Abstract
Gypsy moth (Lymantria dispar L., Lymantriinae) is a major pest of pedunculate oak (Quercus robur) forests in Europe, but how its infections scale with foliage physiological characteristics, in particular with photosynthesis rates and emissions of volatile organic compounds has not been studied. Differently from the majority of insect herbivores, large larvae of L. dispar rapidly consume leaf area, and can also bite through tough tissues, including secondary and primary leaf veins. Given the rapid and devastating feeding responses, we hypothesized that infection of Q. robur leaves by L. dispar leads to disproportionate scaling of leaf photosynthesis and constitutive isoprene emissions with damaged leaf area, and to less prominent enhancements of induced volatile release. Leaves with 0% (control) to 50% of leaf area removed by larvae were studied. Across this range of infection severity, all physiological characteristics were quantitatively correlated with the degree of damage, but all these traits changed disproportionately with the degree of damage. The net assimilation rate was reduced by almost 10-fold and constitutive isoprene emissions by more than 7-fold, whereas the emissions of green leaf volatiles, monoterpenes, methyl salicylate and the homoterpene (3E)-4,8-dimethy-1,3,7-nonatriene scaled negatively and almost linearly with net assimilation rate through damage treatments. This study demonstrates that feeding by large insect herbivores disproportionately alters photosynthetic rate and constitutive isoprene emissions. Furthermore, the leaves have a surprisingly large capacity for enhancement of induced emissions even when foliage photosynthetic function is severely impaired.
Keywords: green leaf volatiles, induced emissions, isoprene emission, large insect herbivores, monoterpene emission, photosynthesis, quantitative responses, volatile organic compounds
Introduction
In field environments, plants are frequently exposed to a multitude of abiotic and biotic stressors. To cope with environmental and biological stressors, more than 100,000 secondary chemical products are synthesized by plants and at least 1,700 of these are known to be volatile (Bauer et al., 1998; Copolovici and Niinemets, 2016; Kesselmeier and Staudt, 1999; Pichersky and Gershenzon, 2002). These biogenic volatile organic compounds (BVOC) serve many functions such as pollinator attraction (Lucas-Barbosa, 2016) and protection of plants against herbivore attacks (Heil, 2014; Pichersky and Gershenzon, 2002; Poelman, 2015), against excess temperatures (Becker et al., 2015; Possell and Loreto, 2013), and oxidative stress, e.g. that generated by ozone exposure (Loreto and Schnitzler, 2010; Possell and Loreto, 2013; Vickers et al., 2009).
While constitutive volatile emissions occur in only a limited number of species (Fineschi et al., 2013), stress-driven volatile emissions can be induced by abiotic and biotic stresses in all plant species (Copolovici and Niinemets, 2016; Harrison et al., 2013; Niinemets, 2010). Key biotic stresses eliciting major volatile emission responses are infestations by fungi, bacteria, herbivores and insects (Copolovici and Niinemets, 2016; Niinemets et al. 2013). Different biotic stressors elicit the same major classes of volatile compounds, green leaf volatiles (such as C5 and C6 alcohols and aldehydes), ubiquitous (e.g., α-pinene, β-pinene, Δ-3-carene) and specific monoterpenes (e.g., β-ocimene, linalool), sesquiterpenes (e.g. β-caryophyllene) and homoterpenes ((3E)-4,8-dimethy-1,3,7-nonatriene (DMNT) and (3E,7E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT)) (Blande et al., 2014; Copolovici and Niinemets, 2016; Holopainen, 2011; Kleist et al., 2012). However, as comparative experiments among different stresses, e.g., among infested, mechanically damaged and jasmonic acid-treated plants (Giorgi et al., 2015), and among leaf wounding and darkening (Brilli et al., 2011), demonstrate, different biotic stresses result in different volatile emission fingerprints.
Stress-induced volatiles can directly protect plants against biotic stress by serving as repellents of herbivores (Lucas-Barbosa et al., 2011) or inhibitors of fungal and bacterial growth (Schmidt et al., 2016). They can also confer indirect defense by attracting predators to their herbivore prey or oviposition host (Arimura et al., 2000a; Dicke and Baldwin, 2010; Johnson and Gilbert, 2015; Koski et al., 2015). For both functions, the composition of the emission blend as well as the total emission rates play important roles, the first determining the specificity of the signal for repelling or attraction, and the second, determining the dispersal of the signal as well as its chemical and physiological activity. Herbivore-induced changes in emissions of volatiles have been shown in numerous papers (Dicke, 2016; Heil, 2014 for reviews), but only a few studies have focused on quantitative relationships between the degree of damage and amount of compounds emitted (Bruce, 2015; Copolovici et al., 2014a; Holopainen and Gershenzon, 2010; Niinemets et al., 2013). Presence of correlations among the degree of herbivory damage and emission of volatiles seems trivial, however, it crucially depends on local and systemic emission responses. While local response in immediately impacted leaf areas is induced rapidly, e.g., emissions of green leaves volatles (GLV) can occur within seconds due to constitutive activity of lipoxygenases (Portillo-Estrada et al., 2015), induction of emissions of mono- and sesquiterpenes that need de novo expression of responsible synthases is more time-consuming, from hours to days (Copolovici et al., 2014a; Copolovici et al., 2011; Pazouki et al., 2016). Also, the induction response is quenched relatively rapidly, within a few days upon relief of herbivory stress (Copolovici et al., 2014a; Copolovici et al., 2011). Thus, the whole leaf response is a complex amalgamate of local and systemic induction and quenching responses. This is biologically highly relevant considering the huge diversity of herbivore impacts plants encounter in the field. While the rate of leaf area consumption is low for small solitary herbivores, leaving plenty of time for systemic responses in non-impacted leaf areas, simultaneous presence of many small herbivores and large solitary herbivores can rapidly consume a major proportion of leaf area, implying that the systemic emission response might not even occur in the major part of the leaf. In addition, while small herbivores mainly consume the intercostal leaf parts, large herbivores can also consume major veins and lead to catastrophic dysfunction of leaf water-, nutrient- and carbohydrate-conducting networks (Sack et al., 2003; Sack et al., 2004). Infections by small herbivores can lead to compensatory increases of leaf photosynthetic activity in remaining leaf parts, but the damage of major veins could mean that feeding by large herbivores can lead to disproportionate reductions in foliage physiological activity, including photosynthetic activity and constitutive isoprenoid emissions as well as hindered elicitation of induced emissions (Copolovici et al., 2014a; Copolovici et al., 2011).
Quantitative understanding of stress-dependent elicitation of volatile organic compound emission is further relevant for large-scale processes in biosphere-atmosphere system. This is because BVOCs affect atmospheric OH radical and O3 concentrations and participate in the formation of secondary organic aerosols (SOA) (Shen et al., 2013; Zhang et al., 2015; Ziemann and Atkinson, 2012). For example, in the Southeastern United States BVOC emissions can have a significant influence on the total aerosol burden due to condensation on acidic sulfate seed aerosols and concomitant growth of particles (Lee et al., 2012; Link et al., 2015). The current estimates of BVOC emissions and their role in atmospheric processes only consider constitutive emissions (Arneth et al., 2011; Guenther, 2013; Guenther et al., 2012), but stress-dependent elicitation of volatiles can significantly modify the overall volatile blend and amount of volatiles released into the atmosphere (Hare, 2011; Holopainen and Blande, 2013; Holopainen and Gershenzon, 2010; Niinemets et al., 2010a). In particular, under major outbreaks of feeding herbivores that regularly occur in nature (Abrams and Orwig, 1996; Dwyer et al., 2004; Mattson and Haack, 1987) induced volatiles can dominate the release of BVOC from vegetation over large areas, underscoring the importance of gaining a better knowledge of quantitative scaling of induced emissions with the degree of herbivory damage.
