Abstract
Past studies have focused on the composition of essential oil of Ocimum basilicum leaves, but data on composition and regulation of its aerial emissions, especially floral volatile emissions are scarce. We studied the chemical profile, within-flower spatial distribution (sepals, petals, pistils with stamina and pedicels), diurnal emission kinetics and effects of exogenous methyl jasmonate (MeJA) application on the emission of floral volatiles by dynamic headspace collection and identification using gas chromatography-mass spectrometry (GC-MS) and proton transfer reaction mass spectrometry (PTR-MS). We observed more abundant floral emissions from flowers compared with leaves. Sepals were the main emitters of floral volatiles among the flower parts studied. The emissions of lipoxygenase compounds (LOX) and monoterpenoids, but not sesquiterpene emissions, displayed a diurnal variation driven by light. Response to exogenous MeJA treatment of flowers consisted of a rapid stress response and a longer-term acclimation response. The initial response was associated with enhanced emissions of fatty acid derivatives, monoterpenoids, and sesquiterpenoids without variation of the composition of individual compounds. The longer-term response was associated with enhanced monoterpenoid and sesquiterpenoid emissions with profound changes in the emission spectrum. According to correlated patterns of terpenoid emission changes upon stress, highlighted by a hierarchical cluster analysis, candidate terpenoid synthases responsible for observed diversity and complexity of released terpenoid blends were postulated. We conclude that flower volatile emissions differ quantitatively and qualitatively from leaf emissions, and overall contribute importantly to O. basilicum flavor, especially under stress conditions.
Keywords: Floral volatiles, Terpenoids, Emission dynamics, Methyl jasmonate treatments, Biosynthesis
Introduction
Sweet basil (Ocimum basilicum L.), native to India and Iran, is an economically important culinary herb with distinctive aroma and flavor. In addition to direct use of the herb as spice, the essential oil of its leaves and flowers is increasingly used as aroma additive in food and cosmetic industry, and there are extensive efforts to enhance the yield of essential oil of O. Basilicum (Lee and others 2005).
The chemical composition of the essential oil of O. basilicum has been studied extensively and it demonstrates considerable variability depending on cultivation conditions and geographical origin of plants (Abdollah and others 2013; Carovič-Stanko and others 2010; Kwee and Niemeyer 2011; Ameneh and others 2013). Benzenoids (eugenol, methyl eugenol, methyl chavicol, and methyl cinnamate) and terpenoids (geraniol, linalool, myrcene, caryophyllene and farnesol) are commonly observed as the dominant volatile compounds in O. basilicum determining its distinctive aroma (Abdollah and others 2013; Calín-Sánchez and others 2012). However, analyses of the endogenous chemical compounds in the essential oil of O. basilicum can be importantly affected by the herbal collection, processing and extraction procedures (Ormeño and others 2011). The volatile compounds in O. basilicum are characteristically determined from dried plant material using hydrophobic solvents at high temperatures that can lead to the degradation and transformation of organic volatile compounds to derivative compounds not biologically synthesized by the plants (Díaz-Maroto and others 2004; Yousif and others 1999; Baritaux and others 1992). Although steam distillation can constitute an alternative method, it only results extraction of relatively low molecular weight volatile compounds present (Ormeño and others 2011). These difficulties can greatly limit the accuracy of identification of endogenous volatile composition of O. basilicum (Díaz-Maroto and others 2004; Baritaux and others 1992). Furthermore, the spectrum of volatiles emitted from living plants under natural conditions can strongly differ from the plant essential oil composition (Steinbrecher 1989; Schürmann and others 1993; Ormeño and others 2007; Soran and others 2014).
The majority of studies on O. basilicum volatiles have used vegetative tissues, in particular, its leaves (Iijima and others 2004b; Xie and others 2008), whereas volatile analyses for reproductive tissues are scarce (Ameneh and others 2013; Chalchat and Özcan 2008).Under natural conditions, the odor from O. basilicum leaves mainly acts as insect repellent, and also has antibacterial, antiviral, antifungaland antioxidant activities (Abdollah and others 2013). The odor of leaves of O. basilicum is often sensed as pungent by people, while flowers smell mildly sweet resembling hyacinth or citrus. Although self-pollination might be the most frequent event, O. basilicum is also known to be entomophilous and presence of flowering individuals in a community can enhance the frequency of pollinator visits to flowers of other adjacent species (Pereira and others 2015). Rather than pollinator attraction, the aerial emissions of floral volatiles from O. basilicum might play other biological roles similar to the volatile emissions from leaves.
Composition of floral scents emitted from plants are regulated by various internal and external factors including light and temperature, flowering stage (pre- and post-pollination ontogeny) and endogenous diurnal rhythms (Dudareva and others 2000; Dudareva and Pichersky 2000; Jiang and others 2011b; Farré-Armengol and others 2014; Farré-Armengol and others 2015). Therefore, the emissions vary qualitatively and quantitatively during anthesis. The diurnal rhythmic patterns of floral volatile release is either directly regulated by light (Jiang and others 2011b) or by circadian clock (Zhuang and others 2008; Jiang and others 2011a; Kong and others 2012), but it is unknown whether O. basilicum exhibits diurnal variations in volatile release and if it does, what factors are responsible for such diurnal variations. Understanding the diurnal variation patterns and their controlling mechanisms is of importance in explaining the biological role of floral volatile emission in O. basilicum.
