Abstract
This study tested whether two doses of remote limb ischemic conditioning (RLIC), induced via blood pressure cuff inflation, enhanced motor and cognitive learning to an equal extent, and explored a panel of blood biomarkers of RLIC. Thirty-two young adults were randomized to 3 groups and underwent a 7 day protocol of RLIC/sham followed by motor and cognitive training, with follow-up. Both RLIC groups had greater motor learning and a trend towards greater cognitive learning compared to the sham group. RLIC at the lower inflation pressure was as effective as RLIC with the higher inflation pressure. No significant candidate blood biomarkers were found. RLIC could be a well-tolerated method to enhance learning and improve rehabilitation outcomes in people with neurological conditions.
Keywords: remote limb ischemic conditioning, motor learning, behavioral training, human learning
INTRODUCTION
Ischemic conditioning refers to a phenomenon in which exposure to sublethal bouts of ischemia followed by reperfusion protects a tissue against future ischemic challenges. These protective effects are seen when ischemic conditioning is applied directly to the target tissue (Hausenloy & Yellon, 2009), or when ischemic conditioning is applied to an organ or tissue remote from the target tissue (Hausenloy & Yellon, 2008). This technique of generating a brief period of tissue ischemia at one site to induce widespread systemic protection of other tissues is referred to as remote ischemic conditioning. Remote limb ischemic conditioning (RLIC) represents a clinically-feasible method of delivering remote ischemic conditioning because it can be achieved through repeated inflation and deflation of a blood pressure cuff on an extremity. In humans, evidence of the cardioprotective (Ali et al., 2007; Botker et al., 2010; Cheung et al., 2006; Crimi et al., 2013; Hausenloy et al., 2007) and neuroprotective (Hougaard et al., 2014; Meng et al., 2012; Meng et al., 2015) effects of RLIC is mounting.
The exact mechanisms by which RLIC affords cardio- and neuroprotection remain unclear and over 100 different signaling molecules and mechanisms have been reported in a variety of experimental preparations (Heusch, 2015). Specifically, there is evidence that the active mechanisms underlying the phenomenon involve inflammatory, oxidative, excitotoxic, metabolic, and vascular and glial pathways (Brooks & Andrews, 2013; Gidday, 2006; Hausenloy & Yellon, 2008; Ishida, Yarimizu, Gute, & Korthuis, 1997; Lim, Yellon, & Hausenloy, 2010; Tapuria et al., 2008). The robust protection obtained in preclinical studies suggests multiple humoral factors are involved and that the conditioning stimulus triggers changes in gene expression secondary to epigenetic modulations of the genome, resulting in transient or long-lasting changes in phenotype (Hess et al., 2015). Human trials investigating the physiological response to RLIC are largely based on preclinical work and include examination of hemostatic, endothelial, and inflammatory pathways (S. Koch, Della-Morte, Dave, Sacco, & Perez-Pinzon, 2014). Given that repeated exposure to RLIC prolonged prothrombin time (PT) and international normalized ratio (INR) in humans with aneurysmal subarachnoid hemorrhage (Mayor et al., 2013), that RLIC led to increased serum vascular endothelial growth factor (VEGF) levels and endothelial progenitor cells in healthy adults (Kimura et al., 2007), and that a positive tumor necrosis factor-α / interleukin-6 (TNF-α / IL-6) ratio may be a marker for ischemic tolerance in humans with prior transient ischemic attack (Castillo et al., 2003), we sought to examine these specific biomarkers in our RLIC study.
Given the multifactorial, epigenetic basis of RLIC-induced neuroprotection, we postulated that RLIC might also induce some of the mechanisms responsible for neural plasticity and therefore facilitate distinct types of learning. This hypothesis is unique in that it proposes RLIC as an enhancer of neuronal plasticity and thus a potential facilitator of learning, not just as a technique that protects neurons from ischemia, the focus of virtually all previous work. Our recent proof-of-concept study showed that RLIC using a single, high (200 mmHg) cuff inflation pressure robustly facilitated motor, but not cognitive, learning in young, healthy adult humans (Cherry-Allen, Gidday, Lee, Hershey, & Lang, 2015). However, the cognitive task we used was psychometrically suboptimal, perhaps interfering with its sensitivity. The current study represents the next step in our investigation of RLIC as a learning-enhancing agent as we strive to move this protocol down the translational pathway toward clinical application.
Here, we: 1) examined the efficacy of lower cuff inflation pressures (inflation to 20 mmHg above individual systolic blood pressure [RLIC+20 group] vs. inflation to 200 mmHg [RLIC200 group]) because the lower cuff inflation pressure may be more tolerable for future patient populations, as well as to ensure that it was not something unique about high pressure skeletal muscle compression that was necessary to induce the response; 2) tested whether RLIC enhanced cognitive learning on a psychometrically improved version of the cognitive task, hypothesizing that the mechanisms underlying RLIC-induced learning enhancement are not be restricted to just the motor system; and 3) explored a panel of potential blood biomarkers of remote limb ischemic conditioning. Successful identification of one or more biomarkers would facilitate individual RLIC dose titration and allow us to more quickly translate this technique into the clinic.
We hypothesized that improvements in motor and cognitive learning would be equivalent for individuals in the RLIC200 group and the RLIC+20 group, and that these improvements would be greater than improvements found in individuals in the sham conditioning group. We also hypothesized that changes in blood coagulation status, as well as the release into the blood of mitogenic factors from vascular endothelial cells and/or inflammatory mediators, all of which could potentially serve as biomarkers for conditioning efficacy, will differ between participants who received RLIC at either dose compared to those who received sham conditioning. The results of our study represent a critical step toward the translation of RLIC for learning enhancement into a clinical population. Ultimately RLIC could be a low-cost, well-tolerated, and clinically-feasible strategy used to enhance outcomes in individuals undergoing neuromuscular rehabilitation for brain injury and other pathological conditions.
