Abstract
The worldwide increase in obesity prevalence is a result of positive energy balance, with energy intake exceeding expenditure. The eating behavior in obesity ranges from mild passive overconsumption to excessive overeating with loss of control observed in binge eating disorder (BED). The signaling systems that underlie appetite control in BED are complex and, at this point, not well understood. The present review highlights the current knowledge of key components of the gut peptide system and examines evidence of defects in signaling that differentiate obese binge eaters from obese non–binge eaters. The signaling network underlying hunger, satiety, and metabolic status includes leptin and insulin from energy stores and cholecystokinin, glucagon-like peptide-1, peptide YY(3-36), and ghrelin from the gastrointestinal tract. Of the many gastrointestinal peptides, ghrelin is the only established appetite-stimulating one, whereas cholecystokinin, glucagon-like peptide-1, and peptide YY(3-36) promote satiety. Adipose tissue provides hormonal signals via leptin and insulin to the brain about energy stores and likely from adiponectin and resistin. Binge eating has been related to a dysfunction in the ghrelin signaling system. Moreover, the larger gastric capacity observed in BED may further reduce satiety signals and contribute to overeating.
Keywords: obesity, binge, eating, BED, hormones, appetite, peptide, ghrelin, CCK, leptin
Background
Obesity, which is associated with the metabolic syndrome1,2 and chronic diseases such as diabetes, hypertension, and heart disease,3 continues to increase in prevalence in developing countries4,5 and the United States,6 where it has reached epidemic proportions.7 Obesity is highly resistant to treatment, with most of the weight lost regained within 5 years after dieting.8,9 The nation’s medical costs for obesity have been estimated at $70 to $100 billion per year and account for 9% of all health costs. Based on the latest criterion for obesity, body mass index >30 kg/m2,10,11 at least 30% of Americans are obese.6
Binge eating is characterized by eating, in a discrete period of time, an amount of food that is definitely larger than most individuals would eat under similar circumstances, accompanied by a sense of lack of control.12 The prevalence of binge eating is disproportionately greater within the obese population,13–15 and binge eaters represent a sizable and distinct subgroup.15–17 The prevalence of some form of binge eating within the overweight population18 has been estimated at 25% or higher.15 Binge eating has also been implicated in the development of obesity19,20 based on prospective longitudinal studies21–23 and is a risk factor for weight regain following weight loss.24
Most research on binge eating has focused on patients with bulimia nervosa (BN),25 who engage in binge eating followed by some form of compensation or purging; therefore, this review also covers some gut peptide literature in BN. Those diagnosed with BN, however, make up only a fraction of individuals who regularly binge eat.26 The much larger group includes those who meet some, but not all, of the research criteria for binge eating disorder (BED).12,25 BED is defined as recurrent episodes of binge eating (at least twice per week for 6 months) in the absence of the regular use of inappropriate compensatory behaviors, such as vomiting or using laxatives.12 As noted by Susan Yanovski, “BED provides an opportunity to study the causes and concomitants of binge eating in the absence of compensatory behaviors.”27 BED is listed in the appendix of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM IV-TR), of the American Psychiatric Association12,15,28 and currently falls into the eating disorder–not otherwise specified category. BED is likely to be included as a recognized eating disorder in the next revision of the DSM (DSM-V).29 BED participants have been observed to consume binge meals, both inside and outside of the laboratory.30–32 They are more resistant to weight loss treatment, have higher dropout rates, and show greater recidivism33 than other obese participants. Unlike the classic eating disorders of anorexia nervosa (AN) and BN, BED is common in men—about 40% of the BED participants in overweight clinical and community samples.15 Many obese individuals, 18% to 46% of those enrolling in weight loss programs, meet the criteria for BED.34 With the rising epidemic of obesity, BED prevalence is also increasing, lending urgency to the study of its pathogenesis.
There is a scientific consensus supporting the clinical validity and utility of the BED diagnosis35,36 and its distinction from nonpurging BN, who may compensate by other means, such as exercise.37 The clinical soundness of BED is evidenced by its chronicity38 and association with overall life impairment and general psychopathology.15 The lifetime prevalence of other psychiatric diagnoses in those with BED is 60% to 72% as compared with 28% to 49% in controls.39 Obese BED participants scored higher than matched non-BED obese controls on depression, anger, disinhibited eating,40 impulsivity,41,42 and overall psychopathology43 and scored lower on self-esteem.44 They had more frequent weight fluctuations and more shape-/weight-related concerns.37 Compared with patients with BN, they had less restrained eating, less body image disturbance, and either less or similar psychological distress and psychiatric comorbidity.45 Eating studies have shown that, in BED, binge size is somewhat smaller and eating rate is slower than in BN. Compared with obese non-BED patients, obese BED participants have greater caloric intake during both binge meals and regular meals.46,47 There is recent evidence for moderate heritability of 0.50 in BED,48 which implies a biological basis. Thus, there is a clear need to identify possible disturbances in peripheral peptides involved in regulating food intake in BED. Currently, the biological substrates and mechanisms underlying BED, including the role of gut peptides, are not well understood.
Appetite-Related Peptides
There are 3 different sets of signals from the periphery: one from adipose tissue that exerts long-term regulatory mechanisms on food intake and the other two from the gastrointestinal (GI) tract that exert primarily short-term effects on food intake (Figure 1). This review focuses on the current literature on the peptides and related GI signals. Other signals and agents, such as blood sugar and insulin, circulating non-esterified fatty acids, catecholamines, and 5-hydroxytryptamine, are also involved in the control of food intake49 but are beyond the scope of this review.
Figure 1.