Emission of volatiles from both vegetative and reproductivetissuesis a key mechanism among the diverse array of responses employed by plants to withstand various biotic and environmental stresses (Niinemets and others 2013; Zheng and Dicke 2008; Peng and others 2011).Exogenous application of methyl jasmonate, MeJA, an important signaling molecule, has been frequently shown to regulate the production and emission of volatiles from vegetative tissues of a broad range of plant species, and is thus, commonly used to simulate defense responses similar to responses elicited by herbivore feeding or physical wounding (Abdollah and others 2014; Martin and others 2003; Semiz and others 2012; Ament and others 2004; Wouter and others 2013). While the majority of elicitor studies have been conducted with leaves, studies on the effects of exogenous application of MeJA on flowers have been rare. Although generally well-protected by large investments in constitutive chemical defenses, there are multiple specialized floral herbivores (Theis 2006; Veromann and others 2013), implying that flowers can be exposed to biotic stresses in natural conditions. While terpenoid synthesis pathways are strongly upregulated by MeJA in the case of leaves (Rodriguez-Saona and others 2001; Martin and others 2003; Degenhardt and Lincoln 2006), the key question is to what extent can flowers respond to such elicitation.
We have used O. Basilicum as a model to gain insight into the regulation of floral volatile emissions. We first compared volatile chemical compositions among flowers and leaves, and among flower parts to elucidate the correlation of the biosynthesis of the volatiles in different organs and flower parts in O. basilicum. Then we determined diurnal kinetics of floral emission both under light and dark conditions to estimate the extend of diurnal variability of emissions and gain insight into the mechanisms of possible emission variations. Ultimately, we explored the effects of MeJA on the emission of floral volatiles at both short- and long-term. Considering the strong constitutive emissions, our working hypothesis was that floral volatile release in O. basilicum exhibits moderate diurnal variations and distinctresponse to MeJA application, underscoring its potential ecological significance in the defense under biotic stress.
Materials and Methods
Plant growth
Seeds of Ocimum basilicum (Lot 8CJ311048, SC AGROSEL SRL, Romania) were sown in 1 L plastic pots filled with commercial potting soil with nutrients (Biolan Oy, Finland) and grown at 12 h light period under a light intensity of 250 μmol m−2 s−1 at the level of plants (Philips HPI/T Plus 400W metal halide lamps) as described before (Copolovici and others 2012). Temperature was maintained at 24°C day/20 °C night and humidity at 60–70%, and plants were watered daily to field capacity. Plants with about 10 inflorescences were used for the experiments. An individual inflorescence with 20 small flowers was carefully detached from the plant in the morning at 10:00, placed in a flask filled with distilled water, and transported to the laboratory for volatile analysis.
Volatile collection and analysis
To sample emitted floral volatiles from O. basilicum, each individual inflorescence was inserted in a glass chamber (3 L volume) of a multi-chamber gas-exchange system described in detail by Toome and others (2010). Each chamber operated with a constant purified and humidified air at a flow rate of 1 L min-1, and turbulent conditions in the chamber were achieved by a fan installed in each individual chamber. The light regime during measurements followed the growth light conditions with the light intensity of 200 µmol m−2 s−1 provided for 12-h day conditions. The temperature inside the chambers was between 24-26 °C during the light period and 22 °C during the dark period.
Volatiles were sampled from each chamber using multibed stainless steel cartridges (10.5 cm length, 3 cm inner diameter, Supelco, Bellefonte, PA, USA) using the method of Kännaste and others(2014). For quantitative adsorption of volatiles, 4 L of chamber exhaust air with emitted volatiles was collected from every chamber at a flow rate of 200 mL min-1 for 20 min through the adsorption cartridge using a constant flow air sample pump (1003-SKC, SKC Inc., Houston, TX, USA). The cartridges were filled with Carbotrap C 20/40 mesh (0.2 g), Carbopack C 40/60 mesh (0.1g) and Carbotrap X 20/40 adsorbents (Supelco, Bellefonte, USA; see Copolovici and others 2012 for further details). These chemically neutral carbon-based adsorbents enable trapping of unsaturated compounds without intervening chemical surface reactions that can for instance happen in oxidative atmospheres when using organic polymer adsorbents such as Tenax TA (Darmais and others 2000; Calogirou and others 1996). Fresh mass of enclosed plant material was determined immediately after volatile collection, and dry mass was determined after oven drying at 70 °C for 48 h. Only dry mass was used for the calculation of emission rates. Dry to fresh mass ratios of flower tissues are provided in Table 1 to allow for interconversion among dry and fresh mass.
Table 1.
Interconversion of the fresh mass and dry mass ( mean ± standard error) of leaves and different floral parts of Ocimum basilicum used for headspace volatile collection.
| Leaves | Intact Inflorescences | Sepals | Petals | Pistils&Stamina | Pedicels | |
|---|---|---|---|---|---|---|
| Dry mass (g) | 0.069±0.021 | 0.081±0.016 | 0.060±0.008 | 0.120±0.010 | 0.080±0.010 | 0.044±0.010 |
| Water content (%) | 83.3±1.3 | 83.2±0.6 | 79.0±0.5 | 75.9±1.0 | 76.3±0.6 | 86.6±2.1 |
| Dry to fresh mass ratio (%) | 18.7±1.3 | 16.8±0.6 | 21.0±0.5 | 24.1±1.0 | 23.7±0.6 | 13.4±2.1 |
| Fractional flower compositiona (%) | 100.0±0.0 | 17.8±1.1 | 7.6±0.4 | 4.1±0.3 | 70.5±1.8 |
Fractional flower composition: ratio of each flower tissue mass divided by total inflorescence mass
Analysis of terpenoid compounds with GC-MS
Adsorbent cartridges were analyzed for volatiles with a combined Shimadzu TD20 automated cartridge desorber and Shimadzu 2010 Plus GC–MS system (Shimadzu Corporation, Kyoto, Japan) as described before (Toome and others 2010). The compounds were identified by comparison of their mass spectra with those in the NIST library and in the custom-generated library and based on the identity of retention times and mass spectra of the authentic standards (GC purity, Sigma–Aldrich, St. Louis, MO, USA). The absolute concentrations of terpenes and fatty acid derivative products were calculated based on calibrations with external authentic standards. The background (blank) VOC concentrations measured with empty chamber were subtracted from the emission samples of the plants.