MATERIALS and METHODS
Experimental Design
This study used a repeated measures design with nine total sessions to examine the effects of two doses of RLIC combined with motor and cognitive training on learning in neurologically-intact adults. This study and consent procedures were approved by the Washington University Human Research Protection Office and the study was conducted in compliance with the Helsinki Declaration. All participants provided written informed consent prior to beginning the study. Consent was documented on a paper form and in the electronic database, with a copy of the signed paper form provided to participants. Participants were compensated for their time.
Participants
Thirty-two neurologically intact adults were recruited for participation in this study. Participants were included if they were 18 to 40 years old. Exclusion criteria were determined by self-report and included: 1) history of neurological condition, balance impairment, or vestibular disorder; 2) history of attentional disorders; 3) history of sleep apnea; 4) history of lower extremity condition, injury, or surgery that would compromise performance on the motor task; 5) any extremity soft tissue, orthopedic, or vascular condition or injury which may contraindicate RLIC (uncontrolled hypertension, peripheral vascular disease, hematological disease, severe hepatic or renal dysfunction); 6) any learning disability, sensory, or communication problem; 7) current use of medication for, or treatment with: systemic inflammation, spasticity, selective serotonin reuptake inhibitors, which could decrease nervous system excitability; 8) current weight lifting or interval training exercise which could confound the effects of RLIC, or 9) current substance abuse or dependence.
Order of Experiment
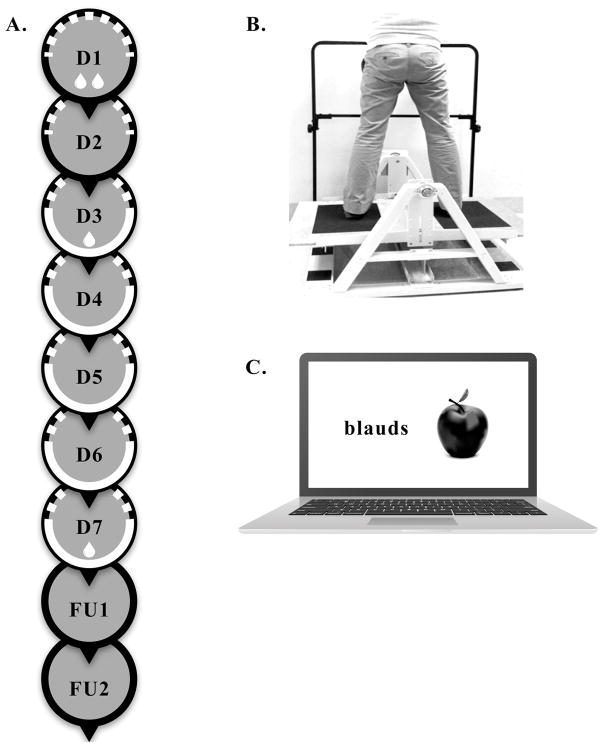
This experiment included 7 consecutive sessions (D1-D7) and two follow-up sessions (FU1, FU2), as previously described (Cherry-Allen et al., 2015) and shown in Figure 1A. All sessions took place on weekdays. The sessions that were separated by the weekend were dependent upon which day of the week a participant began their participation in the study. Nearly all participants began their participation on a Monday, Tuesday, or Wednesday and consequently the weekend usually fell between D5 and D6, between D4 and D5, or between D3 and D4, respectively. During the first session (D1), participants provided informed consent and demographic data. Each participant then completed a blood draw (indicated by drop symbol in Figure 1A), motor task pretest, and cognitive task pretest. The blood draw was completed first to avoid the confounding effects of physical activity on potential blood biomarker values but the sequence of motor and cognitive task performance was randomized. After pretesting, participants were randomly assigned to RLIC200, RLIC+20, or sham conditioning groups via a random numbers generator in MATLAB. Participants were blinded to their group assignment. Still on D1, participants underwent one set of conditioning (indicated by dashed semi-circle in Figure 1A), immediately followed by another blood draw. During the second session (D2) participants underwent one set of conditioning.
Figure 1.
Participants returned for the next 5 consecutive weekdays for sessions (D3-D7). These sessions consisted of conditioning, immediately followed by 15–20 minutes of both motor and cognitive training, in a randomized order (indicated by white semi-circle in Figure 1A). Details of motor and cognitive training are outlined below. Five sessions which included conditioning plus training were selected because this period of time is adequate to assess learning (Cherry-Allen et al., 2015; McNevin, Shea, & Wulf, 2003; Wulf, Weigelt, Poulter, & McNevin, 2003), is unlikely to result in performance plateaus, and does not present an excessive time burden for participants. Learning on the motor and cognitive tasks, as well as potential blood biomarker values were assessed through posttests at the end of the 7th session (D7). Follow-up sessions consisting of motor and cognitive task assessments took place at two (FU1) and four (FU2) weeks after D7 to evaluate the retention of performance gains achieved through training.
Remote Limb Ischemic and Sham Conditioning
Conditioning consisted of inflation of a blood pressure cuff on a participant’s non-dominant upper extremity. Each set of conditioning consisted of 5 cycles of 5 minutes of blood pressure cuff inflation followed by 5 minutes of blood pressure cuff deflation (Cherry-Allen et al., 2015). The blood pressure cuff was inflated to 200 mmHg (RLIC200 group), to 20 mmHg above a participant’s resting systolic blood pressure (RLIC+20 group), or to 10 mmHg below a participant’s resting diastolic blood pressure (sham group). Inclusion of the RLIC+20 group tested the hypothesis that it is limb ischemia, which occurs by inflating the blood pressure cuff to a pressure higher than an individual’s systolic blood pressure, not the specific inflation pressure of 200 mmHg, that triggers a cascade of adaptive signaling events and facilitates learning. In order to ensure that participants in the RLIC+20 group experienced the desired limb ischemia, we specifically selected an inflation pressure that was outside of the typical range of daily systolic blood pressure fluctuations (10 – 15 mmHg) (Mancia et al., 1983; Zakopoulos et al., 2005). The sham group did not experience limb ischemia and was included so that any performance enhancements found in the RLIC groups could be attributed to the effects of limb ischemia rather than other factors (e.g., duration of interaction between the participant and the investigator). We specifically selected blood pressure cuff inflation to 10 mmHg below a participant’s diastolic blood pressure for the sham group because it gives participants the sensation of cuff inflation on their arm but does not occlude blood flow to the distal extremity or cause tissue ischemia. Participants were queried at the end of the study about to which group they thought they were assigned.