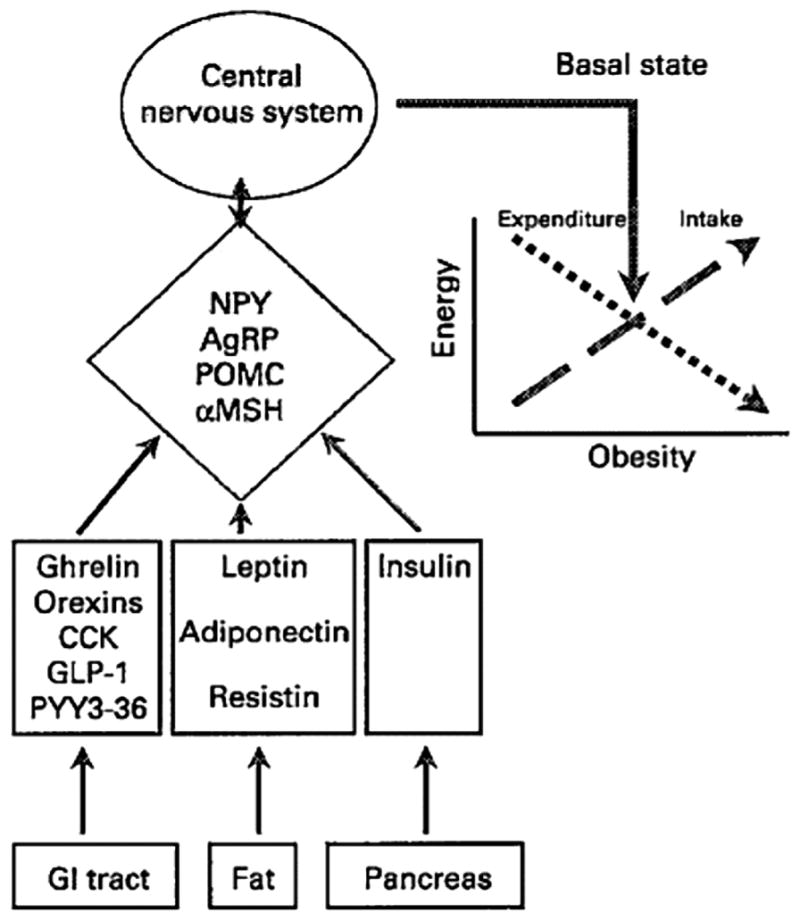
Major neuropeptide and peptide hormone controls of food intake. The graph illustrates the balance between energy intake and expenditure to maintain a stable fat mass.
NPY indicates neuropeptide Y; AgRP, agouti gene-related peptide; POMC, pro-opiomelanocortin; αMSH, alpha-melanocyte stimulating hormone; CCK, cholecystokinin; GLP, glucagon-like peptide; PYY, peptide YY; GI, gastrointestinal. From Hellstrom et al.49 Reproduced with permission from The Nutrition Society.
A number of peripheral peptides have been shown to induce satiety signals that act directly on the brain, indirectly via the vagus nerve, or by slowing the gastric emptying rate.49,50 Such satiety peptides include leptin, cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY(3-36)), which rise after meals and which suppress food intake when administered peripherally51,52 or centrally.49,53 Postprandial CCK levels are apparently lower in individuals with BN.49,54 This may be a consequence of slower gastric emptying,54,55 delaying nutrients from reaching the duodenum to trigger CCK release (see the CCK, gastric emptying, and gastric capacity section).
Leptin
Nearly all released by adipose tissue, leptin is considered to be part of a feedback loop, in which low levels signal to the central nervous system (CNS) that energy stores are depleted.56 Leptin, similar to insulin, decreases appetite when administered centrally by inhibiting CNS neurons that release neuropeptide Y (NPY) and agouti gene-related peptide (AgRP).49 Leptin also acts on pro-opiomelanocortin (POMC) neurons, which regulate energy homeostasis in the hypothalamus.57 Both animals58 and humans57 with POMC deficiency have increased appetite and are obese. Fasting causes a reduction of POMC mRNA in the arcuate nucleus; however, leptin is able to reverse this effect.59 Therefore, it is possible that POMC neurons are responsible for most of leptin’s actions in the CNS.60 Fasting leptin is higher in the obese because of the presence of excess adipose tissue, and during weight and fat loss, leptin decreases.61,62 Leptin increases slowly after meals,63 which may not become apparent until 2 hours afterward.64,65 It has been suggested that leptin plays a role in short-term satiety when released by the stomach,66 a recently discovered small additional source of leptin besides the adipose tissue.67,68 Overweight and obese women with either BED, subthreshold BED, or no binge eating showed an acute rise in leptin levels, which did not, however, correlate with fullness, following consumption of a brief liquid meal.66 The meal was relatively high in carbohydrate (55%), which is known to stimulate leptin more than does fat.69
Despite its role in the regulation of food intake, leptin injections in obese animals70 and humans71 have not been highly efficacious in reducing food intake or body weight. This is likely because of the development of leptin resistance.72,73 Leptin-resistant individuals, with high plasma levels of leptin, do not adequately decrease food intake in response to leptin.73 Fasting leptin is correlated primarily to body fat, regardless of the eating disorder, including AN and BN.74 However, in one report, fasting leptin was higher in BED patients as compared with weight-matched non-BED controls.75
It has been suggested that melanocortin 4 receptor (MC4R) dysfunction may contribute to the development of BED in obese individuals.76 In a 2003 report in the New England Journal of Medicine, obese carriers of an MC4R mutation were all diagnosed with BED, as compared with only 14.2% of obese participants and 0% of normal-weight participants without MC4R mutation.76 It has also been suggested that MC4R dysfunction may be related to the severity of BED.77 However, there is significant controversy surrounding these findings, as recent studies have been unable to replicate them.78–81
GLP-1
A peptide released in the lower gut, GLP-1, acts as an ileal brake for the upper GI tract and slows gastric emptying of liquid and solid meals. GLP-1 could reduce food intake in part by slowing gastric emptying with resulting greater gastric distension. However, there is also evidence that GLP-1 acts more directly via the vagus nerve as well as centrally.49 In humans, most studies show decreased food intake after administration of GLP-1, with reduced hunger and increased fullness ratings, without reports of nausea.49 GLP-1, however, is rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4), resulting in a half-life of active GLP-1 of only 1 to 2 minutes. Studies are being performed to independently inhibit DPP-4 as well as to use DPP-4–resistant GLP-1 receptor agonists to reduce food intake.82
A synthetic analog of GLP-1, which resists DPP-4, exenatide (Byetta) from Amylin Pharmaceuticals, was approved by the Food and Drug Administration in 2005 as an adjunctive therapy in patients with type 2 diabetes already taking either metformin, a sulfonylurea, or a combination of metformin and a sulfonylurea but without achieving adequate glycemic control.83,84 In clinical trials, the drug appeared to also cause modest weight loss and was associated with some side effects, including nausea, diarrhea, dizziness, and headache. About 3% of participants receiving the drug withdrew from the trial because of nausea. The greater incidence of side effects with the analog as compared with the natural peptide is likely due to differences in structure (53% amino acid homology with GLP-1) and function as well as the much longer half-life, 2.4 hours, of the drug, designed to resist DPP-4. The drug is administered by subcutaneous injection 2 or 3 times per day, but a long-acting formulation with once-per-week injections is anticipated.85 Preliminary data show that fasting and postprandial levels of GLP-1 in BED versus non-BED obese groups do not differ.86
PYY(3-36)
Another lower gut peptide, PYY(3-36), is co-localized in the GI tract with GLP-1.87 PYY(3-36), which penetrates the blood-brain barrier,88 is also subject to deactivation by DPP-4 and has a half-life in the blood of 9 minutes.82 Several clinical trials to treat obesity are under way with a PYY(3-36) nasal inhalant.89 To date, however, intra-nasal PYY(3-36) has not been efficacious in inducing weight loss in obese individuals.90 Although peripheral PYY(3-36) injections have been shown to suppress food intake in animals,91 others initially were unable to obtain these results.92 However, a recent study suggests that daily intermittent intravenous injections are required to produce a sustained effect of PYY(3-36) on food intake and adiposity.93 PYY(3-36) is likely to reduce food intake in part by acting on Y2 receptors on vagal afferents, which increase activity in the arcuate nucleus of the hypothalamus. Vagotomy in rats has been shown to abolish the feeding suppression by PYY(3-36),94 although the concentrations of PYY(3-36) in the ileum and colon were not significantly affected by vagotomy in mice.95