Determination of variability of emissions among leaves and flowers and flower parts
Leaves, intact inflorescences, petals, sepals, stamina with pistils, and pedicels were carefully separated and enough plant material was collected. The biomass fractions were weighted and analyzed individually for volatile emissions to gain insight into the fine-scale spatial emission variability within plant organs, inflorescences and individual flowers.
The collection of volatiles emitted from leaves and flowers and flower parts was conducted in a 3 L glass chamber using the same procedures as described in the section Volatile collection and analysis. To minimize the effect of drying of the detached organs, flower parts were kept moist prior to measurement and volatile collection was conducted in humid chamber atmospheres and finished in 20 min since the enclosure of fresh organs. The analysis was replicated 6 times with different plants. We also note that the rate of volatile emission is typically much less sensitive to low water availability than the rate of other physiological processes such as photosynthesis (Niinemets and others 2010 for a review).
Monitoring of the light-dependent kinetics of terpenoids emission with PTR-MS
To analyze the endogenous vs. light-dependent dynamics of emission of floral volatiles of O. basilicum, continuous measurements of volatile emissions from inflorescences of intact plants were carried out. The measurements were started at 10:00 am every day under two light regimes: normal light/dark (12 h/12 h) cycle and continuous darkness. The kinetics of volatile emission were monitored for a total period of 48 h with a proton-transfer reaction quadrupole mass spectrometer (PTR-MS, high sensitivity version, Ionicon Analytik, Innsbruck, Austria) attached to a custom-made gas-exchange system that has opportunity to measure both reference (incoming) and sample (outgoing) gas streams (Copolovici and Niinemets 2010 for system description). The inflorescences were enclosed in the temperature-controlled 1.2 L glass cuvette of the system and the measurements were carried out using ambient air purified by a charcoal filter and humidified by a custom-made humidifier as in other studies (e.g. Bourtsoukidis and others 2014). The air flow rate was maintained at 1.6 L min-1. During measurements of volatile emissions, the surface temperature of flowers was monitored by a thermocouple. The temperature of the measurement system was maintained at a constant level of 30 °C by regulating the chamber temperature (Copolovici and others 2010). Therefore, the effect of any differences in temperature for instance among measurements in the light and in the dark was excluded.
The flow of sample air from the chamber for PTR-MS measurements was 100 mL min−1 and the PTR-MS measurements were conducted using standard measurement procedures (Beauchamp and others 2005; Copolovici and Niinemets 2010). The PTR-MS was operated under standard conditions with drift tube voltage kept at 600 V and drift tube pressure at 2.3 mbar. Optimization of the instrument resulted in high and sustained primary ion signal which enhanced the sensitivity of the measurements. The PTR-MS system was calibrated with a calibration standard mixture including key volatiles from different compound families (Ionimed analytic GmbH, Austria). The accuracy of the measurements was estimated to bebetter than ±15%. The total amount of octadecanoid pathway products (LOX products) was found as the sum of individual protonated mass signals (m/z 57 [(E)-2-hexenal (fragment)] + m/z 83 [hexenol+hexanal (fragments)] + m/z 85 [hexanol (fragment)] + m/z 99 [(Z)-3-hexenal+(E)-3-hexenal (main)] + m/z 101 [(Z)-3-hexenol+(E)-3-hexenol + (E)-2-hexenol+hexanal (main)] + m/z 103 [hexanol (main)]) (Copolovici and Niinemets 2010; Brilli and others 2012). The total monoterpenoid emission was calculated from the sum of individual mass signals (m/z 137 [monoterpene] + m/z 155 [linalool] + m/z 169 [monoterpeneoxide]), while total sesquiterpenoid emission from the sum of individual mass signals (m/z 205 [sesquiterpene] + m/z 237 [sesquiterpene oxide]). As PTR-MS cannot distinguish between the volatile compounds with the same mass, such as different monoterpenoids, the contributions of individual monoterpenoids and sesquiterpenoids with the same mass were quantified by taking samples for GC–MS analyses as explained above. The rate of emission was estimated from the difference between the trace gas concentrations in incoming and outgoing gas streams according to Niinemets and others (2011). The analysis was replicated 6 times with different plants.
The effect of methyl jasmonate (MeJA) on the emission of the floral volatiles over short- and long-term
Inflorescence with approximately 10 small flowers were selected for exogenous MeJA (Sigma-Aldrich) treatment. In the control treatment, detached flowers (with the cut end hold in distilled water) were sprayed with 10 mL 5% ethanol solution in distilled water. In the MeJA treatment, detached flowers were sprayed with the solution of 10 mL 10 mM MeJA solution in 5% ethanol.The treatment conditions were selected based on previous reports that demonstrated greater efficacy and repeatability of treatment with MeJA alcoholic solutions compared with distilled water solutions, reflecting much more uniform dispersion solutions of hydrophobic MeJA in alcohol than in water (Dinh and others 2013; Martin and others 2003). After spraying, the inflorescences were immediately placed in the gas exchange system for the headspace collection.Headspace collections were performed at the time points of 20 min, 2 h, 10 h and 24 h after the initiation of MeJA treatment using PTR-MS. The analysis was replicated 6 times with different plants. Although we used detached flowers, and acknowledge that the detachment itself can affect floral volatile blend due to wounding, control plants processed identically were used in every experiment. Thus, the effects of MeJA on floral scent emission are studied in relation to appropriate control treatments. Furthermore, floral detachment was carried out very carefully trying to minimize the mechanical damage of the rest of the inflorescence. Separate measurements with attached and detached inflorescences demonstrated that the effect of detachment was minor, except for the initial release of fatty acid derivatives immediately after detachment (data not shown).