In order to confirm the presence (RLIC200 and RLIC+20) or absence (sham) of ischemia in the conditioned arm, participants wore a continuous pulse oximeter (Beijing Choice Electronic Technology, M-50 Series SpO2 Sensor) throughout conditioning on the index finger of the conditioned arm, the radial pulse of the conditioned arm was checked during each cycle of conditioning, and intermittent visual inspection of the conditioned limb was performed. An oxygen saturation reading of 0 or ‘error’, the absence of the radial pulse, and a cool, pale or dusky distal limb was indicative of ischemia. An oxygen saturation reading similar to the pre-conditioning measure, the presence of the radial pulse, and unchanged color and temperature of the limb indicated that ischemia was not occurring. If ischemia was not confirmed in a participant in the RLIC200 or the RLIC+20 group at any point during conditioning, the blood pressure cuff was inflated an additional 10 mmHg and a note was made in the electronic record.
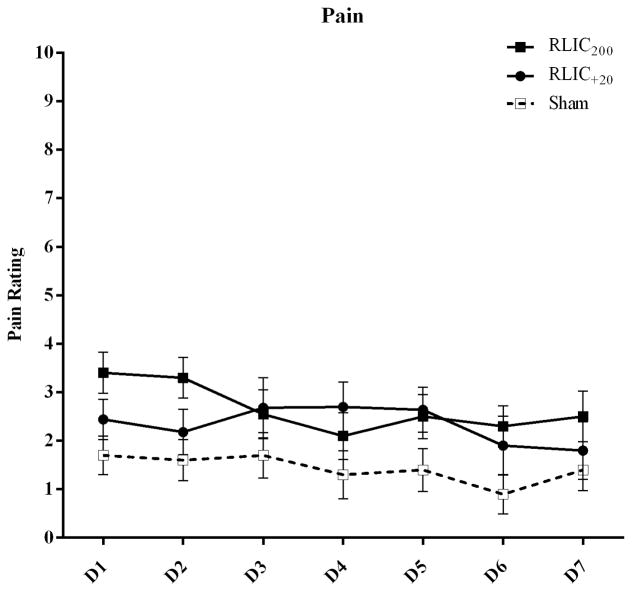
To monitor safety, oxygen saturation, heart rate, and blood pressure were measured before, during, and after each set of conditioning on the arm not undergoing conditioning. Pain was monitored during each cycle of conditioning; participants rated the average pain associated with a set of conditioning on a numerical pain scale ranging from 0 (no pain) to 10 (worst pain imaginable). Conditioning was terminated if oxygen saturation levels were <80%, heart rate was <40 or >110 bpm, systolic blood pressure was <80 mmHg or >150 mmHg, or if pain levels were >6. Following each set of blood pressure cuff inflation/deflation we also monitored for skin changes, defined as red marks, lines, or spots that appeared on the upper arm where the blood pressure cuff was placed.
Motor Task
As utilized previously, the motor task was to maintain balance on a stability platform (Lafayette Instrument, model 16030) shown in Figure 1B (Cherry-Allen et al., 2015; Cherry, Lenze, & Lang, 2014; Taubert et al., 2010). This motor task was selected because of its ecological validity (Toraman & Yildirim, 2010) and because it engages a broad range of brain systems (vestibular, visual, motor, somatosensory, and cognitive) permitting us to distinguish the systemic effects of RLIC from focal changes in the sensorimotor cortex that could result from brief periods of upper limb deafferentation. The general nature of this motor task allows it to serve as a simple probe to test the global response of the motor system to RLIC.
The stability platform generates electronic tilt angle measurement (angle measurement resolution 1.0°) and includes selectable balance thresholds (angle limit setting resolution 1.0°), digital angle readouts, platform re-zero ability, and built-in timing functions for test and rest timing (timing resolution 0.001 sec).
Participants were instructed to stand on the platform with feet facing forward and to keep the platform level for as many seconds as possible during a 30-second trial. Performance was quantified by measuring the cumulative amount of time (to the tenth of a second) that a participant maintained the stability platform ±3° of horizontal during each trial. Participants performed 90 total trials: 5 pretest trials during D1, 15 trials each day on D3-D7, and 5 trials on each of the two follow up sessions (FU1, FU2). The final five trials on D7 served as the posttest measure of performance on the motor task. Trials were separated by 30 seconds of rest. Following each motor task trial, participants were told how many seconds they kept the platform level (±3° of horizontal) but no feedback was given regarding balance strategies, allowing participants to develop their own techniques based on trial and error.
Cognitive Task
The cognitive task was a verbal-visual association learning task (Bunge, Burrows, & Wagner, 2004; Duzel et al., 2003) shown in Figure 1C. In our previous report there was overall poor performance and a lack of improvement with practice on the cognitive task in RLIC and sham conditions indicating that the task was too difficult and not a good test of cognitive learning (Cherry-Allen et al., 2015). To more accurately test the hypothesis that RLIC facilitates cognitive learning, we modified the task with increased stimulus presentation times, increased response times, and different items in an effort to decrease the overall difficulty of the task. Instructions, stimuli, and feedback were presented electronically on a laptop computer screen using the E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Non-words (e.g., crost, meaks) were displayed in Courier New, size 18 font on the left side of the screen while the image of a familiar object (e.g., kite, dog) appeared on the right side of the screen. Pronounceable non-words (5–7 letters long, 1–2 syllables) were selected from a database of non-words provided by the English Lexicon Project (Balota et al., 2007) and familiar, single-item images were selected from a bank of Microsoft Clip Art.
