INTRODUCTION
Pulmonary hypertension (PH) is defined as a resting mean pulmonary artery pressure (mPAP) ≥25mmHg, which is roughly double the normal mPAP.1 The breadth and inclusivity of this definition is problematic for several reasons. First, PH is a heterogeneous syndrome with variable etiologies, therapies and prognosis. Thus, a diagnosis of PH does not guide therapy; although it does portend poor prognosis. Second, mPAP is rarely measured directly, because this requires right heart catheterization. In clinical practice, right ventricular systolic pressure (RVSP) is usually estimated from noninvasive Doppler measurement of the peak velocity of a tricuspid regurgitation jet, using the Bernoulli equation. This estimate is fraught with difficulties including inaccuracy and an intrinsic inability to distinguish whether PH is due to pulmonary vascular disease versus left ventricular (LV) diastolic dysfunction. Although pulmonary vascular disease can be identified and mPAP calculated from the PA Doppler signal (by measuring pulmonary artery acceleration time, PAAT)2 (Figure 1) this simple measurement is often omitted.
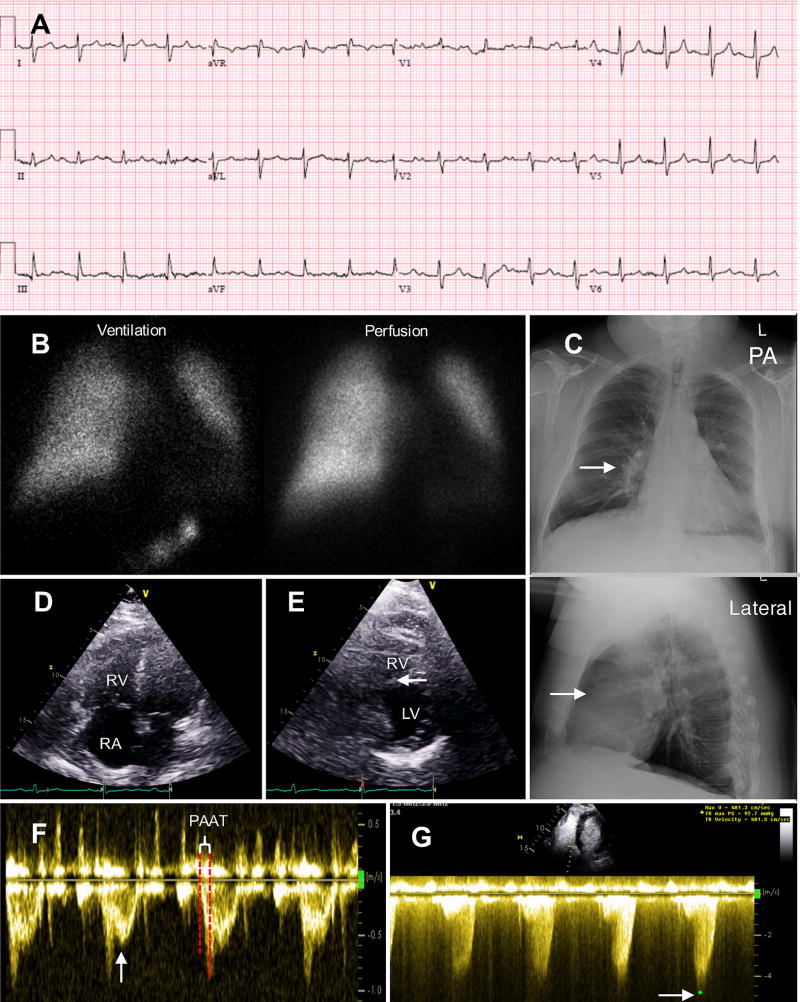
Figure 1. Group 3 Pulmonary Hypertension case: 68 year-old male with moderately severe chronic obstructive pulmonary disease and obstructive sleep apnea.
A. Electrocardiogram showed sinus tachycardia with first degree atrial ventricular block. Note right axis deviation and right ventricular hypertrophy with S1Q3T3 pattern.
B. Ventilation perfusion (VQ) scan did not demonstrate VQ mismatch. No evidence of pulmonary embolism.
C. Posterior anterior (PA) and lateral chest x-ray. Evidence of right ventricle (RV) enlargement (arrows indicate enlarged right pulmonary artery on PA view and loss of retrosternal airspace on lateral view).
D. Apical four chamber view on ultrasound demonstrating RV and right atrial (RA) enlargement.
E. Parasternal short axis view on ultrasound showing mild septal flattening (arrow), a sign of RV pressure/volume overload.
F. Pulse wave Doppler of the pulmonic valve demonstrates severe pulmonary hypertension. The Doppler signal is early peaking and there is a short pulmonary artery acceleration time (PAAT) with mid-systolic notching (arrow), reflective of a noncompliant pulmonary vascular bed.
G. Tricuspid regurgitation (TR) continuous wave Doppler [using Definity® contrast (Lantheus Medical Imaging, N. Billerica, Massachusetts) to enhance the signal]. TR peak velocity (V) (arrow) was used to calculate right ventricular systolic pressure (RVSP) of 93 mmHg using Bernoulli equation (RVSP=4V2).
To facilitate clinical categorization and management of PH patients, the 1998 World Symposia on Pulmonary Hypertension divided PH into five groups. The latest update in 2013 modified the taxonomy; however the five major groups are unchanged.1 Group 1 PH or pulmonary arterial hypertension (PAH) is an orphan disease, with an estimated prevalence of 10–52 cases/million adults.3 Group 1 PH, defined as a mPAP≥25 mmHg with pulmonary capillary wedge pressure (PCWP) ≤15mmHg and pulmonary vascular resistance (PVR) >3 Wood units,4 has received the most attention. Preclinically there are robust animal models of Group 1 disease and a related basic science knowledge base. Clinically these patients are managed in specialized clinics and are treated with 4 classes of approved PH-targeted therapies.3 There has been a remarkable increase in our knowledge of the molecular mechanisms underlying Group 1 PH over the past decade.5–7 In contrast, there are far fewer preclinical models and a less detailed understanding of the molecular mechanisms of the other PH groups. Most WHO Group 2–4 patients are not managed in specialized, Group-focused PH clinics, which is also an impediment to progress.
Group 2 is the most prevalent form of PH and is usually due to valvular or myocardial left heart disease.3 An echocardiography-based epidemiologic study from Australia estimates the prevalence of Group 2 PH at 250 cases/100,000 population8 and Group 3 PH at 37 cases/100,000 population.8 Group 4 PH, also known as chronic thromboembolic pulmonary hypertension (CTEPH), has an incidence similar to Group 1 disease.9 German and French PH registries suggest the annual incidence of CTEPH is 4 and 6 cases/million adults/year, respectively.10 Since Group 5 PH is a heterogeneous collection of PH syndromes, the search for common intra-group mechanisms is most applicable to WHO Groups 1–4.
The WHO PH classification is based on clinical phenotype (hemodynamics, comorbidities and histology); rather than pathological cellular and molecular mechanisms, such as endothelial dysfunction, inflammation/immunity, and mitochondrial dysfunction.11–14 Some molecular mechanisms, such as endothelial dysfunction, are shared across PH groups. For instance, in Group 2 PH caused by mitral stenosis (MS), the initiating pathophysiological stimulus is retrograde transmission of elevated left atrial (LA) pressure and the resulting pulmonary venous hypertension.15 PH occurs in 78% of MS patients.15 PH in MS may be proportional or disproportional. In a proportional PH response, the elevation of mPAP is at the minimum level required for antegrade circulation. In such cases, right heart catheterization (RHC) reveals a minimal gap between PA diastolic pressure (PAd) and PCWP. However, in MS patients with disproportionate PH there is a large PAd-PCWP gap and their hemodynamics have some commonality with Group 1 patients, notably elevated PVR. This reflects adverse pulmonary vascular remodelling and/or pulmonary vasoconstriction. Mitral valve replacement may not immediately cure PH in disproportionate responders because it does not address their residual pulmonary vascular disease. Indeed, 42–64% of MS patients have persistent PH after successful mitral valve surgery (Figure 2).16
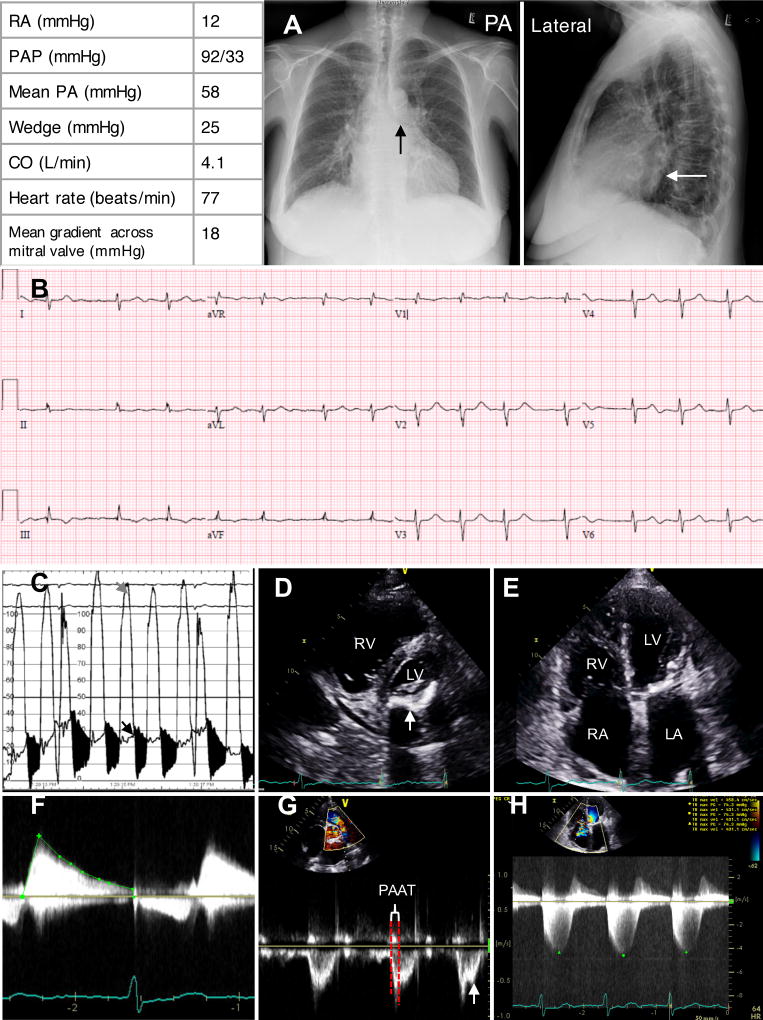
Figure 2. Group 2 pulmonary hypertension case: 73 year-old female with severe mitral valve stenosis and scleroderma.
Table displays typical right heart catheterization parameters of a Group 2 PH patient, which is characterized by increased right atrial (RA) pressure of 12mmHg, elevated pulmonary artery pressure (PAP) of 92/33 mmHg, mean pulmonary artery pressure (mPAP) of 58 mmHg, increased pulmonary capillary wedge pressure (PCWP) of 25 mmHg, cardiac output (CO) of 4.1 L/min, heart rate of 77 beat per minute and mean gradient across the mitral valve of 18 mmHg.
A. Chest x-ray demonstrates left atrial enlargement with elevation of left main stem bronchus and splaying of carina on posterior anterior (PA) view (black arrow) and posterior prominence of left atria on lateral view (white arrows).
B. Electrocardiogram demonstrates atrial fibrillation with right axis deviation, S1Q3T3 pattern and right ventricular hypertrophy. There is also low voltage in limb and precordial leads and poor R wave progression, consistent with lung disease.
C. Left ventricular (grey arrow) and wedge pressure (black arrow) tracings superimposed. Mitral valve was severely stenotic with a gradient (in black) of 18 mmHg (heart rate 77 bpm).
D. Parasternal short axis view on ultrasound with severe posterior mitral annular calcification (arrow).
E. Apical four chamber view on ultrasound shows right ventricle (RV), right atrium (RA), left atrium (LA) enlargement with normal left ventricle (LV).
F. Continuous wave Doppler across the mitral valve displays a mean gradient of 10 mmHg characteristic of severe mitral valve stenosis.
G. Pulse wave Doppler through pulmonic valve demonstrates early peaking signal with short PAAT and mid systolic notching (arrow), consistent with severe PH
H. Continuous wave Doppler across tricuspid valve displayed tricuspid regurgitation permitted calculation of a peak (green dot) RV-RA gradient (averaged) of 75 mmHg.
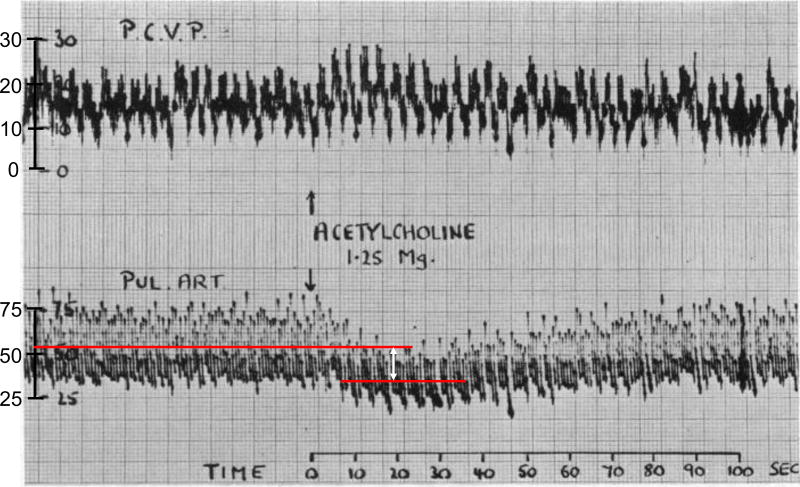
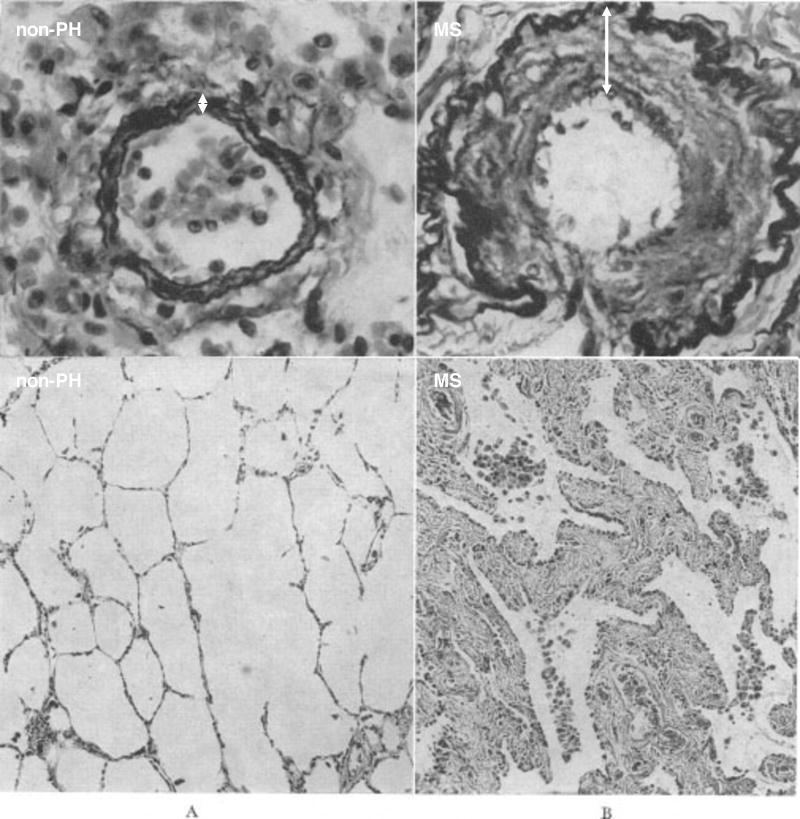
Endothelin-1 (ET-1), a potent endothelial-derived vasoconstrictor that is elevated in Group 1 PH, is also elevated three-fold in patients with severe MS.17 Indeed, Paul Wood showed the important role of pulmonary vasoconstriction in a subset of MS patients. He showed that PVR could be halved acutely with infusion of acetylcholine (Figure 3)18, an endothelium-dependent vasodilator which causes synthesis of nitric oxide (NO)19 and endothelium-derived hyperpolarizing factor20. In addition to vasoconstriction, some MS patients have disproportionate PH due to adverse pulmonary vascular remodelling, with thickening of the intima and media of small PAs (Figure 4)21. Thus, individual variations in endothelial function and vascular remodelling likely contribute to the susceptibility of patients with LHD to Group 2 PH.17 The fact that these “disproportionate responders” have some physiologic similarities to Group 1 patients suggests that it may be profitable to examine molecular pathways that are disordered in Group 1 PH for potential relevance to other PH Groups.