Statistical analyses
Emission rates among organs and flower parts were compared by ANOVA. ANOVA was also used to test for the effects of time-dependent variations in emissions as driven by light and the effects of MeJA treatments. The statistical analyses were conducted with SAS (Version 8.02. SAS Institute, Cary, NC). The statistical tests were considered significant at P ≤ 0.05.
The characteristic feature of O. basilicum volatile blend is the richness of terpenoids. As different terpenoids synthesized by the same enzyme or through the same biochemical pathway often accumulate synchronously (Chen and other 2011; Chen and other 2009), statistical methods can be used to identify putative terpenoid synthases based on correlated emission profiles through time. Hierarchical cluster analysis is widely used to analyze data from DNA microarray experiments and other genomic datasets. Recently, this method has been elaborated to investigate the accumulation pattern of secondary metabolites (Chen and others 2009; Jiang and others 2011). We used the hierarchical cluster analysis to study time-dependent changes in the emission profiles of monoterpenoids and sesquiterpenoids in the inflorescences of O. basilicum. The emission rates of monoterpenoids and sesquiterpenoids identified from the flowers under eight conditions, control (20 min, 2 h, 10 h, 24 h) and MeJA treatment (20 min, 2 h, 10 h, 24 h) were used in this analysis. The cluster analysis was conducted by the Cluster 3.0 software (Version 1.39, Stanford University, Stanford Microarray Database, Stanford, CA, US) using the average linkage analysis with calculated weight as the clustering method as applied before (Chen and others 2009). Before the cluster analysis, procedures of “Filter Data” (with the settings of % Present >= 80, SD (Gene Vector) =2, At least X Observations with abs(Val) >= 2, MaxVal-MinVal >= 2) and “Adjust Data” were carried out (Chen and others 2009). Heat maps were created using the Java TreeView 1.60 software (Stanford University, Stanford, CA, US).
Results
Differences in volatile emissions among vegetative and reproductive tissues and among flower parts
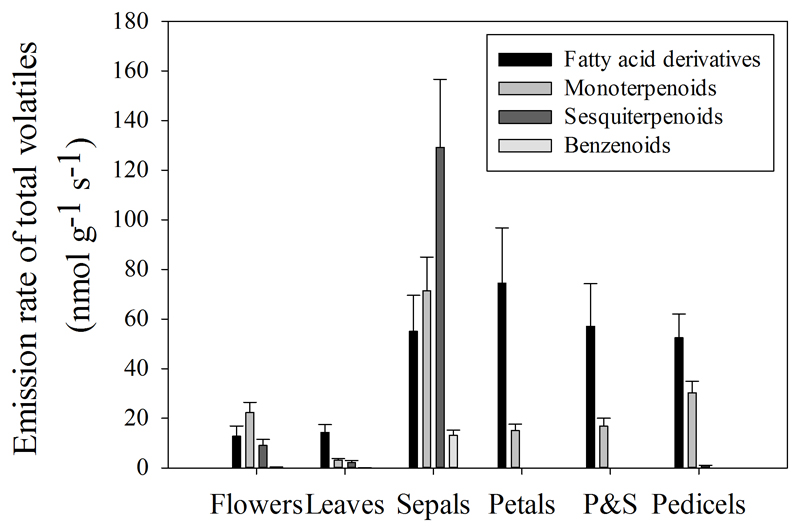
To gain insight into the correlations among volatile emissions between vegetative and reproductive organs, and analyze the variability of emissions among flower parts, emissions of volatiles from leaves, intact inflorescences, petals, sepals, stamina with pistils, and pedicels were analyzed under the same environmental conditions. In total, 29, 39, 37, 10, 9 and 14 volatile chemicals (Table 2) were identified from leaves (total emission 19.7 nmol g-1 s-1), intact inflorescences (66.7 nmol g-1 s-1), petals (89.5 nmol g-1 s-1), sepals (268.2 nmol g-1 s-1), stamina with pistils (73.9 nmol g-1 s-1), and pedicels (83.8 nmol g-1 s-1), respectively (Fig. 1). Reproductive organs (intact inflorescences) exhibited 3.4-fold higher volatile emissions than vegetative organs (leaves). Among the reproductive organs, sepals are the main emitters of volatiles with the composition of emissions being dominated by terpenoids (26.6% monoterpenoids, and 48.1% sesquiterpenoids) and fatty acid derivatives (21.4%), and with a minor proportion (4.9%) of benzenoids. β-pinene was the dominant monoterpene and α-bergamotene was the dominant sesquiterpene in sepals. Eugenol was the only benzenoid compound identified from leaves, intact flowers and sepals. Composition and emission rate of volatiles detected from petals, stamina with pistils, and pedicels were similar with a low amount of sesquiterpenes and with no detectable eugenol emission.
Table 2.