Batterham et al96 showed that PYY (3-36) levels were lower premeal in obese than in lean participants and rose less in the obese postmeal. They also showed that a PYY(3-36) intravenous infusion reduced food intake in both obese and lean individuals and decreased plasma ghrelin without inducing any side effects or nausea. Recently, there have been 2 reports of blunted levels of PYY(3-36) after a meal in the related disorder of BN.97,98 Preliminary data of PYY(3-36) fasting and postprandial levels in BED versus non-BED obese groups, however, showed no differences.86 Combining GLP-1 and PYY(3-36) reduces food intake in an additive fashion.99 The peptides just reviewed as promoting satiety are not all inclusive, and other less established candidates include amylin,100 pancreatic polypeptide, and oxyntomodulin.100
Ghrelin
A more recently discovered gut peptide is ghrelin.101 A ligand for growth hormone secretagogue receptor, ghrelin is downregulated by excess growth hormone as in acromegaly.102 Ghrelin is produced mainly by the stomach and when administered increases food intake in animals103 and humans.104 In animals, ghrelin enhances gut motility and speeds gastric emptying.105 The effect on gastric emptying is less clear-cut in humans, where in one study, no effect was seen, as assessed by an acetaminophen tracer,104 but in another study, fasting ghrelin levels correlated positively with subsequent gastric emptying rate.106 Evidence is also mixed on whether peripherally administered ghrelin in animals acts via vagal afferents. A vagotomy abolished ghrelin-induced feeding in one study107 but not in another.108 Injection of ghrelin centrally109 stimulates the release of the orexigenic neuropeptides NPY and AgRP in a number of brain areas, especially the arcuate nucleus of the hypothalamus.110 Ghrelin-producing neurons have been found in the cerebral cortex and in the hypothalamus, where they stimulate NPY neurons.111 Double-knockout mice for NPY and AgRP do not show the feeding-enhancing effect of ghrelin administration.112
In humans, ghrelin rises before meals and falls following meals.112 The higher the energy value of a meal, the larger the postprandial decline in ghrelin.113 Ghrelin also follows a diurnal pattern, increasing from the morning to the evening and reaching a higher peak before dinner than before breakfast.112 This is consistent with the meal pattern in the United States, where dinner is usually the largest meal.114 The rise in ghrelin from morning to evening appears to be greater in the obese than in the lean.112 For example, obese participants showed a ghrelin rise of 63 pg/mL from 8 am before breakfast to 5:30 pm just before dinner, while the lean participants showed a rise of only 10 pg/mL during this period. In another study,115 following several days of fasting, ghrelin rose more during the first 24 hours from morning to night in the obese than in the lean participants. Although BED was not assessed, it is likely that some of the obese were binge eaters, which may have contributed to the findings.
Surprisingly, fasting ghrelin has been found to be 27% lower in obese than in normal-weight individuals.116 Ghrelin rises following weight loss in obese participants117 and also rises in animals during starvation.118 There are contradictory findings on whether there is a smaller fall in ghrelin after a meal in obese as compared with lean humans.112,119 The authors reporting the smaller fall in ghrelin propose that the smaller fall in ghrelin maintains hunger.114 The lower fasting levels in obesity suggest that ghrelin is downregulated in response to overeating or excess body weight.114,116 Indeed, fasting ghrelin is negatively correlated with percentage body fat, fasting insulin, and leptin, all of which are higher in obesity.116,120,121 Ghrelin is lower in insulin-resistant obese individuals relative to insulin-sensitive controls.122 Ghrelin, however, is elevated in Prader-Willi syndrome (PWS), the result of a genetic deletion in chromosome 15, which also leads to mental retardation and short stature and is associated with childhood onset of severe obesity.123 High ghrelin levels may not account for the increased appetite seen in PWS, as administration of somatostatin to PWS adults reduces ghrelin levels with no effect on appetite.124 However, somatostatin also inhibits the release of satiety peptides, which may cancel any of the effects on ghrelin. A ghrelin receptor antagonist125 or antighrelin Spiegelmer, which inactivates ghrelin in the blood circulation,126 may have the potential to reduce food intake.114 These approaches have been shown to reduce food intake and body weight in animals.126,127
Fasting levels of ghrelin were found to be higher in BN128 compared with normal participants and highest in AN.121 In theory, AN participants who continue to starve themselves could develop resistance to their high ghrelin levels.120 AN patients with the binging/purging subtype had somewhat higher ghrelin than the restricting subtype129 in one report but not in another.130 Fasting ghrelin was found to be lower in nonpurging bulimics than in purging bulimics.130 In 2 recent articles, there was a smaller drop in ghrelin following a meal in BN patients than in controls.98,131 Ghrelin levels have recently been reported in BED, indicating lower morning fasting ghrelin and a smaller postprandial decline in BED than in non-BED obese individuals.66,132 The lower ghrelin levels in BED compared with non-BED individuals, although unexpected, are consistent with lower ghrelin levels in obese compared with lean participants and suggest down-regulation of ghrelin by habitual overeating. In another recent report, morning fasting ghrelin was found to be lower in both lean and obese female BED participants, suggesting that BED status, rather than overweight category, was the relevant characteristic.133
Obestatin
Obestatin, a 23-amino-acid peptide with a glycine residue at the C-terminal, was reported to be co-expressed with ghrelin, with opposite actions by reducing food intake and weight in animals.134 Obestatin is postulated to become active by post-translational amidation at its carboxyl terminus.135 Serum concentrations appear to be unaffected by fasting or refeeding,134 and it is rapidly degraded when injected into the blood circulation in rats.136 However, multiple studies have been unable to replicate the anorexigenic property initially reported, the ability to oppose ghrelin-induced stimulation of food intake, or obestatin’s inhibition of gastric emptying and jejunal contractile activity in rodents.137,138 Thus, the precise role of obestatin in controlling appetite and weight remains to be determined.