Pedunculate oak (Quercus robur L.) is a widely distributed constitutively isoprene-emitting tree species that is one of the most economically important broad-leaved forest trees in Europe. Oak forests currently exhibit declining productivity and crown dieback in several sites throughout Europe. Various abiotic and biotic factors, including herbivory by the larvae of phyllophagous insects have been shown to importantly contribute to the oak decline (Batos et al., 2014; Thomas et al., 2002; Tonioli et al., 2001). The most important defoliating insects feeding on Q. robur are winter moth (Operophtera brumata L.), tortrix moth (Tortrix viridana L.) and European gypsy moth (Lymantria dispar dispar L.) (Thomas et al., 2002). Among these, L. dispar is a species with large larvae that can grow to the size of 50-90 mm (Milanovic et al., 2014) and that are capable of consuming 10 cm2 leaf area per day per larva, including the second order veins and the terminal part of the mid-rib (Milanovic et al., 2014). In the current study, we used L. dispar larvae as a model to characterize the influence of large insect herbivore on constitutive and induced volatile release in Q. robur. We tested the hypothesis that the physiological activity of Q. robur leaves infected with the large herbivore L. dispar is quantitatively associated with the degree of damage, in particular, that the infection leads to a major decline in leaf photosynthetic activity and isoprene emissions and a modest increase in induced emissions.
Materials and methods
Plant material
The field measurements were performed in Lipova forest at Arad county, Romania (46° 5′ 30″ N, 21° 41′ 30″ E) in May 2014. The site supports a broad-leaved deciduous forest that is mainly dominated by Quercus robur (canopy height 4-5 m), whereas Q. petraea, Alnus glutinosa, and Q. rubra are minor canopy components. The forest expands more than 6,300 ha and more than half of the trees (51%) were infected at the time of the study. The infection was spatially highly heterogeneous, and forest patches with infested (more than 50 different patches with most trees infected observed) and non-infested patches with almost all trees lacking herbivores were interspersed. The weather conditions at the time of measurements were: average air temperature of 26 ± 2 °C, relative humidity of 62% and atmospheric pressure of 102.4 kPa. The plants of Q. robur included for measurements were 10-12 years old and 4-5 m tall. At the time of the measurements, the length of L. dispar larvae was about 30-40 mm. We took all measurements with control leaves from the clean plots with healthy trees. These control leaves were further checked for presence of eggs and small larvae, and discarded if there was evidence of biotic interactions. As all experiments were performed in natural conditions, the past and current larval damage as well as the actual number of herbivores that had been feeding in the leaf could not be controlled. Therefore, we only report the relationships of leaf physiological traits with the degree of leaf damage.
Photosynthetic measurements
Foliage photosynthetic characteristics during larval feeding were determined in the field with a portable gas exchange system GFS-3000 (Waltz, Effeltrich, Germany) as in Niinemets et al. (2010b), except for minor modifications in environmental conditions as stated below. This system has an environmental-controlled cuvette with 8 cm2 window area and a full-window leaf chamber fluorimeter for sample illumination and fluorescence measurements. Each time, a leaf fully filling the cuvette area was enclosed and standard measurement conditions (light intensity of 1000 nmol m-2 s-1, leaf temperature of 25°C, chamber air humidity of 70%, and CO2 concentration of 385 µmol mol-1) were established. The leaf was stabilized under the standard conditions until stomata opened and steady-state CO2 and water vapor exchange rates were reached. Steady-state values of net assimilation (A) and transpiration (E) rates, and stomatal conductance to water vapor (gs) were calculated according to von Caemmerer and Farquhar (1981).
Volatile sampling and GC-MS analyses
Volatile organic compounds (VOC) were sampled via the outlet of the gas-exchange cuvette at a flow rate of 200 ml min−1 for 20 min with a constant flow air sample pump 210-1003MTX (SKC Inc., Houston, TX, USA). The leaf chamber air was drawn through a multibed stainless steel cartridge (10.5 cm length, 4 mm inner diameter, Supelco, Bellefonte, USA) filled with Carbotrap C 20/40 mesh (0.2 g), Carbopack B 40/60 mesh (0.1 g) and Carbotrap X 20/40 mesh (0.1 g) adsorbents (Supelco, Bellefonte, USA) to quantitatively sample all volatiles in C5-C15 range (Kännaste et al., 2014). Volatiles were also collected using L. dispar larvae without plants in the laboratory conditions using a 3 L glass chambers with a flow rate of 2 L min-1 similarly as in Copolovici et al. (2011b). To estimate the background VOC concentrations (blank samples), air samples were taken from empty chambers before and after enclosure of leaves or larvae.
The adsorbent cartridges were analyzed for volatile lipoxygenase (LOX) pathway products (also called green leaf volatiles, GLV), terpenes and methyl salicylate using a Shimadzu TD20 automated cartridge desorber integrated with a Shimadzu 2010 Plus GC-MS instrument (Shimadzu Corporation, Kyoto, Japan) according to the method of Toome et al. (2010) and Kännaste et al. (2014). Briefly, for tube desorption, He purge flow was set at 40 ml min-1, primary desorption temperature at 250 °C, and primary desorption time was 6 min. The mass spectrometer was operated in electron-impact (EI) mode at 70 eV, with the transfer line temperature set at 240 °C and ion-source temperature at 150 °C. The identification of terpenes, green leaf volatiles and isoprene was done using NIST spectral library ver. 14 and authentic standards (Sigma-Aldrich, Taufkirchen, Germany). The absolute concentrations of compounds were calculated based on an external authentic standard consisting of known amount of VOCs as described in full detail in Kännaste et al. (2014). Briefly, 1 μl of calibration sample has been injected into the multi-bed tube followed by passing a nitrogen gas at 300 ml min-1 through the tube for 5 min in order to evaporate the solvent and trap the volatiles on the adsorbent. Finally, the tube has been analyzed in GC-MS using the same program as for the samples. The background (blank) VOC concentrations were subtracted from the concentrations with leaf samples and volatile emission rates were calculated according to Niinemets et al (2011).
Leaf pigment analysis
Pigment extraction was performed according to the method of Opris et al. (2013) with minor modifications. Briefly, leaf samples of 4 cm2 were taken after leaf gas-exchange measurements and volatile sampling and immediately frozen in liquid nitrogen. The pigments were extracted in ice-cold 100% acetone with calcium carbonate (Sigma-Aldrich, Steinheim, Germany), and centrifuged with a Hettich centrifuge (Hettich 320 R Universal, Hettich GmbH, Tuttlingen, Germany) at 0 ºC and 9500 g for 3 min. Then the supernatant was decanted, and the extraction was repeated till the supernatant remained colorless, but at least three times. The extracts were pooled and brought to a final volume of 10 mL and then filtered through a 0.45 µm PTFE membrane filter (VWR International, Radnor, PA, USA). The obtained extracts were analyzed for carotenoid and chlorophyll pigments by a high pressure liquid chromatograph (HPLC-MS Shimadzu 2010, Kyoto, Japan) equipped with a diode array detector (DAD) according to Niinemets et al. (1998) using a Lichrosorb® RP-18 column (length 125 mm, inner diameter 4 mm, film thickness 5 μm; Hichrom, UK). The column temperature was maintained at 10 ºC and the flow rate at 1.5 ml min–1. The solvents used for the chromatographic elution consisted of ultra-pure water (A) and HPLC grade acetone (B) (Sigma-Aldrich, Steinheim, Germany). The chromatographic elution was started by isocratically running a mixture of 25% A and 75% B for the first 7.5 min, followed by a 9.5 min linear gradient to 100% B, which was run isocratically for 3 min. Further, the eluent was changed to the initial composition of 25% A and 75% B by a 2 min linear gradient. The HPLC was calibrated using commercially available chlorophyll a, chlorophyll b, and β-carotene standards (Sigma-Aldrich, Steinheim, Germany), and the calibration curves were developed at corresponding spectral maxima (430 nm for chlorophyll a, and 455 nm for chlorophyll b and β-carotene).
Estimation of the degree of leaf damage
The leaves were scanned at 200 dpi, and the total leaf area and the leaf area damaged by L. dispar larvae were estimated with the “Leaf Area Measurement” software (www.plant-image-analysis.org). Leaf dry mass was estimated after oven-drying at 70 ºC, and leaf dry mass to leaf area was calculated. Foliage photosynthetic rates, volatile emissions and pigment contents per unit leaf area were estimated after correction for the consumed leaf area.
Statistical analysis and data handling
All measurements have been done in triplicates and data points correspond to averages of three replicate leaves in each individual plant (±SE). The data were analyzed by linear and non-linear regression analyses, and the herbivory treatment effect at a certain level of damage severity relative to non-infected leaves was also tested by ANOVA. All statistical analyses were conducted with ORIGIN 10.0 (OriginLab Corporation, MA, USA) and the statistical tests were considered significant at P < 0.05.