Figure 3. Impaired pulmonary vasodilation in response to acetylcholine in a patient with mitral stenosis-induced Group 2 PH.
Simultaneous tracing of pulmonary capillary venous pressure (PCVP; upper trace) and pulmonary artery pressure (PAP; lower trace) of group 2 PH patient with mean PAP (mPAP) around 50 mmHg and PCVP of 25 mmHg. PCVP is an indirect measure of left atrial pressure. Acetylcholine (Ach) injection (1.25 mg) significantly decrease PAP and increase PCVP. The red lines indicate the mPAP pre- and post-Ach injection. The white arrow indicates the drop in mPAP around 20 mmHg. Reproduced from Wood et al. with permission.18
Figure 4. Structural remodeling of pulmonary circulation in mitral stenosis-induced Group 2 PH.
Pulmonary arteriole (top row) and section of the lung (bottom row) from a non-pulmonary hypertension (non-PH) patient (A) compared with that from a patient with mitral stenosis (MS) (B). Top row: both vessels being less than 100 microns in diameter (Verhoeff Elastic-tissue Stain × 500). Note how marked intimal thickening (arrow) partially occludes the lumen in B. Bottom row: showing severe thickening of the alveolar walls (Hematoxylin and Eosin Stain × 125). Reproduced from Goodale et al. with permission.21
This review explores our current understanding of the preclinical models, underlying molecular pathways, and potential new therapeutic targets for Group 2, 3, and 4 PH. We will begin with a review of the preclinical models for each PH Group.
PRECLINICAL RODENT PH MODELS
Rodent models of PH are most frequently used due to their low cost, convenience, rapidity, and the greater societal acceptance of the ethics of using rodents versus large animals in research. Thus, most of our understanding of the molecular mechanisms responsible for PH is derived from rodent PH models.
Models of Group 1 PH
Although this review is focused on Group 2–4 PH, we briefly discuss the most widely used Group 1 rodent PH models, because Group 2–4 PH models share disorders of some of the same molecular pathways.
Monocrotaline-induced PAH
Monocrotaline (MCT) is derived from the plant Crotalaria spectabilis. CYP3A4-dependent hepatic metabolism creates a reactive MCT pyrrole, which causes endothelial damage. A single dose of MCT (60–100 mg/kg, subcutaneously) reliably causes PH in rats within 3–4 weeks and leads to death from RV failure within 6–8 weeks.22 The MCT rodent model recapitulates many features of human PAH, such as early endothelial injury, generation of reactive oxygen species, adverse pulmonary vascular remodeling, increased mitochondria fission, and mitochondrial metabolic abnormalities in the pulmonary vasculature and RV.22 However, critics of the model point out that MCT’s systemic toxicities (hepatotoxicity, cardiac toxicity, immunotoxicity, and DNA crosslinking)23 and the absence of plexiform lesions on lung histology, make it is an imperfect approximation of human PAH.22 However, the MCT model does display all other histologic features of Group 1 PH, such as medial hypertrophy and intimal hyperplasia, and the animals die from RV failure, as do Group 1 patients.
Sugen 5416-hypoxia (SuHx) model
Sugen 5416 (SU) is an inhibitor of vascular endothelial growth factor (VEGF) receptor 2. A PAH model was initially created by subcutaneous injection of adult male Sprague-Dawley rats with SU5416 (200 mg/kg, 3-times per week for 3-weeks). At Denver, Colorado’s altitude (~1600m) these rats developed PH (mPAP 32±2 mmHg).24 The model has evolved to combine a single, lower dose, subcutaneous injection of SU5416 (20 mg/kg) with 3-weeks of hypoxia (10% oxygen). This reliably generates severe PAH (RVSP 96±11mmHg) that persist upon return to normoxia.25 This model develops plexiform arteriopathy that resembles the histology in human PAH; however, it does not lead to RV failure as reliably as the MCT model.
Furthermore, gender and stain differences are important factors to consider when creating the SuHx model. Dean et al. showed that metformin reverses development of pulmonary hypertension via aromatase inhibition. However, this therapeutic response only occurs in female SuHx rats, suggesting the importance of sex difference in the pathophysiology of PAH.26 Jiang et al. showed marked strain-specific differences in the SU rat model.27 Surprisingly, ~75% Sprague-Dawley (SD) rats from Charles River Laboratories (Montreal, QC, Canada) exhibit a severe PAH phenotype with SU5416 (20 mg/kg) subcutaneous (SC) injection alone, whereas the other 25% showed no response.27 In comparison, SD rats from Harlan Laboratories (Indianapolis, Ind, USA) do not develop PAH phenotype with SU5416 (20 mg/kg, SC) alone.27
Models of Group 2 PH
Supracoronary aortic banding (SAB) and transverse aortic constriction (TAC) models
The SAB rat or TAC mice models are used to study Group 2 PH. The SAB model is created by banding the ascending aorta of a juvenile rat, using either a suture or a titanium clip, placed approximately 1 cm distal to the aortic valve.28–30 Hemodynamic assessment is done 4–9 weeks after SAB28–30. Our preliminary data show that 8 weeks after banding and administration of Nω-Nitro-L-arginine methyl ester (L-NAME) 1.85 mM in drinking water, rats develop marked increase in pulmonary arteriole wall thickness (Figure 5 A & B) and decreased pulmonary artery acceleration time (PAAT) (Figure 5 C & D). (see Online Supplement for the materials and methods section.) In the murine TAC model a ligature is placed around the aortic arch between the brachiocephalic trunk and left common carotid artery11, 31 and hemodynamic assessment is performed 1–4 weeks later31, 32. In animals that are not in heart failure, tighter aortic banding yields higher TBPG and LVSP (Figure 6); however, if the aortic banding is too severe animals die acutely of heart failure. Moreover, if the model progresses to the point of heart failure and cardiac output declines TBPG will fall in parallel. Although most studies using the SAB model do not report the TBPG28–30, our preliminary data indicates that rise of TBPG is associated with increased RVSP. For example, a TBPG of approximately 100 mmHg generates a RVSP of ~30 mmHg 7–8 weeks post banding with an operative mortality of 5–10%. Chen et al. used a 26-gauge needle for banding and noted that this produced a mixed group of TAC mice, some with moderate and others with severe heart failure.33 Subsequently, the same group used a smaller needle (27-gauge) to create more severe TAC and noted that these mice consistently developed severe heart failure.34 This more severe TAC also caused greater pulmonary inflammatory changes and leukocyte infiltration.33, 34 These data suggest that a tighter band does indeed create more severe left heart failure and a more severe PH phenotype.
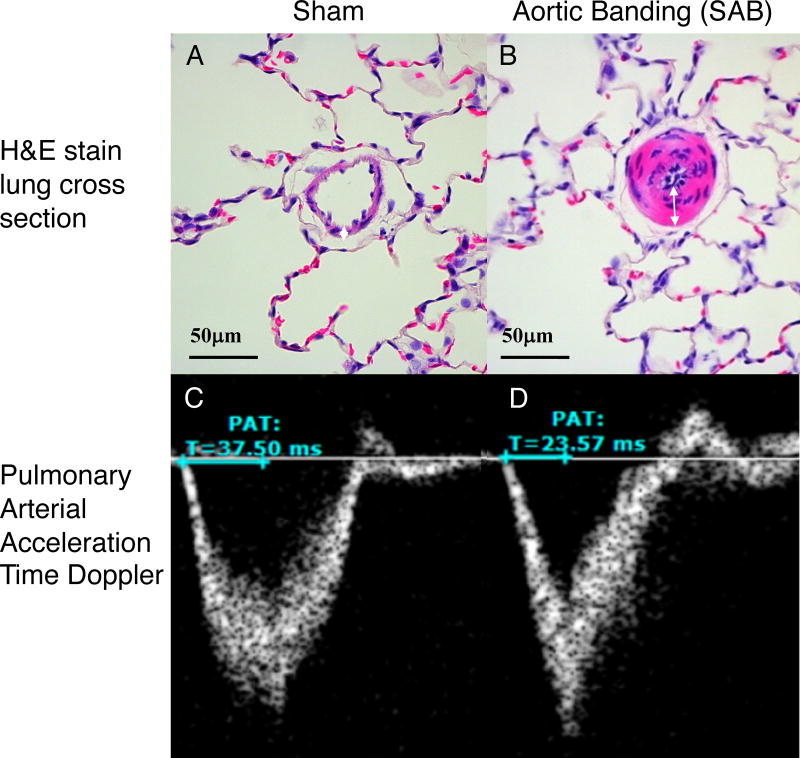
Figure 5. Increased pulmonary arteriole wall thickness and decreased pulmonary arterial acceleration time (PAAT) in rats 8 weeks post supra-coronary aortic banding (SAB) surgery.
(A–B) Haemotoxylin and Eosin (H&E) stain of lung cross-sections show increased pulmonary arteriole wall thickness (white arrows) in SAB animal (B) compared to sham (A). (C–D) Pulse wave Doppler through pulmonic valve demonstrates decreased pulmonary artery acceleration time (mentioned as PAT) in SAB rats (D) compared to sham rat (C). Both sham rats and aortic banding rats were fed with Nω-Nitro-L-arginine methyl ester (L-NAME) 1.85 mM in drinking water for 7–8 weeks, before tissue collection.
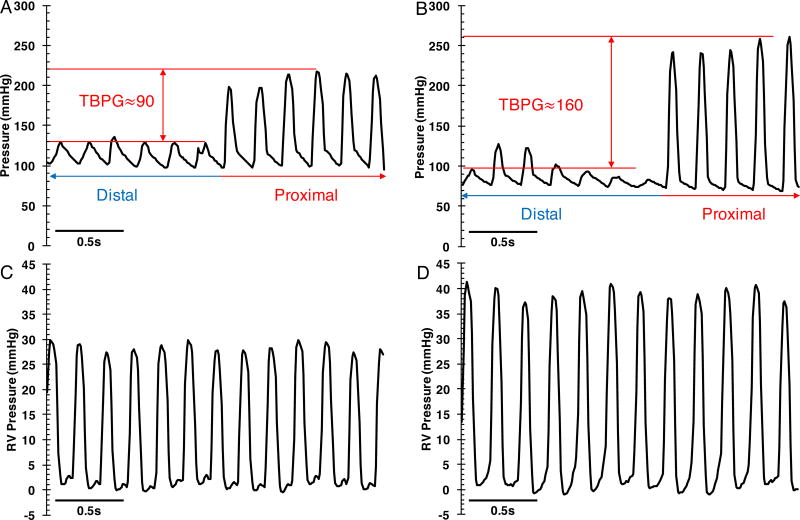
Figure 6. Hemodynamic assessment of the left and right heart in supra-coronary aortic banding (SAB) rats.
(A) and (C) are the left and right heart pressure traces respectively from one rat. (B) and (D) are the left and right heart pressure traces respectively from another rat. Trans-banding pressure gradient (TBPG) is equal to systolic pressure proximal to the band (red arrow) minus systolic pressure distal to the band (blue arrow). Higher TBPG is associated with higher right ventricle (RV) pressure.
The SAB and TAC rodent models have clinical relevance, mimicking aortic stenosis and aortic coarctation; however, they require technical expertise, develop only modest PH and do so relatively slowly. The modest PH that develops in SAB and TAC model is similar in magnitude to that seen in patients with isolated LV pressure overload due to diseases such as hypertension. A second, unidentified abnormality, likely some form of endothelial dysfunction, appears to be needed for the generation of severe PH.
Left atrial stenosis (LAS) model
Fujimoto et al. recently generated a novel Group 2 PH rat model by partially banding the left atrium with a metal clip in male Sprague-Dawley rat (5 weeks old) to increase the left atrial and pulmonary venous pressure.35 The LAS model, relevant to mitral stenosis, displayed an increased RV/LV pressure ratio (0.52 vs. sham 0.22), increased RV/body weight ratio (0.54 vs. sham 0.39 mg/g), and marked medial hypertrophy of the pulmonary veins (1.6 times that of sham rats).35 Immunoblots revealed significantly elevated expression of transforming growth factor-β (TNF-β) and ET-1 levels in the lung of the LAS vs. sham rats.35
Models of Group 3 PH
Chronic hypoxia model
Exposing rodents to either normobaric or hypobaric hypoxia generates a Group 3 PH model. Rodents are acclimated to decreasing inspired oxygen concentrations over 3–5 days and then maintained at 10% oxygen for 3–4 weeks. They develop pulmonary artery medial hypertrophy, elevated mPAP, PVR, and right ventricular hypertrophy (RVH).22 However, the chronic hypoxia model does not fully recapitulate Group 3 PH in humans, which usually results from chronic obstructive pulmonary disease (COPD).36, 37 Specifically, chronic hypoxia does not cause abnormal lung parenchyma or obstructive PA intimal lesions and induces only mild PH.36, 37 Both the PH and histologic changes reverse when the rodents are returned to normoxia.22 Many preclinical therapies are effective in preventing and regressing PH in the chronic hypoxic rat model but most of these have failed to benefit Group 3 patients.