Average (± SE) emission rates per unit dry mass (nmol g-1 s-1) of the volatiles identified from leaves and different floral parts of Ocimum basilicum
| Compounds | Retention Time (min) | Leaves | Intact Inflorescences | Sepals | Petals | Pistils &Stamina | Pedicels |
|---|---|---|---|---|---|---|---|
| Aliphatic derivatives | |||||||
| (Z)-3-Heptene | 9.737 | 1.82±0.37a | ND | ND | ND | ND | ND |
| Pentanal | 10.777 | NDb | 0.37±0.12 | ND | ND | ND | ND |
| n-Hexanal | 13.895 | 0.230±0.050 | 1.88±0.32 | 1.67±0.31 | ND | ND | 3.8±0.6 |
| cis-3-Hexen-1-ol | 16.512 | 1.93±0.27 | 1.52±0.43 | 1.92±0.34 | 0.13±0.04 | ND | 2.4±0.5 |
| Heptanal | 18.262 | ND | ND | 1.5±0.5 | ND | ND | ND |
| 2-ethyl,1-Hexanol | 25.665 | 2.3±0.6 | 3.71±0.41 | 3.7±0.6 | 8.3±2.1 | 7.6±3.2 | 4.5±0.6 |
| Nonanal | 30.115 | 4.1±0.7 | 3.62±0.32 | 21.6±2.3 | 41.3± 2.3 | 31.7±4.3 | 10±2 |
| Decanal | 36.837 | 4.2±0.6 | 1.65±0.05 | 24.7±2.4 | 24.7± 4.2 | 19.6±2.4 | 31±5 |
| Monoterpenoids | |||||||
| α-Pinene | 18.672 | 0.250±0.040 | 0.53±0.06 | 1.17±0.23 | ND | ND | 0.44±0.11 |
| Camphene | 19.777 | 0.170±0.030 | 0.190±0.020 | ND | ND | ND | ND |
| α-Myrcene | 20.704 | ND | 0.42±0.06 | ND | 2.3±0.9 | 3.2 ±2.0 | 3.2±1.0 |
| β-Pinene | 21.187 | 0.46±0.12 | 6.4±1.3 | 25.3±5.2 | 5.2± 0.8 | 6.4 ± 3.1 | 0.85± 0.26 |
| γ-Terpinene | 22.81 | 0.33±0.07 | 0.220±0.040 | 0.52±0.05 | 1.57±0.32 | 1.81±0.07 | 1.14±0.23 |
| α-Pinene epoxide | 23.19 | ND | 1.85±0.37 | 2.1±1.4 | 3.0±0.7 | 3.2±1.0 | 5.8±1.3 |
| trans-β-Ocimene | 23.735 | 0.140±0.040 | 1.46±0.31 | 4.2±0.7 | 1.23±0.14 | 1.52±0.34 | 1.16±0.34 |
| Limonene | 23.94 | 0.430±0.06 | 2.32±0.47 | 7.0±2.1 | ND | ND | ND |
| cis-β-Ocimene | 24.606 | 0.050±0.010 | 2.23±0.32 | 6.8±0.8 | ND | ND | 16.0±3.1 |
| 1,8-Cineole | 24.77 | 0.130±0.040 | 0.39±0.07 | 3.2±0.6 | 1.88±0.32 | 1.5±0.5 | ND |
| Terpinolene | 27.537 | 0.39±0.05 | 0.28±0.06 | 0.91±0.23 | ND | ND | ND |
| Linalool | 29.889 | 0.42±0.09 | 5.2±1.5 | 17.3±4.4 | ND | ND | 1.72±0.44 |
| Camphor | 35.036 | 0.33±0.05 | 0.45±0.07 | 1.64± 0.27 | ND | ND | ND |
| α-Terpineol | 37.145 | 0.060±0.010 | 0.42±0.11 | 0.63±0.15 | ND | ND | ND |
| Limonene oxide | 47.789 | ND | ND | 0.81±0.27 | ND | ND | ND |
| Sesquiterpenoids | |||||||
| α-Copaene | 47.094 | 0.120±0.040 | 0.060±0.010 | 0.68±0.09 | ND | ND | ND |
| β-Elemene | 48.322 | ND | 0.080±0.020 | 5.6±0.8 | ND | ND | ND |
| α-Bergamotene | 50.41 | 0.170±0.020 | 4.02±0.37 | 57.1±4.0 | ND | ND | 0.92±0.12 |
| α-Guaiene | 51.095 | 0.120±0.020 | 0.210±0.030 | 3.9±0.6 | ND | ND | ND |
| α-Cubebene | 52.116 | ND | ND | 0.56±0.16 | ND | ND | ND |
| α-Caryophyllene | 53.397 | ND | ND | 0.91±0.26 | ND | ND | ND |
| α-Curcumene | 53.909 | ND | 0.070±0.010 | 2.89±0.33 | ND | ND | ND |
| β-Farnesene | 54.17 | ND | 0.140±0.030 | 4.2±0.8 | ND | ND | ND |
| β-Bisabolene | 54.907 | ND | 0.110±0.030 | 6.3±0.7 | ND | ND | ND |
| α-Selinene | 55.218 | ND | 0.180±0.05 | 1.91±0.39 | ND | ND | ND |
| α-Bulnesene | 55.551 | 0.160±0.040 | 0.36±0.06 | 9.5±2.1 | ND | ND | ND |
| Cedrene | 56.1 | 0.81±0.13 | 0.91±0.08 | 16.4±3.2 | ND | ND | ND |
| α-Bisabolol | 56.477 | 0.170±0.030 | 0.200±0.040 | 0.82±0.13 | ND | ND | ND |
| α-Muurolene | 57.17 | 0.090±0.020 | 0.40±0.19 | 0.93±0.17 | ND | ND | ND |
| Viridiflorol | 60.564 | 0.190±0.030 | 0.34±0.06 | 1.0±0.6 | ND | ND | ND |
| Cubenol | 61.9 | 0.050±0.010 | 1.35±0.08 | 5.22±0.26 | ND | ND | ND |
| δ-Cadinol | 62.898 | 0.28±0.06 | 0.59±0.15 | 11.8±4.3 | ND | ND | ND |
| Benzenoids | |||||||
| Eugenol | 49.33 | 0.050±0.010 | 0.36±0.05 | 13.1± 2.1 | ND | ND | ND |
All data are averages of three independent measurements with different plants.