Results
Effects of herbivory on foliage photosynthetic characteristics
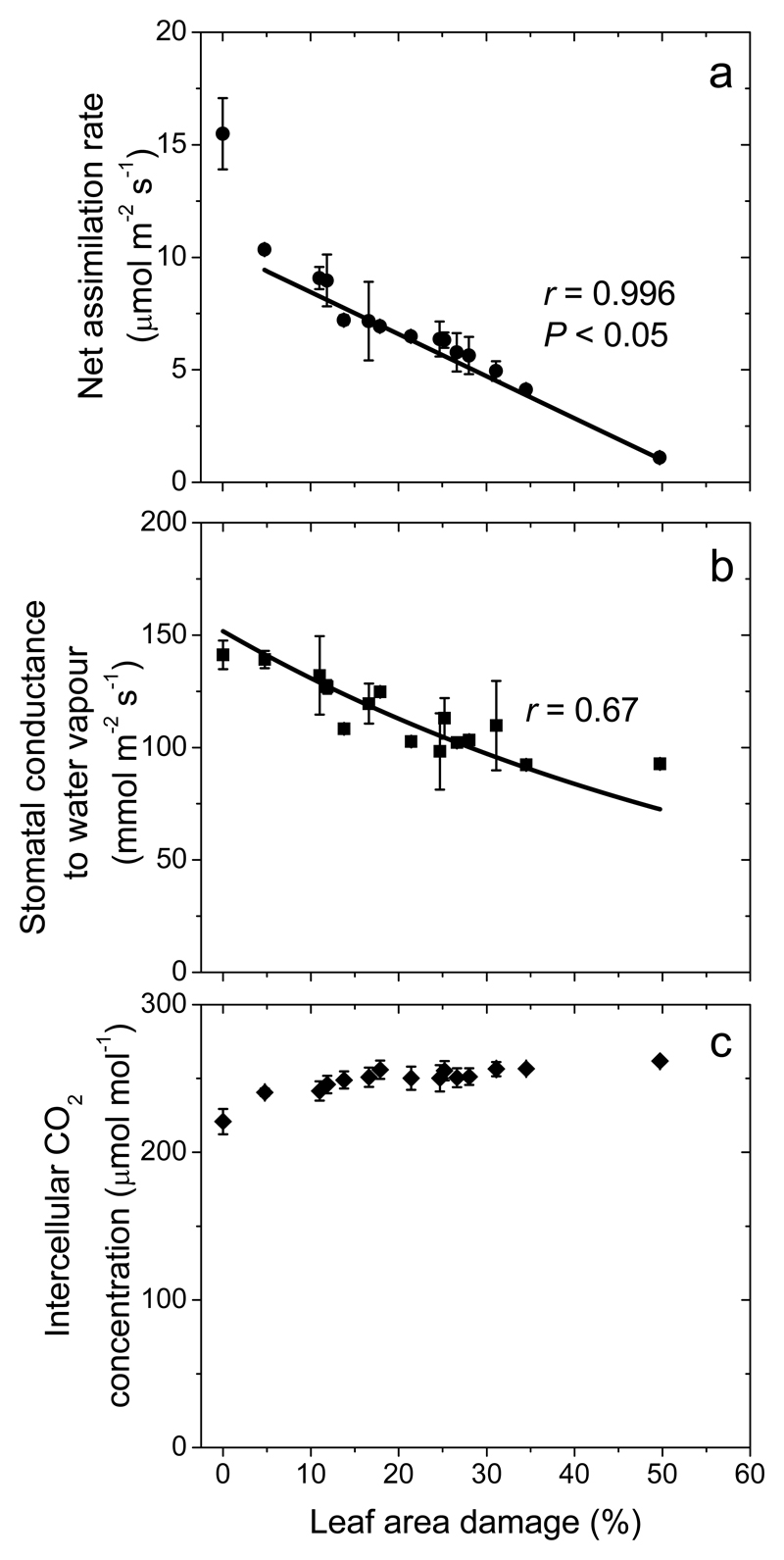
Herbivore feeding by L. dispar reduced leaf net CO2 assimilation rate (A), and even a moderate feeding, ca. 10% of leaf area removed, resulted in ca. 12% reduction of net assimilation rate, i.e. resulting in values of about 10 μmol m−2 s−1 (Fig. 1a). Net assimilation rate decreased with further increases in insect feeding, reaching values of 1-2 μmol m−2 s−1 in leaves with ca. 50% of leaf area eaten (Fig. 1a).
Fig. 1.
Foliage net assimilation rate (a), stomatal conductance to water vapor (b) and intracellular CO2 concentration per unit projected leaf area in Quercus robur plants in relation to the degree of damage by the larvae of the lymantriid moth Lymantria dispar (percentage of leaf area consumed). Data points correspond to averages of three replicate leaves in each individual plant (± SE). Data were fitted by linear (a) and non-linear regression in the form y = abx (b).
Differently from A, the stomatal conductance to water vapor (gs) was only moderately affected by larval feeding (Fig. 1b). In fact, the average (± SE) gs of 109 ± 20 mmol m-2 s-1 for strongly damaged leaves with 30-35% leaf area eaten was not different from the average gs in non-damaged leaves (141 ± 6 mmol m−2 s−1; P = 0.18 for the comparison among the means; Fig. 1b). Nevertheless, the regression analysis indicated that through the entire damage range of 0-50%, gs was reduced by ca. 30%. Given the smaller gs than A response to herbivory, the intercellular CO2 concentration (Ci) increased with increasing the degree of herbivory damage (Fig. 1c).
Elicitation of volatile emissions in herbivory-infected leaves
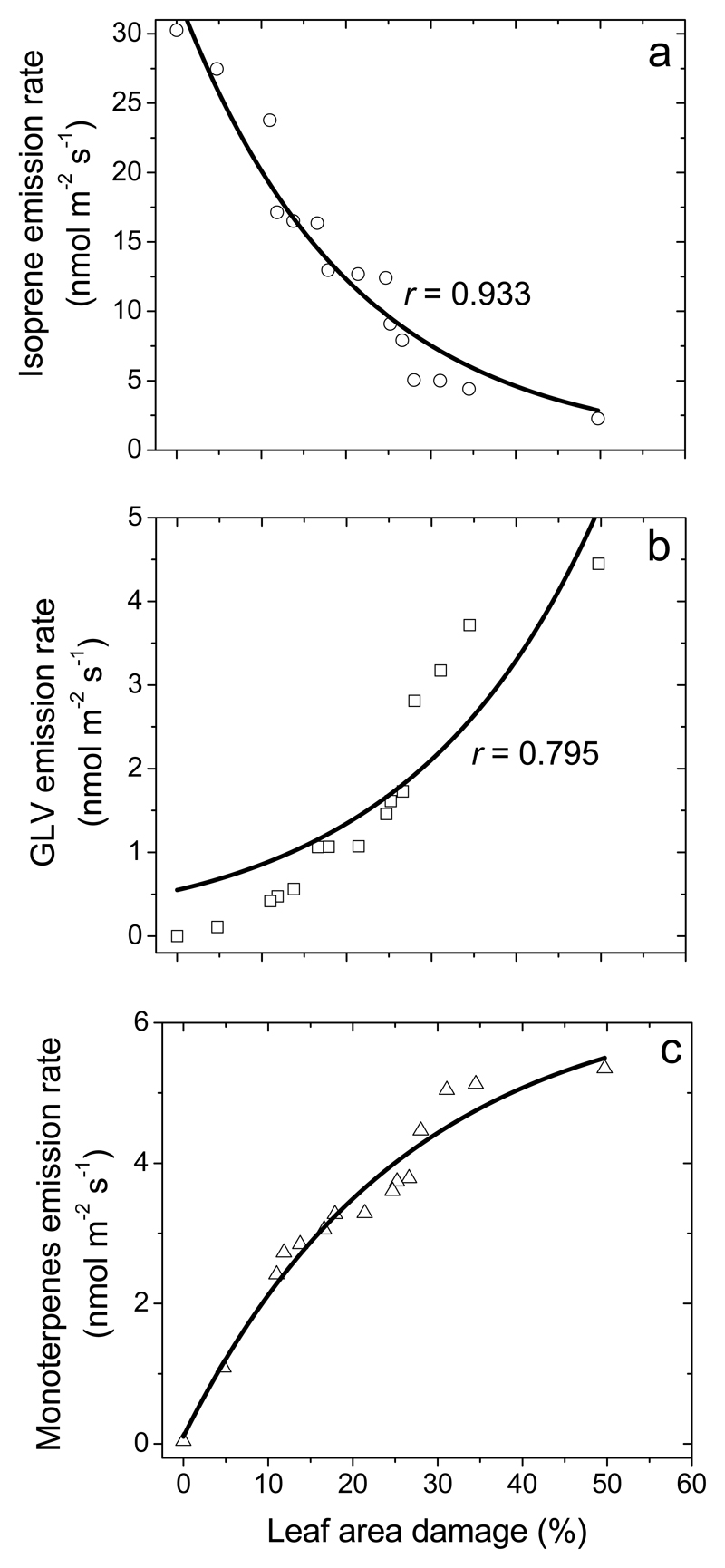
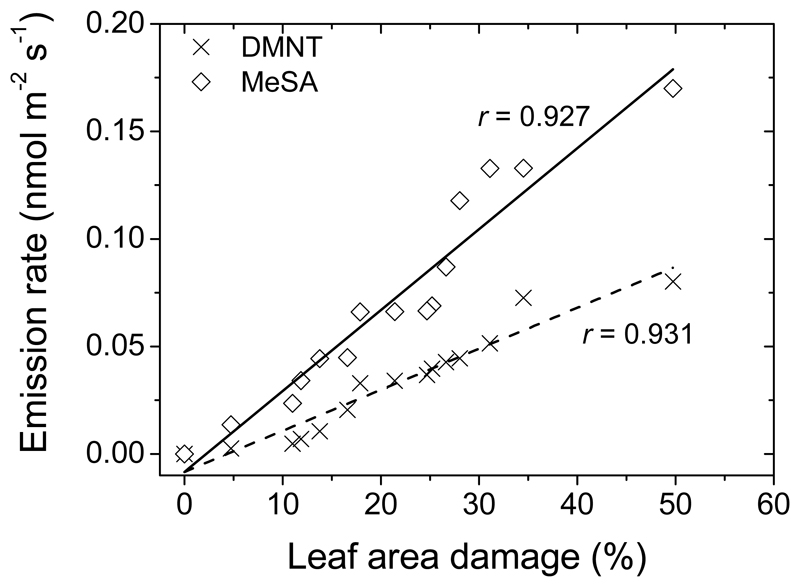
Constitutive isoprene emission in herbivore-fed leaves was reduced from 30.3 ± 0.7 nmol m-2 s-1 (average ± SE) in healthy leaves to 4-5 nmol m-2 s-1 in heavily infected leaves (40-50% of leaf area eaten by insects), and a strong negative correlation between isoprene emission rate and percentage of leaf area eaten was observed (Fig. 2a). Larval feeding induced emissions of green leaf volatiles [GLV; primarily, (Z)-3-hexenol, (E)-2-hexenal, (Z)-3-hexenyl acetate and 1-hexanol), Fig. 2b], that increased with the degree of damage, reaching very high values of 3.0-4.4 nmol m-2 s-1 (sum of all GLV) in heavily eaten leaves (Fig. 2b). Herbivory also induced emissions of ubiquitous monoterpenes (α-pinene, camphene, Δ-3-carene, limonene and β-phellandrene) and typical stress-marker monoterpenes such as (E)-β-ocimene and linalool (Table 1). The total emission rate of monoterpenes increased with the degree of leaf damage from close to zero level in control leaves to values as high as ca. 5.3 nmol m-2 s-1 in strongly infected leaves (Fig. 2c). Herbivory feeding also led to low-level emissions of the benzenoid methyl salicylate (MeSA) and the homoterpene (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), whereas the emission rates increased curvilinearly with the percentage of leaf damage to maximum values of 0.17 nmol m-2 s-1 for MeSA and 0.08 nmol m-2 s-1 for DMNT (Figure 3).