Bleomycin-induced PH
Bleomycin is a chemotherapeutic agent that creates DNA strand breaks. A rodent model of Group 3 PH due to pulmonary fibrosis is produced by endotracheal instillation of bleomycin (2–4 unit/kg) into rats38 or mice,39 respectively. Bleomycin causes fibrogenic cytokine release, resulting in pulmonary fibrosis, increased PA medial thickness, RVH, and PH.38, 39 In a model using adult male C57/BL6 mice, survival is 74% 2-weeks post bleomycin.39 Male Wistar rats instilled with bleomycin developed significant pulmonary fibrosis, accompanied by increased lung expression of hydroxproline, collagen, and connective tissue growth factor.38
Model of Group 4 PH
A Group 4 PH model has recently been created by tail vein injection of male Sprague-Dawley rats with 85µm polystyrene microspheres plus subcutaneous SU5416 (20 mg/kg).40 All rats treated with microspheres+SU5416 developed sustained PH (RVSP of 62±13 mmHg and 53±14 mmHg at 3 and 6 weeks, respectively). They also developed significant RV dysfunction, RVH and RV fibrosis and had decreased exercise capacity. Lung sections of the microspheres+SU5416 rats demonstrated marked medial hypertrophy in small arterioles and intimal proliferation with obliteration of the lumen; however, as in humans with Group 4 disease there were no plexiform lesions. Computed tomography pulmonary vascular imaging showed lack of neovascularization and Ki-67 immunohistochemistry showed decreased cellular proliferation in the microspheres+SU5416 group vs. either microspheres or sham group. The microspheres+SU5416 rats also had evidence of increased inflammation and platelet activation.40 In contrast, with microspheres alone only 10% of rats developed PH. Hence, a combined insult (pulmonary embolism plus inhibition of endothelial function) is required to routinely induce Group 4 PH.40
WHO GROUP 2–4 PH in PATIENTS
We will next discuss the definitions and disordered molecular pathways associated with Group 2–4 PH in patients.
WHO Group 2 (Table 1)
Table 1.
Molecular pathways implicated in Group 2 pulmonary hypertension.
| Pathway | Molecular Mechanism | Consequence | Animal Model- Species |
Author (year)ref |
|---|---|---|---|---|
| ET-1 | A functional shift of ET receptor subtype towards ETA occurs in Group 2 PH | In CHF, responses via the ETB receptor are reduced and responses via the ETA receptors are enhanced in LV and PA | CHF induced by rapid ventricular pacing-dogs | Tadano (2004)57 |
| NO-sGC-cGMP | Impaired endothelial [Ca2+]i oscillation | Reduced NO synthesis, decreased endothelium-dependent vasodilation without alteration of eNOS expression | SAB-rats | Kerem (2010)73 |
| PDE5 dysregulation | Despite down-regulation of PDE5 in SAB rats, chemical inhibition of PDE5 preserves endothelial function | Sildenafil, a PDE5 inhibitor, attenuates PH, RVH, and dysfunction in SAB rats | SAB-rats | Yin (2011)29 |
| Mitochondrial dysfunction | Smaller mitochondria (area <0.4µm2) are more abundant and larger ones (area>1.6µm2) rarer in cardiac myocytes of CHF dogs | Profound mitochondria structural abnormalities are present throughout cardiomyocytes in dogs with CHF. Smaller mitochondria portend worse H, indicated by lower EF, higher LVEDP, higher PAWP, and higher plasma NE level | CHF induced by intracoronary multiple embolization with microsphere-dogs | Sabbah (1992)90 |
| Mitochondrial dysfunction | Inhibition of mitochondrial fission, promotes angiogenesis, and decreases mitophagy | Mdivi-1 improves cardiac function and decreases cardiac fibrosis in TAC mice | TAC-mice | Givvimani (2012)12 |
| RhoA/Rho-kinase inhibition (direct) | Fasudil decreased Rho-kinase activity, enhanced eNOS expression, increased NO and cGMP, and decreased ET-1 level | Fasudil 30 mg/kg/day decreases mPAP, attenuates RVH and pulmonary arteriolar medial hypertrophy | SAB-rats | Dai (2011)28 |
| RhoA/Rho-kinase inhibition (indirect) | Inhibition of HMG-CoA reductase decreases isoprenoids production and subsequent isoprenylation (and inhibition) of Rho-kinase | Atorvastatin 10 mg/kg/day decreases mPAP, RVH, and pulmonary arteriolar medial thickness by down-regulating RhoA/Rho kinase expression | SAB-rats | Wang (2016)30 |
| Metabolic syndrome | Genome-Wide association study identified increased EGFR expression in lungs of mice that develop the most severe PH after exposure to a high fat diet | Increased EGFR expression is associated in the susceptibility to develop PH with metabolic syndrome (increased RVSP) | High fat diet-mice | Kelly (2017)105 |
Abbreviations: ET=Endothelin; CHF=congestive heart failure; LV=left ventricle; PA=pulmonary artery; NO=nitric oxide; sGC=soluble guanylate cyclase; cGMP=cyclic guanosine monophosphate; [Ca2+]I=intracellular calcium concentration; eNOS=endothelial nitric oxide synthase; SAB=supracoronary aortic banding; PDE5=phosphodiesterase 5; PH=pulmonary hypertension; RVH=right ventricle hypertrophy; EF=ejective fraction; LVEDP=left ventricle end-diastolic pressure; PAWP=pulmonary artery wedge pressure; NE=norepinephrine; TAC=transverse aortic constriction; mPAP=mean pulmonary arterial pressure; HMG-CoA=3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; EGFR=endothelin growth factor receptor; RVSP=right ventricle systolic pressure;
PH-LHD is defined as a mPAP ≥25 mmHg with elevated left heart filling pressures (PCWP >15 mmHg or LVEDP >18 mmHg).41 PH-LHD is sub-classified as isolated post-capillary PH (IpcPH) or combined post- and pre-capillary PH (CpcPH). In IpcPH the diastolic pulmonary vascular gradient (DPG), defined as PAPdiastolic–PCWP, is <7mmHg and the PVR≤3WU; whereas in CpcPH the DPG is ≥7mmHg and the PVR>3WU.42 The major causes of elevated left heart filling pressures and heart failure that give rise to PH are: 1) mitral and aortic valvular disease and 2) LV myocardial diseases. Heart failure is further subdivided into heart failure with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF).41 (Figure 7) Most patients with HFrEF or HFpEF develop PH. A community-based study showed that 83% of HFpEF patients (n=244; mean age 76±13 years; 45% male) have PH.43 The incidence of PH in HFrEF patients is similar (72–79%).44, 45
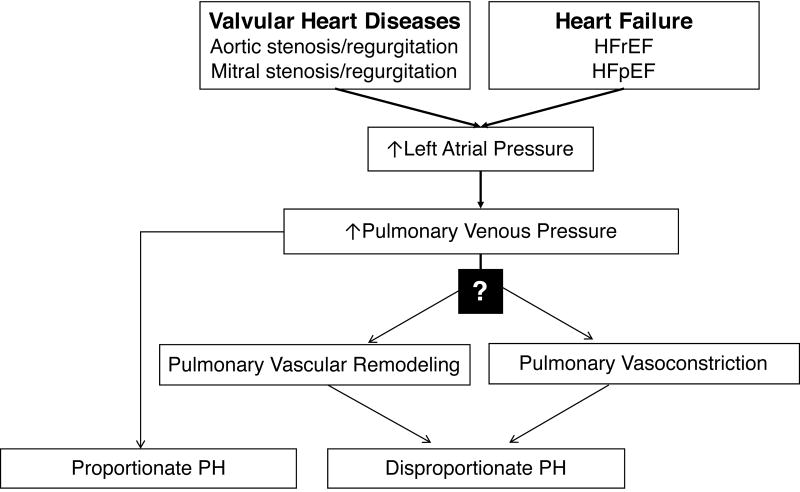
Figure 7. Pathophysiology of Group 2 Pulmonary Hypertension (PH).
In most patients left heart disease increases pulmonary venous pressure and causes a proportionate PH (left). However, for unknown reasons, in a minority of patients with LHD, elevation of pulmonary venous pressure leads to adverse pulmonary vascular remodeling, and/or pulmonary vasoconstriction. This causes disproportionate PH, which may not fully resolve with relief of elevated pulmonary venous pressure. HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction.
The treatment of Group 2 PH focuses on symptomatic care (diuretics, sodium restriction) and correction of the underlying LHD. For HFpEF this often involves control of systemic hypertension to a target pressure of 120/80 mmHg. For HFrEF this involves the use of angiotensin converting enzyme inhibitors or angiotensin receptor antagonists as well as β-adrenergic receptor antagonists. For patients with valvular LHD surgical correction of the valve abnormality is recommended. However, Group 2 PH cannot always be completely reversed by treating the LHD16, as discussed previously (Figure 2). We next review molecular mechanisms that may be responsible for the pathological remodeling and vasoconstriction of the pulmonary vasculature that underlies disproportionate PH in some Group 2 patients.
Disordered Pathways in Group 2 PH
Endothelial Dysfunction
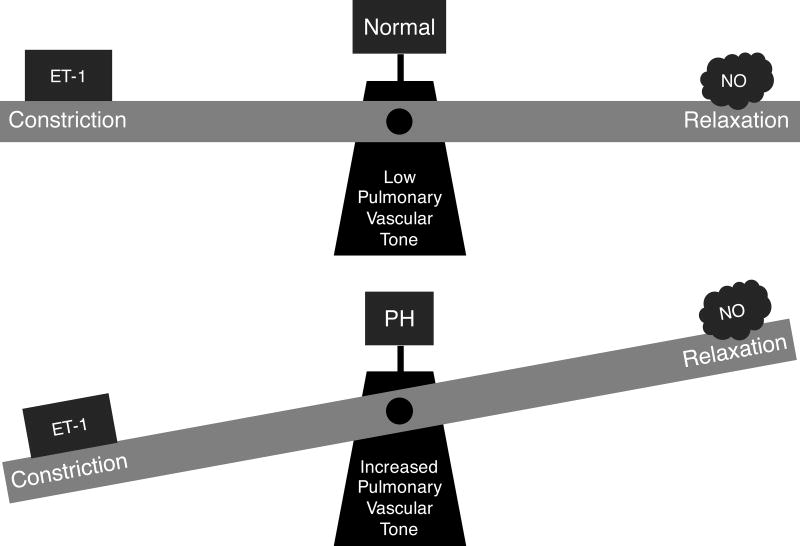
PH-LHD is associated with endothelial dysfunction, characterized by an increase in levels of vasoconstrictors, such as endothelin-1 (ET-1) and a decrease in vasodilators, such as nitric oxide (NO).46, 47 (Figure 8)
Figure 8. Imbalance in endothelin-1 (ET-1) and nitric oxide (NO) homeostasis in pulmonary hypertension favours pulmonary vasoconstriction.
Endothelin-1 (ET-1)
ET-1 is a potent vasoconstrictor identified by Yanagisawa in 1988.48 ET-1 levels are markedly elevated in PH, including Group 2 patients.49 Exposing endothelial cells to increased pressure increases ET-1 production by a phospholipase C (PLC) and protein kinase C (PKC)-dependent mechanism.50 Pressure-induced elevations of ET-1 likely contribute to Group 2 PH.
ET-1 binds to ETA and ETB receptors.51 ETA receptors are expressed mainly on vascular smooth muscle cells (VSMCs) and cardiac myocytes. Binding of ET-1 to ETA receptors activates PLC, which generates diacylglycerol (DAG) and inositol triphosphate (IP3).52 These secondary messengers trigger the release of intracellular calcium, which leads to activation of myosin light chain kinase, phosphorylation of myosin light chain, and vasoconstriction.52 ET-1 also activates the RhoA/Rho kinase pathway, which leads to calcium sensitization and sustained vasoconstriction.53 ETB receptors are expressed on vascular endothelial cells, and upon binding of ET-1, promote production of NO and prostacyclin, resulting in vasodilation.54 The ratio of ETA to ETB receptors in humans is approximately 9:1 and the net effect of ET-1 on pulmonary arteries is vasoconstriction.55
In heart failure, there is not only elevation of ET-1 levels but also increased vasoconstrictor responsiveness to ET-1.56 In a canine HF model induced by rapid ventricular pacing, Tadano et al. demonstrated a functional shift of ET receptor subtypes favoring vasoconstriction.57 While there is biological plausibility that ETRAs (endothelin receptor antagonists), such as bosentan or macitentan, might benefit PH-LHD patients many clinical trials indicate a lack of benefit.58–61 In examining putative PH-targeted therapies one must not only consider their pulmonary vascular interactome but also their effect on the RV, which is a key determinant of prognosis in PH. One potential mechanism for the lack of benefit from ETRA in Group 2 PH is the preclinical observation that some ETRAs are negative RV inotropea.62
NO-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway
NO is an endothelium-derived vasodilator produced from L-arginine by the endothelial NO-synthase (eNOS).63 NO is a nitrogen based radical and has a very short half-life of only 0.05–1 second.64 NO is synthesized in the endothelium and diffuses to subjacent SMC.65 The effects of this gaseous vasodilator are largely exerted by activation of its molecular receptor, sGC and subsequent generation of cGMP.19 NO production can be triggered by various endogenous factors, such as acetylcholine (Ach) and bradykinin, or mechanical stimuli, such as shear stress and stretch.66–68 sGC stimulators, such as riociguat, compensate for deficiency in biologically active NO and for sGC dysfunction, resulting from sGC oxidation or loss of sGC’s heme moiety.69 Riociguat is approved for treatment of Group 1 and Group 4 PH.70 However, in the LEPHT trial, which studied 201 Group 2 patients, riociguat (2mg three-times daily for 16-weeks) failed to reduce mPAP, the study’s primary endpoint.71 Riociguat did improve cardiac index and reduced pulmonary and systemic vascular resistance.71
Both decreased NO production and reduced vasodilator responsiveness to NO occur in PH-LHD.72 SAB rats have impaired pulmonary endothelium-dependent vasodilation and reduced lung capillary endothelial NO synthesis.73 The impaired NO synthesis reflects a decrease in intracellular calcium oscillations in endothelial cells, rather than down regulation of eNOS.73 Calcium oscillations were attenuated in SAB rats in response to mechanical stress, ACh, histamine, and thapsigargin, an inhibitor of sarcoplasmic reticulum Ca2+ ATPase.73 The Ca2+ ionophore, A-23187 restored endothelial calcium oscillations and NO production.73 Thus, the underlying cause of endothelial dysfunction and decreased NO production in this preclinical Group 2 PH model is dysfunction of endothelial calcium signaling pathways. The cause of impaired calcium signaling in Group 2 PH and its relevance to human PH requires further study.
The endothelium has many functions beyond NO synthesis. Simply eliminating NO, by exposing rats to Nω-Nitro-L-arginine methyl ester (L-NAME ,1.85 mM in drinking water for 3-weeks) failed to cause Group 2 PH, despite induction of severe systemic hypertension.74
Phosphodiesterase 5 (PDE5) Dysregulation
PDE5 catalyzes the hydrolysis of cGMP. Although PDE5 inhibitors, such as sildenafil, are approved therapy for Group 1 PH, off-label use of sildenafil in Group 2 PH patients shows some potential clinical and hemodynamic benefits.75, 76
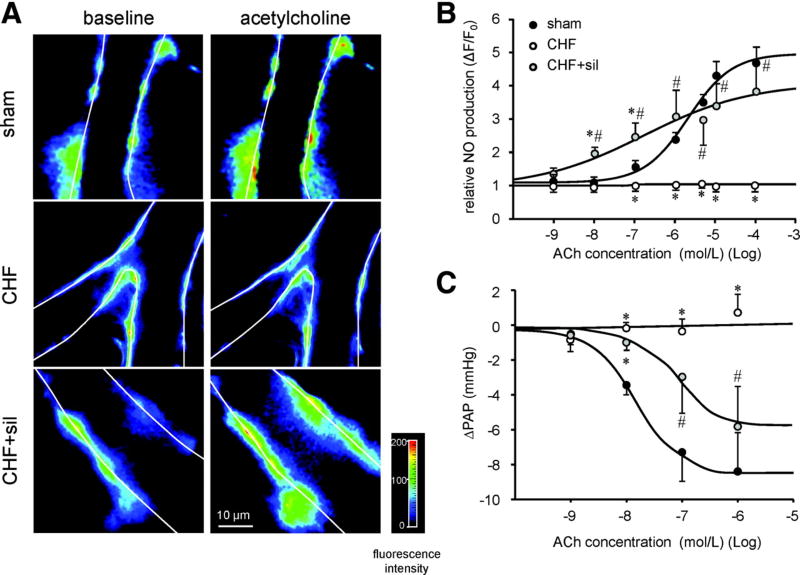
In the SAB rat model, sildenafil (60 mg/kg daily for 8-weeks) decreased endothelial dysfunction (Figure 9A), restored NO production (Figure 9B), and restored pulmonary arteriole vasorelaxation (Figure 9C).29 Interestingly, the PDE5 expression in SAB rats lung homogenate was decreased by ~50%.29 This differs from Group 1 PH rat models in which lung PDE5 expression is increased.77, 78 Nonetheless, plasma cGMP levels were reduced in SAB rats and sildenafil significantly increased cGMP levels.29
Figure 9. Impaired endothelial function in SAB (Group 2) PH.