Participants were shown 100 ‘target’ non-word-image pairs (stimulus presentation = 1000 ms, interstimulus interval = 500 ms) which they were instructed to associate and remember. Then participants were shown 50 pairs (25 target pairs, 25 foil pairs) and were instructed to press the ‘1’ key if the pair was a target pair or press the ‘2’ key if the pair was a ‘foil’ pair (stimulus presentation + response time = 1000 ms, interstimulus interval = 500 ms). Foil pairs were pairs in which the non-word, the image, or both were not target items, or in which the non-word and the image were incorrectly paired target items. Each participant performed 1 pretest (D1), 5 training (D3-D7), and 2 follow-up trial(s) (FU1, FU2) and the trial on D7 served as the posttest measure of performance on the cognitive task. Participants were not given the opportunity to review the targets prior to the follow-up assessments and thus were required to remember target pairs for approximately 2 and 4 weeks.
Performance on the cognitive task was evaluated by assessing discriminability (Pr; accuracy with which a participant is able to discriminate between target pairs and foil pairs) and correct response reaction time (CRRT; time between stimulus presentation and participant response, averaged across all correct responses) (Snodgrass & Corwin, 1988). Higher Pr values indicate better performance and a Pr of zero reflects responding correctly half of the time. Participants received immediate feedback about the accuracy of each response as the word(s) “correct,” “incorrect,” or “no response detected” appeared on the screen for 500 ms after each stimulus. Response times shorter than 150 ms were considered anticipatory responses, and were excluded from CRRT analysis.
Potential Blood Biomarkers of RLIC
Based on evidence from the preclinical and early phase human trials investigating tissue protection in the setting of cerebral ischemia, it appears that ischemic conditioning leads to important effects on hemostatic, endothelial, and inflammatory pathways (S. Koch et al., 2014). In order to correlate changes in learning with one or more blood biomarkers of RLIC, we measured changes in two hemostatic variables, and changes in the plasma concentration of several proteins representative of vascular endothelial mitogenic and inflammatory activity. These included prothrombin time and international normalized ratio (PT, INR; hemostatic pathway) (Mayor et al., 2013; Roesner et al., 2007; Stenzel-Poore et al., 2003; Warzecha et al., 2007), vascular endothelial growth factor (VEGF; endothelial pathway) (Kawata et al., 2001; Kimura et al., 2007), and tumor necrosis factor alpha and interleukin 6 (TNF-α, IL-6; inflammatory pathway) (Castillo et al., 2003; Ginis et al., 2002). Each participant underwent a blood draw before and after conditioning on D1, before conditioning on D3, and after conditioning at on D7. A comparison of D1 pre- and D1 post-conditioning values allowed us to evaluate the early-phase response to conditioning, a comparison of D1 preconditioning and D3 values allowed us to evaluate the effects of two days of conditioning alone, and a comparison of D1 preconditioning and D7 values allowed us to evaluate the later-phase response to conditioning and the effects of repeated sessions of conditioning plus training.
A 10.5 mL blood withdrawal via aseptic technique from the antecubital vein was performed by a trained patient care technician from the Washington University Clinical Research Unit. In order to account for the potential diurnal variation in these blood biomarkers, blood was drawn at approximately the same time of day. The sample was drawn into three different tubes (BD Vacutainer), 1.5 mL into a standard PT/INR tube with sodium citrate anticoagulant, 3 mL for VEGF into a tube with an EDTA anticoagulant and a protease inhibitor cocktail, and 6 mL for TNF-α and IL-6 into a tube with EDTA anticoagulant. PT/INR samples were kept at room temperature for 30 minutes and then transferred to the Quest Diagnostics laboratory where they were processed using Siemen’s reagents and hemostasis equipment (BCS XP System) following standard procedures. Following sample collection, VEGF, TNF-α, and IL-6 samples were put on ice, centrifuged (Eppendorf 5804R) for 15 minutes at 5000 rpm, and placed in a −80°C freezer. The separated samples were stored in the freezer until analysis which was performed by Washington University CORE Laboratory staff. VEGF, TNF-α, and IL-6 plasma concentrations were detected in an antibody sandwich format by using the appropriate Enzyme-linked immunoassay (ELISA) kit (R&D Systems, Inc.). PT represents the time (seconds) for a participant’s blood to clot after the addition of calcium and an activator of the extrinsic pathway (thromboplastin). INR is a mathematical conversion of PT that accounts for the sensitivity of the thromboplastin used in a given laboratory by factoring in the international sensitivity index value supplied by its manufacturer. VEGF, TNF-α, and IL-6 values represent plasma concentrations (pg/mL).
Data Analysis
Data were managed and stored in a secure REDCap database (Harris et al., 2009) and statistical analyses were done with SPSS Statistics 21 (IBM, Armonk, NY). Criterion for statistical significance was set at α ≤ 0.05. A repeated measures ANOVA was used to analyze pain data, with a within-subject factor of time (7 levels: D1-D7) and a between-subject factor of conditioning group (RLIC200 vs. RLIC+20 vs. sham). As before (Cherry-Allen et al., 2015), we computed change scores and their 95% confidence intervals (CI) in order to directly test our specific hypotheses about learning (difference; D7 - D1) and retention (difference; FU1 - D7 and FU2 - D7) on the motor and cognitive tasks. Group differences in change scores were tested using a one-way ANOVA. Planned Tukey HSD post-hoc comparisons were used to analyze significant group differences. Our hypothesis that RLIC200 and RLIC+20 enhance motor and cognitive learning would be supported by a significant group difference between RLIC200 /RLIC+20 and sham. Retention of performance gains at 2 and 4 week follow-ups would be shown by 95% CIs that contain zero. The same approach was also used to explore whether RLIC influenced values for each of the potential blood biomarkers of ischemic condoning from D1 post-conditioning – D1 pretest evaluating an initial conditioning effect of RLIC and changes from D3 – D1 pretest and D7 – D1 pretest evaluating an overall across-sessions effect. Finally, Cohen’s d effect sizes of change scores were calculated for RLIC (merged RLIC200 and RLIC+20 data) and sham conditioning on motor and cognitive learning.