Adiponectin and resistin
Adiponectin, a peptide produced and released exclusively by adipose tissue,139 may be an additional adipocyte signaling factor. There are similarities between adiponectin and leptin; however, plasma levels of adiponectin remain relatively constant throughout the day, are not affected by food intake,140 and correlate negatively with body mass index.141 Obese individuals with diabetes have even lower plasma levels of adiponectin than nondiabetic obese individuals.140,142–145 Diminished adiponectin may be a factor in the development of insulin resistance. Resistin, also known as adipose tissue–specific secretory factor, is another peptide secreted by adipocytes and acts on myocytes of skeletal muscle, hepatocytes, and adipocytes. Opposite in most of its actions to adiponectin, resistin is positively correlated with insulin resistance.100 Adiponectin and resistin have yet to be studied in BED.
CCK, gastric emptying, and gastric capacity
A number of different GI peptides are known to be anorexigenic, promoting early termination of a meal. Of these, CCK is the most widely studied.147 After a meal, CCK is released from endocrine I cells of the duodenum and the jejunum.148 Studies in humans have shown that CCK administration inhibits food intake,149,150 although a preload is generally necessary to demonstrate a satiation effect.151 Peripherally administered CCK also acts on CCKA receptors in the gastric antrum, which are involved in the CCK-mediated inhibition of gastric emptying.152 CCKA receptors are also found in the abdominal section of the vagus nerve,153 and vagotomy abolishes the anorexigenic effect of the peptide in rats. A relationship between decreased plasma levels of CCK and increased hunger, as well as decreased fullness, has been reported in humans,154 supporting a satiety role for CCK.
Gastric emptying rate influences the release of CCK, which is triggered by nutrients reaching the duodenum. In BN, fixed liquid meals empty more slowly,54,55 delaying the duodenal release of CCK,54,155 which may lead to less satiation.156 With acetaminophen employed as a tracer,157,158 gastric emptying of liquids in obese BED participants was not slower, and consistent with this, no significant reduction in CCK levels was observed, unlike that seen in BN.66
The capacity of the stomach can also influence satiation and affect the release of peptides. Distention of the stomach activates gastric stretch receptors and mechanoreceptors that transmit satiety signals.159 A stomach with a large capacity may require a larger than usual meal to generate satiety signals, and consistent with this, gastric capacity correlates highly with test-meal intake.55 Surgical reduction in gastric capacity to treat obesity reduces meal size, leads to marked weight loss,160 and usually eliminates binge eating in BED,161 confirming that a large stomach capacity helps maintain binge eating. Moreover, nonsurgical reduction of stomach capacity by external abdominal pressure162 or by filling an intragastric balloon also reduces meal intake, especially in the short term.163 A stomach with increased capacity may also empty fixed liquid meals more slowly, as has been found in BN.155,164 When gastric capacity was compared between normal-weight individuals with BN, obese individuals, and normal non–binge-eating lean participants, gastric capacity was largest in those with BN, intermediate in the obese participants, and smallest in the lean participants.164
When the obese participants were further subdivided into binge eaters and non–binge eaters, the obese binge eaters were similar in stomach capacity to BN patients, while the obese non–binge eaters were similar to the normal lean participants,132 even though few of the obese binge eaters met the full criteria for BED. In a more recent study of BED,132 gastric capacity and emptying as well as levels of glucose, insulin, leptin, CCK, and ghrelin were assessed while fasting and following a test meal. Although not as large as in BN,55 gastric capacity was greater in obese full-fledged BED than in subthreshold BED or non-BED groups. Gastric capacity was assessed by filling an intra-gastric balloon until maximal tolerance as well as by measuring changes in intra-gastric pressure to determine compliance. Test-meal size correlated with gastric capacity across all groups, and the gastric emptying rate did not differ. The only fasting peptide concentration to differ significantly between groups was ghrelin, which during fasting was lowest in BED, intermediate in subthreshold BED, and highest in the non-BED group. Ghrelin decreased after a fixed meal as expected but decreased the least in BED from a lower baseline. Ghrelin at 30 minutes correlated inversely with gastric capacity: r = −0.36, P < .05, implying that the large gastric capacity may be responsible for the lower ghrelin level. The lower fasting ghrelin level suggests that binge eating results in downregulation of ghrelin, consistent with lower levels in obese as compared with lean participants.116 If the extent of the fall in ghrelin after a meal is itself a signal for satiety, the smaller relative decrease in ghrelin postprandially may actually contribute to the binge eating in BED. Overall, these results demonstrate a greater gastric capacity and abnormal ghrelin pattern in BED.
Conclusion
In sum, the major peripheral peptides influencing appetite under normal conditions can be divided into those that help initiate food intake (ghrelin) and decline following meals and those that help terminate food intake and rise following meals (CCK, leptin, amylin, GLP-1, and PYY(3-36)). Except for CCK,89,165 these appetite-regulating gut peptides can cross the blood-brain barrier, suggesting a central action.68,166–168 Many of the gut peptide studies comparing lean versus obese groups did not examine or control for binge eating, which may account for some of the differences between these groups, given that BED is much more common in obese than lean populations.15 Binge eating has been related to a dysfunction in the ghrelin signaling system and to enlarged gastric capacity, which may contribute to the lower ghrelin. Further identifying the dysfunctional gastric and peptide signaling mechanisms in BED may lead to new therapeutic approaches.