A. Representative images of 4-amino-5-methylamino-2’,7’-difluorofluorescein diacetate (DAF-FM)–loaded capillaries show endothelial cells color-coded for NO production in response to acetylcholine (ACh)-note that CHF=SAB rats.
B. Impaired capillary DAF-FM fluorescence in response to stimulation with ACh in lungs from SAB rats.
C. Endothelium-dependent vasorelaxation response to ACh in isolated, perfused rat lungs preconstricted by U46619 (is impaired in SAB rats and this is partially restored by sildenafil. Reproduced from Yin et al. with permission.29
Clinical trials of sildenafil in Group 2 PH are promising. A review by Hansdottir et al. summarized three small trials showing patients with PH secondary to HFrEF and HFpEF treated with sildenafil (25 to 75 mg orally 2 to 3 times daily for 12 weeks to 1 year) had reduced hospitalizations for HF, improved exercise capacity, enhanced quality of life, reduced mPAP, and decreased breathlessness score versus control subjects.79 Two multicenter clinical trials testing PDE5 inhibitors in Group 2 PH are currently underway (PITCH-HF trial for Tadalafil; NCT01910389 and the Sil-HF trial80 for sildenafil; NCT01616381).
Mitochondrial dysfunction
Mitochondria are not only generators of ATP but also regulate processes such as cell proliferation and apoptosis.81 Mitochondria have noncanonical function that in aggregate are referred to as mitochondrial dynamics.81 These properties include division (fission), union (fusion), movement (translocation), biogenesis and quality control (mitophagy). Using confocal imaging, and mitochondrial-targeted potentiometric dyes and fluorescent probes, one can visualize dynamic networks of mitochondria in live cells.81 Acquired disorders of mitochondrial metabolism and dynamics contributes to the pathogenesis of complex diseases such as cardiovascular disease, neurodegenerative disease, and cancer.82
In Group 1 PH, there are acquired abnormalities of mitochondrial metabolism and dynamics81 in both the pulmonary vasculature83 and RV84. The mitochondrial phenotype in PAH includes suppression of glucose oxidation, due to increased expression and activation of pyruvate dehydrogenase kinase (PDK)81, 84, 85, and mitochondrial fragmentation, due to increased mitochondrial fission and impaired mitochondrial fusion.86
The metabolism of glucose normally involves close coupling of cytosolic glycolysis with mitochondrial glucose oxidation (which relies on the pyruvate generated by glycolysis). However, PDK activation can disrupt this coupling by phosphorylating and inhibiting pyruvate dehydrogenase (PDH)85. This metabolic shift (called the Warburg phenomenon) is reversed by PDK inhibitors, such as dichloroacetate, with therapeutic benefit to the pulmonary vasculature and RV.85, 87, 88 Uncoupled glycolysis promotes cell proliferation and suppresses mitochondria-mediated apoptosis.85 This favors survival of abnormal cells in the pulmonary circulation and impairs the bioenergetic reserve of the RV. In models of Group 1 PH, such as the fawn hooded rat (a Group 1 PH model caused by epigenetic silencing of superoxide dismutase 2, SOD2) and MCT rats, PDK inhibition regresses established pulmonary vascular disease.87, 88 In the PAH RV, the metabolic shift to Warburg metabolism causes RV hypokinesis, and this too is improved by dichloroacetate.84, 85
Although there are parallels in the metabolic alterations in the PASMC and RV cardiomyocytes the initiating factors and their functional consequences differ. In PASMC, the mitochondrial morphologic changes reflect enhanced mitotic fission, which is initiated by the kinases that regulate both the activation of the fission mediator, dynamin related protein 1 (Drp1) and mitosis83 (e.g. cyclin B-CDK1), as well as by elevation of cytosolic calcium86. The consequences of the mitochondrial metabolic phenotype in the vasculature is accelerated cell proliferation and migration and apoptosis-resistance.81 In the RV myocytes changes in metabolism and dynamics are likely initiated by RV ischemia and result in myocardial hibernation and reduced RV function.84
Cell division requires a coordination of nuclear and mitochondria division.81. Mitochondrial fission that is coordinated with mitosis is called mitotic fission. The coordination reflects a shared reliance on mitosis promoting Drp1-activating kinases, including cyclin B1-CDK1.81 Mitochondria in PAH PASMC are fragmented due to increased fission (due to activation of dynamin related protein 1 (Drp1)) and impaired fusion (due to decreased function of the fusion mediator, mitofusion-2).85 This fission/fusion imbalance promotes excessive cell proliferation and impairs apoptosis.81 Preventing mitotic fission, by inhibiting Drp1 or enhancing mitofusin-2, causes cell cycle arrest and can regress vascular disease and improve hemodynamics in experimental models of PAH.81
In PAH, RV ischemia, related to microvascular rarefaction and impaired coronary perfusion pressure, promotes Drp1 activation and mitochondrial fission, which increases ROS production.81 Inhibiting fission, using the Drp1 inhibitor, mitochondrial division inhibitor-1, Mdivi-1, or P110 (a competitive peptide inhibitor of the interaction between Drp1 and its binding partner, fission protein 1, Fis1), preserves RV function in an ischemia-reperfusion injury model.89
Interaction between the changes in metabolism and altered mitochondrial dynamics in PH is poorly understood. In Group 1 PH impaired expression and function of the mitochondrial calcium uniporter complex (MCUC) constitutes one link between metabolism and dynamics.86 The MCUC is the major route of calcium entry into the mitochondrial matrix.86 Loss of MCUC function (due to a downregulation of the pore forming subunit, called MCU, and upregulation of the negative regulatory element MICU1) impairs mitochondrial calcium uptake and causes accumulation of calcium in the cytosol.86 Reduced intramitochondrial calcium inhibits calcium-sensitive dehydrogenases within the mitochondria, including PDH. This inactivates Krebs’ cycle, contributing to the shift to uncoupled glycolysis.86 The elevation of cytosolic calcium, resulting from impaired MCUC function promotes vasoconstriction and mitochondrial fission.86 The down regulation of MCUC in PAH results from increased expression of microRNAs (miRs) miR-138 and miR-25. Anti-miRs against these epigenetic mediators reduces PASMC proliferation, migration, and apoptosis-resistance and regress experimental PAH.86 The potential role of the MCUC is Group 2 PH has not been assessed.
Data regarding mitochondria in PH-LHD is scarce. In dogs with CHF produced by intracoronary microembolizations the number of mitochondria in cardiomyocytes was increased but their average size was smaller, both in the LV and RV (Table 1).90 These findings are consistent with increased fission. Although RVSP or mPAP was not reported, mean LVEDP (23±3 mmHg) and PAWP (13±1 mmHg) were markedly elevated compared to control. Dogs with the highest plasma norepinephrine (>600 pg/mL), had smaller mitochondria and worse heart failure.90
In the TAC model, Mdivi-1 improves LV function and prevents ventricular remodeling by preventing cardiomyocyte apoptosis and promoting angiogenesis.12 Inhibiting mitochondrial fission also preserves LV function in a global ischemia model and, in a rodent cardiac arrest model, enhances the success of resuscitation.91, 92 However, the pulmonary hemodynamics were not reported in these studies. Thus, it is uncertain whether inhibiting mitochondria fission in Group 2 PH can improve RV function or clinical outcomes.
RhoA/Rho-kinase pathway
The RhoA/Rho-kinase pathway is implicated in PH-LHD.28, 30 Rho-kinase (ROCK) is a serine/threonine kinase that its activated when it binds GTP-bound RhoA.93 RhoA, a small GTPase, is activated by guanine nucleotide exchange factor (GEF) and inactivated by GTPase-activating protein (GAP).93 Normally a balance between GEF and GAP determine RhoA activity, which regulates the two ROCK isoforms.94 ROCK1 is expressed in circulating inflammatory cells whilst Rho-kinase 2 (ROCK 2) is found in VSMC.95, 96 The ROCKs produce sustained vasoconstriction due to calcium sensitization, a result of their interactions with substrates, including myosin light chain (MLC) and myosin phosphatase target subunit-1 (MYPT-1).97 Relevant to PH, ROCK activation also increases VSMC proliferation, inflammatory cell migration, platelet activation, ROS production, and endothelial dysfunction.97 MYPT-1 is activated in the lungs of rats with SU5416-hypoxia induced PAH and this is reversed by the rho kinase inhibitor, fasudil.98 Rho kinase inhibition regresses SU5416-hypoxia-induced PAH, suggesting that severe sustained vasoconstriction is important.98 RhoA/Rho-kinase inhibition also attenuate PH-LHD induced by SAB.28, 30 Fasudil (30 mg/kg/day administered from day 29–42 post-banding), decreased mPAP by 65%, RVH by 30%, and PA medial thickness by 50%.28 On a molecular level, fasudil decreased expression of ROCK2 expression by 28% (without altering ROCK1) while increasing lung eNOS.28
Drugs that indirectly inhibit the RhoA/Rho-kinase pathway also attenuate PH-LHD in rodent models. Atorvastatin (10 mg/kg/day orally for 9-weeks), an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase30, significantly reduced RhoA and ROCK2 expression in SAB rats by impairing production of isoprenoids, which are required for isoprenylation of Rho-kinase.30 However, this same statin failed to regress Group 1 PH in both rats and humans99, 100.
Metabolic Syndrome
Metabolic syndrome (MetS) has emerged as a risk factor for both Group 1101, 102 and Group 2 PH103. In mice, there is a link between genetic background and susceptibility to PH induced by a high fat diet (HFD). In a genome-wide association study (GWAS) of 36 mouse strains challenged with 20-weeks of HFD104, variants in the endothelial growth factor receptor (EGFR) gene were associated with increased susceptibility to Group 2 PH.105 EGFR expression was increased in the lungs of mice with the most severe HFD-PH (although the severity of PH in this study was mild).105 Previous studies have identified the critical role of EGFR in PASMC growth, vascular remodeling, and survival in experimental model of Group 1 PH.106 Based on the GWAS and supporting data, Kelly et al. concluded that higher baseline level of EGFR expression may increase susceptibility to HFD-induced Group 2 PH.105
Type 2 diabetes mellitus (type 2 DM) is a component of MetS. Chronic hyperglycemia inhibits adenosine monophosphate-activated protein kinase (AMPK).107 AMPK is a heterotrimeric enzyme with α, β and γ subunits. AMPK serves as a fuel gauge, generating ATP as required to correct energy depletion. When AMP levels are increased AMPK is activated. There are 12 AMPK variants with tissue specific distribution.108 AMPK is activated by phosphorylation of its α-subunit at threonine 172. This phosphorylation is regulated by competitive binding of adenosine monophosphate (AMP) versus adenosine triphosphate (ATP) to the γ subunit.108 Metformin, which is used to treat type 2 DM, is an AMPK activator.109 In experimental Group 2 PH metformin decreases RVSP and adverse vascular remodeling.110 Lai et al. developed a model of MetS and PH-HFpEF by injecting an obese rat strain (obese ZSF1) with SU5416.110 In this model, metformin decreased hyperglycemia, activated AMPK in PASMC and caused beneficial vascular remodeling. Metformin111 and a more selective AMPK activator, 5’-aminoimidazole-4-arboxamide ribonucleoside (AICAR),112 also decrease PASMC proliferation and improve survival in rodent PAH models.
Lung Inflammation and Fibrosis
Chen et al. showed that male TAC mice (C57B6J) have a ≤5.3 -fold increase in lung wet weight that reflects massive pulmonary fibrosis and significant pulmonary leukocyte infiltration, rather than pulmonary edema. Thus, lung fibrosis and inflammation may play an important role in the pathophysiology of Group 2 PH.33 Wang et al. used either CD28 or B7 knockout mice, which have impaired T-lymphocyte activation, to show that activated effector T cells (CD3+CD44high cells) play a central role in the inflammatory response associated with TAC-induced HF.34 Knockout mice lacking either CD28 or B7 had dramatic reduction in the accumulation of activated effector T cells in both the heart and the lung, and had better preserved LV function post-TAC. Although RVSP was not measured in this study, the ratio of RV/body weight was significantly reduce in both the CD28 or B7 knockout TAC mice34 These data suggest targeting T cell activation may be useful in treating Group 2 PH.34
WHO Group 3 PH (Table 2)
Table 2.