Fig. 2.
Emissions rates of isoprene (a), green leaf volatiles (b; GLV, volatiles of lipoxygenase pathway, LOX volatiles) and monoterpenes (c) from Q. robur leaves with different degrees of feeding by L. dispar larvae (replicates and data presentation as in Fig. 1). Total LOX product emission was calculated as the sum of emissions of 1-hexanol, (Z)-3-hexenol, (Z)-2-hexenal, and (Z)-3-hexenyl acetate and the total monoterpene emission as the sum of emissions of α-pinene, β-pinene, camphene, limonene, Δ-3-carene, p-cymene, and β-phellandrene. Data were fitted by non-linear regressions in the form of y = abx.
Table 1.
Average ± SD emission rates (nmol m-2 s-1) of different volatiles released from leaves of Q. robur in response to insect damage
| Compound | Control | Average (±SD) degree of damage (%) |
||
|---|---|---|---|---|
| 12.2±0.6 | 18.6±1.0 | 38.4±9.9 | ||
| (Z)-3-hexenol | nd | 0.107±0.005 | 0.213±0.007 | 0.90±0.13 |
| (E)-2-hexenal | nd | 0.100±0.003 | 0.320±0.010 | 0.82±0.17 |
| (Z)-3-hexenyl acetate | nd | 0.054±0.007 | 0.080±0.010 | 0.223±0.023 |
| 1-hexanol | nd | 0.223±0.022 | 0.454±0.017 | 1.84±0.33 |
| isoprene | 30.3±3.2 | 19.1±3.4 | 14.0±1.7 | 3.9±1.4 |
| α–pinene | 0.0092±0.0012 | 0.622±0.011 | 0.848±0.027 | 1.58±0.11 |
| camphene | nd | 0.033±0.002 | 0.043±0.001 | 0.111±0.010 |
| Δ-3-carene | 0.011±0.006 | 0.504±0.004 | 0.663±0.009 | 1.07±0.07 |
| limonene | 0.0094±0.003 | 0.305±0.032 | 0.412±0.008 | 1.02±0.05 |
| β-phellandrene | 0.0106±0.007 | 1.12±0.14 | 1.09±0.13 | 0.98±0.15 |
| (E)-β-ocimene | nd | 0.052±0.001 | 0.098±0.001 | 0.297±0.044 |
| linalool | nd | 0.029±0.001 | 0.053±0.002 | 0.128±0.024 |
| DMNT | nd | 0.007±0.002 | 0.021±0.006 | 0.068±0.015 |
| methyl salicylate | nd | 0.034±0.006 | 0.045±0.011 | 0.145±0.021 |
nd = not detected
Fig. 3.
Emissions of the benzenoid methyl salicylate (MeSA) and the homoterpene (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) from leaves of Q. robur in relation to the degree of herbivory by L. dispar larvae. Data presentation and replication as in Fig. 1. The relationships were fitted by linear regressions.
Responses of foliage chlorophyll and β-carotene contents to herbivory
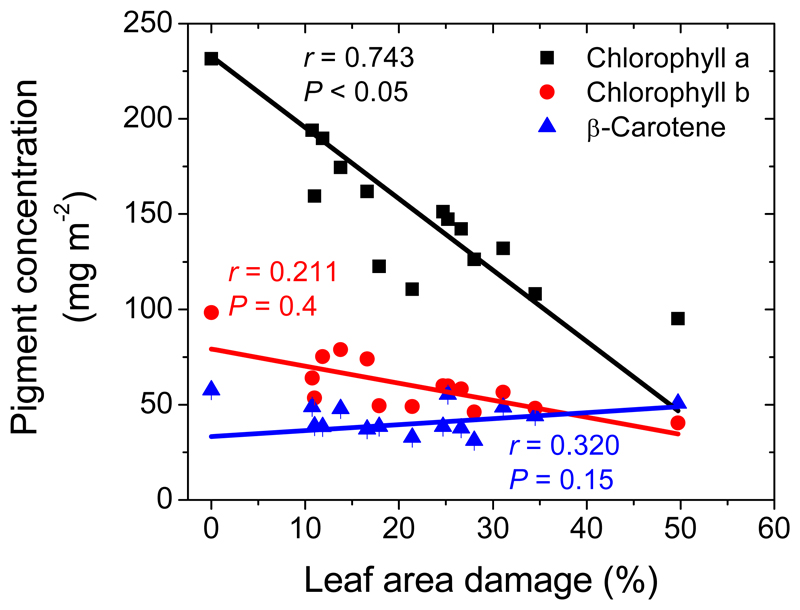
Both chlorophyll a and b contents decreased strongly with the area eaten by the larvae, e.g., the average chlorophyll a content decreased from 231 ± 2 mg m-2 in non-infected leaves to 95 ± 3 mg m-2 in leaves with 30-50% damage (Fig. 4). The chlorophyll a/b ratio was on average 2.48 ± 0.26 and did not depend on the degree of leaf damage (r= 0.5, P > 0.05). β-Carotene content did not significantly correlate with the degree of larval damage (Fig. 4).
