Visual Abstract
Key Words: bioprosthesis, therapy, ultrasonic
Abbreviations and Acronyms: CPB, cardiopulmonary bypass; PCU, pulsed cavitational ultrasound; PHT, pressure half time
Highlights
-
•
Bioprosthetic heart valves have limited durability, with a progressive deterioration of the bioprosthesis after 12 to 15 years, mainly due to intravalvular calcifications.
-
•
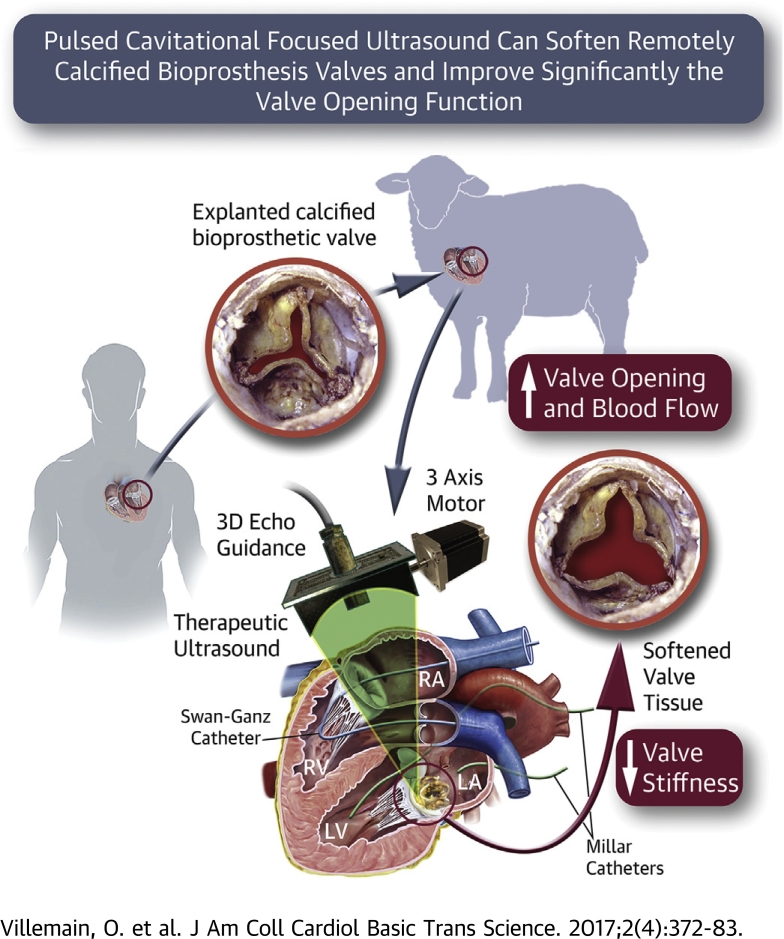
In this proof-of-concept study, we demonstrated in vivo using an ovine model and in vitro that pulsed cavitational focused ultrasound can be used to remotely soften human degenerative calcified bioprosthetic valves and significantly improve the valve opening function.
-
•
This new noninvasive approach has the potential to improve the outcome of patients with severe bioprosthesis stenosis.
Summary
The authors propose a novel noninvasive therapeutic approach for degenerative calcified bioprosthetic heart valves based on pulsed cavitational ultrasound (PCU) to improve the valvular function by remotely softening calcified stiff cusps. This study aims to demonstrate both in vivo, using an ovine model with implanted human calcified bioprosthesis, and in vitro that PCU can significantly improve the bioprosthesis function. A 50% decrease of the transvalvular gradient was found, demonstrating a strong improvement of the valve opening function. This new noninvasive approach has the potential to improve the outcomes of patients with severe bioprosthesis stenosis.
Bioprosthetic valves are becoming increasingly common in patients with valvular heart diseases. They are often favored over mechanical valves because of a lower risk of thrombotic or bleeding events, as well as the desire to avoid lifetime anticoagulation medications (1). However, bioprosthetic valves have limited durability, with a progressive deterioration of the bioprosthesis after 12 to 15 years, mainly due to intravalvular calcifications (2). The need for a redo surgery due to the incidence of structural valve deterioration is expected to increase (3). Nevertheless, redo valve surgery is associated with significant morbidity and mortality (4). Transcatheter valve-in-valve implantation has emerged as a promising, less invasive alternative to redo surgery; however, it still comes with its own various set of complications (5).
In parallel, for about 30 years, other therapeutic strategies 6, 7 have been investigated to treat calcified valves (native or bioprosthetic). One promising approach was ultrasound-based (8), but it remained limited at the time by the need for open surgery with cardiopulmonary bypass (CPB). Nevertheless, this limitation must be challenged again with the recent improvement of technologies and concepts of pulsed cavitational focused ultrasound (PCU) or histotripsy. Histotripsy is a noninvasive, cavitation-based therapy (mechanical effect) based on very short, high-pressure ultrasound pulses focused in tissues to generate a dense, energetic, lesion-producing bubble cloud. Although histotripsy can be used to produce sharp lesions in soft tissues 9, 10, recent studies have suggested that cavitation activity can also soften biological tissues (11).
The objective of our study was to evaluate the efficacy of PCU to significantly improve valve opening of severe degenerative calcified bioprosthetic valves. First, we demonstrated in vitro the efficacy of PCU on a human explanted calcified bioprosthesis mounted on an artificial heart pump and quantified the improvement of the valvular function. Then, we demonstrated its feasibility and efficacy in vivo in the beating heart of an ovine model with implanted calcified bioprosthesis.
Methods
Model of heart calcified valve
We used Carpentier-Edwards Perimount Magna (stented bovine pericardial bioprosthesis, Edwards Lifesciences, Irvine, California), explanted on a human, as a model of heart calcified valve stenosis. For all patients, the indication of explant was a severe stenosis with calcification. Each valve was fixed in glutaraldehyde 0.6% immediately after explant. Before each experiment, the valve was immersed for 5 min in saline serum (0.9% NaCl) 3 consecutive times. This protocol was in agreement with institutional guidelines (national reference number of the 143 study: 02255.02).