References
- 1.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109(6):706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 2.Pritchett AM, Foreyt JP, Mann DL. Treatment of the metabolic syndrome: the impact of lifestyle modification. Curr Atheroscler Rep. 2005;7(2):95–102. doi: 10.1007/s11883-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Caballero B. Introduction. Symposium: obesity in developing countries: biological and ecological factors. J Nutr. 2001;131(3):866S–870S. doi: 10.1093/jn/131.3.866S. [DOI] [PubMed] [Google Scholar]
- 5.Klein S. The national obesity crisis: a call for action. Gastroenterology. 2004;126(1):6. doi: 10.1053/j.gastro.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 7.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 8.Foster GD, Wadden TA, Kendall PC, Stunkard AJ, Vogt RA. Psychological effects of weight loss and regain: a prospective evaluation. J Consult Clin Psychol. 1996;64(4):752–757. doi: 10.1037//0022-006x.64.4.752. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31(suppl 11):S663–S667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 11.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 13.de ZM, Nutzinger DO, Schoenbeck G. Binge eating in overweight women. Compr Psychiatry. 1992;33(4):256–261. doi: 10.1016/0010-440x(92)90050-z. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL. Binge eating disorder: a multi-site field trial of the diagnostic criteria. J Eat Disord. 1992;11:191–203. [Google Scholar]
- 15.Spitzer RL, Yanovski S, Wadden T, et al. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord. 1993;13(2):137–153. [PubMed] [Google Scholar]
- 16.Yanovski SZ, Nelson JE, Dubbert BK, Spitzer RL. Association of binge eating disorder and psychiatric comorbidity in obese subjects. Am J Psychiatry. 1993;150(10):1472–1479. doi: 10.1176/ajp.150.10.1472. [DOI] [PubMed] [Google Scholar]
- 17.Pinaquy S, Chabrol H, Simon C, Louvet JP, Barbe P. Emotional eating, alexithymia, and binge-eating disorder in obese women. Obes Res. 2003;11(2):195–201. doi: 10.1038/oby.2003.31. [DOI] [PubMed] [Google Scholar]
- 18.Marcus M. Binge-eating in obesity. In: Fairburn CWG, editor. Binge-Eating: Nature, Assessment, and Treatment. New York, NY: Guilford; 1993. pp. 77–96. [Google Scholar]
- 19.Mussell MP, Mitchell JE, Weller CL, Raymond NC, Crow SJ, Crosby RD. Onset of binge eating, dieting, obesity, and mood disorders among subjects seeking treatment for binge eating disorder. Int J Eat Disord. 1995;17(4):395–401. doi: 10.1002/1098-108x(199505)17:4<395::aid-eat2260170412>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Yanovski SZ. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? Int J Eat Disord. 2003;34(suppl):S117–S120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]
- 21.Stice E, Killen JD, Hayward C, Taylor CB. Age of onset for binge eating and purging during late adolescence: a 4-year survival analysis. J Abnorm Psychol. 1998;107(4):671–675. doi: 10.1037//0021-843x.107.4.671. [DOI] [PubMed] [Google Scholar]
- 22.Stice E. Predicting onset and cessation of bulimic behaviors during adolescence: a longitudinal grouping analysis. Behav Ther. 1998;29:257–297. [Google Scholar]
- 23.Leon GR, Keel PK, Klump KL, Fulkerson JA. The future of risk factor research in understanding the etiology of eating disorders. Psychopharmacol Bull. 1997;33(3):405–411. [PubMed] [Google Scholar]
- 24.McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67(2):177–185. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Lynch WC, Everingham A, Dubitzky J, Hartman M, Kasser T. Does binge eating play a role in the self-regulation of moods? Integr Physiol Behav Sci. 2000;35(4):298–313. doi: 10.1007/BF02688792. [DOI] [PubMed] [Google Scholar]
- 26.Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Subst Abuse. 1992;4(4):341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- 27.Yanovski SZ. Biological correlates of binge eating. Addict Behav. 1995;20(6):705–712. doi: 10.1016/0306-4603(96)00092-5. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer RL. Nonpurging bulimia nervosa and binge eating disorder. Am J Psychiatry. 1991;148(8):1097–1098. doi: 10.1176/ajp.148.8.aj14881097. [DOI] [PubMed] [Google Scholar]
- 29.Ochner CN, Geliebter A. Binge eating disorder. Obes Manage. 2007;3(4):161–164. [Google Scholar]
- 30.Goldfein JA, Walsh BT, LaChaussee JL, Kissileff HR, Devlin MJ. Eating behavior in binge eating disorder. Int J Eat Disord. 1993;14(4):427–431. doi: 10.1002/1098-108x(199312)14:4<427::aid-eat2260140405>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Yanovski SZ, Leet M, Yanovski JA, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992;56(6):975–980. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- 32.Geliebter A, Hassid G, Hashim SA. Test meal intake in obese binge eaters in relation to mood and gender. Int J Eat Disord. 2001;29(4):488–494. doi: 10.1002/eat.1047. [DOI] [PubMed] [Google Scholar]
- 33.Yanovski SZ. Binge eating disorder: current knowledge and future directions. Obes Res. 1993;1(4):306–324. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 34.de ZM, Mitchell JE, Raymond NC, Spitzer RL. Binge eating disorder: clinical features and treatment of a new diagnosis. Harv Rev Psychiatry. 1994;1(6):310–325. doi: 10.3109/10673229409017098. [DOI] [PubMed] [Google Scholar]
- 35.Agras S. Course of BED: classification among eating disorders: AN, BN, BED, and partial syndromes, treatment response, similarities and differences among BED and other EDs. Paper presented at: Eating Disorders Research Society Annual Meeting; 1999. [Google Scholar]
- 36.Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. Int J Eat Disord. 2003;34(suppl):S96–S106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt U. Binge eating and binge eating disorder. Eur Eat Disord Rev. 2000;8:340–343. [Google Scholar]
- 38.Wilfley DE, Cohen LR. Psychological treatment of bulimia nervosa and binge eating disorder. Psychopharmacol Bull. 1997;33(3):437–454. [PubMed] [Google Scholar]
- 39.Specker S, de ZM, Raymond N, Mitchell J. Psychopathology in subgroups of obese women with and without binge eating disorder. Compr Psychiatry. 1994;35(3):185–190. doi: 10.1016/0010-440x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell JE, Mussell MP. Comorbidity and binge eating disorder. Addict Behav. 1995;20(6):725–732. doi: 10.1016/0306-4603(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 41.Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43(3):303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. 2007;4(8):727–732. doi: 10.1002/eat.20441. [DOI] [PubMed] [Google Scholar]