Molecular pathways implicated in Group 3 pulmonary hypertension.
| Pathway | Molecular Mechanism | Consequence | Animal Model- Species |
Author (year)ref |
|---|---|---|---|---|
| Endothelial AMPK inhibition | Inducible knockdown of eAMPK (generated by crossing AMPK floxed mice with Tie2-Cre mice on a C57BL/6 background) increases VEGF, IL-6, PDGF-BB, and FGF-2; decreases expression of p53 and eNOS in hypoxic lung | eAMPK knockdown mice are more susceptible to hypoxia induced PH than WT mice. Metformin up-regulates eAMPK and ameliorates hypoxia-induced PH | Chronic hypoxia-mice | Omura (2016)121 |
| PDE5 activation | Sildenafil decreases RhoA/ROCK, iNOS activity, and ROS generation whilst increasing PKG and eNOS activity | Sildenafil improves survival, reduces RVH and fibrosis, and pulmonary vascular medial hypertrophy in bleomycin-induced pH | Bleomycin-induced PH -mice | Hemnes (2008)39 |
| HIF-1α activation | Genetic haploinsufficiency of HIF-1α decreases NHE1 and TRPC1 expression, which prevents hypoxia induced alkalinization and elevation of [Ca2+]I in PASMC, respectively | Reduced HIF-1α activation decreases polycythemia, RV hypertrophy, PH, and adverse pulmonary vascular remodeling in HIF1a+/− mice | Chronic hypoxia-mice and rats | Shimoda (2006)132 Wang (2006)133 |
| HIF-1α activation | inhibition of HIF-1α transcriptional activity by digoxin has therapeutic benefit | Treatment with digoxin (0.2 or 1 mg/kg/d, IP) prevented attenuates RV hypertrophy, prevented increases in RV pressure, PASMC [Ca2+]i and pHi | Chronic hypoxia-mice | Abud (2012)134 |
| Mitochondria-HIF-1α-Kv | Hypoxia decreases mitochondria ROS production and activatesHIF-1α, which in turn suppresses PASMC and cardiac myocytes Kv channel expression and alters metabolism (favoring uncoupled glycolysis). This increases cell proliferation and vasoconstriction (in vessels) and promotes hypertrophy and impairs contractility (in cardiomyocytes). | Dysfunction in this pathway promotes adverse pulmonary vascular remodeling and RV dysfunction in PH. DCA, a mitochondrial PDK inhibitor, shifts redox balance towards oxidized state and restored Kv channel expression and activates PDH restoring glucose oxidation. This latter change regresses vascular obstruction by increasing PASMC apoptosis and enhances RV function. | Chronic hypoxia-rats and the spontaneously pulmonary hypertensive fawn hooded rat | Michelakis (2002)140 Bonnet (2006)88 |
| Mitochondria-HIF-1α | Chronic hypoxia decreases mitochondria ROS production and promotes HIF-1α activation | HIF-1α activation facilitates development of PH and exacerbated the associated pulmonary vascular remodeling | Chronic hypoxia-mice | Nakada (2017)139 |
| CyPA-Basigin | CyPA promotes inflammatory cytokine release and Bsg increases CyPA, PDGF-BB, and ERK1/2 phosphorylation | Mice engineered to have partial CyPA or Bsg deficiency in CyPA+/− or Bsg+/− have reduced RVSP, pulmonary arterial remodeling, and RV hypertrophy compared CyPA+/+ or Bsg+/+ mice in response to chronic hypoxia. | Chronic hypoxia-mice | Satoh (2014)14 |
Abbreviations: AMPK: adenosine monophosphate-activated protein kinase; eAMPK: endothelial AMPK; PDE5=phosphodiesterase 5; ROCK=Rho-associated protein kinase; iNOS=inducible nitric oxide kinase; PKG=cGMP-dependent protein kinase; eNOS=endothelial nitric oxide kinase; HIF=hypoxia inducible factor; NHE1=sodium-hydrogen antiporter 1; TRPC1=transient receptor potential channel 1; IP=intraperitoneal; RV=right ventricle; PASMC=pulmonary arterial smooth muscle cell; [Ca2+]I=intracellular calcium concentration; pHi=intracellular pH; ROS=reactive oxygen species; CyPA=cyclophilin A; Bsg=basigin; PDGF-BB=platelet-derived growth factor-BB; ERK1/2=extracellular signal-regulated kinase 1 and 2; Kv=voltage-gated potassium channel; FHR=Fawn Hooded Rats: DCA=dichloroacetate; PDK=pyruvate dehydrogenase kinase
Group 3 PH is defined as mPAP ≥25mmHg and PCWP pressure ≤15 mmHg associated with COPD, interstitial lung disease (ILD), sleep disordered breathing, alveolar hypoventilation disorders, or chronic exposure to high altitude.113 RV failure associated with Group 3 PH is referred to as “cor pulmonale”. Group 3 patients may receive long-term supplemental oxygen and supportive care with diuretics; however, PH-targeted therapies are not recommended.42, 113 The nonselective nature of the vasodilatation caused by these agents often impairs hypoxic pulmonary vasoconstriction and exacerbates hypoxemia. For example, when Group 3 patients (mPAP >30mmHg) with COPD exacerbations requiring mechanical ventilation, were randomized to receive flolan (prostacyclin, 2–12 ng/kg/min, intravenously) vs placebo PVR was reduced but there was no improvement in respiratory status.114 Patients receiving flolan developed more severe hypoxemia due to the nonselective vasodilatation. Likewise, ETRAs are not recommended for Group 3 PH.115, 116 Although small open-label studies suggest that PDE5 inhibitors,117, 118 sGC stimulators,119 and intravenous prostacyclins120 may be beneficial in selected Group 3 patients larger, double-blinded trials would be required to change practice.
Disordered Pathways in Group 3 PH
Endothelial AMPK
AMPK is a putative therapeutic target in PH.26, 121 Dean et al. showed that metformin can reduce the development of PH in the SU5416/hypoxia female rat model.26 While this is considered a Group 1 model there is some relevance to Group 3 PH, in that hypoxia is a key contributor to the model. Metformin’s mechanism of action is complex. They propose that the metformin activates AMPK, which in turn inhibits aromatase.26 Aromatase converts testosterone to estrogen, which in turn is metabolized via cytochrome p450 1B1 to generate 16a-hydroxyestrone, a metabolite known to cause PH.26 Interestingly, inhibiting aromatase activity is only effective in the female PH rat models.26 Epidemiological studies reveal a higher percentage of female than male COPD patients have PH.122, 123 Thus, both in humans and in rodent models there are important sex difference in the pathophysiology of Group 3 PH.
In addition to responding to bioenergetic perturbations AMPK can be activated by inhibition of Rho-kinase.124 AMPK activation has differential effects in endothelial versus SMC. In endothelial cells AMPK activates eNOS and has an antiapoptotic effect; whereas, in VSMCs, it reduces secretion of various growth factors and has a proapoptotic effect.125–127
Omura et al. showed that AMPK is downregulated in a hypoxia-induced Group 3 PH mouse model.121 Moreover, they created endothelial-specific AMPK-knockout mice (eAMPK−/−).121 The eAMPK−/− mice have no basal increase in % muscularized distal PA; however, compared to control mice, they develop more severe PH after 4 weeks of hypoxia.121 The eAMPK−/− mice have impaired endothelial function (due to reduced eNOS activity) and increased PASMC proliferation due to upregulation of growth factors [platelet derived growth factor-BB (PDGF-BB) and fibroblast growth factor-2 (FGF-2)].121 Metformin, attenuates hypoxia-induced PH in these mice.121
Li et al. showed that AMPK activation in endothelial cells prevents Drp1-mediated mitochondrial fission and improves endothelial function.128 Pretreating rat aortic endothelial cells with AMPK activators, salicylate or AICAR, prevents palmitate-induced mitochondrial fission, with an efficacy that is comparable to Mdivi-1.128 This study suggests that endothelial AMPK activation inhibits Drp1 recruitment, prevents mitochondrial fission and provides an intriguing link between the disorders of mitochondrial dynamics and bioenergetics that may be relevant to Group 3 PH. However, metformin has many actions in addition to AMPK activation, including an anti-inflammatory effect evident in its ability to inhibit of endoplasmic reticulum stress-associated inflammasome activation in the adipose tissue of diabetic mice.129
However, there are also data suggesting AMPK activation may be harmful in Group 3 PH. Ibe et al. showed that inhibition of AMPK with Compound C significantly reduced RVH, RVSP, and pulmonary vascular remodeling in chronically hypoxic C57BL/6 mice.130 The opposing data on the role of AMPK inhibition may reflect the different animal models used, off target effects of AMPK modulating drugs, the cellular compartment in which the AMPK dysregulation occurs (SMC vs. endothelium) and/or tissue heterogeneity in AMPK isoform expression.
RhoA/Rho-kinase pathway
Hemnes et al. showed that sildenafil inhibits ROS generation and RhoA/Rho-kinase activation thereby attenuating bleomycin-induced PH.39 Male C57/BL6 mice were treated with intratracheal bleomycin (4 U/kg).39 Sildenafil (100 mg/kg/day orally) improved survival by 10.5%, reduced RVH and fibrosis, and pulmonary vascular medial hypertrophy.39 PDE5 inhibition decreased RhoA and ROCK activity, increased PKG activity, enhanced eNOS activity and inhibited inducible nitric oxide synthase (iNOS).39
Hypoxia-induced PH is attenuated in heterozygous ROCK2-deficient (ROCK2+/−) mice and exacerbated in VSMC-specific ROCK2-overexpressing transgenic mice (ROCK2-Tg).96 ROCK activity exacerbates PH by increasing cell proliferation. In ROCK2+/− mice both the expression of the activated form of the mitosis-promoting kinase extracellular signal-regulated kinase (ERK1/2) and the number of Ki67-positive proliferating cells in the lung were reduced; whereas, in the ROCK2-Tg mice opposite results were found.96
Hypoxia-inducible factor (HIF-1α)
The transcription factor, HIF-1α, plays an important role in the pathogenesis of PAH.88 Patients with Group 3 PH are exposed to chronic hypoxia either secondary to diseased lung parenchyma (COPD), intermittent apnea (sleep apnea) or environmental hypoxia (altitude). Thus, HIF-1α also plays a key role in the pathogenesis of Group 3 PH.131 HIF-1α is activated in the rodent hypoxia PH model.6 HIF-1α haploinsufficiency protects mice from Group 3 PH, reducing polycythemia, RVH, mPAP, and adverse pulmonary vascular remodeling.
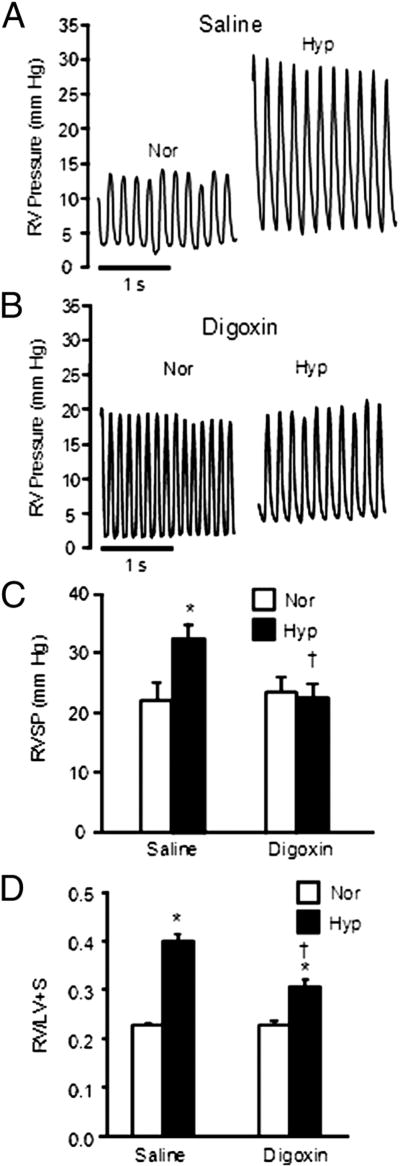
In chronic hypoxia HIF-1α-dependent transcriptional activation of the TRPC1 (transient receptor potential channel 1) and NHE1 (sodium-hydrogen antiporter 1) genes increases intracellular calcium concentration ([Ca2+]i) and intracellular pH (pHi) in PASMC, respectively.132, 133 Inhibiting HIF-1α, using digoxin, decreases [Ca2+]i and pHi, reduces RVH, and attenuates pulmonary vascular remodeling in this model (Figure 10).134
Figure 10. Digoxin reduces Group 3 PH in rats.
Representative tracings (A-B) and mean data (C) showing that digoxin (1 mg/kg digoxin) attenuates PH induced by chronic hypoxia (3 weeks at 10% O2) and (D) reduces RVH. Bar graphs (mean±SEM) Reproduced from Abud et al. with permission.134
HIF-1α also regulates the expression of voltage-gated, oxygen-sensitive potassium channels.88 Normoxic activation of HIF-1α in fawn hooded rat inhibits the expression of Kv1.5. This depolarizes PASMC, which activates the voltage-dependent, large conductance calcium channel and elevates [Ca2+]i. Conversely, augmenting SOD2 inhibits HIF-1α, restores Kv1.5 expression and regresses PH.88 Consistent with this, Whitman et al. found that Kv1.5 and Kv2.1 expression is preserved in HIF-1α haploinsufficient mice exposed to chronic hypoxia.135 These ionic mechanisms are relevant both for control of pulmonary vascular tone and structural remodeling.6
Mitochondria-ROS-HIF-1α-Kv channel pathway
Hypoxia stabilizes HIF-1α, increasing its accumulation in the nucleus and its activity. The regulation of HIF-1α involves oxygen-sensitive prolyl hydroxylases and von Hippel-Lindau factor-mediated ubiquitination.136 However, the cell’s redox state, which is, largely determined by mitochondria, also regulates HIF-1α.6 SOD2, converts toxic, short-lived ROS, produced by the mitochondrial electron transport chain, into diffusible H2O2, a redox-signaling molecule that regulates HIF-1α.6 Mitochondrial ROS can be regulated by pO2 or by alteration in SOD2 expression. Hypoxia alters mitochondrial ROS production.137–139 Controversy exists as to whether hypoxia increases or decrease mitochondrial ROS production. However, we consistently find that ROS production in whole lungs, PASMC and PASMC mitochondria decreases in response to physiologic hypoxia. In FHR PASMC, a deficiency in normoxic H2O2 production, triggered by epigenetic SOD2 downregulation, creates a “pseudo hypoxic milieu, in which normoxic activation of HIF-1α promotes PH88
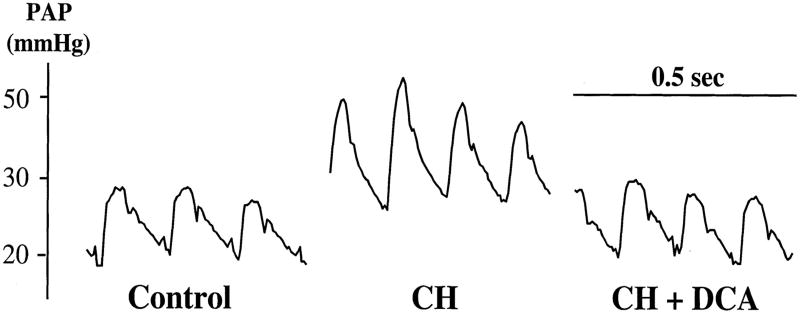
HIF-1α activation increases transcription of PDK, which creates a pathologic metabolic shift from oxidative glucose metabolism to reliance on uncoupled glycolytic metabolism. This form of metabolism is also observed in cancer and is called the Warburg effect.6, 85 Hence, there is biologic plausibility that drugs that modulate mitochondrial metabolism may have therapeutic potential in Group 1 and Group 3 PH. Michelakis et al. showed that dichloroacetate, a metabolic modulator that inhibits all 4 isoforms of PDK, regresses chronic hypoxic PH in rats (Figure 11).140 PDK inhibition increases glucose oxidation and mitochondrial NADH (nicotinamide adenine dinucleotide) thereby increasing mitochondrial electron flux and restoring physiologic ROS production. Dichloroacetate also regresses PH in the FHR model.88
Figure 11.
Representative trace PAP in 3 rat groups with Group 3 PH treated with the PDK inhibitor Dichloroacetate (DCA). DCA in drinking water reverses rise in PA pressure caused by CH. Reproduced from Michelakis et al. with permission.140
Cyclophilin A (CyPA)-Basigin (Bsg) Pathway
Bsg, also known as CD147 or extracellular matrix metalloproteinase (MMP) inducer (EMMPRIN), is a ubiquitous transmembrane glycoprotein.14, 32 Bsg is activated by CyPA (an immunophilin), mechanical stretch, soluble Bsg (sBsg), or angiotensin II.14, 32 Bsg regulates many intracellular pathways involved in VSMC proliferation, including MMP activation, autophagy, and platelet activation.14, 32
Haploinsufficiency of CyPA (CyPA+/−) or Bsg+/− reduces chronic hypoxic PH and pulmonary artery remodeling in mice.14 Furthermore, Bsg+/− PASMC had reduced rates of proliferation.14 CyPA+/− mice exposed to hypoxia had lower levels of inflammatory cytokines, especially chemokine ligand 2, macrophage colony-stimulating factor, interleukin (IL)-2 and IL18.14 Proliferative signaling was also reduced in PASMC from hypoxic Bsg+/− mice, with reduced expression of PDGF-BB (platelet derived growth factor BB) and decreased phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2).14 These data suggest CyPA/Bsg signaling regulates inflammatory cell recruitment and growth factor secretion and may be a novel therapeutic target for treating Group 3 PH. In PAH patients, plasma CyPA levels directly correlate with PVR.14
WHO Group 4 PH (CTEPH, Table 3)
Table 3.