Ultrasound generation and PCU acoustic parameters
A 1.25-MHz focused single-element transducer (Imasonic, Besançon, France), called a therapy transducer, was used to generate PCU. This transductor had a 100-mm focal length (f-number = 1) and was driven by a high-voltage amplifier (GA-2500A, RITEC, Warwick, Rhode Island). We produced PCU using 10-cycle pulses, each 8 μs long, delivered at a pulse repetition frequency of 100 Hz. We estimated the pressure peak amplitudes at the focal spot to be 70 and −19 MPa, respectively, for the positive and negative peak pressure.
Ultrasound cavitational treatment guidance and monitoring
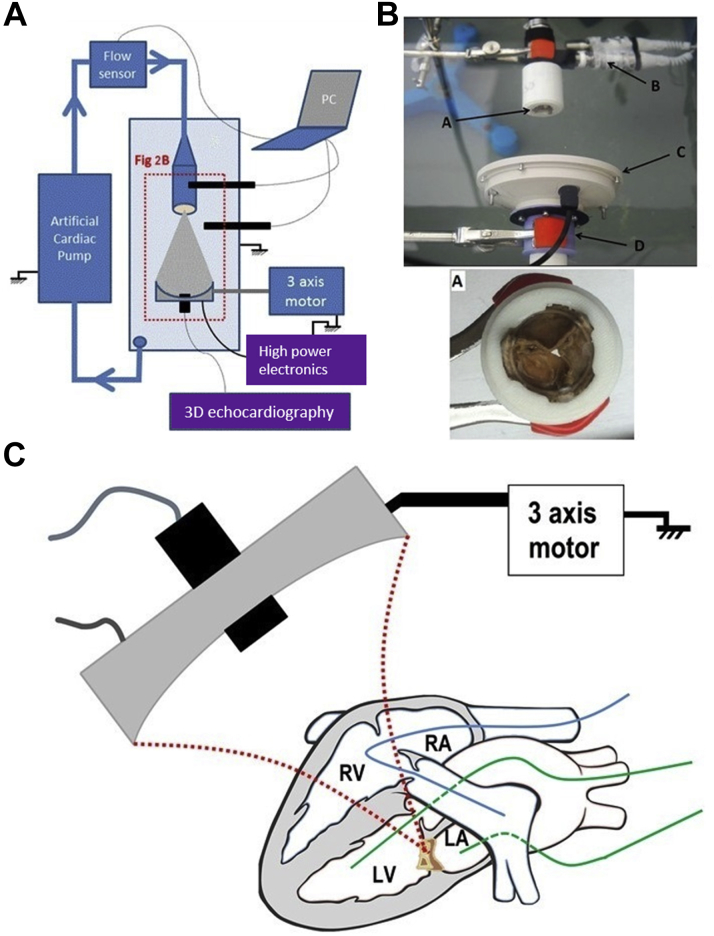
Three-dimensional echocardiography was used to guide and monitor the treatment. An IE33 scanner and X5-1 probe (xMATRIX array, 3 MHz, 3,040 elements with microbeam-forming) (Philips Healthcare, Bothell, Washington) were used. The imaging probe was fixed through a hole in the center of the therapy transducer (Figure 1). The focal spot of the therapy transducer was positioned on the central axis of the imaging probe at a depth of 100 mm. A biplane imaging mode with 2 imaging planes set at 90° was used during the entire procedure. The cavitation bubble cloud generated at the PCU focal spot was visible within the 2 imaging planes. The combination of therapy transducer and imaging probe was called the “therapy device.” The same material was used for in vitro and in vivo procedures.
Figure 1.
Image-Guided Therapy Setup
The imaging probe was fixed through a hole in the center of the pulsed cavitational ultrasound therapy transducer.
For all the procedures, 10 min sequences of PCU were applied and repeated until the transvalvular gradient was stabilized for 3 consecutive sequences (considered as the ineffective threshold for PCU procedures). The therapy device was controlled by a 3-axis motor to scan the PCU continuously and uniformly over the entire valve.
Shear wave elastography evaluation
The assessment of valve leaflet biomechanical properties remains challenging and requires destructive strain-stress mechanical tests. To noninvasively assess the modification of the biomechanical properties induced by PCU, we used shear wave elastography, an ultrasound-based tool for noninvasive evaluation of soft tissue’s stiffness. We used the Aixplorer ultrasound imaging system (Aixplorer, Supersonic Imagine, Aix-en-Provence, France) with a linear probe (SL10-2) to evaluate the stiffness of each valvular leaflet. Three acquisitions were made for each leaflet, using the shear wave elastography imaging mode (SWE) of the Aixplorer scanner in the “penetration” setting. A ‘‘QBox’’ region of interest (mean diameter 1 mm) was positioned inside the elasticity image after each acquisition to obtain a mean stiffness value.
In vitro procedure
The objective of the in vitro procedure was to analyze the effect of the PCU on the anterograde transvalvular flow, with a pulsatile flow equivalent to the cardiac flow (Figures 2A and 2B). The flow was generated by an artificial heart pump (Pulsatile Blood Pump, Harvard Apparatus, Holliston, Massachusetts) with flow controlled variation. The flow rates were applied at 3, 4, and 5 l/min, and were monitored by a flow sensor (Small Flow Meter Kit, Atlas Scientific, Jacksonville, Florida) (accuracy ± 1 ml/min). The valve and the therapy device were immersed in degassed water. A total of 8 bioprostheses were explanted and used for this procedure. The transvalvular pressure gradient was estimated by:
-
•
A pulsed Doppler ultrasound assessment by applying the Bernoulli equation: ΔP = 4 (Vmax)2
-
•
Hemodynamic assessment by pressure sensor before and after the valve (sensor IXIAN 0 to 7.5 PSI Industrial Control Pressure Sensor, Atlas Scientific; accuracy ± 1 mm Hg).
Figure 2.