- 43.Antony MM, Johnson WG, Carr-Nangle RE, Abel JL. Psychopathology correlates of binge eating and binge eating disorder. Compr Psychiatry. 1994;35(5):386–392. doi: 10.1016/0010-440x(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 44.de ZM, Mitchell JE, Seim HC, et al. Eating related and general psychopathology in obese females with binge eating disorder. Int J Eat Disord. 1994;15(1):43–52. doi: 10.1002/1098-108x(199401)15:1<43::aid-eat2260150106>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Abbott DW, de ZM, Mussell MP, et al. Onset of binge eating and dieting in overweight women: implications for etiology, associated features and treatment. J Psychosom Res. 1998;44(3–4):367–374. doi: 10.1016/s0022-3999(97)00261-4. [DOI] [PubMed] [Google Scholar]
- 46.Guss JL, Kissileff HR, Devlin MJ, Zimmerli E, Walsh BT. Binge size increases with body mass index in women with binge-eating disorder. Obes Res. 2002;10(10):1021–1029. doi: 10.1038/oby.2002.139. [DOI] [PubMed] [Google Scholar]
- 47.Geliebter A. Binge eating disorder (BED) from laboratory to clinic. Paper presented at: International Conference on Eating Disorders; April 2002; Boston, MA. [Google Scholar]
- 48.Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge eating. Int J Eat Disord. 2003;33(3):293–298. doi: 10.1002/eat.10140. [DOI] [PubMed] [Google Scholar]
- 49.Hellstrom PM, Geliebter A, Naslund E, et al. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br J Nutr. 2004;92(suppl 1):S47–S57. doi: 10.1079/bjn20041142. [DOI] [PubMed] [Google Scholar]
- 50.Hellstrom PM, Naslund E. Interactions between gastric emptying and satiety, with special reference to glucagon-like peptide-1. Physiol Behav. 2001;74(4–5):735–741. doi: 10.1016/s0031-9384(01)00618-7. [DOI] [PubMed] [Google Scholar]
- 51.Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol. 1992;262(6 pt 2):R975–R980. doi: 10.1152/ajpregu.1992.262.6.R975. [DOI] [PubMed] [Google Scholar]
- 52.Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R992–R998. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- 53.Woods SC, Seeley RJ. Insulin as an adiposity signal. Int J Obes Relat Metab Disord. 2001;25(suppl 5):S35–S38. doi: 10.1038/sj.ijo.0801909. [DOI] [PubMed] [Google Scholar]
- 54.Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;65(1):114–120. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- 55.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56(4):656–661. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 57.Wardlaw SL. Clinical review 127: obesity as a neuroendocrine disease: lessons to be learned from proopiomelanocortin and melanocortin receptor mutations in mice and men. J Clin Endocrinol Metab. 2001;86(4):1442–1446. doi: 10.1210/jcem.86.4.7388. [DOI] [PubMed] [Google Scholar]
- 58.Huang XF, Han M, South T, Storlien L. Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res. 2003;992(1):9–19. doi: 10.1016/j.brainres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55(3):567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 60.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 61.Campfield LA. Leptin and body weight regulation. In: Fairburn CG, Brownell KD, editors. Eating Disorders and Obesity: A Comprehensive Handbook. 2. New York, NY: Guilford; 2002. pp. 32–36. [Google Scholar]
- 62.Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr. 2000;130(12):3127S–3131S. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 63.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A pre-prandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 64.Taylor AE, Hubbard J, Anderson EJ. Impact of binge eating on metabolic and leptin dynamics in normal young women. J Clin Endocrinol Metab. 1999;84(2):428–434. doi: 10.1210/jcem.84.2.5502. [DOI] [PubMed] [Google Scholar]
- 65.Elimam A, Marcus C. Meal timing, fasting and glucocorticoids interplay in serum leptin concentrations and diurnal profile. Eur J Endocrinol. 2002;147(2):181–188. doi: 10.1530/eje.0.1470181. [DOI] [PubMed] [Google Scholar]
- 66.Geliebter A, Gluck ME, Hashim SA. Plasma ghrelin concentrations are lower in binge-eating disorder. J Nutr. 2005;135(5):1326–1330. doi: 10.1093/jn/135.5.1326. [DOI] [PubMed] [Google Scholar]
- 67.Pico C, Oliver P, Sanchez J, Palou A. Gastric leptin: a putative role in the short-term regulation of food intake. Br J Nutr. 2003;90(4):735–741. doi: 10.1079/bjn2003945. [DOI] [PubMed] [Google Scholar]
- 68.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394(6695):790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 69.Romon M, Lebel P, Velly C, Marecaux N, Fruchart JC, Dallongeville J. Leptin response to carbohydrate or fat meal and association with subsequent satiety and energy intake. Am J Physiol. 1999;277(5 pt 1):E855–E861. doi: 10.1152/ajpendo.1999.277.5.E855. [DOI] [PubMed] [Google Scholar]
- 70.Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1011–R1020. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- 71.Zelissen PM, Stenlof K, Lean ME, et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2005;7(6):755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 72.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26(4):504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 73.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 74.Calandra C, Musso F, Musso R. The role of leptin in the etiopathogenesis of anorexia nervosa and bulimia. Eat Weight Disord. 2003;8(2):130–137. doi: 10.1007/BF03325002. [DOI] [PubMed] [Google Scholar]
- 75.Adami GF, Campostano A, Cella F, Scopinaro N. Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord. 2002;26(8):1125–1128. doi: 10.1038/sj.ijo.0802010. [DOI] [PubMed] [Google Scholar]
- 76.Branson R, Potoczna N, Kral JG, Lentes KU, Hoehe MR, Horber FF. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;348(12):1096–1103. doi: 10.1056/NEJMoa021971. [DOI] [PubMed] [Google Scholar]
- 77.Potoczna N, Branson R, Kral JG, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg. 2004;8(8):971–981. doi: 10.1016/j.gassur.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 78.Inui A. Melanocortin signalling, single nucleotide polymorphism, and eating disorders. Int J Psychiatry Med. 2004;34(4):399–403. doi: 10.2190/XV07-WJJJ-FHAG-M60V. [DOI] [PubMed] [Google Scholar]
- 79.Tao YX, Segaloff DL. Functional analysis of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab. 2005;90(10):5632–5638. doi: 10.1210/jc.2005-0519. [DOI] [PubMed] [Google Scholar]
- 80.Hebebrand J, Geller F, Dempfle A, et al. Binge-eating episodes are not characteristic of carriers of melanocortin-4 receptor gene mutations. Mol Psychiatry. 2004;9(8):796–800. doi: 10.1038/sj.mp.4001491. [DOI] [PubMed] [Google Scholar]
- 81.Lubrano-Berthelier C, Dubern B, Lacorte JM, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab. 2006;91(5):1811–1818. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- 82.Ahren B. Inhibition of dipeptidyl peptidase-4 (DPP-4)—a novel approach to treat type 2 diabetes. Curr Enzyme Inhibition. 2005;1:65–73. [Google Scholar]