Molecular pathways implicated in Group 4 pulmonary hypertension.
| Pathway | Molecular Mechanism | Consequence | Species | Author (year)ref |
|---|---|---|---|---|
| NO-sGC-cGMP | CTEPH patients have elevated plasma ADMA levels, a potent endogenous NOS inhibitor and lower eNOS expression compared to controls | Endogenous NOS inhibition by ADMA is associated with development of CTEPH | Human | Skoro-Sajer (2007)149 |
| ET-1 | Plasma ET-1 levels are increased in dogs with CTEPH induced by repeated embolization with ceramic beads | Treatment with bosentan, an endothelin receptor antagonist, decreases PAP, PVR, and pulmonary arterial medial hypertrophy in dogs with CTEPH | CTEPH embolization model-dogs | Kim (2000)150 |
| ET-1 | CTEPH patients have elevated plasma ET-1 levels | Plasma ET-1 levels correlated positively with severity of CTEPH. ET-1 levels > 1.77 pg/mL are associated with a poor PTE postoperative outcomes | Human | Reesink (2006)151 |
| Platelet dysfunction | Platelets in CTEPH patients are hypersensitive to thrombin stimulation compared to platelets in controls | Activated platelets have higher expression of VCAM-1, which is an important mediator of immune cells adhesion to vascular endothelium and is associated with development of PH | Human | Yaoita (2014)157 |
| Fibrinolytic abnormalities | TAFI, a plasma carboxypeptidase, decreases binding capacity of plasminogen activator and plasmin to fibrin by removing the C-terminal lysines from the fibrin | TAFI plasma levels are significantly higher in CTEPH patients compared to controls. Impairment of fibrinolysis is improved with a TAFI inhibitor, CPI-2KR | Human | Yaoita (2016)158 |
Abbreviations: NO=nitric oxide; sGC=soluble guanylate cyclase; cGMP=cyclic guanosine monophosphate; CTEPH=chronic thromboembolic pulmonary hypertension; ADMA=asymmetric dimethylarginine; N/A=not applicable (human subjects); ET=endothelin; PTE=pulmonary thromboendarterectomy; PAP=pulmonary arterial pressure; PVR=pulmonary vascular resistance; VCAM-1=vascular cell adhesion protein-1; PH=pulmonary hypertension; TAFI=thrombin activatable fibrinolysis inhibitor; CPI-2KR=carboxypeptidase R inhibiting peptide-2KR;N/A=not applicable
CTEPH occurs when pulmonary emboli fail to resolve and an obstructive neo-intima reduces perfusion to a critical volume of the pulmonary circulation.141 The incidence of CTEPH following acute pulmonary embolism ranges from 0.1–9.1% (average 4%).9, 142–147 A clinically diagnosed pulmonary embolic event is absent in 1/3 of CTEPH patients.141 Once established CTEPH gradually worsens, even without additional clinical embolic events.141 The preferred treatment is pulmonary thromboendarterectomy (PTE).141 In the United States, the estimated annual incidence of CTEPH is around 5000 cases, but only 300–400 PTEs/year are performed, suggesting patients are being deprived of an effective therapy.141 In 2013, riociguat, a soluble guanylate cyclase stimulator, was approved for inoperable CTEPH patients.70 In CHEST-2, riociguat improved 6-min walk test duration and reduced PVR and N-terminal pro-brain natriuretic peptide in PTE ineligible patients.70, 148
Disordered Pathways in Group 4 PH
Endothelial Dysfunction
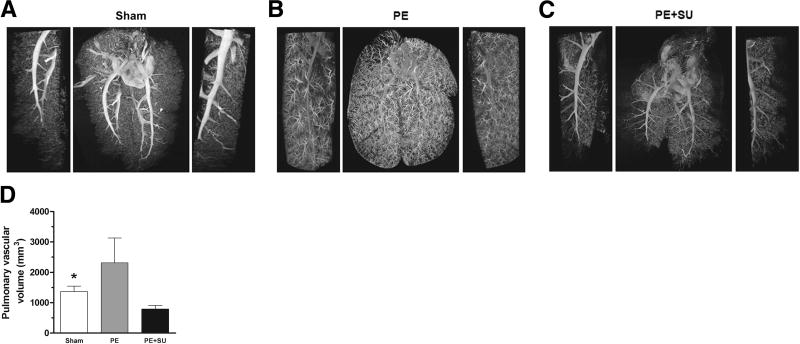
In creating a novel CTEPH animal model, Neto-Neves et al. showed embolization with polystyrene microspheres (PE) alone was usually insufficient to create CTEPH.40 However, combining embolization with SU5416 reliably produced sustained PH (Figure 12).40 Likewise, plasma levels of asymmetric dimethylarginine (ADMA), a potent endogenous NOS inhibitor, are significantly elevated in CTEPH patients and eNOS expression is reduced.149 Thus, there is an important synergistic, role of endothelial dysfunction in promoting CTEPH in PE patients.
Figure 12. Lung computed tomography imaging in experimental CTEPH.
A. Representative three-dimensional micro–computed tomographic images and magnifications (left and right sides) of sham rats injected with vehicle only.
B. Pulmonary embolism (PE) rats injected with microspheres only.
C. PE + SU6416 rats injected with both microspheres and SU5416 (20 mg/kg).
D. Quantification of the pulmonary vascular volume in sham, PE, and PE+SU groups. N-4 per group. *P<0.05 for sham versus PE+SU group. Reproduced from Neto-Neves et al. with permission.40
ET-1
ET-1 levels are increased in dogs with CTEPH (induced by repeated embolization with ceramic beads).141 Bosentan attenuated pulmonary vascular remodeling, reduced mPAP and PVR in this model.150 Reesink et al. found serum ET-1 levels were increased in 35 CTEPH patients (1.62±0.21 pg/mL) compared with controls (0.75±0.06 pg/mL).151 Furthermore, ET-1 levels correlated well with measures of CTEPH severity, including mPAP (r=0.70), cardiac index (r=−0.76), total pulmonary resistance (r=0.72), and 6-min walk test (r=0.59). ET-1 levels>1.77 pg/mL are associated with a bad postoperative outcome from PTE.151 Recently, Southwood et al. found there was a greater expression of ETA than ETB receptors in the chronic thrombus from PTE patients.152
However, clinical trials do not support the use of ETRAs for CTEPH. In the BENEFiT trial, patients with inoperable CTEPH were randomized to placebo (80) or bosentan (77), (62.5 mg twice a day for 4-weeks, then 125 mg twice a day for 12-weeks). Bosentan significantly reduced PVR (−24.1) and improved cardiac index (+0.3 L/min/m2) but did not improve 6-min walk distance (6MWD) (+2.2 m) or reduce mPAP (−2.5 mmHg).153 In MERIT-1 (NCT02021292), a randomized, double-blind, placebo-controlled study, macitentan (10 mg/daily for 24-weeks) significantly improved PVR and 6MWD in CTEPH patients.154 However, the validity of the unpublished data awaits peer review.
Platelets in CTEPH
Surprisingly, the prevalence of hereditary thrombophilia, such as factor V Leiden, factor II mutations, protein C and S deficiencies is not significantly different in PAH or CTEPH, versus control subjects155, reviewed by Mehta et al..156 However, other prothrombotic mechanisms, including enhanced platelets activation and reduced fibrinolytic function, occur in CTEPH.157, 158 Platelets from CTEPH patients are hypersensitive to thrombin stimulation.157 P-selectin and PAC-1 are markers of activated platelets. After thrombin stimulation, the percentage of P-selectin-positive and PAC-1-positive platelets in CTEPH patients (14.7% and 53.9% respectively) was significantly higher versus controls (2.09% and 20.86% respectively).157 The activated platelets induce the expression of vascular cell adhesion molecule-1 (VCAM-1)157, an important mediator of immune cells adhesion to the vascular endothelium. Increases in VCAM-1 are associated with development of PH.159 After thrombin stimulation, the relative increase in platelet VCAM-1 expression was significantly higher in CTEPH patients (17.6 fold) than controls (2.4 fold).157 These findings suggest platelet dysfunction may be involved in the pathogenesis of CTEPH. If confirmed this would change the therapeutic approach to this PH Group, which currently focuses on inhibiting of the clotting cascade.
Fibrinolytic abnormalities
Thrombin also activates a plasma carboxypeptidase inhibitor, TAFI, the thrombin-activatable fibrinolysis inhibitor.160 TAFI removes the C-terminal lysines from fibrin and decreases its binding by tissue-type plasminogen activator and plasmin.161 Thus, TAFI activation impairs fibrinolysis.162 Plasma TAFI levels were significantly higher in CTEPH patients versus controls.158 Furthermore, impairment of fibrinolysis in patients was improved with a TAFI inhibitor, CPI-2KR, suggesting TAFI as a potential therapeutic target in CTEPH.158 TAFI can also be released from activated platelets.157, 163 In CTEPH patients, TAFI released from activated platelets was markedly higher compared to controls.158 Yaoita et al. demonstrated that an inhibitor of platelet activation, prostaglandin E1, indirectly lowers TAFI levels in CTEPH patients.158
CONCLUSION
There remains inadequate preclinical focus on basic mechanisms and novel therapeutics for Group 2–4 PH. The human experience to date suggests little therapeutic overlap of existing PH-targeted therapeutic agents amongst groups. Thus, the search for animal models that accurately recapitulate the disease phenotype for each PH group is important as a means of discovering new Group specific therapies and biomarkers. We have identified some knowledge gaps and listed corresponding future research directions in Table 4.
Table 4.
Key knowledge gaps and future directions for Group 2, 3, and 4 pulmonary hypertension research.
| Key knowledge gaps |
|---|
| • Optimal pre-clinical models that recapitulate many forms of Group 2 PH (e.g. mitral stenosis), Group 3 PH (e.g obstructive sleep apnea), and Group 4 PH do not exist or are not well standardized and easy to use. |
| • Preclinical therapeutic trials require greater rigour in terms of duration, endpoints and characterization of cardiopulmonary function and structure. Optimal assessment of PH should include characterization of the pulmonary vasculature and right ventricle and careful measurement of diastolic function of the left ventricle. This requires advanced hemodynamic studies in closed-chest animals. Broader application of high fidelity ultrasound, cardiac catheterization, micro-CT, positron emission tomography and magnetic resonance imaging is required to better phenotype Groups 2–4 PH and measure response to experimental therapeutics. |
| • The importance of age, sex, diet and strain differences on hemodynamics, lung and heart histology, and mortality in preclinical models of Group 2–4 PH is poorly understood. |
| • The mechanism by which disproportional PH develops in Group 2 and 3 patients is not well understood. It is unclear, for example, why similar elevations of LVEDP or LA pressure elicits severe PH in some patients and experimental animals while only eliciting a modest increase in PVR in others. |
| • Can disordered molecular pathways that are shared by several PH groups be therapeutically exploited to create more universally applicable PH-targeted therapies? |
| • The importance of changes in mitochondrial metabolism and mitochondrial dynamics in Group 2–4 PH is unknown. |
| • The role of genetic and epigenetic dysregulation in Group 2–4 PH is largely unknown. |
| • It is poorly understood why a second insult, in addition to aortic banding, hypoxia, and pulmonary embolism, is often needed to generate severe PH in preclinical models. The role of adverse stimuli, such as endothelial dysfunction, inflammation, impaired angiogenesis or metabolic syndrome, in the genesis of Group-4 PH patients remains poorly characterized. These PH-promoting abnormalities are not factored into the WHO classification system nor are they included in the standard operating procedures for creation of most preclinical PH models. |
| • The prevalence and prognostic impact of adaptive versus maladaptive right ventricular responses to Group 2–4 PH is poorly understood. |
| • Most preclinical studies of PH mechanisms focus on a single molecular pathway. A systems biology approach that addresses the interaction of pathways is needed. |
| • Therapies for Group 1 PH are ineffective in Group 2–3 PH and the optimal molecular targets for Group 2–4 PH remain poorly defined. Therefore, better understanding of the basic mechanisms underlying each of these PH groups is required to identify new therapeutic targets. This will require integrated use of preclinical models and human cells. The value of blood out growth endothelial cells (BOECs) as readily accessible, disease-relevant cells remains unknown. |
| • The potential value of modifying the current PH classification system based on better understanding of molecular mechanisms is unexplored. Could knowledge of pathologic mutations, epigenetic modifications and altered signaling pathway function refine the WHO classification system to better support a more personalized medicine approach to diagnosis and treatment? |
Abbreviations: PH=pulmonary hypertension; PAH=pulmonary arterial hypertension; LVEDP=left ventricle end diastolic pressure; LA=left atrium;
Supplementary Material
Acknowledgments
We would like to acknowledge Ms. Monica Neuber-Hess for performing the SAB surgery.
SOURCES OF FUNDING
This study was supported in part by U.S. National Institutes of Health (NIH) grants NIH 1R01HL113003-01A1 (S.L.A.) and NIH 2R01HL071115-08 (S.L.A.), Canada Foundation for Innovation (S.L.A.), Tier 1 Canada Research Chair in Mitochondrial Dynamics and Translational Medicine (S.L.A.), the American Heart Association (A.H.A.) (S.L.A.), and the William J. Henderson Foundation (S.L.A.).
Footnotes
DISCLOSURES
None.