Experimental and In Vivo Procedures
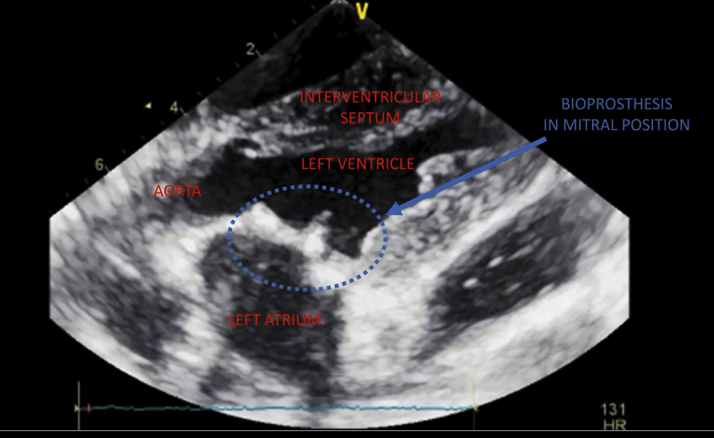
(A) In vitro procedure setup. (B) Photography of a part of the in vitro setup. The bioprosthesis (A) was placed in front of the therapy device composed of a therapy transducer (C) and an imaging transducer (D). The 2 pressure sensors (B) were positioned before and after the valve. (C) In vivo procedure, in the beating heart. The bioprosthesis was implanted in the mitral position, between the LA and the LV. The Swan-Ganz catheter (blue, to estimate the cardiac flow in real time), was positioned into the right heart (RV and RA) and the pulmonary artery. The Millar catheters (green), were positioned into the LA and LV to estimate the transvalvular gradient in real time. PCU was then applied all over the bioprosthesis by moving the therapy device using the 3-axis motorized stage. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
The pump operated during 2 h at a 4 l/min flow rate (70 cycles/min, ejection volume: 57 ml) to control the variation of the gradient before PCU, after which sequences of PCU were applied. All post-PCU transvalvular gradients were reassessed 1 month after the procedure (each valve was fixed in glutaraldehyde between these evaluations). Elastography was performed before and after the procedure on each valve.
Finally, the bioprostheses were sent to the department of pathology for histopathological analysis.
In vivo procedure: Sheep model
The in vivo procedure is shown in Figure 2C. The animal procedure was approved by the Institutional Animal Care and Use Committee of the Hôpital Européen Georges Pompidou (PARCC) according to the European Commission guiding principles (2010/63/EU).
The sheep were anesthetized with thiopentothal (0.5 ml/kg), intubated, ventilated at 15 ml/kg with 2% isoflurane, and given glycopyrrolate (0.4 mg intravenous) and vancomycin (0.5 g intravenous). A sterile sternotomy was performed. The calcified bioprosthesis was implanted in the mitral position after CPB. Vital signs (including heart rate, oxygen saturation, and arterial blood pressure), left atrial and ventricle pressure (by 2 Mikro-Tip Millar Catheter Transducers, to have the transvalvular pressure gradient in real time), and cardiac flow (by a Swan-Ganz CCOmbo Pulmonary Artery Catheter, Edwards Lifesciences) were monitored. The CPB was stopped and removed to restore independent cardiac activity. Sternotomy was maintained and the thorax was filled with degassed saline water. A complete echocardiography was performed, especially to evaluate the calcified bioprosthesis 12, 13, 14. A total of 14 explanted bioprostheses were used for this procedure.
We then applied the sequences of PCU. An echocardiographic evaluation was performed between each 10-min sequence, concurrent with the catheter’s evaluation (pressure and cardiac flow).
At the end of the procedure, the animal was sacrificed (Dolethal intravenous injection, 1 ml/kg) and an anatomic macroscopic evaluation of the cardiac structure was performed. Elastography examination of the bioprosthesis was performed before and after each procedure. The bioprosthesis was then explanted and sent after elastography to the department of pathology for histopathological analysis.
Microscopic analysis
Histological exploration
Immediately after the procedure (in vitro and in vivo), the bioprosthesis was dissected, fixed in formalin, and embedded in individual paraffin blocks. Regions of interest, such as macroscopic calcification on leaflet, were labelled with tattoo ink. Serial sections were stained with hematoxylin and eosin for histopathological analysis.
In addition, we also analyzed 5 calcified bioprostheses directly after their explantation from humans, without any PCU procedure. The objective was to perform a histopathological comparison between bioprostheses with or without PCU.
Micro-computed tomography imaging
The micro–computed tomography (CT) device used in this study was the Quantum FX Caliper (Life Sciences, Perkin Elmer, Waltham, Massachusetts). Samples up to a 10-mm diameter field of view and 3-dimensional acquisitions were performed using an isotropic voxel size of 20 × 20 × 20 μm3. Full 3-dimensional, high-resolution raw data were obtained by rotating both the x-ray source and the flat panel detector 360° around the sample, with a rotation step of 0.1°. The corresponding 3,600 image projections were then automatically reconstructed (RigakuSW software, Caliper, Newton, Massachusetts) into a DICOM (Digital Imaging and Communications in Medicine) stack of 512 files, using standard back-projection techniques.
Statistical analysis
Continuous variables were presented as mean ± SD. A Wilcoxon matched-pairs signed-ranks test was performed to evaluate the SD between individual mean values before and after therapy. The level of significance was set at an alpha level of ≤0.05. The analysis was conducted using MedCalc software (version 14.10.2, MedCalc, Mariakerke, Belgium).
Results
All of our results consistently showed a softening of the valve leaflets, allowing a decrease of the anterograde gradient after PCU. This decrease persisted 1 month after the procedure. The decrease of transvalvular gradient measured by Doppler echocardiography was confirmed by invasive pressure sensors in both the in vitro and in vivo setup.
