prevented in the higher risk secondary prevention group compared with the primary prevention participants, and in both cohorts the number of excess amputation events was numerically lower than the number of cardiorenal outcomes that were prevented. These data may be helpful to clinicians and patients for shared clinical decisions in the management of diabetes mellitus to reduce cardiovascular and renal outcomes.
Limitations
These analyses have several limitations. The trial was not designed with appropriate statistical power to show definitive treatment differences in the outcomes in primary and secondary prevention participants. The primary prevention cohort was smaller, was lower-risk, and accrued fewer events than the secondary prevention cohort, and therefore the ability to exclude heterogeneity between the primary and secondary prevention cohorts is limited. The primary and secondary prevention participants were categorized based on investigator-reported inclusion and exclusion criteria and were not confirmed. We did not screen patients for subclinical atherosclerotic vascular disease in this large international trial, so patients with asymptomatic cardiovascular disease or clinically silent prior cardiovascular events could have been included in the primary prevention cohort. We followed participants for ≈3.5 years; however, glucose-lowering agents are often used for a much longer duration, well beyond the horizon of this study. Further study with longer follow-up in a primary prevention population could potentially identify more long-term benefits because of greater life expectancy.
Conclusions
In the CANVAS Program, which evaluated patients with type 2 diabetes mellitus and elevated cardiovascular risk, participants with prior cardiovascular events (secondary prevention) compared with those without prior cardiovascular events (primary prevention) had greater absolute rates of cardiovascular, renal, and death outcomes. Canagliflozin reduced cardiovascular and renal outcomes overall, with no statistical evidence of heterogeneity of canagliflozin effects between the primary and secondary prevention participants.
Acknowledgments
The authors thank all investigators, study teams, and patients for participating in these studies. Medical writing support was provided by Kimberly Dittmar, PhD, of MedErgy.
Sources of Funding
The CANVAS Program was supported by Janssen Research & Development, LLC. Medical writing support was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corp.
Disclosures
Dr Mahaffey’s financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. Dr Neal reports receiving research support from the Australian National Health and Medical Research Council Principal Research Fellowship and from Janssen, Roche, Servier, and Merck Schering Plough; and serving on advisory boards or involvement in continuing medical education programs for Abbott, Janssen, Novartis, Pfizer, Roche, and Servier, with any consultancy, honoraria, or travel support paid to his institution. Dr Perkovic reports receiving research support from the Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant); serving on Steering Committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Novartis, and Pfizer; and serving on advisory boards or speaking at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, and Vitae. Dr de Zeeuw reports serving on advisory boards or as a speaker for AbbVie, Astellas, Eli Lilly, Fresenius, Janssen, Boehringer Ingelheim, Bayer, and Mitsubishi-Tanabe, with all consultancy honoraria paid to his institution. Dr Fulcher reports receiving research support from Novo Nordisk and serving on advisory boards and as a consultant for Janssen, Novo Nordisk, Boehringer Ingelheim, and Merck Sharp and Dohme. Dr Li reports being a full-time employee of the George Institute for Global Health. Drs Erondu, Shaw, Fabbrini, Sun, and Desai report being full-time employees of Janssen Research & Development, LLC. Dr Matthews reports receiving research support from Janssen; serving on advisory boards and as a consultant for Novo Nordisk, Novartis, Eli Lilly, Sanofi-Aventis, Janssen, and Servier; and giving lectures for Novo Nordisk, Servier, Sanofi-Aventis, Eli Lilly, Novartis, Janssen, Mitsubishi Tanabe, and Aché Laboratories.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.032038/-/DC1.
Circulation is available at http://circ.ahajournals.org.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, Meininger G, Matthews D. Rationale, design, and baseline characteristics of the CANagliflozin cardioVascular Assessment Study (CANVAS): a randomized placebo-controlled trial. Am Heart J. 2013;166:217.e11–223.e11. doi: 10.1016/j.ahj.2013.05.007. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, Erondu N, Desai M, Shaw W, Vercruysse F, Yee J, Deng H, de Zeeuw D CANVAS-R Trial Collaborative Group. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19:387–393. doi: 10.1111/dom.12829. doi: 10.1111/dom.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR CANVAS Program Collaborative Group. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19:926–935. doi: 10.1111/dom.12924. doi: 10.1111/dom.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 10.Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Haahr PM, Lange M, Frandsen KB, Rabøl R, Buse JB. Design of DEVOTE (Trial Comparing Cardiovascular Safety of Insulin Degludec vs Insulin Glargine in Patients With Type 2 Diabetes at High Risk of Cardiovascular Events) - DEVOTE 1. Am Heart J. 2016;179:175–183. doi: 10.1016/j.ahj.2016.06.004. doi: 10.1016/j.ahj.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Green JB, Bethel MA, Paul SK, Ring A, Kaufman KD, Shapiro DR, Califf RM, Holman RR. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166:983.e7–989.e7. doi: 10.1016/j.ahj.2013.09.003. doi: 10.1016/j.ahj.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. doi: 10.1016/S2213-8587(16)00052-8. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 15.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]