ND: not detected.
Fig. 1.
Emission rates of volatiles from vegetative (leaves) and reproductive tissues including intact inflorescence and individual flower components (sepals, petals, stamina with pistils, and pedicels) in Ocimum basilicum. The values are averages of three independent measurements. The emission rates are expressed per unit dry mass.
Emission dynamics of floral volatiles
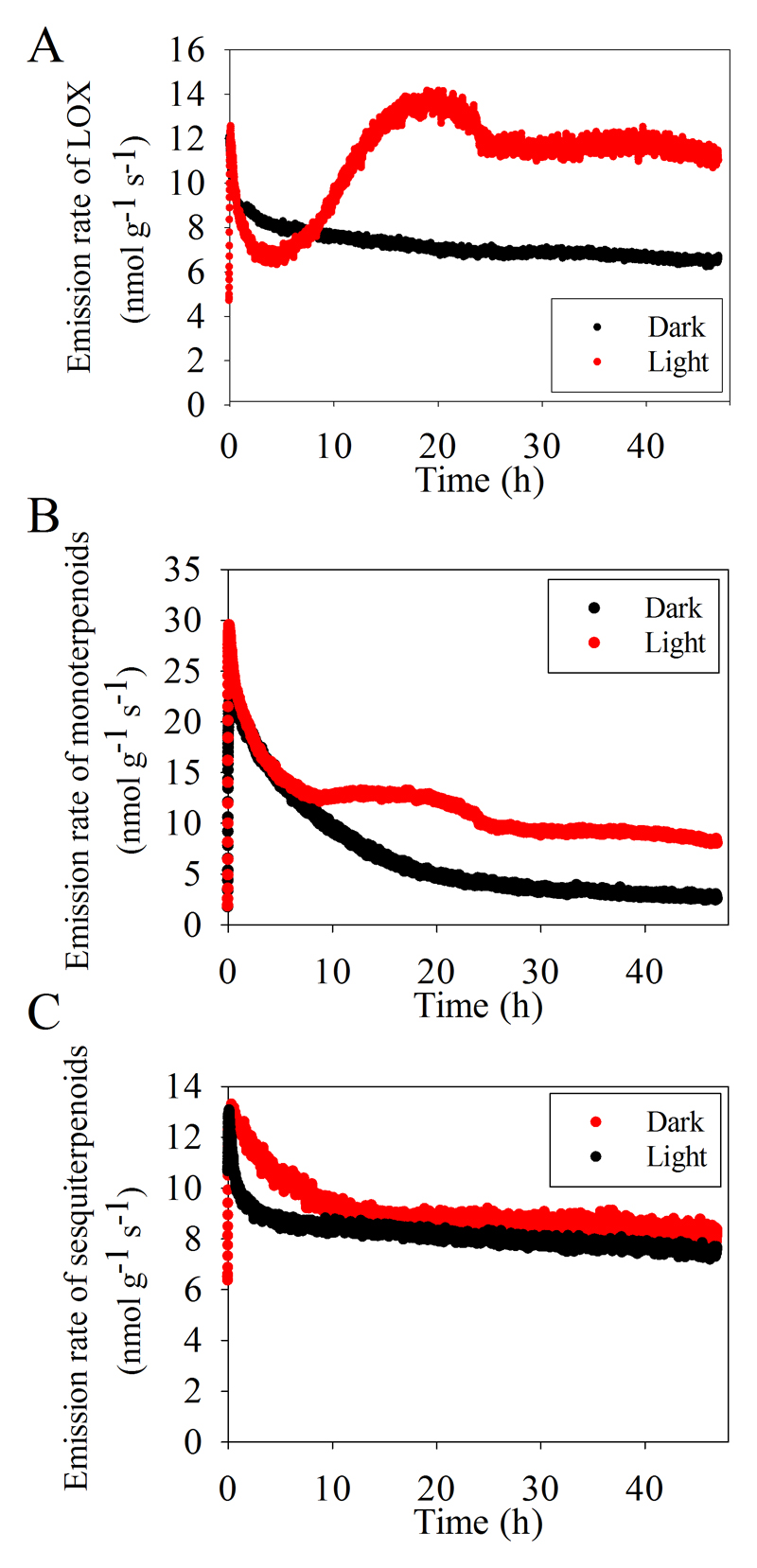
The emissions of different volatiles groups exhibited contrasting temporal patterns. The emission of LOX compounds decreased first with the minimum emission observed at about 4 h after enclosure of inflorescences in the chamber (14:00 pm) and increased continuously with the maximum emission observed at about 18 h after start of measurements the next day at 4:00 am (Fig. 2A). The emission of monoterpenoids decreased continuously and showed a second moderate elevation in 10 h after enclosure at about 20:00 pm (Fig. 2B). The emission of sesquiterpenoids declined continuously during the whole 48 h measurement period without any rhythmic pattern (Fig. 2C).