In vitro procedure
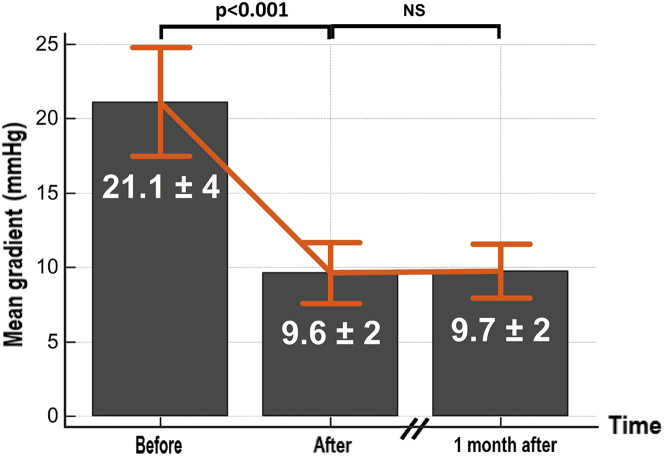
Eight bioprosthesis were explanted and used for the in vitro procedure (Figure 3). At a flow rate of 4 l/min, the mean transvalvular gradient over the set of valves was 21.1 ± 3.9 mm Hg (maximum 38 mm Hg, minimum 10 mm Hg) (Figure 3), and the maximum gradient was 39 ± 6.9 mm Hg (maximum 73 mm Hg, minimum 22 mm Hg). After 2 h of controlled pulsatile flow without PCU, we observed no statistically significant change of the transvalvular gradients. The mean duration of PCU was 70 ± 12 min with a maximum duration of 90 min and a minimum of 50 min. After the procedure, the mean transvalvular gradient was 9.6 ± 1.7 mm Hg (maximum 19 mm Hg; minimum 4 mm Hg), corresponding to a decrease of 55 ± 10% (p < 0.001). The maximum gradient was 19.6 ± 3.5 mm Hg (maximum 37 mm Hg; minimum 10 mm Hg), corresponding to a decrease of 51 ± 9% (p < 0.001). The results of each individual bioprosthesis are shown in Supplemental Figure 1.
Figure 3.
In Vitro Results
Mean transvalvular gradient measured before and after pulsed cavitational ultrasound.
Hemodynamic parameters were also measured at 3 and 5 l/min before and after procedure. The gradients also showed a significant decrease at the different flow rates (p < 0.001) (Supplemental Figure 2). At 3 l/min, the mean gradient varied from 14.2 ± 2.5 mm Hg to 7.1 ± 1.2 mm Hg (p < 0.001) and the maximum gradient from 29.1 ± 5.1 mm Hg to 14.9 ± 2.6 mm Hg (p < 0.001). At 5 l/min, the mean gradient varied from 23.8 ± 4.2 mm Hg to 13.0 ± 2.3 mm Hg (p < 0.001) and the maximum gradient from 42.3 ± 7.5 mm Hg to 24.1 ± 4.3 mm Hg (p < 0.001).
The gradient dependence with flow rate allowed us to assess the pliability of the valve cusps before and after the procedure. Pliability is indeed inversely linked to the slope of the gradient–flow rate relationship. The slope of the maximum gradient–flow rate relationship was found to decrease from 9 ± 4.3 mm Hg/l/min to 4.9 ± 2.9 mm Hg/l/min (p < 0.001), which confirmed an improved pliability of the valve cusps after the procedure.
All post-PCU transvalvular gradients were reassessed 1 month after the procedure, and no statistically significant difference was observed (Figure 3).
In vivo procedure
Fourteen explanted bioprosthesis were used for this procedure (Table 1, Figures 4 and 5, Supplemental Video 1). A total of 7 animals experienced a massive acute pulmonary edema with severe heart failure just after the implantation of the valve and the cessation of the CPB. These animals died before the PCU procedure. The other animals tolerated the implantation. Thus, 7 valves were treated and analyzed. The mean weight of the animals was 37.8 ± 4.6 kg (minimum 29 kg; maximum 43 kg).
Table 1.
In Vivo Results
| Before Therapy | After Therapy | Variation (%)∗ | p Value | |
|---|---|---|---|---|
| Shear wave imaging | ||||
| Elastography (kPa) | 76.1 ± 23.7 | 35.6 ± 7.2 | −52 ± 7 | <0.001 |
| Echocardiography | ||||
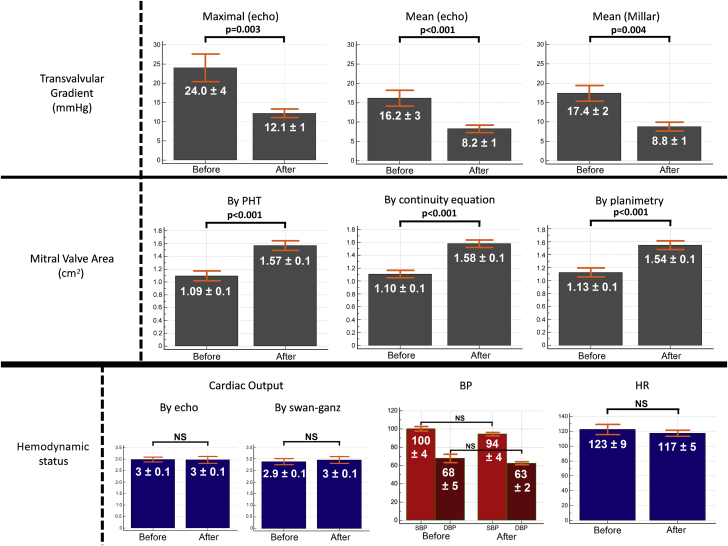
| Doppler mitral valve (Figure 5) | ||||
| Maximal velocity, m/s | 2.41 ± 0.50 | 1.73 ± 0.21 | −28 ± 6 | 0.007 |
| Maximal pressure gradient, mm Hg | 24.0 ± 4.4 | 12.1 ± 1.4 | −49 ± 11 | 0.003 |
| Mean velocity, m/s | 1.95 ± 0.36 | 1.38 ± 0.24 | −29 ± 6 | 0.001 |
| Mean pressure gradient, mm Hg | 16.2 ± 3.2 | 8.2 ± 1.3 | −48 ± 7 | <0.001 |
| Mitral valve area, cm2 | ||||
| By pressure half time (Figure 5) | 1.09 ± 0.09 | 1.57 ± 0.08 | +143 ± 18 | <0.001 |
| By continuity equation | 1.10 ± 0.15 | 1.58 ± 0.15 | +142 ± 15 | <0.001 |
| By planimetry (Figure 5) | 1.13 ± 0.13 | 1.54 ± 0.14 | +137 ± 14 | <0.001 |
| Pulmonary artery pressure, mm Hg | ||||
| Maximal (tricuspid valve) | 64.7 ± 12.8 | 34.1 ± 10.2 | −47 ± 12 | <0.001 |
| Cardiac output,† l/min | 2.98 ± 0.10 | 2.96 ± 0.14 | −1.0 ± 0.2 | 0.83 |
| Pressure sensors (Millar), mm Hg | ||||
| Mean diastolic LAP | 36.8 ± 6.2 | 20.2 ± 5.1 | −44 ± 11 | 0.004 |
| Mean diastolic LVP | 17.4 ± 2.7 | 11.4 ± 1.9 | −35 ± 10 | 0.014 |
| Mean diastolic gradient LVP-LAP | 17.4 ± 2.4 | 8.8 ± 1.2 | −50 ± 13 | 0.002 |
| Cardiac flow captor (l/min) | ||||
| Swan-Ganz catheter | 2.87 ± 0.11 | 2.96 ± 0.14 | +3.0 ± 0.4 | 0.74 |
Values are mean ± SD.