RESULTS
Thirty-two adults were enrolled and randomized to RLIC200 (n=10), RLIC+20 (n=12), and sham conditioning (n=10) groups. Two participants from the RLIC+20 group did not complete all sessions of the experiment (n = 1 unknown reason for study discontinuation, n = 1 discontinued study secondary to arm discomfort) and are not included in the subsequent analysis. Data from one participant (sham group) were excluded from cognitive task analysis due to multiple anticipatory responses and a default strategy of identifying all stimuli as targets (only pushed the 1 key). There were no significant demographic differences among groups that underwent RLIC200 vs. RLIC+20 vs. sham conditioning (Table 1). Ischemia and sham conditioning were confirmed when intended. Most participants reported that they thought they were in a RLIC group (n = 21; 6/9 who reported sham were RLIC). The results of a numerical pain rating scale are presented in Figure 2. We detected a main effect of time (pain decreasing over time; F(6,162) = 3.239, p = 0.005) on pain rating, but no effect of group (F(2,27) = 2.485, p = 0.102) and no group-by-time interaction effect (F(12,162) = 1.517, p = 0.123). Skin changes that resulted from conditioning were minimal and included superficial red dots or lines and occurred in 5 participants (RLIC200 = 4; RLIC+20 = 1).
Table 1.
Demographic Data
| Characteristics | Participants | |||
|---|---|---|---|---|
|
| ||||
| RLIC 200 Group (n = 10) | RLIC +20 Group (n = 10) | Sham Group (n = 10) | Main Effect of Group (P value) | |
| Age, yr | 27.2 ± 4.5 | 26.4 ± 5.0 | 26.2 ± 4.2 | 0.876 |
| Sex (female/male) | 7/3 | 6/4 | 9/1 | 0.303 |
| Dominant side (right/left) | 10/0 | 8/2 | 9/1 | 0.329 |
| Height, cm | 173.5 ± 9.4 | 171.7 ± 11.9 | 167.6 ± 9.7 | 0.447 |
| Weight, kg | 67.1 ± 17.4 | 72.8 ± 15.3 | 69.4 ± 19.0 | 0.761 |
| Race | ||||
| Caucasian | 10 | 7 | 7 | 0.441 |
| African American | 0 | 2 | 2 | |
| Asian | 0 | 1 | 1 | |
Values represent counts or means ± SD.
Figure 2.
Motor Learning and Retention
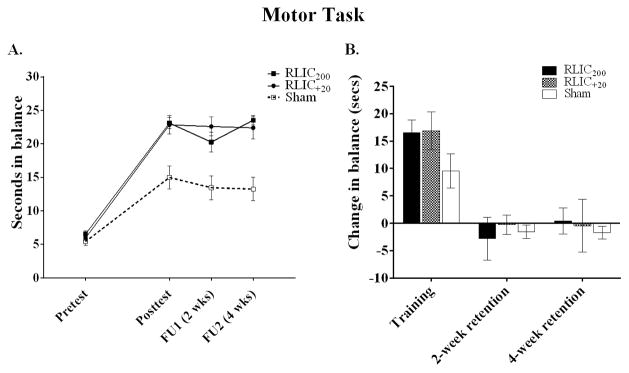
RLIC, with cuff inflation pressure either to 200 mmHg, or to 20 mmHg above a participant’s systolic blood pressure, enhanced responses to motor training by approximately 70%. All three groups improved on the trained motor task over time (Figure 3), as can be seen in absolute values (Figure 3A) and change scores (Figure 3B). The two RLIC groups had greater changes than the sham group, as determined by one-way ANOVA (F(2,27) = 9.761, p = .001) and post-hoc testing (p = .002 RLIC200 and RLIC+20 vs. sham). There were no statistically significant differences between the RLIC200 and RLIC+20 groups (p = .984). The RLIC groups demonstrated retention of motor task performance at two- and four-weeks as indicated by change scores from D7 to follow up that included zero in the 95% CI (Figure 3B). Improvements were not fully retained at follow-up in the sham group based on 95% CIs that did not include zero (Figure 3B). There were, however, no statistical differences in retention among the three groups either at 2-week (F(2,27) = 1.286, p = .293) or 4-week (F(2,27) = 0.594, p = .559) follow-up. The effect size of RLIC for the motor task was d = 1.71.
Figure 3.
Cognitive Learning and Retention
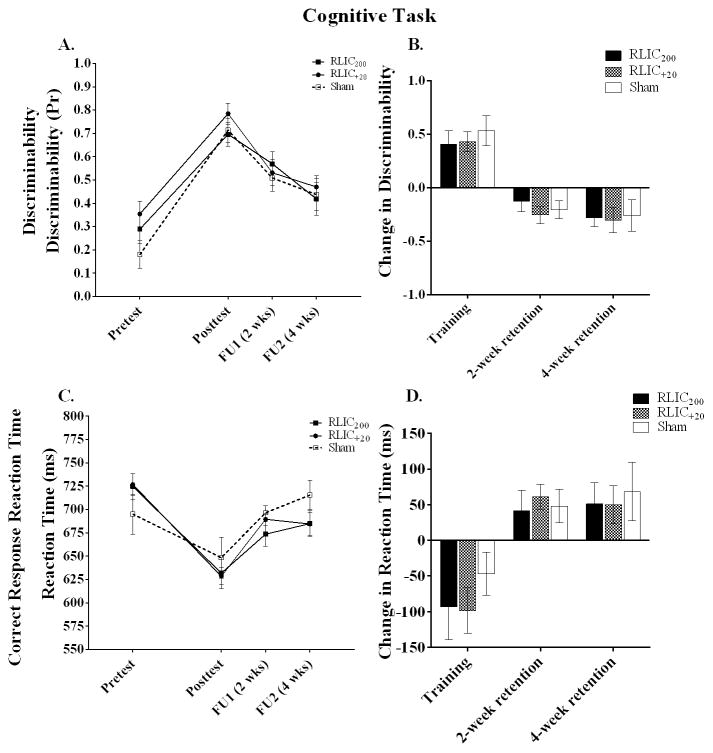
RLIC with either cuff inflation pressure resulted in a trend toward improved cognitive learning as measured by correct response reaction time, but not by discriminability, as shown in Figure 4. For discriminability (Figures 4A and 4B), all three groups improved from pretest to posttest, but RLIC at either cuff inflation pressure did not enhance discriminability (F(2,26) = 1.549, p = .231). For correct response reaction time, all three groups had faster reaction times from pretest to posttest and the two RLIC groups had reaction time improvements that were nearly twice as large as the sham group (Figure 4D). A one-way ANOVA showed a trend toward a group difference in CRRT change scores (F(2,26) = 2.874, p = .075). When RLIC200 and RLIC+20 CRRT change scores were combined and compared to the sham group CRRT change score, this difference reached statistical significance (t(27)= −2.43, p = 0.02). The effect size of RLIC for the cognitive task was 0.68 for discriminability and 1.04 for CRRT.