Fig. 2.
Emission dynamics of total lipoxygenase pathway (LOX) volatiles (A), monoterpenoids (B) and sesquiterpenoids (C) from O. basilicum flowers under both light and dark conditions during a time-period of 48 h. The emission rates were measured by a proton transfer reaction mass spectrometer (PTR-MS). Three independent replicates yielded similar results, and thus, data are demonstrated for representative individual samples. Table 2 provides detailed information of the composition of the three analyzed compound classes.
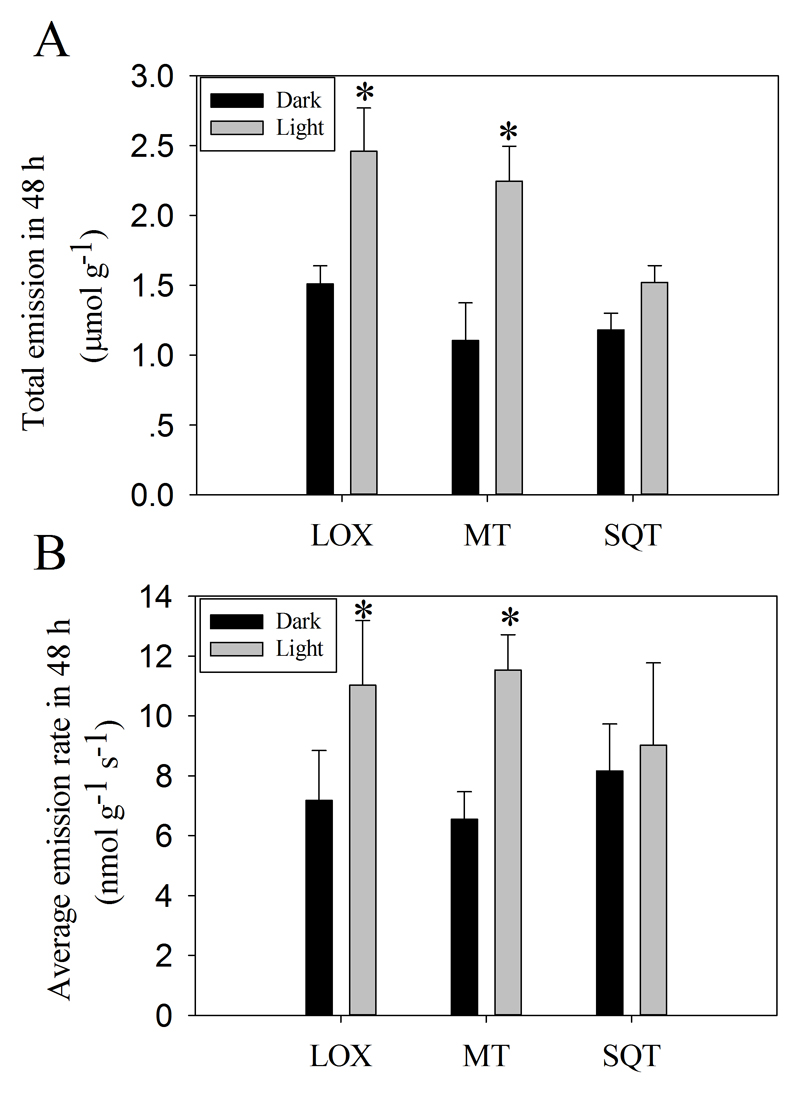
To determine whether the observed rhythmic emission pattern of floral volatiles is light-dependent, flowers of O. basilicum were placed in continuous darkness for 48 h. In dark, the emissions of LOX compounds, monoterpenoids and sesquiterpenoids decreased continuously without fluctuations (Fig. 2A, B, C). The total emissions of LOX and monoterpenoids and average emission rate of monoterpenoids under constant light condition were significant higher than those under constant darkness, while differences in total emission and average emission rate among light and dark were negligible for sesquiterpenoids (Fig. 3A, B). This evidence suggests that the emission kinetics of LOX compounds and monoterpenoids, but not sesquiterpenoids, is regulated by light.
Fig. 3.
Total (A) and average emission rate (B) of LOX compounds, monoterpenoids (MT) and sesquiterpenoids (SQT) from O. basilicum flowers under both light and dark conditions during a time-period of 48 h. The values are averages of three independent measurements. The emission rates are expressed per unit dry mass. “*” denotes statistically significant differences at P ≤ 0.05 according to ANOVA analyses.