Fig. 4.
Effects of feeding by the larvae of L. dispar on the contents of chlorophyll a and b, and carotene in Q. robur leaves. Statistical replicates and data presentation as in Fig. 1. Data were fitted by linear regressions.
Negative scaling among constitutive and induced isoprenoids
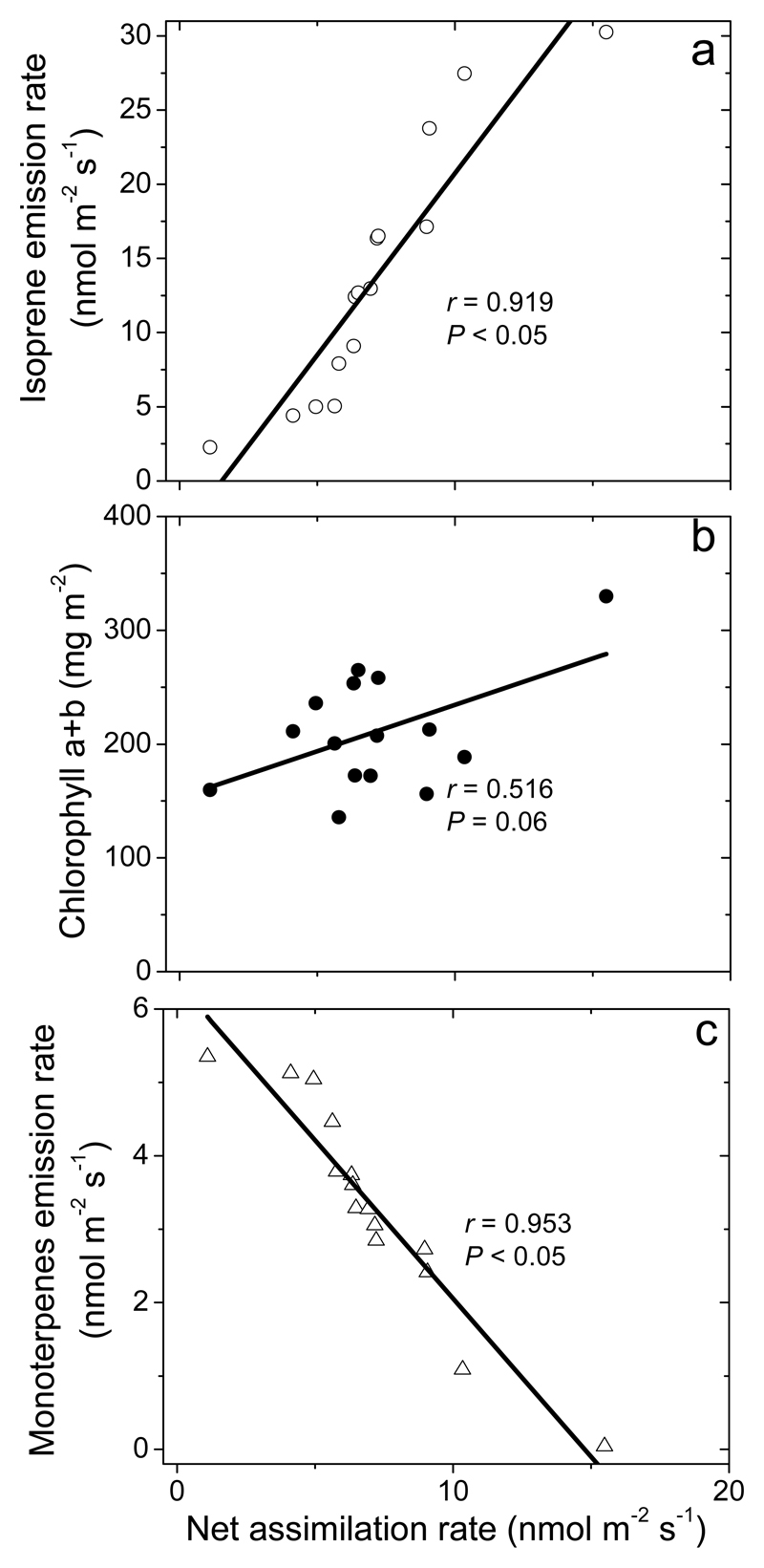
Through different damage severities, foliage net assimilation rate scaled negatively with the emissions of induced volatiles (Fig. 5a for total monoterpenes, 5b for isoprene and 5c for total chlorophylls, r= 0.83, P < 0.01 for GLV; r= 0.89, P < 0.01 for MeSA; r= 0.87, P < 0.01 for DMNT, for linear regression used). Constitutive isoprene emissions were negatively correlated with induced emissions (r= 0.97, P < 0.001 for total monoterpenes; r= 0.88, P < 0.01 for GLV; r= 0.93, P < 0.01 for MeSA; r= 0.91, P < 0.01 for DMNT).
Fig. 5.
Correlations of net assimilation rate (a), isoprene emission rate (b) and chlorophyll (a+b) content (c) with monoterpene emission rate in Q. robur leaves with different degrees of damage by L. dispar larvae (Fig. 1a, 2a,c and 4 for the correlations of given traits with the degree of damage). Data were fitted by non-linear regressions.
Discussion
Changes in photosynthetic function in leaves infected by large larvae
Plant photosynthetic responses to biotic stresses such as herbivory can range from stimulating effects, no effect or even significant impairment (Attaran et al., 2014; Gog et al., 2005; Nabity et al., 2009, 2013; Roslin et al., 2006; Zhou et al., 2015). Especially, colonization by solitary small herbivores can lead to compensatory enhancement of photosynthesis in remaining leaf parts (Copolovici et al., 2014a; Copolovici et al., 2011; Delaney et al., 2008). In Quercus robur in our study, we observed a drastic, disproportionate reduction in net assimilation rate (Fig. 1a), consistent with the hypothesis that herbivory by large larvae capable of biting through and consumption of also major veins can seriously impair photosynthesis. Yet, stomatal conductance was surprisingly little affected, seemingly inconsistent with this hypothesis (Fig. 1b). However, the low change in calculated stomatal conductance might be an artifact due to evaporation of water from free water surfaces generated upon herbivory, as well as due to transient increases in stomatal conductance upon relaxation of epidermal tension due to damage and concomitant opening of stomata (so-called Ivanov’s effect, Moldau et al., 1993).
On the other hand, longer-term reductions in photosynthesis in herbivore-fed leaves have also been associated with impaired electron transport rate (Nabity et al., 2013). In our study, the chlorophyll content decreased with leaf damage severity (Fig. 4). Such a reduction has been observed in some studies looking at diffuse massive leaf infection, e.g. mustard (Brassica juncea) leaf infection by phloem-sucking mustard aphid (Lipaphis erysimi) (Rehman et al. (2014) and tomato (Solanum lycopersicum) infection by cotton mealybug (Phenacoccus solenopsis) (Huang et al. (2013)), but not necessarily upon infection by tissue-removing herbivores. However, damage of major veins and concomitant reduction in water availability of isolated mesophyll areas can well lead to the start of senescence processes (Munné-Bosch, 2007, 2008). Given the reduction of leaf pigment content (Fig. 4), changes characteristic to programmed cell death such as inhibition of photosynthetic electron transport and reductions in the amount or activity of photosynthetic rate-limiting enzymes were also likely responsible for reduction in net-assimilation rate in larval-eaten leaves.