References
- 1.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol. 2013;62:D1–D3. doi: 10.1016/j.jacc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Urboniene D, Haber I, Fang Y-H, Thenappan T, Archer SL. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L401–L412. doi: 10.1152/ajplung.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper MM, Simon RGJ. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev. 2014;23:450–457. doi: 10.1183/09059180.00007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated Clinical Classification of Pulmonary Hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JXJ. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2012;302:H1546–H1562. doi: 10.1152/ajpheart.00944.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JGN, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Lung Cell Mol Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, Lau EMT, Montani D, Jais X, Sitbon O, Simonneau G. Advances in Therapeutic Interventions for Patients With Pulmonary Arterial Hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 8.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98:1805–1811. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gall H, Hoeper MM, Richter MJ, Cacheris W, Hinzmann B, Mayer E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26:160121. doi: 10.1183/16000617.0121-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160112. doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitling S, Ravindran K, Goldenberg NM, Kuebler WM. The Pathophysiology of Pulmonary Hypertension in Left Heart Disease. Am J Physiol Lung Cell Mol Physiol. 2015;309:L924–L941. doi: 10.1152/ajplung.00146.2015. [DOI] [PubMed] [Google Scholar]
- 12.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) Ameliorates Pressure Overload Induced Heart Failure. PLoS ONE. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, Miyamichi-Yamamoto S, Miura M, Shimizu T, Ikeda S, Yaoita N, Fukumoto Y, Minami T, Miyata S, Nakamura K, Ito H, Kadomatsu K, Shimokawa H. Basigin Mediates Pulmonary Hypertension by Promoting Inflammation and Vascular Smooth Muscle Cell Proliferation. Circ Res. 2014;115:738–750. doi: 10.1161/CIRCRESAHA.115.304563. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer TL, Bashore TM. Pulmonary hypertension related to left-sided cardiac pathology. Pulm Med. 2011;2011:381787. doi: 10.1155/2011/381787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briongos Figuero S, Moya Mur JL, García-Lledó A, Centella T, Salido L, Aceña Navarro Á, García Martín A, García-Andrade I, Oliva E, Zamorano JL. Predictors of persistent pulmonary hypertension after mitral valve replacement. Heart and Vessels. 2016;31:1091–1099. doi: 10.1007/s00380-015-0700-2. [DOI] [PubMed] [Google Scholar]
- 17.Snopek G, Pogorzelska H, Rywik TM, Browarek A, Janas J, Korewicki J. Usefulness of endothelin-1 concentration in capillary blood in patients with mitral stenosis as a predictor of regression of pulmonary hypertension after mitral valve replacement or valvuloplasty. Am J Cardiol. 2002;90:188–189. doi: 10.1016/s0002-9149(02)02451-7. [DOI] [PubMed] [Google Scholar]
- 18.Wood P, Besterman EM, Towers MK, McIlroy MB. The effect of acetylcholine on pulmonary vascular resistance and left atrial pressure in mitral stenosis. Br Heart J. 1957;19:279–286. doi: 10.1136/hrt.19.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furchgott RF, Jothianandan D. Endothelium-Dependent and -Independent Vasodilation Involving Cyclic GMP: Relaxation Induced by Nitric Oxide, Carbon Monoxide and Light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 20.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 21.Goodale F, Sanchez G, Friedlich AL, Scannell JG, Myers GS. Correlation of Pulmonary Arteriolar Resistance with Pulmonary Vascular Changes in Patients with Mitral Stenosis before and after Valvulotomy. N Engl J Med. 1955;252:979–983. doi: 10.1056/NEJM195506092522303. [DOI] [PubMed] [Google Scholar]
- 22.Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: harmonisation with the world health organisation’s categorisation of human PH. Int J Clin Pract Suppl. 2011;65:15–34. doi: 10.1111/j.1742-1241.2011.02710.x. [DOI] [PubMed] [Google Scholar]
- 23.Das M, Fessel J, Tang H, West J. A process-based review of mouse models of pulmonary hypertension. Pulm Circ. 2012;2:415–433. doi: 10.4103/2045-8932.105030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 25.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of Plexiform Lesions in Experimental Severe Pulmonary Arterial Hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 26.Dean A, Nilsen M, Loughlin L, Salt IP, MacLean MR. Metformin Reverses Development of Pulmonary Hypertension via Aromatase Inhibition. Hypertension. 2016;68:446–454. doi: 10.1161/HYPERTENSIONAHA.116.07353. [DOI] [PubMed] [Google Scholar]
- 27.Jiang B, Deng Y, Suen C, Taha M, Chaudhary KR, Courtman DW, Stewart DJ. Marked Strain-Specific Differences in the SU5416 Rat Model of Severe Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol. 2016;54:461–468. doi: 10.1165/rcmb.2014-0488OC. [DOI] [PubMed] [Google Scholar]
- 28.Dai Z-K, Wu B-N, Chen IC, Chai C-Y, Wu J-R, Chou S-H, Yeh J-L, Chen I-J, Tan M-S. Attenuation of pulmonary hypertension secondary to left ventricular dysfunction in the rat by Rho-kinase inhibitor fasudil. Pediatr Pulmonol. 2011;46:45–59. doi: 10.1002/ppul.21323. [DOI] [PubMed] [Google Scholar]
- 29.Yin J, Marian K, Julia H, Anja S-K, Juergen B, E HW, Hermann K, M KW. Sildenafil preserves lung endothelial function and prevents pulmonary vascular remodeling in a rat model of diastolic heart failure. Circ Heart Fail. 2011;4:198–206. doi: 10.1161/CIRCHEARTFAILURE.110.957050. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Guo Y-Z, Zhang Y-T, Xue J-J, Chen Z-C, Cheng S-Y, Ou M-D, Cheng K-L, Zeng W-J. The Effects and Mechanism of Atorvastatin on Pulmonary Hypertension Due to Left Heart Disease. PLOS ONE. 2016;11:e0157171. doi: 10.1371/journal.pone.0157171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Chien KR, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki K, Satoh K, Ikeda S, Sunamura S, Otsuki T, Satoh T, Kikuchi N, Omura J, Kurosawa R, Nogi M, Numano K, Sugimura K, Aoki T, Tatebe S, Miyata S, Mukherjee R, Spinale FG, Kadomatsu K, Shimokawa H. Basigin Promotes Cardiac Fibrosis and Failure in Response to Chronic Pressure Overload in MiceSignificance. Arterioscler Thromb Vasc Biol. 2016;36:636–646. doi: 10.1161/ATVBAHA.115.306686. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left Ventricular Failure Produces Profound Lung Remodeling and Pulmonary Hypertension in Mice. Heart Failure Causes Severe Lung Disease. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Kwak D, Fassett J, Hou L, Xu X, Burbach BJ, Thenappan T, Xu Y, Ge J-b, Shimizu Y, Bache RJ, Chen Y. CD28/B7 Deficiency Attenuates Systolic Overload-Induced Congestive Heart Failure, Myocardial and Pulmonary Inflammation, and Activated T Cell Accumulation in the Heart and Lungs. Hypertension. 2016;68:688–696. doi: 10.1161/HYPERTENSIONAHA.116.07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto Y, Urashima T, Kawachi F, Akaike T, Kusakari Y, Ida H, Minamisawa S. Pulmonary hypertension due to left heart disease causes intrapulmonary venous arterialization in rats. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2017.06.053. [published online ahead of print July 5, 2017] [DOI] [PubMed] [Google Scholar]
- 36.Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest. 2000;106:733–738. doi: 10.1172/JCI11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campian ME, Hardziyenka M, Michel MC, Tan HL. How valid are animal models to evaluate treatments for pulmonary hypertension? Naunyn-Schmiedeberg’s Arch Pharmacol. 2006;373:391–400. doi: 10.1007/s00210-006-0087-9. [DOI] [PubMed] [Google Scholar]
- 38.Schroll S, Arzt M, Sebah D, Nuchterlein M, Blumberg F, Pfeifer M. Improvement of bleomycin-induced pulmonary hypertension and pulmonary fibrosis by the endothelin receptor antagonist Bosentan. Respir Physiol Neurobiol. 2010;170:32–36. doi: 10.1016/j.resp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–L33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 40.Neto-Neves EM, Brown MB, Zaretskaia MV, Rezania S, Goodwill AG, McCarthy BP, Persohn SA, Territo PR, Kline JA. Chronic Embolic Pulmonary Hypertension Caused by Pulmonary Embolism and Vascular Endothelial Growth Factor Inhibition. Am J Pathol. 2017;187:700–712. doi: 10.1016/j.ajpath.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult--a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31:913–933. doi: 10.1016/j.healun.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2015;37:61–119. [Google Scholar]
- 43.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 46.Moraes DL, Colucci WS, Givertz MM. Secondary Pulmonary Hypertension in Chronic Heart Failure: the role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–1723. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 47.Driss AB, Devaux C, Henrion D, Duriez M, Thuillez C, Levy BI, Michel J-B. Hemodynamic Stresses Induce Endothelial Dysfunction and Remodeling of Pulmonary Artery in Experimental Compensated Heart Failure. Circulation. 2000;101:2764–2770. doi: 10.1161/01.cir.101.23.2764. [DOI] [PubMed] [Google Scholar]
- 48.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 49.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased Plasma Endothelin-1 in Pulmonary Hypertension: Marker or Mediator of Disease? Ann Ital Med Int. 1991;114:464–464. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 50.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Pressure Enhances Endothelin-1 Release From Cultured Human Endothelial Cells. Hypertension. 1995;25:449–452. doi: 10.1161/01.hyp.25.3.449. [DOI] [PubMed] [Google Scholar]
- 51.Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 52.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J. 1995;9:1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 53.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1- induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol. 2002;283:H983–H989. doi: 10.1152/ajpheart.00141.2002. [DOI] [PubMed] [Google Scholar]
- 54.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuroda T, Kobayashi M, Ozaki S, Yano M, Miyauchi T, Onizuka M, Sugishita Y, Goto K, Nishikibe M. Endothelin receptor subtypes in human versus rabbit pulmonary arteries. J Appl Physiol. 1994;76:1976–1982. doi: 10.1152/jappl.1994.76.5.1976. [DOI] [PubMed] [Google Scholar]
- 56.Parker JD, Thiessen JJ. Increased endothelin-1 production in patients with chronic heart failure. Am J Physiol Heart Circ Physiol. 2004;286:H1141–H1145. doi: 10.1152/ajpheart.00239.2001. [DOI] [PubMed] [Google Scholar]
- 57.Tadano K, Hosokawa A, Fukuroda T, Nishikibe M. The Functional Shift of Endothelin Receptor Subtypes in Dogs With Heart Failure Produced by Rapid Ventricular Pacing - Journals - NCBI. J Cardiovasc Pharmacol. 2004;44:S350–S353. doi: 10.1097/01.fjc.0000166291.72324.eb. [DOI] [PubMed] [Google Scholar]
- 58.Kalra PR, Moon JCC, Coats AJS. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85:195–197. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 59.Lüscher TF, Enseleit F, Pacher R, Mitrovic V, Schulze MR, Willenbrock R, Dietz R, Rousson V, Hürlimann D, Philipp S, Notter T, Noll G, Ruschitzka F. Hemodynamic and Neurohumoral Effects of Selective Endothelin A (ETA) Receptor Blockade in Chronic Heart Failure. Circulation. 2002;106:2666–2672. doi: 10.1161/01.cir.0000038497.80095.e1. [DOI] [PubMed] [Google Scholar]
- 60.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 61.McMurray JJV, Teerlink JR, Cotter G, Bourge RC, Cleland JGF, Jondeau G, Krum H, Metra M, O’Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I Veritas Investigators ft. Effects of Tezosentan on Symptoms and Clinical Outcomes in Patients With Acute Heart Failure. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 62.Nagendran J, Sutendra G, Paterson I, Champion HC, Webster L, Chiu B, Haromy A, Rebeyka IM, Ross DB, Michelakis ED. Endothelin Axis Is Upregulated in Human and Rat Right Ventricular Hypertrophy. Circ Res. 2013;112:347–354. doi: 10.1161/CIRCRESAHA.111.300448. [DOI] [PubMed] [Google Scholar]
- 63.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 64.Kelm M. Nitric oxide metabolism and breakdown. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 65.Denninger JW, Marletta MA. Guanylate cyclase and the ·NO/cGMP signaling pathway. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 66.Kellogg DL, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 67.Chi DS, Qui M, Krishnaswamy G, Li C, Stone W. Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric Oxide. 2003;8:127–132. doi: 10.1016/s1089-8603(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 68.Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S. Stretch Activates Nitric Oxide Production in Pulmonary Vascular Endothelial Cells In Situ. Am J Respir Crit Care Med. 2003;168:1391–1398. doi: 10.1164/rccm.200304-562OC. [DOI] [PubMed] [Google Scholar]
- 69.Das Gupta A, Bowman L, D'Arsigny CL, Archer SL. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther. 2015;97:88–102. doi: 10.1002/cpt.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghofrani H-A, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 71.Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis ED, Mitrovic V, Oudiz RJ, Boateng F, Scalise A-V, Roessig L, Semigran MJ. Riociguat for Patients with Pulmonary Hypertension due to Systolic Left Ventricular Dysfunction: A Phase IIb Double-Blind, Randomized, Placebo-Controlled, Dose-Ranging Hemodynamic Study. Circulation. 2013;128:502–511. doi: 10.1161/CIRCULATIONAHA.113.001458. [DOI] [PubMed] [Google Scholar]
- 72.Ontkean M, Gay R, Greenberg B. Diminished endothelium-derived relaxing factor activity in an experimental model of chronic heart failure. Circ Res. 1991;69:1088–1096. doi: 10.1161/01.res.69.4.1088. [DOI] [PubMed] [Google Scholar]
- 73.Kerem A, Yin J, Kaestle SM, Hoffmann J, Schoene AM, Singh B, Kuppe H, Borst MM, Kuebler WM. Lung Endothelial Dysfunction in Congestive Heart Failure: Role of Impaired Ca2+ Signaling and Cytoskeletal Reorganization. Circ Res. 2010;106:1103–1116. doi: 10.1161/CIRCRESAHA.109.210542. [DOI] [PubMed] [Google Scholar]
- 74.Hampl V, Archer SL, Nelson DP, Weir EK. Chronic EDRF inhibition and hypoxia: effects on pulmonary circulation and systemic blood pressure. J Appl Physiol. 1993;75:1748–1757. doi: 10.1152/jappl.1993.75.4.1748. [DOI] [PubMed] [Google Scholar]
- 75.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–2348. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 76.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil Improves Exercise Capacity and Quality of Life in Patients With Systolic Heart Failure and Secondary Pulmonary Hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 77.Abman SH. Abnormal Vasoreactivity in the Pathophysiology of Persistent Pulmonary Hypertension of the Newborn. Pediatr Rev. 1999;20:e103–e109. [PubMed] [Google Scholar]
- 78.Sanchez LS, de la Monte SM, Filippov G, Jones RC, Zapol WM, Bloch KD. Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development. Pediatr Res. 1998;43:163–168. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Hansdottir S, Groskreutz DJ, Gehlbach BK. WHO's in Second?: A Practical Review of World Health Organization Group 2 Pulmonary Hypertension. Chest. 2013;144:638–650. doi: 10.1378/chest.12-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper TJ, Guazzi M, Al-Mohammad A, Amir O, Bengal T, Cleland JG, Dickstein K. Sildenafil in Heart Failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail. 2013;15:119–122. doi: 10.1093/eurjhf/hfs152. [DOI] [PubMed] [Google Scholar]