Figure 4.
Potential Blood Biomarkers of RLIC
There was no effect of RLIC at either cuff inflation pressure on our panel of hemostatic, endothelial, and inflammatory biomarkers either in the early phase (D1 pretest – D1 post-conditioning) or late phase (D1 – D3; D1 – D7). PT, INR, and VEGF values were within standard ranges. As might be expected in this population of healthy, young adults, TNF-α and IL-6 concentrations were low and detection required analysis with high-sensitive assays. The same statistical approach that was used to evaluate changes in motor and cognitive task performance was used to evaluate changes in values of blood biomarker levels. All statistical comparisons were insignificant (p = 0.072 – p = 0.944) with the exception of the change in TNF-α concentration within D1, which was greater in the RLIC200 group (0.06 pg/mL) compared to the RLIC+20 group (−0.03 pg/mL) (F(2,27) = 3.587, p = .042). The means and standard deviations (SD) for all biomarkers at all time points are reported in Table 2.
Table 2.
Potential Blood Biomarkers of Remote Limb Ischemic Conditioning
|
|
|||||
|---|---|---|---|---|---|
| D1 Pretest | D1 Posttest | D3 | D7 | ||
| PT, seconds | RLIC200 | 11.4 ± 0.7 | 11.4 ± 0.9 | 11.1 ± 0.5 | 11.2 ± 0.5 |
| RLIC+20 | 11.3 ± 0.6 | 11.1 ± 0.6 | 11.2 ± 0.4 | 11.2 ± 0.7 | |
| Sham | 11.0 ± 0.6 | 11.0 ± 0.7 | 10.8 ± 0.6 | 10.8 ± 0.9 | |
|
| |||||
| INR | RLIC200 | 1.08 ± 0.08 | 1.08 ± 0.08 | 1.03 ± 0.05 | 1.06 ± 0.05 |
| RLIC+20 | 1.07 ± 0.07 | 1.08 ± 0.06 | 1.06 ± 0.05 | 1.06 ± 0.07 | |
| Sham | 1.02 ± 0.08 | 1.02 ± 0.08 | 1.03 ± 0.07 | 0.99 ± 0.07 | |
|
| |||||
| Plasma VEGF, pg/mL | RLIC200 | 30.4 ± 25.6 | 20.4 ± 9.2 | 26.6 ± 16.4 | 27.3 ± 23.7 |
| RLIC+20 | 19.7 ± 6.6 | 29.5 ± 16.4 | 20.6 ± 6.5 | 27.6 ± 23.0 | |
| Sham | 30.8 ± 20.3 | 23.7 ± 9.6 | 28.2 ± 19.7 | 24.1 ± 13.1 | |
|
| |||||
| Plasma TNF-α, pg/mL | RLIC200 | 0.74 ± 0.24 | 0.82 ± 0.24 | 0.79 ± 0.38 | 0.66 ± 0.19 |
| RLIC+20 | 0.77 ± 0.24 | 0.74 ± 0.28 | 0.82 ± 0.38 | 0.71 ± 0.20 | |
| Sham | 0.62 ± 0.35 | 0.62 ± 0.38 | 0.74 ± 0.34 | 0.66 ± 0.40 | |
|
| |||||
| Plasma IL-6, pg/mL | RLIC200 | 1.25 ± 1.49 | 1.21 ± 1.17 | 1.14 ± 0.92 | 0.77 ± 0.55 |
| RLIC+20 | 0.76 ± 0.26 | 0.76 ± 0.30 | 0.78 ± 0.26 | 0.80 ± 0.33 | |
| Sham | 1.62 ± 1.48 | 1.63 ± 1.75 | 1.72 ± 1.43 | 1.40 ± 1.24 | |
Values represent means ± SD.
DISCUSSION
Results from this study indicate that remote limb ischemic conditioning (RLIC) with cuff inflation pressures to 20 mmHg above a participant’s systolic blood pressure was equally effective in enhancing learning when compared with RLIC using cuff inflation pressures to 200 mmHg. Compared to the sham group, both RLIC groups showed robust facilitation of motor learning and a trend toward enhanced cognitive learning, as measured by improved correct response reaction time, but not by discriminability. The exploration of potential blood biomarkers of RLIC related to hemostasis, inflammation, and endothelial mitogens did not reveal any candidates that changed significantly at the times we sampled, relative to baseline values.
This study represents a successful replication of the robust findings from our original proof-of-concept study (Cherry-Allen et al., 2015). The replication is notable given that reproducibility is key to the advancement of clinic research and recent reports suggest that only a small percentage of original scientific findings are able to be fully reproduced (Ioannidis, 2005; Open Science, 2015; Prinz, Schlange, & Asadullah, 2011). The results extend beyond replication, however, to support our hypothesis that crossing the systolic blood pressure threshold to induce limb ischemia is a sufficient stimulus for RLIC to facilitate learning, as indicated by equivalent responses in RLIC200 and RLIC+20 groups. In addition to supporting the hypothesis that it is skeletal muscle ischemia rather than the effect of a high-pressure compression of skeletal muscle and/or its recovery that is necessary to promote learning enhancement, there are also important clinical implications of the effects generated with the lower cuff pressure. Specifically, the likelihood of better tolerability and the lower incidence of side effects as we witnessed herein at cuff inflation pressures just slightly beyond the threshold to induce tissue ischemia will help facilitate the translation of RLIC to older adult and other patient populations who may be at an increased risk of bruising due to greater skin fragility and/or medications (e.g., blood thinners) that are used to treat medical conditions common to these patient subgroups.