HR = heart rate; LAP = left atrial pressure; LVOT = left ventricular outflow tract; LVP = left ventricular pressure; VTI = velocity time integral.
Variation = ([result after – result before]/result before) × 100.
Cardiac output = HR × LVOT area × LVOT VTI.
Figure 4.
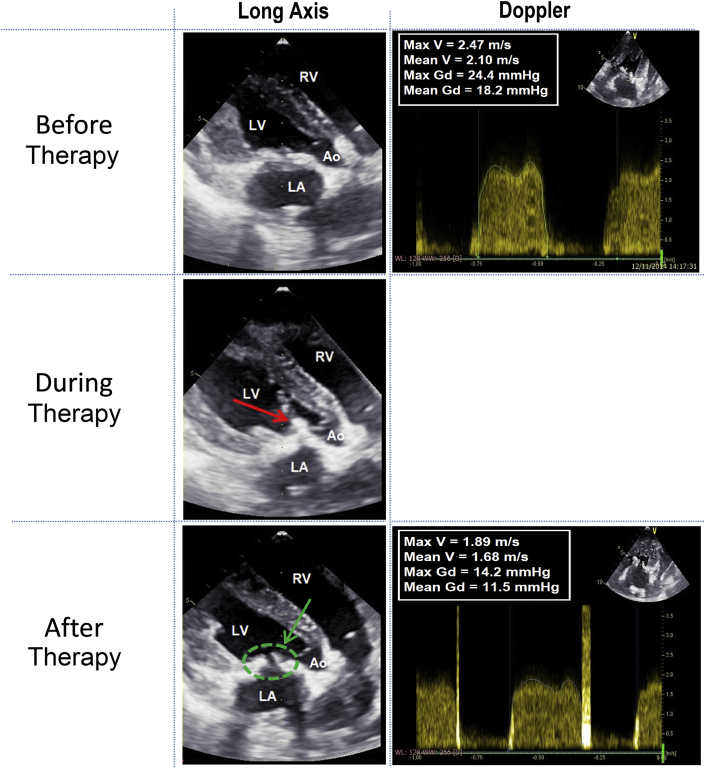
Images of Echocardiography During Procedure
During PCU a “cavitation bubble cloud” is visible as a hyperechogenic zone (red arrow). After PCU, the improvement of the bioprosthesis opening is confirmed thanks to the echocardiography (green arrow). The images are realized during diastasis (closed aortic valve). See Supplemental Video 1. Ao = aorta; other abbreviations as in Figure 2.
In vivo procedure of pulsed cavitational ultrasound therapy
Figure 5.
Results In Vivo, Before and After Pulsed Cavitational Focused Ultrasound
BP = blood pressure; HR = heart rate; PHT = pressure half-time method.
Just after the valve implantation, we monitored all parameters for 1 h before any PCU procedure. From the beginning (just after the valve implantation) to 1 h after implantation (before any PCU procedure), there was no statistical significant difference of the transvalvular gradients (p = 0.45) and mitral valve areas (planimetry, p = 0.38; continuity equation, p = 0.74; pressure half time, p = 0.51).
The mean procedure duration was 60 ± 13 min (maximum 100 min; minimum 40 min) (see Supplemental Video 1 of an in vivo procedure). We observed an important decrease of the transvalvular gradient after PCU (Table 1), which was on average reduced by 50%. The results of elastography, echocardiography, and of pressure/flow cardiac catheters after the PCU procedures are synthesized in Table 1. The mean cardiac frequency was 123 ± 9 (minimum 94; maximum 154), and all hemodynamic parameters were stable during the procedures: heart rate (p = 0.24), blood pressure (p = 0.27), and O2 saturation (p = 0.42).
No mitral valve regurgitation was observed at the end of the procedures.
We observed isolated ventricular extrasystoles in 2 animals, without any repercussion in hemodynamic parameters. As long as the focal spot of the therapy device remained on the bioprosthesis, no arrhythmia was visible.
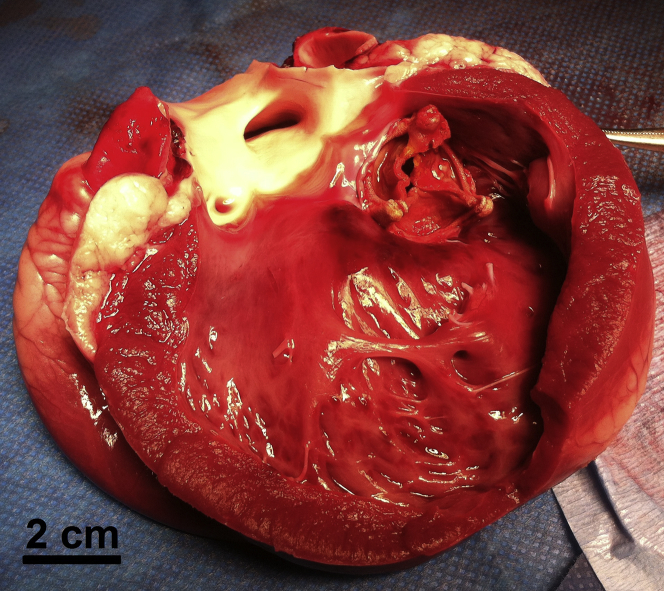
After the threshold was reached and all parameters were re-evaluated, the animal was sacrificed and the heart was explanted. Macroscopic analysis showed that all cardiac structures were intact (Figure 6), except in 1 animal in which a 7-mm diameter superficial hematoma (epicardium) was visible at the lateral left ventricle wall (on the path of the ultrasound beam). This animal was also 1 of 2 animals that presented isolated ventricular extrasystoles. At the end of the procedure, the bioprosthesis was sent to the department of pathology for histopathological analysis.