- 81.Archer SL. Mitochondrial Dynamics — Mitochondrial Fission and Fusion in Human Diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 82.Wai T, Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol Metab. 2016;27:105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-Related Protein 1-Mediated Mitochondrial Mitotic Fission Permits Hyperproliferation of Vascular Smooth Muscle Cells and Offers a Novel Therapeutic Target in Pulmonary Hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2010;88:1011–1020. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sutendra G, Michelakis Evangelos D. The Metabolic Basis of Pulmonary Arterial Hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Hong Z, Chen K-H, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, Wu D, Mewburn J, Ormiston ML, Archer SL. MicroRNA-138 and MicroRNA-25 Down-regulate Mitochondrial Calcium Uniporter, Causing the Pulmonary Arterial Hypertension Cancer Phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMurtry MS, Bonnet S, Wu X, Dyck JRB, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate Prevents and Reverses Pulmonary Hypertension by Inducing Pulmonary Artery Smooth Muscle Cell Apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 88.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An Abnormal Mitochondrial–Hypoxia Inducible Factor-1α–Kv Channel Pathway Disrupts Oxygen Sensing and Triggers Pulmonary Arterial Hypertension in Fawn Hooded Rats. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 89.Tian L, Neuber-Hess M, Mewburn J, Das Gupta A, Dunham-Snary K, Wu D, Chen K-H, Hong Z, Sharp WW, Kutty S, Archer SL. Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J Mol Med (Berl) 2017;95:381–393. doi: 10.1007/s00109-017-1522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial Abnormalities in Myocardium of Dogs with Chronic Heart Failure. J hlol Ikll. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 91.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting Mitochondrial Fission Protects the Heart Against Ischemia/Reperfusion Injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 92.Sharp WW, Beiser DG, Fang YH, Han M, Piao L, Varughese J, Archer SL. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit Care Med. 2015;43:e38–e47. doi: 10.1097/CCM.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 94.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 95.Hartmann S, Ridley AJ, Lutz S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front Pharmacol. 2015;6:276. doi: 10.3389/fphar.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimizu T, Fukumoto Y, Tanaka S, Satoh K, Ikeda S, Shimokawa H. Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arterioscler Thromb Vasc Biol. 2013;33:2780–2791. doi: 10.1161/ATVBAHA.113.301357. [DOI] [PubMed] [Google Scholar]
- 97.Yaoita N, Satoh K, Shimokawa H. Novel Therapeutic Targets of Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2016;36:e97–e102. doi: 10.1161/ATVBAHA.116.308263. [DOI] [PubMed] [Google Scholar]
- 98.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 99.Kawut SM, Bagiella E, Lederer DJ, Shimbo D, Horn EM, Roberts KE, Hill NS, Barr RG, Rosenzweig EB, Post W, Tracy RP, Palevsky HI, Hassoun PM, Girgis RE. Randomized Clinical Trial of Aspirin and Simvastatin for Pulmonary Arterial Hypertension. Circulation. 2011;123:2985–2993. doi: 10.1161/CIRCULATIONAHA.110.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 101.Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, Pickworth J, Kiely DG, Crossman DC, Francis SE. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011;179:1693–1705. doi: 10.1016/j.ajpath.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, Watkins SC, Gor S, Cantu-Medellin N, Weidert ER, Frisbee JC, Gladwin MT, Champion HC, Freeman BA, Khoo NKH. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ussavarungsi K, Thomas CS, Burger CD. Prevalence of metabolic syndrome in patients with pulmonary hypertension. Clin Respir J. doi: 10.1111/crj.12406. [published online ahead of print October 23, 2015] [DOI] [PubMed] [Google Scholar]
- 104.Meng Q, Lai Y-C, Kelly NJ, Bueno M, Baust JJ, Bachman TN, Goncharov D, Vanderpool RR, Radder JE, Hu J, Goncharova E, Morris AM, Mora AL, Shapiro SD, Gladwin MT. Development of a Mouse Model of Metabolic Syndrome, Pulmonary Hypertension, and Heart Failure with Preserved Ejection Fraction. Am J Respir Cell Mol Biol. 2017;56:497–505. doi: 10.1165/rcmb.2016-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelly NJ, Radder JE, Baust JJ, Burton CL, Lai Y-C, Potoka KC, Agostini BA, Wood JP, Bachman TN, Vanderpool RR, Dandachi N, Leme AS, Gregory AD, Morris A, Mora AL, Gladwin MT, Shapiro SD. Mouse Genome-Wide Association Study of Preclinical Group II Pulmonary Hypertension Identifies Epidermal Growth Factor Receptor. Am J Respir Cell Mol Biol. 2017;56:488–496. doi: 10.1165/rcmb.2016-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal Growth Factor Receptor Blockade Mediates Smooth Muscle Cell Apoptosis and Improves Survival in Rats With Pulmonary Hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 107.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lai Y-C, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocaña A, Goncharova EA, Tofovic SP, Mora AL, Gladwin MT. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G, Pacaud P. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol. 2009;158:1285–1294. doi: 10.1111/j.1476-5381.2009.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen M, Cai H, Yu C, Wu P, Fu Y, Xu X, Fan R, Xu C, Chen Y, Wang L, Huang X. Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKalpha1-dependent pathways. Am J Transl Res. 2016;8:12–27. [PMC free article] [PubMed] [Google Scholar]
- 113.Klinger JR. Group III Pulmonary Hypertension. Cardiol Clin. 2016;34:413–433. doi: 10.1016/j.ccl.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Archer SL, Mike D, Crow J, Long W, Weir EK. A placebo-controlled trial of prostacyclin in acute respiratory failure in COPD. Chest. 1996;109:750–755. doi: 10.1378/chest.109.3.750. [DOI] [PubMed] [Google Scholar]
- 115.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, de Andrade J, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O'Riordan TG. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 116.Corte TJ, Keir GJ, Dimopoulos K, Howard L, Corris PA, Parfitt L, Foley C, Yanez-Lopez M, Babalis D, Marino P, Maher TM, Renzoni EA, Spencer L, Elliot CA, Birring SS, O’Reilly K, Gatzoulis MA, Wells AU, Wort SJ. Bosentan in Pulmonary Hypertension Associated with Fibrotic Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2014;190:208–217. doi: 10.1164/rccm.201403-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Network TIPFCR. A Controlled Trial of Sildenafil in Advanced Idiopathic Pulmonary Fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corte TJ, Gatzoulis MA, Parfitt L, Harries C, Wells AU, Wort SJ. The use of sildenafil to treat pulmonary hypertension associated with interstitial lung disease. Respirology. 2010;15:1226–1232. doi: 10.1111/j.1440-1843.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- 119.Hoeper MM, Halank M, Wilkens H, Günther A, Weimann G, Gebert I, Leuchte HH, Behr J. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41:853–860. doi: 10.1183/09031936.00213911. [DOI] [PubMed] [Google Scholar]
- 120.Saggar R, Khanna D, Vaidya A, Derhovanessian A, Maranian P, Duffy E, Belperio JA, Weigt SS, Dua S, Shapiro SS, Goldin JG, Abtin F, Lynch JP, Ross DJ, Forfia PR, Saggar R. Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax. 2014;69:123–129. doi: 10.1136/thoraxjnl-2013-204150. [DOI] [PubMed] [Google Scholar]
- 121.Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, Sunamura S, Tatebe S, Aoki T, Sugimura K, Miyata S, Hoshikawa Y, Okada Y, Shimokawa H. Protective Roles of Endothelial AMP-Activated Protein Kinase Against Hypoxia-Induced Pulmonary Hypertension in MiceNovelty and Significance. Circ Res. 2016;119:197–209. doi: 10.1161/CIRCRESAHA.115.308178. [DOI] [PubMed] [Google Scholar]
- 122.Sharma KK, Chaturvedi H, Pandey RD, Choyal T. Gender differences of echocardiographic characteristics in patients with acute exacerbation of COPD. European Respiratory Journal. 2015;46:PA2527-PA2527. [Google Scholar]
- 123.Sertogullarindan B, Gumrukcuoglu HA, Sezgi C, Akil MA. Frequency of pulmonary hypertension in patients with COPD due to biomass smoke and tobacco smoke. Int J Med Sci. 2012;9:406–12. doi: 10.7150/ijms.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noda K, Nakajima S, Godo S, Saito H, Ikeda S, Shimizu T, Enkhjargal B, Fukumoto Y, Tsukita S, Yamada T, Katagiri H, Shimokawa H. Rho-Kinase Inhibition Ameliorates Metabolic Disorders through Activation of AMPK Pathway in Mice. PLoS ONE. 2014;9:e110446. doi: 10.1371/journal.pone.0110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fisslthaler B, Fleming I. Activation and Signaling by the AMP-Activated Protein Kinase in Endothelial Cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 126.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 127.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine Monophosphate-Activated Protein Kinase Suppresses Vascular Smooth Muscle Cell Proliferation Through the Inhibition of Cell Cycle Progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 128.Li J, Wang Y, Wang Y, Wen X, Ma X-N, Chen W, Huang F, Kou J, Qi L-W, Liu B, Liu K. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 129.Li A, Zhang S, Li J, Liu K, Huang F, Liu B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol Cell Endocrinol. 2016;434:36–47. doi: 10.1016/j.mce.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 130.Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX, Raj JU, Zhou G. Adenosine monophosphate-activated protein kinase is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:609–618. doi: 10.1165/rcmb.2012-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bryant AJ, Scott EW. “A small leak will sink a great ship”: hypoxia-inducible factor and group III pulmonary hypertension. Receptors Clin Investig. 2016;3:e1213. doi: 10.14800/rci.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 133.Wang J, Weigand L, Lu W, Sylvester JT, L Semenza G, Shimoda LA. Hypoxia Inducible Factor 1 Mediates Hypoxia-Induced TRPC Expression and Elevated Intracellular Ca2+ in Pulmonary Arterial Smooth Muscle Cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 134.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci USA. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2008;294:L309–L318. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
- 136.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 137.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 138.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 140.Michelakis ED, McMurtry MS, Wu X-C, Dyck JRB, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a Metabolic Modulator, Prevents and Reverses Chronic Hypoxic Pulmonary Hypertension in Rats Role of Increased Expression and Activity of Voltage-Gated Potassium Channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 141.Kim NH. Group 4 Pulmonary Hypertension. Cardiol Clin. 2016;34:435–441. doi: 10.1016/j.ccl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 142.Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 143.Poli D, Grifoni E, Antonucci E, Arcangeli C, Prisco D, Abbate R, Miniati M. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis. 2010;30:294–299. doi: 10.1007/s11239-010-0452-x. [DOI] [PubMed] [Google Scholar]
- 144.Klok FA, van Kralingen KW, van Dijk APJ, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95:970–975. doi: 10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang S, Yang Y, Zhai Z, Kuang T, Gong J, Zhang S, Zhu J, Liang L, Shen YH, Wang C. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis. 2015;7:1927–1938. doi: 10.3978/j.issn.2072-1439.2015.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, Pontal D, Guégan M, Simonneau G, Meyer G, Sanchez O. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost. 2014;112:598–605. doi: 10.1160/TH13-07-0538. [DOI] [PubMed] [Google Scholar]
- 147.Martí D, Gómez V, Escobar C, Wagner C, Zamarro C, Sánchez D, Sam A, Briongos S, Gaudó J, Sueiro A, Jiménez D. Incidence of Symptomatic and Asymptomatic Chronic Thromboembolic Pulmonary Hypertension. Arch Bronconeumol. 2010;46:628–633. doi: 10.1016/j.arbres.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 148.Simonneau G, D’Armini AM, Ghofrani H-A, Grimminger F, Hoeper MM, Jansa P, Kim NH, Wang C, Wilkins MR, Fritsch A, Davie N, Colorado P, Mayer E. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2) Eur Respir J. 2015;45:1293–1302. doi: 10.1183/09031936.00087114. [DOI] [PubMed] [Google Scholar]
- 149.Skoro-Sajer N, Mittermayer F, Panzenboeck A, Bonderman D, Sadushi R, Hitsch R, Jakowitsch J, Klepetko W, Kneussl MP, Wolzt M, Lang IM. Asymmetric Dimethylarginine Is Increased in Chronic Thromboembolic Pulmonary Hypertension. Am J Respir Crit Care Med. 2007;176:1154–1160. doi: 10.1164/rccm.200702-278OC. [DOI] [PubMed] [Google Scholar]
- 150.Kim H, Yung GL, Marsh JJ, Konopka RG, Pedersen CA, Chiles PG, Morris TA, Channick RN. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J. 2000;15:640–648. doi: 10.1034/j.1399-3003.2000.15d04.x. [DOI] [PubMed] [Google Scholar]
- 151.Reesink HJ, Meijer RC, Lutter R, Boomsma F, Jansen HM, Kloek JJ, Bresser P. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J. 2006;70:1058–1063. doi: 10.1253/circj.70.1058. [DOI] [PubMed] [Google Scholar]
- 152.Southwood M, MacKenzie Ross RV, Kuc RE, Hagan G, Sheares KK, Jenkins DP, Goddard M, Davenport AP, Pepke-Zaba J. Endothelin ETA receptors predominate in chronic thromboembolic pulmonary hypertension. Life Sciences. 2016;159:104–110. doi: 10.1016/j.lfs.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jais X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 154.Ghofrani HA, Simonneau G, D'Armini AM, Fedullo P, Martin N, Howard L, Jais X, Jenkins DP, Jing Z-C, Madani M. Efficacy And Safety Of Macitentan For Inoperable Chronic Thromboembolic Pulmonary Hypertension: Results From The Randomized Controlled MERIT Study. B14 CLINICAL TRIALS ACROSS PULMONARY DISEASE: Am Thoracic Soc. 2017:A7601–A7601. [Google Scholar]
- 155.Wolf M, Boyer-Neumann C, Parent F, Eschwege V, Jaillet H, Meyer D, Simonneau G. Thrombotic risk factors in pulmonary hypertension. Eur Respir J. 2000;15:395–399. doi: 10.1034/j.1399-3003.2000.15b28.x. [DOI] [PubMed] [Google Scholar]
- 156.Mehta S, Helmersen D, Provencher S, Hirani N, Rubens FD, De Perrot M, Blostein M, Boutet K, Chandy G, Dennie C, Granton J, Hernandez P, Hirsch AM, Laframboise K, Levy RD, Lien D, Martel S, Shoemaker G, Swiston J, Weinkauf J. Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: A clinical practice guideline. Can Respir J. 2010;17:301–334. doi: 10.1155/2010/704258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yaoita N, Shirakawa R, Fukumoto Y, Sugimura K, Miyata S, Miura Y, Nochioka K, Miura M, Tatebe S, Aoki T, Yamamoto S, Satoh K, Kimura T, Shimokawa H, Horiuchi H. Platelets Are Highly Activated in Patients of Chronic Thromboembolic Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2014;34:2486–2494. doi: 10.1161/ATVBAHA.114.304404. [DOI] [PubMed] [Google Scholar]
- 158.Yaoita N, Satoh K, Satoh T, Sugimura K, Tatebe S, Yamamoto S, Aoki T, Miura M, Miyata S, Kawamura T, Horiuchi H, Fukumoto Y, Shimokawa H. Thrombin-Activatable Fibrinolysis Inhibitor in Chronic Thromboembolic Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2016;36:1293–1301. doi: 10.1161/ATVBAHA.115.306845. [DOI] [PubMed] [Google Scholar]
- 159.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 160.Schadinger SL, Lin JHH, Garand M, Boffa MB, Miyata S, Miura Y, Nochioka K, Miura M, Tatebe S, Aoki T, Yamamoto S, Satoh K, Kimura T, Shimokawa H, Horiuchi H. Secretion and antifibrinolytic function of thrombin-activatable fibrinolysis inhibitor from human platelets. J Thromb Haemost. 2010;8:2523–2529. doi: 10.1111/j.1538-7836.2010.04024.x. [DOI] [PubMed] [Google Scholar]
- 161.Bouma BN, Meijers JCM. Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) J Thromb Haemost. 2003;1:1566–1574. doi: 10.1046/j.1538-7836.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 162.Eichinger S, Schönauer V, Weltermann A, Minar E, Bialonczyk C, Hirschl M, Schneider B, Quehenberger P, Kyrle PA. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood. 2004;103:3773–3776. doi: 10.1182/blood-2003-10-3422. [DOI] [PubMed] [Google Scholar]
- 163.Carrieri C, Galasso R, Semeraro F, Ammollo CT, Semeraro N, Colucci M. The role of thrombin activatable fibrinolysis inhibitor and factor XI in platelet-mediated fibrinolysis resistance: a thromboelastographic study in whole blood. J Thromb Haemost. 2011;9:154–162. doi: 10.1111/j.1538-7836.2010.04120.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.