- 83.Exenatide: AC 2993, AC002993, AC2993A exendin 4, LY2148568. Drugs R D. 2004;5(1):35–40. doi: 10.2165/00126839-200405010-00007. [DOI] [PubMed] [Google Scholar]
- 84.Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10(22):2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30(6):1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 86.Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED) Physiol Behav. doi: 10.1016/j.physbeh.2008.04.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stanley S, Wynne K, Bloom S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G693–G697. doi: 10.1152/ajpgi.00536.2003. [DOI] [PubMed] [Google Scholar]
- 88.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3–36 in the mouse. J Pharmacol Exp Ther. 2003;306(3):948–953. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- 89.Small CJ, Bloom SR. Gut hormones as peripheral anti obesity targets. Curr Drug Targets CNS Neurol Disord. 2004;3(5):379–388. doi: 10.2174/1568007043336950. [DOI] [PubMed] [Google Scholar]
- 90.Gantz I, Erondu N, Mallick M, et al. Efficacy and safety of intranasal peptide YY3–36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92(5):1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 91.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 92.Tschop M, Castaneda TR, Joost HG, et al. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature. 2004;430(6996):1. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- 93.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R298–R305. doi: 10.1152/ajpregu.00674.2005. [DOI] [PubMed] [Google Scholar]
- 94.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 95.El-Salhy M, Danielsson A, Axelsson H, Qian BF. Neuroendocrine peptide levels in the gastrointestinal tract of mice after unilateral cervical vagotomy. Regul Pept. 2000;88(1–3):15–20. doi: 10.1016/s0167-0115(99)00107-x. [DOI] [PubMed] [Google Scholar]
- 96.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 97.Monteleone P, Martiadis V, Rigamonti AE, et al. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry. 2005;57(8):926–931. doi: 10.1016/j.biopsych.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Kojima S, Nakahara T, Nagai N, et al. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol (Oxf) 2005;62(1):74–78. doi: 10.1111/j.1365-2265.2004.02176.x. [DOI] [PubMed] [Google Scholar]
- 99.Neary NM, Small CJ, Druce MR, et al. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 100.Reda TK, Geliebter A, Pi-Sunyer FX. Amylin, food intake, and obesity. Obes Res. 2002;10(10):1087–1091. doi: 10.1038/oby.2002.147. [DOI] [PubMed] [Google Scholar]
- 101.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 102.Freda PU, Reyes CM, Conwell IM, Sundeen RE, Wardlaw SL. Serum ghrelin levels in acromegaly: effects of surgical and long-acting octreotide therapy. J Clin Endocrinol Metab. 2003;88(5):2037–2044. doi: 10.1210/jc.2002-021683. [DOI] [PubMed] [Google Scholar]
- 103.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 104.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86(12):5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 105.Peeters TL. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol. 2003;54(suppl 4):95–103. [PubMed] [Google Scholar]
- 106.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180(2):273–281. doi: 10.1677/joe.0.1800273. [DOI] [PubMed] [Google Scholar]
- 107.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 108.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24(4):597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 110.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 111.Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 112.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 113.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89(3):1319–1324. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 114.Geliebter A. Weight loss and plasma ghrelin levels. N Engl J Med. 2002;347(17):1379–1381. [PubMed] [Google Scholar]
- 115.Espelund U, Hansen TK, Hojlund K, et al. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab. 2005;90(2):741–776. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- 116.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 117.Hansen TK, Dall R, Hosoda H, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56(2):203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 118.Asakawa A, Inui A, Kaga T, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 119.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87(6):2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 120.Zigman JM, Elmquist JK. Minireview: from anorexia to obesity—the yin and yang of body weight control. Endocrinology. 2003;144(9):3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 121.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 122.McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89(4):1630–1635. doi: 10.1210/jc.2003-031572. [DOI] [PubMed] [Google Scholar]
- 123.Goldstone AP, Thomas EL, Brynes AE, et al. Elevated fasting plasma ghrelin in Prader-Willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89(4):1718–1726. doi: 10.1210/jc.2003-031118. [DOI] [PubMed] [Google Scholar]
- 124.Tan TM, Vanderpump M, Khoo B, Patterson M, Ghatei MA, Goldstone AP. Somatostatin infusion lowers plasma ghrelin without reducing appetite in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2004;89(8):4162–4165. doi: 10.1210/jc.2004-0835. [DOI] [PubMed] [Google Scholar]
- 125.Depoortere I, Thijs T, Peeters T. The contractile effect of the ghrelin receptor antagonist, D-Lys3-GHRP-6, in rat fundic strips is mediated through 5-HT receptors. Eur J Pharmacol. 2006;537(1–3):160–165. doi: 10.1016/j.ejphar.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 126.Kobelt P, Helmling S, Stengel A, et al. Anti-ghrelin Spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut. 2006;55(6):788–792. doi: 10.1136/gut.2004.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beck B, Richy S, Stricker-Krongrad A. Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci. 2004;76(4):473–478. doi: 10.1016/j.lfs.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka M, Naruo T, Muranaga T, et al. Increased fasting plasma ghrelin levels in patients with bulimia nervosa. Eur J Endocrinol. 2002;146(6):R1–R3. doi: 10.1530/eje.0.146r001. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka M, Naruo T, Yasuhara D, et al. Fasting plasma ghrelin levels in subtypes of anorexia nervosa. Psychoneuroendocri nology. 2003;28(7):829–835. doi: 10.1016/s0306-4530(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 130.Otto B, Tschop M, Cuntz U. Letter to the editor: similar fasting ghrelin levels in binge eating/purging anorexia nervosa and restrictive anorexia nervosa. Psychon euroendocrinology. 2004;29(5):692–693. doi: 10.1016/j.psyneuen.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Monteleone P, Martiadis V, Fabrazzo M, Serritella C, Maj M. Ghrelin and leptin responses to food ingestion in bulimia nervosa: implications for binge-eating and compensatory behaviours. Psychol Med. 2003;33(8):1387–1394. doi: 10.1017/s0033291703008316. [DOI] [PubMed] [Google Scholar]