Figure 6.
Macroscopic Postmortem Analysis
Macroscopic analysis showed that all cardiac structures were intact. The whole hearts appeared intact from the outside, with no visible damage on the epicardial surfaces (except in 2 animals in which a 7-mm diameter superficial hematoma [epicardium] was visible). After dissection, no damage was observed inside of the heart.
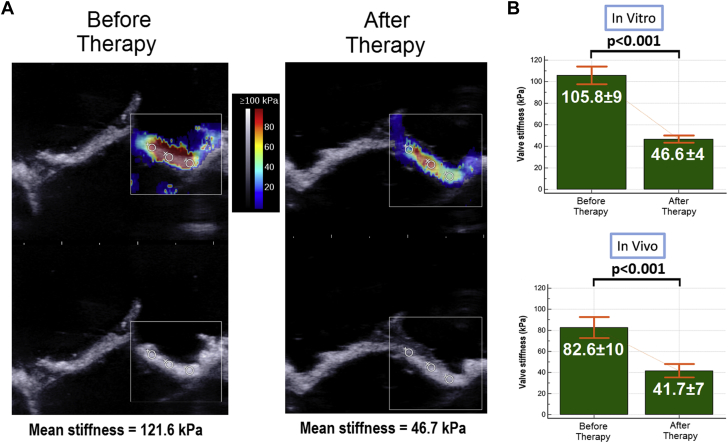
Elastography
Elastography results are displayed in Figures 7A and 7B. In vitro, before PCU, the mean stiffness of the valve leaflets measured by elastography was 105.8 ± 9 kPa. After the procedure, the mean stiffness of the valve leaflets measured by elastography was 46.6 ± 4 kPa, corresponding to a decrease of 55 ± 8% (p < 0.001).
Figure 7.
Elastography
(A) Example of bioprosthesis elastography in vitro by shear wave elastography. (B) Elastrography results (in vitro and in vivo).
We observed a similar stiffness decrease for the bioprosthesis used in vivo (82.6 ± 10 kPa before the procedure and 41.7 ± 7 kPa after PCU, 49 ± 7% decrease; p < 0.001). The results of each individual bioprosthesis are shown in Supplemental Figure 3.
Microscopic analysis
Histological exploration
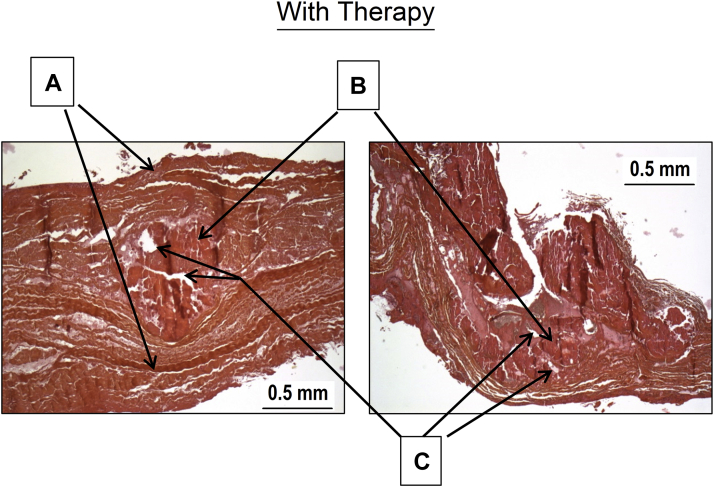
The histological exploration is described in Figure 8. All superficial structures of the leaflets were intact (fibrosa and ventricularis). In comparison with the 5 bioprosthesis explanted without PCU procedure, we observed:
-
•
A fragmentation of the calcification.
-
•
Presence of vacuoles inside the calcification.
Figure 8.
Histological Analysis
After pulsed cavitational focused ultrasound, the superficial structures remain intact (A). An aspect of calcification fragmentation is visible (B). Some vacuoles are also found inside of the calcifications (C).
There was no histological evidence for acute inflammation or acute thrombosis on the bioprosthesis.
Micro-CT imaging
Micro-CT imaging did not show modifications of the calcification shapes (Supplemental Figure 4). However, we did observe qualitatively multiple fragmentations inside of the calcification after PCU. These micro-fragmentations are visible as subvoxel modifications of the CT image (size <20 μm) so that quantitative analysis remains limited.
The estimation of calcification volume shows no statistical difference before PCU (mean volume = 294.9 mm3 [minimum 45.7 mm3; maximum 662.3 mm3]) and after PCU (mean volume = 288.9 mm3 [minimum 42.9 mm3; maximum 659.9 mm3]), with a mean diminution of 2.02% (p = 0.44).
Discussion
In this study, we demonstrated in vitro and in vivo that PCU can remotely decrease the transvalvular gradients of calcified bioprosthetic valves. The mean and maximal transvalvular gradients decreased by 2-fold on average in both the in vitro and in vivo setups. Moreover, these hemodynamic modifications persisted after 1 month (evaluated only in vitro). The evolution of other echocardiographic parameters measured in vivo (valve area, pulmonary artery pressure) confirmed a consistent decrease of the valvular stenosis. Finally, we showed that PCU induced a decrease of the valves leaflet stiffness.