In contrast, β-carotene content was weakly affected by larval feeding (Fig. 4). Differently from chlorophylls, β-carotene primarily functions as antioxidant and its sustained level might serve protective function. Although carotenoids can be destroyed under severe abiotic stress conditions (Ashraf and Harris, 2013), carotenoids are maintained longer than leaf chlorophylls in leaf tissues though senescence (Garcia-Plazaola et al., 2003; Niinemets et al., 2012).
Modification of constitutive isoprene emissions by herbivory
Concomitant reductions of net assimilation rates and constitutive isoprene emissions as observed in herbivore-fed leaves in our study (Fig. 1b) have been reported in several other herbivory studies (Laothawornkitkul et al., 2008; Loivamaeki et al., 2008; Loreto et al., 2014) as well as in fungal-infected leaves (Copolovici et al., 2014b; Jiang et al., 2016). Such a simultaneous reduction might indicate that limited plastidic carbon availability or delayed activation of alternative carbon sources can have resulted in the reduction of the pool size of the immediate isoprene precursor, dimethylallyl diphosphate (DMADP) in chloroplasts (Rasulov et al., 2011; Rasulov et al., 2009), thereby reducing the emission rate. In addition, simultaneous reduction of isoprene synthase activity and isoprene emission can indicate overall decreases in foliage primary metabolism and constitutive isoprene synthesis in non-consumed leaf areas. Such a reduction is supported by significant declines in foliage chlorophyll contents in infected leaves (Fig. 4).
On the other hand, chloroplastic isoprene and monoterpene syntheses rely on the same plastidic DMADP pool, but the in vivo effective Michaelis-Menten constant for DMADP (Km) is much smaller for monoterpenes than for isoprene (Rasulov et al., 2014). Lower Km for monoterpene synthesis implies that the competition for DMADP is one-sided, and thus, elicitation of monoterpene synthesis upon herbivory feeding, can also partly explain the decline in isoprene emission rates (Fig. 5b).
Induction of green leaf volatile emissions in larval-eaten leaves
As a result of an attack by insects, specific elicitor molecules are generated by chemical or physical damage to plant membranes (Heil, 2014). Our study demonstrated elicitation of all key stress-induced volatile compound classes, green leaf volatiles (GLV), mono- and sesquiterpenes, homoterpenes and methyl salicylate in herbivory-infected leaves (Table 1). GLV are synthesized in a process where free octadecanoid fatty acids (linoleic acid = 18:2 and linolenic acid = 18:3) are released from plant membranes by phospholipases. Upon release of these free fatty acids, lipoxygenases (LOX) then produce 9- or 13-hydroperoxylinoleic or -linolenic acid or a mixture of both (Matsui, 2006). A hydroperoxide lyase further catalyzes the breakdown of 13-hydroperoxylinole(n)ic acid to a C6-compound, (Z)-3-hexenal, and a C12-product (12-oxo-(Z)-9-dodecenoic acid). (Z)-3-Hexenal can further give rise to (Z)-3-hexenol, (E)-2-hexenol, (E)-3-hexenol or (E)-2-hexenal in consequent reactions (Feussner and Wasternack, 2002; Matsui, 2006). The emission of green leaf volatiles is a reliable marker of oxidative stress and membrane-level damage (Porta and Rocha-Sosa, 2002). GLV are rapidly released upon herbivory feeding due to constitutive activity of LOX, and their almost immediate release has been typically associated with mechanical damage upon wounding (Matsui et al., 2012; Portillo-Estrada et al., 2015; Scala et al., 2013). In our study, we observed a strong correlation between the emissions of green leaf volatiles and the percentage of leaf area damaged (Figure 2b). Similarly to our study, increases in the emission of GLV with the degree of herbivore feeding were also observed in experiments with Caberia pusaria feeding on grey alder (Alnus incana) (Copolovici et al., 2011) and in experiments with Epirrita autumnata feeding on hybrid aspen (Populus tremula x P. tremuloides) (Schaub et al., 2010). Given that in these studies and in our study, the degree of damage quantified as the percentage of leaf area removed includes both fresh and somewhat older damage, the question is how such a correlation can occur if GLV release is exclusively associated with immediate damage. However, conversion of (Z)-3-hexenal to more reduced and less toxic volatiles can also occur in non-impacted leaf areas (Matsui et al., 2012), and GLV release can continue for a certain period of time after the immediate biotic impact (Copolovici et al., 2011; Jiang et al., 2016). Thus, scaling of GLV emissions with the degree of damage (Fig. 2b) might be explained by the sustained GLV release from non-impacted leaf areas.
There is evidence that GLV release is higher upon damage of veins than upon damage of intercostal tissues (Portillo-Estrada et al., 2015). Interestingly, the increase of GLV release with the degree of damage in leaves with minor to moderate damage of 5-20% was much less than in leaves with moderate to extensive damage of 20-50% (Fig. 2b). Such a difference might reflect the circumstance that in leaves with 5-20% damage, there was limited big vein severance by herbivores, while herbivores also bit through major veins in leaves with extensive damage. This result is in a marked contrast with the linear relationship of GLV release vs. leaf area damage in the study with small herbivores Caberia pusaria (Copolovici et al., 2011) and Monsoma pulveratum (Copolovici et al., 2014a) that are incapable of biting through major veins.
Elicitation of emissions of terpenoids and MeSA
As a widespread consensus, isoprene and monoterpenes are thought to be synthesized via 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in plastids (Copolovici et al., 2014a; Fineschi et al., 2013; Vranova et al., 2012) and sesquiterpenes via mevalonate (MVA) pathway in cytosol (Lombard and Moreira, 2011), although there is recent evidence of possible monoterpene synthesis in cytosol depending on substrate availability (Pazouki and Niinemets, 2016). DMNT is synthesized from the sesquiterpene (E)-nerolidol likely in the cytosol (Baldwin et al., 2006; Tholl et al., 2011). Although present in different cellular compartments, both isoprenoid synthesis pathways are upregulated upon herbivory stress and several terpene synthase genes involved have already been identified (Arimura et al., 2000a; Arimura et al., 2000b; Baldwin et al., 2006; Loreto et al., 2014). Thus, elicitation of emissions of mono-, sesqui-, and homoterpenes has been found in different deciduous trees under herbivore stress (Stam et al., 2014; Zhu et al., 2014 for reviews). The interplay between different terpene synthase pathways is still somewhat unclear, especially given that different compounds serve different ecological functions. In Q. robur infected by the moth Tortrix viridana, Ghirardo et. al (2012) demonstrated that the larvae were attracted to the plants releasing higher amounts of homoterpene DMNT and monoterpene (E)-β-ocimene, while sesquiterpenes α-farnesene and germacrene D acted as a repellent.
We observed that the monoterpene emission rate increased with increasing leaf damage (Fig. 2c), indicating that the activity of monoterpene synthases increased upon herbivore feeding. In addition, as discussed above, monoterpene synthesis could have been further favored by greater competitive capacity for chloroplastic DMADP compared with isoprene synthesis (Fig. 5b) and inhibition of pigment synthesis as evident in the reduction in leaf pigment content (Fig. 5c). Scaling of mono- and sesquiterpene emissions with the degree of damage has been observed in several experiments looking at lepidopteran larval feeding effects on volatile release (for example Copolovici et al., 2014a; Copolovici et al., 2011). As we hypothesized, there was evidence of leveling off of monoterpene and DMNT vs. damage severity relationships at higher severity of damage (Fig. 2c, Fig. 3). In fact, the ratios of monoterpene to GLV emissions and DMNT to GLV emissions decreased with increasing the degree of damage, indicating that the induction response was relatively less prominent in more severely damaged leaves compared with the immediate stress response (or with the rapidly induced stress response). This is different from infection by small larvae (Copolovici et al., 2014a; Copolovici et al., 2011) or from infection by slowly developing biotic stresses such as fungal infections (Copolovici et al., 2014b; Jiang et al., 2016), where GLV and monoterpene and GLV emissions are almost proportional.
The emission of shikimate pathway derived compound, methyl salicylate (MeSA), is typically observed for sap-sucking herbivores such as aphids or whiteflies (Li et al., 2006; Zarate et al., 2007) and is not usually considered as part of chewing herbivore response. However, as in our study, several previous studies have demonstrated the release of methyl salicylate upon chewing herbivore attacks (Cardoza et al., 2002; Dicke et al., 1999), indicating a complex interplay between jasmonate- and salicylate-dependent signalling pathways upon herbivore infestations.