- 132.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81(5):735–740. doi: 10.1016/j.physbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 133.Monteleone P, Fabrazzo M, Tortorella A, Martiadis V, Serritella C, Maj M. Circulating ghrelin is decreased in non-obese and obese women with binge eating disorder as well as in obese non-binge eating women, but not in patients with bulimia nervosa. Psychoneuroendocr inology. 2005;30(3):243–250. doi: 10.1016/j.psyneuen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 134.Zhang JV, Ren PG, vsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310(5750):996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 135.Gualillo O, Lago F, Casanueva FF, Dieguez C. One ancestor, several peptides: post-translational modifications of preproghrelin generate several peptides with antithetical effects. Mol Cell Endocrinol. 2006;256(1–2):1–8. doi: 10.1016/j.mce.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 136.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27(4):911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 137.Gourcerol G, St-Pierre DH, Tache Y. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Regul Pept. 2007;141(1–3):1–7. doi: 10.1016/j.regpep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 138.Gourcerol G, Tache Y. Obestatin—a ghrelin-associated peptide that does not hold its promise to suppress food intake and motility. Neurogastroenterol Motil. 2007;19(3):161–165. doi: 10.1111/j.1365-2982.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 139.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 140.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 141.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147(2):173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 142.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 143.Hug C, Lodish HF. Diabetes, obesity, and Acrp30/adiponectin. Biotechniques. 2002;33(3):654, 656, 658. doi: 10.2144/02333dd01. [DOI] [PubMed] [Google Scholar]
- 144.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440(2–3):213–221. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 145.Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002;80(11):696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 146.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 147.Ritter RC, Covasa M, Matson CA. Cholecystokinin: proofs and prospects for involvement in control of food intake and body weight. Neuropeptides. 1999;33(5):387–399. doi: 10.1054/npep.1999.0051. [DOI] [PubMed] [Google Scholar]
- 148.Buchan AM, Polak JM, Solcia E, Capella C, Hudson D, Pearse AG. Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut. 1978;19(5):403–407. doi: 10.1136/gut.19.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr. 1981;34(2):154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 150.Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological post-prandial plasma concentrations. Clin Sci (Lond) 1995;89(4):375–381. doi: 10.1042/cs0890375. [DOI] [PubMed] [Google Scholar]
- 151.Lieverse RJ, Jansen JB, Lamers CB. Cholecystokinin and satiation. Neth J Med. 1993;42(3–4):146–152. [PubMed] [Google Scholar]
- 152.Reubi JC, Waser B, Laderach U, et al. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997;112(4):1197–1205. doi: 10.1016/s0016-5085(97)70131-8. [DOI] [PubMed] [Google Scholar]
- 153.Mercer JG, Lawrence CB. Selectivity of cholecystokinin (CCK) receptor antagonists, MK-329 and L-365,260, for axonally-transported CCK binding sites on the rat vagus nerve. Neurosci Lett. 1992;137(2):229–231. doi: 10.1016/0304-3940(92)90410-9. [DOI] [PubMed] [Google Scholar]
- 154.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Is cholecystokinin a satiety hormone? Correlations of plasma cholecystokinin with hunger, satiety and gastric emptying in normal volunteers. Appetite. 1993;21(2):95–104. doi: 10.1016/0195-6663(93)90002-2. [DOI] [PubMed] [Google Scholar]
- 155.Geracioti TD, Jr, Liddle RA. Impaired cholecystokinin secretion in bulimia nervosa. N Engl J Med. 1988;319(11):683–688. doi: 10.1056/NEJM198809153191105. [DOI] [PubMed] [Google Scholar]
- 156.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 157.Sanaka M, Koike Y, Yamamoto T, et al. A reliable and convenient parameter of the rate of paracetamol absorption to measure gastric emptying rate of liquids. Int J Clin Pharmacol Ther. 1997;35(11):509–513. [PubMed] [Google Scholar]
- 158.Sanaka M, Kuyama Y, Yamanaka M. Guide for judicious use of the paracetamol absorption technique in a study of gastric emptying rate of liquids. J Gastroenterol. 1998;33(6):785–791. doi: 10.1007/s005350050177. [DOI] [PubMed] [Google Scholar]
- 159.Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol. 1993;264(3 pt 2):R630–R637. doi: 10.1152/ajpregu.1993.264.3.R630. [DOI] [PubMed] [Google Scholar]
- 160.Brolin RE. Critical analysis of results: weight loss and quality of data. Am J Clin Nutr. 1992;55(2 suppl):577S–581S. doi: 10.1093/ajcn/55.2.577s. [DOI] [PubMed] [Google Scholar]
- 161.Adami GF, Meneghelli A, Scopinaro N. Night eating and binge eating disorder in obese patients. Int J Eat Disord. 1999;25(3):335–338. doi: 10.1002/(sici)1098-108x(199904)25:3<335::aid-eat12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 162.Geliebter A, Westreich S, Pierson RN, Jr, Van Itallie TB. Extra-abdominal pressure alters food intake, intragastric pressure, and gastric emptying rate. Am J Physiol. 1986;250(4 pt 2):R549–R552. doi: 10.1152/ajpregu.1986.250.4.R549. [DOI] [PubMed] [Google Scholar]
- 163.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48(3):592–594. doi: 10.1093/ajcn/48.3.592. [DOI] [PubMed] [Google Scholar]
- 164.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav. 2001;74(4–5):743–746. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- 165.Zhu XG, Greeley GH, Jr, Lewis BG, Lilja P, Thompson JC. Blood-CSF barrier to CCK and effect of centrally administered bombesin on release of brain CCK. J Neurosci Res. 1986;15(3):393–403. doi: 10.1002/jnr.490150310. [DOI] [PubMed] [Google Scholar]
- 166.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 167.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19(5):883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 168.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18(1–2):7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]