We believe that PCU can have a real clinical effect on the treatment of calcified bioprosthesis stenosis. Its 2 main advantages are: 1) it could be potentially applied in a transthoracic configuration completely noninvasively; and 2) it would allow the preservation of the bioprosthesis valve ad integrum.
Effect of PCU on calcified bioprosthesis valve
Using quantitative shear wave elastography, we demonstrated that the biomechanical properties of calcified leaflets were modified by PCU. The stiffness was decreased on average by 2-fold. The softening mechanism induced by PCU may be complex and needs to be further investigated; however, we can hypothesize that calcifications are mostly affected by the treatment. The histological analyses showed a fragmentation of calcifications with the preservation of the leaflet superficial structures. Micro-CT imaging, which can be considered a gold standard for valvular calcification evaluation 15, 16, confirmed that microfragmentations appeared inside the calcification after PCU. This microfragmentation of large calcifications could explain the overall change of biomechanical properties, which leads to the improvement of the leaflet motion. Further investigation is required to better understand other potential mechanisms of PCU and, in particular, its effect on soft valvular tissues.
Safety
Accuracy of therapy
For 2 animals, we observed a few nonpersistent ventricular extrasystoles, and post-mortem anatomic exploration showed bruising of the cardiac wall due to off-target cavitation. These 2 undesirable effects are mostly induced by the actual complexity of the target positioning and motion, and could be greatly reduced with some technical improvements. As our therapeutic transducer is readily focused at a single location, the target position can only be changed by mechanically and slowly moving the therapy device with millimetric motors. To solve this problem, a multielement transducer could be used to steer the focal spot electronically in real time. Three-dimensional motion correction would then be feasible in real-time, based on an accurate ultrasonic speckle-tracking method that has been demonstrated for high-intensity focused ultrasound applications in 2004 by our team (17) and, more recently, for histotripsy (18). With such a motion correction technique, we would be able to track the valve motion all along the cardiac cycle and avoid off-target cavitation. An easier strategy would be to trigger PCU exposures by electrocardiogram (19). We could, for instance, select specific moments in the cardiac cycle when the valve is closed. Thus, its whole surface would be equally exposed and far away from the cardiac wall.
Emboli
Our study aimed to demonstrate proof of concept of efficacy. We have thus not precisely evaluated the clinical risk of emboli during and after PCU by endovascular filter or brain magnetic resonance imaging/CT, and these explorations should be performed during specific risk evaluation studies. Nevertheless, our histological exploration after in vitro and in vivo procedures have shown the preservation of the leaflet superficial structures (fibrosa and ventricularis). These are fundamental structures around the calcification (20), meaning that our action seems to be confined within the leaflet. This result was confirmed by the micro-CT explorations, which found no statistical difference in calcification volumes before and after PCU. This local intravalvular effect is a reassuring argument against the major risk of emboli. Moreover, the specific histological lesions due to PCU noted inside the calcification (vacuole) seem to preserve the overall architecture of the valve leaflet.
Concerning cardiac application of PCU, in 2009, Xu et al. (21) showed that the size of myocardium debris obtained after histotripsy was inferior to the size of a red blood cell for >99% of the debris. As of now, no studies have been conducted specifically on valvular calcifications and PCU.
Study limitations
In vivo follow-up
We observed stability of the transvalvular gradient in vitro 1 month after the procedure. However, this was not confirmed for the in vivo procedure, because we sacrificed the animals directly after the procedure. It is, therefore, difficult to evaluate the persistence of the therapeutic effect.
Shear wave elastography evaluation
Shear wave elastography has never been used for the stiffness evaluation of valve leaflets; therefore, this application needs to be validated in further studies. In particular, previous studies have shown the limitation of shear wave elastography in thin layers such as in the arterial wall. In such a medium, the shear waves are guided inside the 2-dimensional leaflet, and correspond to leaky lamb wave propagation (22). This affects the elastography measures, which prohibits direct derivation of the absolute values of leaflet stiffness. Nevertheless, semiquantitative evaluation of leaflet stiffness remains possible by performing elastography before and after PCU on the same leaflet 22, 23.
Transthoracic approach
Transthoracic treatment should be feasible but requires further development of the therapy device. The main challenge of the transthoracic approach is to overcome the effect of the deep heterogeneous tissue layers on the path of the ultrasound beam. The ribs are a particularly strong barrier for ultrasound, allowing the ultrasonic beam to propagate only through the intercostal space. This limited aperture reduces the energy at the focal point as well as the quality of the focus. However, previous work in the field of histotripsy 24, 25 and high-intensity focused ultrasound (26) have shown the feasibility of transcostal focusing.
Clinical applications
The treatment of calcified bioprosthesis stenosis in the mitral or aortic position is currently still a challenge. Redo surgery or transapical transcatheter valve-in-valve implantation expose the patient to significant risks, especially for patients with comorbidities 5, 27. The results presented in this study may provide a new treatment option for these patients. From a general point of view, the PCU could be applied to a calcified bioprosthesis whatever its localization (aortic, mitral, tricuspid, or pulmonary), and this should be confirmed in further animal and human studies. If these results are confirmed and stable in the long-term, the indication of bioprosthesis could be expanded to a wider and younger population with the acceptable perspective of noninvasive redux therapy in the long-term. This application, however, still needs to be confirmed by further prospective studies and significant follow-up.
Conclusions
We have demonstrated in vitro and in vivo that PCU can significantly decrease the transvalvular gradient of a calcified bioprosthesis stenosis by softening the leaflets remotely and improving valve opening. We believe that this novel ultrasound therapy could become a noninvasive therapeutic strategy in cardiology. This new noninvasive approach has the potential to improve the outcome of patients with severe calcified bioprosthesis stenosis by avoiding risky surgical or transcatheter reintervention.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Heart valves are being increasingly replaced by bioprostheses. These bioprostheses have a limited lifespan, and degenerative calcified bioprosthesis carries a dire prognosis despite recent advances in surgery and transcatheter valve-in-valve implantation. Less-invasive strategies to treat calcified valves are needed. The results of this study show that PCU can soften calcified leaflets noninvasively and improve their opening function.