Conclusions
The results of the current study highlight a major negative scaling of foliage photosynthetic rates and constitutive isoprene emissions, and concomitant increase in induced volatile emissions upon feeding by large herbivore larvae (Fig. 1, 2, 5). While the damage-dependent increase of green leaf volatiles was disproportionately greater in leaves with extensive degree of damage than in moderately damaged leaves (Fig. 2b), emission rates of terpenoids leveled off at higher degrees of leaf damage (Fig. 2c). This suggests that faster and more severe damage, especially major vein severance by large herbivores can much more strongly influence foliage physiological activity than feeding by smaller herbivores. These contrasting herbivore responses need consideration in modeling herbivore elicited emissions in large-scale biosphere-atmosphere models.
Acknowledgements
Funding for this study has been provided by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the European Commission through the European Regional Fund (the Center of Excellence EcolChange), the European Research Council (advanced grant 322603, SIP-VOL+) and the European Commission and the Romanian Government (project POSCCE 621/2014). This work was also supported by a grant of the Romanian National Authority for Scientific Research, CNCS – UEFISCDI (project number PN-II-RU-TE-2011-3-0022). We thank the Forestry Directorate Arad (Lipova branch) for all their support during the time of sample collection.
References
- Abrams MD, Orwig DA. A 300-year history of disturbance and canopy recruitment for co-occurring white pine and hemlock on the Allegheny Plateau, USA. Journal of Ecology. 1996;84:353–363. [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000a;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- Arimura GI, Tashiro K, Kuhara S, Nishioka TOR, Takabayashi J. Gene responses in bean leaves induced by herbivory and by herbivory-induced volatiles. Biochemical and Biophysical Research Communications. 2000b;277:305–310. doi: 10.1006/bbrc.2000.3672. [DOI] [PubMed] [Google Scholar]
- Arneth A, Schurgers G, Lathière J, Duhl T, Beerling DJ, Hewitt CN, Martin M, Guenther A. Global terrestrial isoprene emission models: sensitivity to variability in climate and vegetation. Atmospheric Chemistry and Physics. 2011;11:8037–8052. [Google Scholar]
- Ashraf M, Harris PJC. Photosynthesis under stressful environments: An overview. Photosynthetica. 2013;51:163–190. [Google Scholar]
- Attaran E, Major IT, Cruz JA, Rosa BA, Koo AJK, Chen J, Kramer DM, He SY, Howe GA. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiology. 2014;165:1302–1314. doi: 10.1104/pp.114.239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- Batos B, Seslija Jovanovic D, Miljkovic D. Spatial and temporal variability of flowering in the pedunculate oak (Quercus robur L.) Sumarski List. 2014;138:371–379. [Google Scholar]
- Bauer K, Garbe D, Surburh H. The CD-ROM edition, 5th ed. Wiley-VCH Verlag; Berlin: 1998. Flavors and fragrances, Ullmann's encyclopedia of industrial chemistry. [Google Scholar]
- Becker C, Desneux N, Monticelli L, Fernandez X, Michel T, Lavoir A-V. Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. Biomed Research International. 2015 doi: 10.1155/2015/342982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande JD, Holopainen JK, Niinemets U. Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell and Environment. 2014;37:1892–1904. doi: 10.1111/pce.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Mueller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by Proton Transfer Reaction “Time-of-Flight” Mass Spectrometry (PTR-TOF) Plos One. 2011:6. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TJA. Interplay between insects and plants: dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. Journal of Experimental Botany. 2015;66:455–465. doi: 10.1093/jxb/eru391. [DOI] [PubMed] [Google Scholar]
- Cardoza YJ, Alborn HT, Tumlinson JH. In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. Journal of Chemical Ecology. 2002;28:161–174. doi: 10.1023/a:1013523104853. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kannaste A, Remmel T, Niinemets U. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environmental and Experimental Botany. 2014a;100:55–63. doi: 10.1016/j.envexpbot.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kannaste A, Remmel T, Vislap V, Niinemets U. Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Environmental impacts on plant volatile emission. In: Blande J, Glinwood R, editors. Deciphering chemical language of plant communication. Springer International Publishing; Berlin: 2016. pp. 35–59. [Google Scholar]
- Copolovici L, Vaartnou F, Portillo Estrada M, Niinemets U. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiology. 2014b;34:1399–1410. doi: 10.1093/treephys/tpu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KJ, Haile FJ, Peterson RKD, Higley LG. Impairment of Leaf Photosynthesis After Insect Herbivory or Mechanical Injury on Common Milkweed, Asclepias syriaca. Environmental Entomology. 2008;37:1332–1343. doi: 10.1603/0046-225x(2008)37[1332:iolpai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dicke M. Plant phenotypic plasticity in the phytobiome: a volatile issue. Current Opinion in Plant Biology. 2016;32:17–23. doi: 10.1016/j.pbi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the 'cry for help'. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. Journal of Chemical Ecology. 1999;25:1907–1922. [Google Scholar]
- Dwyer G, Dushoff J, Yee SH. The combined effects of pathogens and predators on insect outbreaks. Nature. 2004;430:341–345. doi: 10.1038/nature02569. [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annual Review of Plant Biology. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. [Google Scholar]
- Giraldo A, Heller W, Fladung M, Schnitzler J-P, Schroeder H. Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana. Plant Cell and Environment. 2012;35:2192–2207. doi: 10.1111/j.1365-3040.2012.02545.x. [DOI] [PubMed] [Google Scholar]
- Giorgi A, Manzo A, Nanayakkara NNMC, Giupponi L, Cocucci M, Panseri S. Effect of biotic and abiotic stresses on volatile emission of Achillea collina Becker ex Rchb. Natural Product Research. 2015;29:1695–1702. doi: 10.1080/14786419.2014.997725. [DOI] [PubMed] [Google Scholar]
- Gog L, Berenbaum MR, DeLucia EH, Zangerl AR. Autotoxic effects of essential oils on photosynthesis in parsley, parsnip, and rough lemon. Chemoecology. 2005;15:115–119. [Google Scholar]
- Guenther A. Upscaling biogenic volatile compound emissions from leaves to landscapes. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 391–414. [Google Scholar]
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1) an extended and updated framework for modeling biogenic emissions. Geosci Model Dev. 2012;5:1471–1492. [Google Scholar]
- Hare JD. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annual Review of Entomology. 2011;56:161–180. doi: 10.1146/annurev-ento-120709-144753. [DOI] [PubMed] [Google Scholar]
- Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, Niinemets Ü, et al. Volatile isoprenoid emissions from plastid to planet. New Phytologist. 2013;197:49–57. doi: 10.1111/nph.12021. [DOI] [PubMed] [Google Scholar]
- Heil M. Herbivore- induced plant volatiles: targets, perception and unanswered questions. New Phytologist. 2014;204:297–306. [Google Scholar]
- Holopainen JK. Can forest trees compensate for stress-generated growth losses by induced production of volatile compounds? Tree Physiology. 2011;31:1356–1377. doi: 10.1093/treephys/tpr111. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Blande JD. Where do herbivore-induced plant volatiles go? Frontiers in Plant Science. 2013:4. doi: 10.3389/fpls.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends in Plant Science. 2010;15:176–184. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang P-J, Zhang J, Lu Y-B, Huang F, Li M-J. Chlorophyll content and chlorophyll fluorescence in tomato leaves infested with an invasive mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae) Environmental Entomology. 2013;42:973–979. doi: 10.1603/EN12342. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Veromann L-L, Niinemets Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiology. 2016;38:856–872. doi: 10.1093/treephys/tpw035. [DOI] [PubMed] [Google Scholar]
- Johnson D, Gilbert L. Interplant signalling through hyphal networks. New Phytologist. 2015;205:1448–1453. doi: 10.1111/nph.13115. [DOI] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant isoprenoids: methods and protocols. Humana Press; New York: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- Kleist E, Mentel TF, Andres S, Bohne A, Folkers A, Kiendler-Scharr A, Rudich Y, Springer M, Tillmann R, Wildt J. Irreversible impacts of heat on the emissions of monoterpenes, sesquiterpenes, phenolic BVOC and green leaf volatiles from several tree species. Biogeosciences. 2012;9:5111–5123. [Google Scholar]