There is mounting evidence that RLIC-facilitated protection is mediated by humoral factors (Dickson et al., 1999; Hess et al., 2015; Sebastian Koch, 2010; S. Koch et al., 2014; Konstantinov et al., 2005). For example, similar protection against infarction following coronary artery ligation was found in rabbits that underwent RLIC themselves and those that were pre-treated with plasma from donor rabbits who underwent RLIC (Shimizu et al., 2009). There is some suggestion in the literature that there may also be neurogenic mechanisms although the evidence is not as strong (Hausenloy & Yellon, 2008; Lim et al., 2010; Ren et al., 2009; Wei, Ren, Chen, & Zhao, 2012). Our finding that RLIC applied on the upper arm significantly enhanced motor learning on a motor task that did not directly involve the use of the conditioned arm indicates that the mechanisms through which RLIC enhances motor learning are also likely to be humoral. If the mechanisms are partially neurogenic, then our results indicate the neurogenic response must be effector-independent.
We predicated that, if enhanced motor learning secondary to RLIC is primarily humorally-mediated, then RLIC may also improve other forms of learning, such as establishing new verbal-visual associations on a cognitive task. In spite of the strong effect on motor learning observed in this study, RLIC’s impact on verbal-visual association learning over time was more subtle. Accuracy and correct response reaction time improved over the training period in all groups. RLIC modestly enhanced the improvement in reaction time, but not accuracy. In addition, although the differential enhancement by RLIC was retained over the entire follow-up period, the modest cognitive learning effect in all groups and the modest enhancement in reaction time induced by RLIC appeared to decay quickly. This difference in motor vs. cognitive task learning rate and strength of retention in even the sham conditioning cohort indicates that these two systems (motor vs. verbal-visual association learning) have quantitative differences in their sensitivity to experience and time. Therefore, longer or deeper cognitive task training and/or more robust RLIC paradigms may be necessary to achieve the same degree of response to RLIC as with the motor task. Another consideration is that we were powered to detect a change on the motor task and not on the cognitive task, which is consistent with our finding that when CRRT change scores are combined for the two RLIC groups, the difference between RLIC+20/200 and sham reaches statistical significance and by our finding that the effect size of RLIC was greater for the motor task compared to the cognitive task due to more variability in performance on the cognitive task.
This study included exploration of a panel of blood-borne biomarkers of RLIC because there is evidence that the underlying factors responsible for the protective effects of ischemic conditioning are present in the blood (Dickson et al., 1999; Hess et al., 2015; Meller & Simon, 2015). Moreover, a recent study found that a global proteomic response was stimulated in the serum of healthy volunteers who had undergone RLIC (Hepponstall et al., 2012). Following suggestions from a comprehensive review (S. Koch et al., 2014), candidate blood biomarkers in the hemostatic, endothelial and inflammatory pathways that have been implicated in previous investigations of ischemic conditioning were selected. Our findings that RLIC did not change the hemostasis metrics PT, INR, nor the concentrations of circulating VEGF, TNF-α, and IL-6, add to a body of literature that is conflicted regarding blood biomarkers that are responsive to ischemic limb conditioning. With respect to measures of homeostasis, ischemic conditioning can influence the expression of genes involved in hemostasis and fibrinolysis time in animals (He, Karabiyikoglu, Hua, Keep, & Xi, 2012; Stenzel-Poore et al., 2003; Warzecha et al., 2007) and result in prolonged PT and INR in humans with subarachnoid hemorrhage (Mayor et al., 2013). Others have found, however, that RLIC did not affect serum levels of tissue plasminogen activator (tPA), nor tPA inhibitor antigen and activity, in healthy humans (Pedersen et al., 2012). For VEGF, one study found that RLIC increased plasma VEGF levels and the number of circulating endothelial progenitor cells (Kimura et al., 2007), while another reported that VEGF concentrations did not change 0.5, 12, or 24 hours after conditioning (Czeiger et al., 2011), in healthy adults. Regarding inflammatory biomarkers, RLIC altered levels of IL-6 and TNF-α in infants undergoing cardiac surgery (Zhou et al., 2010), but caused no change in IL-6 or TNF-α levels in children undergoing surgery for a congenital heart defect (Cheung et al., 2006). The reason for these conflicting findings is unclear but may relate to the timing of the measurements relative to the RLIC stimulus, animal versus human differences, subject age, relative ischemic muscle mass rendered ischemic by the cuff, and any number of baseline co-morbid differences relating to disease versus healthy cohorts. Although blood biomarkers explored in this study of young healthy subjects did not change at the post-RLIC times we measured them, continued exploration of other potential blood biomarkers of RLIC is warranted. The ease of serial blood sampling and the high value in titrating individualized RLIC dosing, based on such measures, will benefit efforts to translate RLIC into a clinical population. It is likely that a dynamic repertoire of protein changes occur in the circulation following RLIC, and that a much more comprehensive proteomic approach and a combinational analysis may be needed. Values we obtained for each biomarker are provided in Table 2 to serve as a reference for other investigators pursuing similar questions.
In conclusion, RLIC enhances motor performance and produces a trend toward enhanced cognitive task reaction times in learning paradigms in healthy, young adults at cuff inflation pressures as low as 20 mmHg above the participant’s systolic blood pressure. These findings set the stage for future translational research. A next step will be to determine how responses to RLIC may vary between individuals; future studies can test the effects of age, gender, common medical conditions (e.g., hypertension, diabetes), and widely-prescribed medications on the learning responses elicited by RLIC. While the RLIC protocol used in this study were successful in enhancing learning, it remains to be determined if this stimulus has been optimized, particularly for non-motor learning. Protocol optimization could be achieved through manipulations of the frequency and duration of RLIC bouts and the timing of the behavioral training sessions, as well as by assessing different types of motor and cognitive paradigms. Ultimately, RLIC could serve as a powerful neurorecovery agent that could enhance learning and rehabilitation outcomes in persons suffering from a variety of neurological conditions.