TRANSLATIONAL OUTLOOK: The present study is a proof of concept of the efficacy of PCU to soften bioprosthesis valves. This is an important initial step in translating PCU softening toward clinical applications. Future experimental and translational studies are required to investigate the feasibility and the safety of this new therapeutic approach in human patients.
Acknowledgments
The authors thank Hicham Serroune (Institut Langevin), Julie Piquet, and the staff of the Fondation Alain Carpentier and the Laboratoire biochirurgical.
Footnotes
This work was supported by the LABEX WIFI (Laboratory of Excellence ANR-10-LABX-24) and the French Program “Investments for the Future” under reference ANR-10-IDEX-0001-02 PSL* Research University. Drs. Tanter, Messas, and Pernot are cofounders and shareholders of Cardiawave SAS. Dr. Rémond is an employee of Cardiawave SAS. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Messas and Pernot contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167–214. [DOI] [PubMed]
- 2.Jamieson W.R.E., Rosado L.J., Munro A.I. Carpentier-Edwards standard porcine bioprosthesis: primary tissue failure (structural valve deterioration) by age groups. Ann Thorac Surg. 1988;46:155–162. doi: 10.1016/s0003-4975(10)65888-2. [DOI] [PubMed] [Google Scholar]
- 3.Thourani V.H., Weintraub W.S., Guyton R.A. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement. Circulation. 2003;108:298–304. doi: 10.1161/01.CIR.0000079169.15862.13. [DOI] [PubMed] [Google Scholar]
- 4.Potter D.D., Sundt T.M., Zehr K.J. Risk of repeat mitral valve replacement for failed mitral valve prostheses. Ann Thorac Surg. 2004;78:67–72. doi: 10.1016/j.athoracsur.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Ye J., Cheung A., Yamashita M. Transcatheter aortic and mitral valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol Intv. 2015;8:1735–1744. [Google Scholar]
- 6.Williamson W.A., Aretz H.T., Weng G. In vitro decalcification of aortic valve leaflets with the Er:YSGG laser, Ho:YAG laser, and the cavitron ultrasound surgical aspirator. Lasers Surg Med. 1993;13:421–428. doi: 10.1002/lsm.1900130405. [DOI] [PubMed] [Google Scholar]
- 7.Hutcheson J.D., Aikawa E., Merryman W.D. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman W.K., Schaff H.V., Orszulak T.A., Tajik A.J. Ultrasonic aortic valve decalcification: serial Doppler echocardiographic follow-up. J Am Coll Cardiol. 1990;16:623–630. doi: 10.1016/0735-1097(90)90352-p. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z., Owens G., Gordon D., Cain C.A., Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121:742–749. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villemain O., Kwiecinski W., Bel A. Pulsed cavitational ultrasound for non-invasive chordal cutting guided by real-time 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1101–1107. doi: 10.1093/ehjci/jew145. [DOI] [PubMed] [Google Scholar]
- 11.Ebbini E.S., ter Haar G. Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia. 2015;31:77–89. doi: 10.3109/02656736.2014.995238. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi W.A., Chambers J.B., Dumesnil J.G. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. quiz 1082–4. [DOI] [PubMed] [Google Scholar]
- 13.Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H., Hung J., Bermejo J. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 15.Baker M. Whole-animal imaging: the whole picture. Nature. 2010;463:977–980. doi: 10.1038/463977a. [DOI] [PubMed] [Google Scholar]
- 16.Gillis K., Bala G., Roosens B. Quantification of calcium amount in a new experimental model: a comparison between ultrasound and computed tomography. PLoS One. 2016;11:e0148904. doi: 10.1371/journal.pone.0148904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pernot M., Tanter M., Fink M. 3-D real-time motion correction in high-intensity focused ultrasound therapy. Ultrasound Med Biol. 2004;30:1239–1249. doi: 10.1016/j.ultrasmedbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Miller R.M., Kim Y., Lin K.-W., Cain C.A., Owens G.E., Xu Z. Histotripsy cardiac therapy system integrated with real-time motion correction. Ultrasound Med Biol. 2013;39:2362–2373. doi: 10.1016/j.ultrasmedbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe Y., Otsuka R., Muratore R. In vitro mitral chordal cutting by high intensity focused ultrasound. Ultrasound Med Biol. 2008;34:400–405. doi: 10.1016/j.ultrasmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z., Fan Z., Hall T.L., Winterroth F. Size measurement of tissue debris particles generated from pulsed ultrasound cavitational therapy–histotripsy. Ultrasound Med. 2009;35:245–255. doi: 10.1016/j.ultrasmedbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.-M., Couade M., Bercoff J., Tanter M. Assessment of viscous and elastic properties of sub-wavelength layered soft tissues using shear wave spectroscopy: theoretical framework and in vitro experimental validation. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:2305–2315. doi: 10.1109/TUFFC.2011.2088. [DOI] [PubMed] [Google Scholar]
- 23.Couade M., Pernot M., Prada C. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol. 2010;36:1662–1676. doi: 10.1016/j.ultrasmedbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y., Wang T.-Y., Xu Z., Cain C.A. Lesion generation through ribs using histotripsy therapy without aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:2334–2343. doi: 10.1109/TUFFC.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y., Vlaisavljevich E., Owens G.E., Allen S.P., Cain C.A., Xu Z. In vivo transcostal histotripsy therapy without aberration correction. Phys Med Biol. 2014;59:2553–2568. doi: 10.1088/0031-9155/59/11/2553. [DOI] [PubMed] [Google Scholar]
- 26.Marquet F., Aubry J.F., Pernot M., Fink M., Tanter M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys Med Biol. 2011;56:7061–7080. doi: 10.1088/0031-9155/56/22/005. [DOI] [PubMed] [Google Scholar]
- 27.Maciejewski M., Piestrzeniewicz K., Bielecka-Dąbrowa A., Piechowiak M., Jaszewski R. Redo surgery risk in patients with cardiac prosthetic valve dysfunction. Arch Med Sci. 2011;7:271–277. doi: 10.5114/aoms.2011.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.