- Koski T-M, Laaksonen T, Mantyla E, Ruuskanen S, Li T, Giron-Calva PS, Huttunen L, Blande JD, Holopainen JK, Klemola T. Do insectivorous birds use volatile organic compounds from plants as olfactory foraging cues? Three experimental tests. Ethology. 2015;121:1131–1144. [Google Scholar]
- Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM, Hewitt CN. Isoprene emissions influence herbivore feeding decisions. Plant Cell and Environment. 2008;31:1410–1415. doi: 10.1111/j.1365-3040.2008.01849.x. [DOI] [PubMed] [Google Scholar]
- Lee AKY, Hayden KL, Herckes P, Leaitch WR, Liggio J, Macdonald AM, Abbatt JPD. Characterization of aerosol and cloud water at a mountain site during WACS 2010: secondary organic aerosol formation through oxidative cloud processing. Atmospheric Chemistry and Physics. 2012;12:7103–7116. [Google Scholar]
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Molecular Plant-Microbe Interactions. 2006;19:655–664. doi: 10.1094/MPMI-19-0655. [DOI] [PubMed] [Google Scholar]
- Link M, Zhou Y, Taubman B, Sherman J, Morrow H, Krintz I, Robertson L, Cook R, Stocks J, West M, Sive BC. A characterization of volatile organic compounds and secondary organic aerosol at a mountain site in the Southeastern United States. Journal of Atmospherical Chemistry. 2015;72:81–104. [Google Scholar]
- Loivamaeki M, Mumm R, Dicke M, Schnitzler J-P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17430–17435. doi: 10.1073/pnas.0804488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard J, Moreira D. Origins and early evolution of the mevalonate pathway of isoprenoid Biosynthesis in the three domains of life. Molecular Biology and Evolution. 2011;28:87–99. doi: 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- Loreto F, Dicke M, Schnitzler J-P, Turlings TCJ. Plant volatiles and the environment. Plant Cell and Environment. 2014;37:1905–1908. doi: 10.1111/pce.12369. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Lucas-Barbosa D. Integrating studies on plant-pollinator and plant-herbivore interactions. Trends in plant science. 2016;21:125–133. doi: 10.1016/j.tplants.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Lucas-Barbosa D, van Loon JJA, Dicke M. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry. 2011;72:1647–1654. doi: 10.1016/j.phytochem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Current Opinion in Plant Biology. 2006;9:274–280. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Matsui K, Sugimoto K, Mano Ji, Ozawa R, Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PloS ONE. 2012;7:e36433. doi: 10.1371/journal.pone.0036433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ, Haack RA. The role of drought in outbreaks of plant-eating insects. BioScience. 1987;37:110–118. [Google Scholar]
- Milanovic S, Lazarevic J, Popovic Z, Miletic Z, Kostic M, Radulovic Z, Karadzic D, Vuleta A. Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. European Journal of Entomology. 2014;111:371–378. [Google Scholar]
- Moldau H, Wong S-C, Osmond CB. Transient depression of photosynthesis in bean leaves during rapid water loss. Australian Journal of Plant Physiology. 1993;20:45–54. [Google Scholar]
- Munné-Bosch S. Ageing in perennials. Critical Reviews in Plant Sciences. 2007;26:123–138. [Google Scholar]
- Munné-Bosch S. Do perennials really senesce? Trends Plant Sci. 2008;13:216–220. doi: 10.1016/j.tplants.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Annals of Botany. 2009;103:655–663. doi: 10.1093/aob/mcn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. Herbivore induction of jasmonic acid and chemical defences reduce photosynthesis in Nicotiana attenuata. Journal of Experimental Botany. 2013;64:685–694. doi: 10.1093/jxb/ers364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science. 2010;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–2223. [Google Scholar]
- Niinemets U, Bilger W, Kull O, Tenhunen JD. Acclimation to high irradiance in temperate deciduous trees in the field: changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell and Environment. 1998;21:1205–1218. [Google Scholar]
- Niinemets Ü, Copolovici L, Hüve K. High within-canopy variation in isoprene emission potentials in temperate trees: implications for predicting canopy-scale isoprene fluxes. Journal of Geophysical Research - Biogeosciences. 2010b;115:G04029. [Google Scholar]
- Niinemets Ü, García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. Frontiers in Plant-Microbe Interaction. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Opris O, Copaciu F, Soran ML, Ristoiu D, Niinemets U, Copolovici L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicology and Environmental Safety. 2013;87:70–79. doi: 10.1016/j.ecoenv.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Pazouki L, Kanagendran A, Li S, Kännaste A, Rajabi Memari H, Bichele R, Niinemets Ü. Mono- and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: from gene expression to emission responses. Environmental and Experimental Botany. 2016;132:1–15. doi: 10.1016/j.envexpbot.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazouki L, Niinemets Ü. Multi-substrate terpenoid synthases: their occurrence and physiological significance. Frontiers in Plant Science. 2016;7:1019. doi: 10.3389/fpls.2016.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002a;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Porta H, Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiology. 2002;130:15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different damage treatments in aspen (Populus tremula) Journal of Chemical Ecology. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell & Environment. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü. Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiology. 2011;156:816–831. doi: 10.1104/pp.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiology. 2009;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman F, Khan FA, Anis SB. Assessment of aphid infestation levels in some cultivars of mustard with varying defensive traits. Archives of Phytopathology and Plant Protection. 2014;47:1866–1874. [Google Scholar]
- Roslin T, Gripenberg S, Salminen JP, Karonen M, O'Hara RB, Pihlaja K, Pulkkinen P. Seeing the trees for the leaves - oaks as mosaics for a host-specific moth. Oikos. 2006;113:106–120. [Google Scholar]
- Sack L, Cowan PD, Holbrook NM. The major veins of mesomorphic leaves revisited: testing for conductive overload in Acer saccharum (Aceraceae) and Quercus rubra (Fagaceae) American Journal of Botany. 2003;90:32–39. doi: 10.3732/ajb.90.1.32. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. International Journal of Molecular Sciences. 2013;14:17781–17811. doi: 10.3390/ijms140917781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum. 2010;138:123–133. doi: 10.1111/j.1399-3054.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Etalo DW, de Jager V, Gerards S, Zweers H, de Boer W, Garbeva P. Microbial small talk: volatiles in fungal-bacterial interactions. Frontiers in Microbiology. 2016;6 doi: 10.3389/fmicb.2015.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Zhao Y, Chen Z, Huang D. Heterogeneous reactions of volatile organic compounds in the atmosphere. Atmospheric Environment. 2013;68:297–314. [Google Scholar]
- Stam JM, Kroes A, Li Y, Gols R, van Loon JJA, Poelman EH, Dicke M. Plant interactions with multiple insect herbivores: from community to genes. Annual Review of Plant Biology. 2014;65:689–713. doi: 10.1146/annurev-arplant-050213-035937. [DOI] [PubMed] [Google Scholar]
- Tholl D, Sohrabi R, Huh J-H, Lee S. The biochemistry of homoterpenes – common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry. 2011;72:1635–1646. doi: 10.1016/j.phytochem.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Thomas FM, Blank R, Hartmann G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology. 2002;32:277–307. [Google Scholar]
- Tonioli M, Escarre J, Lepart J, Speranza M. Facilitation and competition affecting the regeneration of Quercus pubescens Willd. Ecoscience. 2001;8:381–391. [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vranova E, Coman D, Gruissem W. Structure and dynamics of the isoprenoid pathway network. Molecular Plant. 2012;5:318–333. doi: 10.1093/mp/sss015. [DOI] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiology. 2007;43:866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, McVay RC, Huang DD, Dalleska NF, Aumont B, Flagan RC, Seinfeld JH. Formation and evolution of molecular products in alpha-pinene secondary organic aerosol. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14168–14173. doi: 10.1073/pnas.1517742112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lou Y-R, Tzin V, Jander G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiology. 2015;169:1488–1498. doi: 10.1104/pp.15.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Poelman EH, Dicke M. Insect herbivore- associated organisms affect plant responses to herbivory. New Phytologist. 2014;204:315–321. [Google Scholar]
- Ziemann PJ, Atkinson R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chemical Society Reviews. 2012;41:6582–6605. doi: 10.1039/c2cs35122f. [DOI] [PubMed] [Google Scholar]