Acknowledgments
This study was partially supported by the Foundation For Physical Therapy, HealthSouth Corporation, and NIH R01 HD085930.
References
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SAR, Akthar AM, … Gaunt ME. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair - A randomized controlled trial. Circulation. 2007;116(11):I98–I105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, … Treiman R. The English Lexicon Project. Behav Res Methods. 2007;39(3):445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, … Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- Brooks MJ, Andrews DT. Molecular mechanisms of ischemic conditioning: translation into patient outcomes. Future Cardiol. 2013;9(4):549–568. doi: 10.2217/fca.13.30. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56(2):141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, Davalos A. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54(6):811–819. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- Cherry-Allen KM, Gidday JM, Lee JM, Hershey T, Lang CE. Remote limb ischemic conditioning enhances motor learning in healthy humans. J Neurophysiol. 2015;113(10):3708–3719. doi: 10.1152/jn.01028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry KM, Lenze EJ, Lang CE. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. J Neurophysiol. 2014;111(12):2516–2524. doi: 10.1152/jn.00882.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, … Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47(11):2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, … Ferrario M. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv. 2013;6(10):1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Czeiger D, Dukhno O, Douvdevani A, Porat Y, Shimoni D, Fulga V, … Shaked G. Transient extremity ischemia augments CD34+ progenitor cell availability. Stem Cell Rev. 2011;7(3):639–645. doi: 10.1007/s12015-011-9234-x. [DOI] [PubMed] [Google Scholar]
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. Journal of thrombosis and thrombolysis. 1999;8(2):123–129. doi: 10.1023/a:1008911101951. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. Journal of Neuroscience. 2003;23(28):9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature Reviews Neuroscience. 2006;7(6):437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22(2):142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, … Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370(9587):575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovascular Research. 2008;79(3):377–386. doi: 10.1093/Cvr/Cvn114. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis. 2009;204(2):334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- He Y, Karabiyikoglu M, Hua Y, Keep RF, Xi G. Ischemic preconditioning attenuates brain edema after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3(1 Suppl 1):180–187. doi: 10.1007/s12975-012-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, … Konstantinov IE. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS One. 2012;7(11):e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11(12):698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116(4):674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, … Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. Plos Medicine. 2005;2(8):696–701. doi: 10.1371/journal.pmed.0020124. ARTN e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8(2):86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Kawata H, Yoshida K, Kawamoto A, Kurioka H, Takase E, Sasaki Y, … Dohi K. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res. 2001;88(7):696–704. doi: 10.1161/hh0701.088842. [DOI] [PubMed] [Google Scholar]
- Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, … Higashi Y. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(6):1403–1410. doi: 10.1161/ATVBAHA.107.143578. [DOI] [PubMed] [Google Scholar]
- Koch S. Preconditioning the human brain: practical considerations for proving cerebral protection. Transl Stroke Res. 2010;1(3):161–169. doi: 10.1007/s12975-010-0025-5. [DOI] [PubMed] [Google Scholar]
- Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA. Biomarkers for ischemic preconditioning: finding the responders. J Cereb Blood Flow Metab. 2014;34(6):933–941. doi: 10.1038/jcbfm.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79(12):1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Research in Cardiology. 2010;105(5):651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, … Zanchetti A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53(1):96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- Mayor F, Bilgin-Freiert A, Connolly M, Katsnelson M, Dusick JR, Vespa P, … Gonzalez NR. Effects of remote ischemic preconditioning on the coagulation profile of patients with aneurysmal subarachnoid hemorrhage: a case-control study. Neurosurgery. 2013;73(5):808–815. doi: 10.1227/NEU.0000000000000098. discussion 815. [DOI] [PubMed] [Google Scholar]
- McNevin NH, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res. 2003;67(1):22–29. doi: 10.1007/s00426-002-0093-6. [DOI] [PubMed] [Google Scholar]
- Meller R, Simon RP. A critical review of mechanisms regulating remote preconditioning-induced brain protection. J Appl Physiol (1985) 2015;119(10):1135–1142. doi: 10.1152/japplphysiol.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, … Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, … Ji X. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics. 2015;12(3):667–677. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Open Science, C. PSYCHOLOGY. Estimating the reproducibility of psychological science. Science. 2015;349(6251):aac4716. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- Pedersen CM, Barnes G, Schmidt MR, Botker HE, Kharbanda RK, Newby DE, Cruden NL. Ischaemia-reperfusion injury impairs tissue plasminogen activator release in man. European Heart Journal. 2012;33(15):1920–1927. doi: 10.1093/eurheartj/ehr380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery. 2011;10(9):712–U781. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesner JP, Petzelbauer P, Koch A, Mersmann J, Zacharowski PA, Boehm O, … Zacharowski K. The fibrin-derived peptide Bbeta15–42 is cardioprotective in a pig model of myocardial ischemia-reperfusion injury. Crit Care Med. 2007;35(7):1730–1735. doi: 10.1097/01.CCM.0000269035.30231.76. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, … Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117(5):191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, … Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362(9389):1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150(2):304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. Journal of Neuroscience. 2010;30(35):11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. 30/35/11670 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraman A, Yildirim NU. The falling risk and physical fitness in older people. Arch Gerontol Geriatr. 2010;51(2):222–226. doi: 10.1016/j.archger.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Kusnierz-Cabala B, … Tomaszewska R. Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. Journal of Physiology and Pharmacology. 2007;58(2):303–319. [PubMed] [Google Scholar]
- Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7(2):e30892. doi: 10.1371/journal.pone.0030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G, Weigelt M, Poulter D, McNevin N. Attentional focus on suprapostural tasks affects balance learning. Q J Exp Psychol A. 2003;56(7):1191–1211. doi: 10.1080/02724980343000062. [DOI] [PubMed] [Google Scholar]
- Zakopoulos NA, Tsivgoulis G, Barlas G, Papamichael C, Spengos K, Manios E, … Moulopoulos SD. Time rate of blood pressure variation is associated with increased common carotid artery intima-media thickness. Hypertension. 2005;45(4):505–512. doi: 10.1161/01.HYP.0000158306.87582.43. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu P, Zhou X. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31(1):22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]