Effect of exogenous MeJA on the emission of floral volatiles
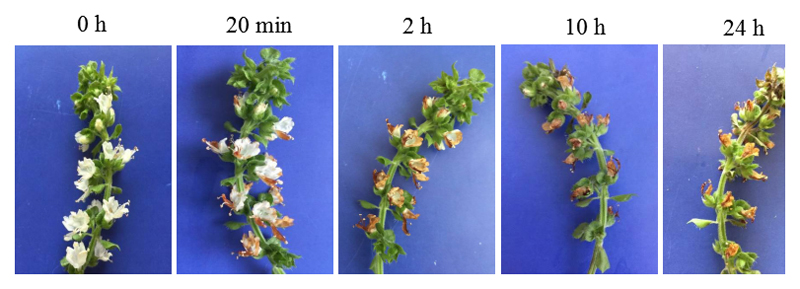
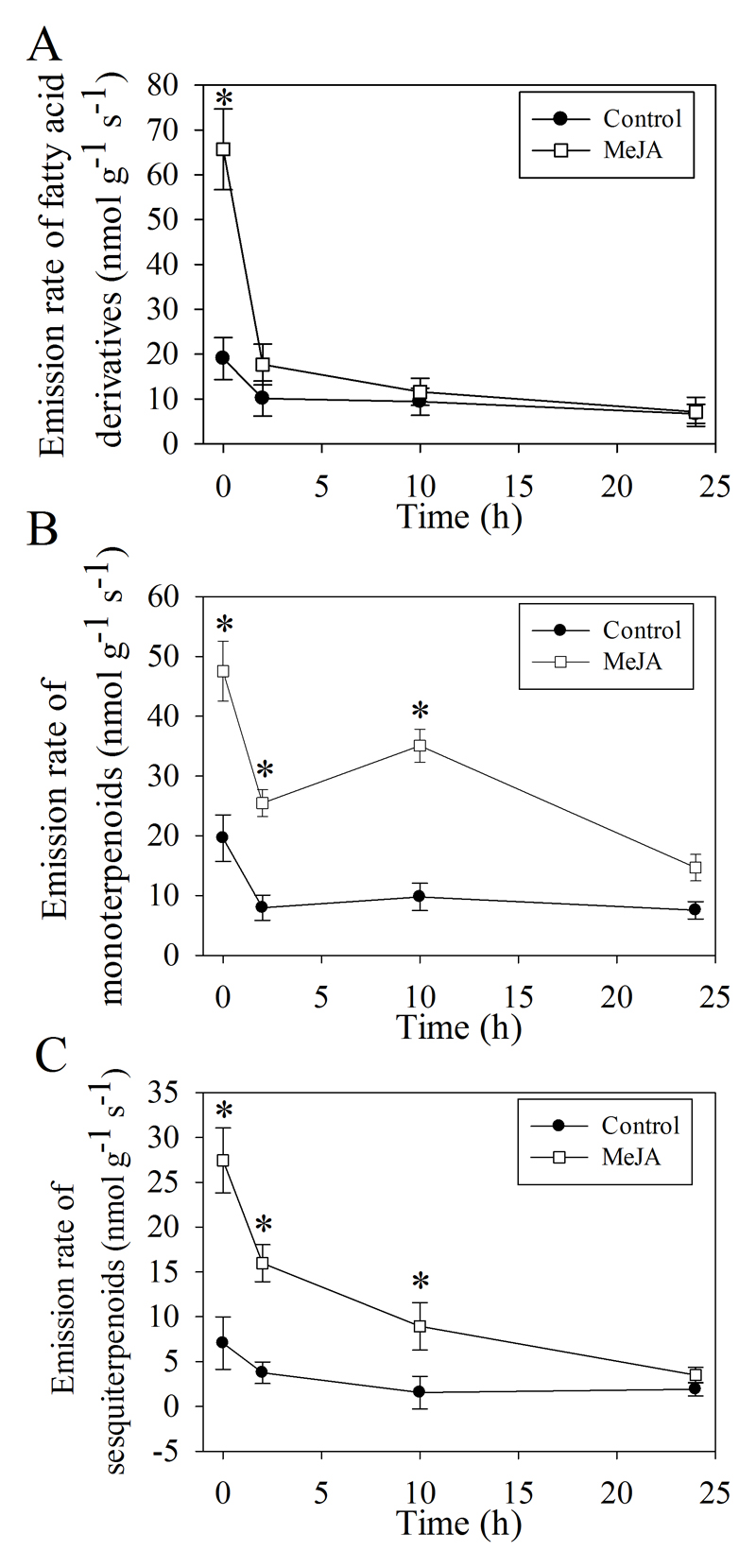
To simulate effects of biotic stress, in particular, herbivore feeding, on floral volatile release, inflorescences were sprayed with MeJA, the plant hormone synthesis of which is elicited upon herbivore attack. After treatment, dynamics of leaf damage and volatile emissions over short-and long-term was monitored. As the visual damage symptoms, wilting, and progression of desiccation and necrosis of flower parts, especially on the petal surfaces were observed in the course of 24 h MeJA treatment (Fig. 4). The emissions of different volatile classes displayed distinctive patterns after the application of MeJA. In the short term, the emission of total fatty acid derivatives (3.5-fold greater than in controls; Fig. 5A), monoterpenoids (2.4-fold greater; Fig. 5B) and sesquiterpenoids (3.9-fold greater; Fig. 5C) were all significantly increased already in 20 min after initiation of MeJA treatment. However, after 2 h and 10 h, the total emission rate of fatty acid derivatives was no longer different from the control treatment (Fig. 5A), while total monoterpenoids reached to 3.6-fold and 3.2-fold higher values compared with the controls, respectively (Fig. 5B). Sesquiterpenoid emissions reached to 5.8-fold and 4.3-fold higher values compared with the controls after 2h and 10h respectively (Fig. 5C). After 24 h, no significant differences among MeJA-treated and non-treated plants were observed for any volatile class.
Fig. 4.
Illustration of propagation of the damage of O. basilicum inflorescences since the application of the elicitor methyl jasmonate (MeJA) with time (0 h, 2 h, 10 h, 24 h). MeJA was applied at a concentration of 10 mM (in 5% aqueous ethanol solution).
Fig. 5.
Emission of total fatty acid derivatives (A), monoterpenoids (B) and sesquiterpenoids (C) from untreated (Control) and MeJA-treated O. basilicum flowers through 24 h since MeJA application. Volatiles were collected at four time points (20 min, 2 h, 10 h and 24h) after the initiation of the treatments. “*” denotes statistically significant differences at P ≤ 0.05 according to ANOVA analyses.
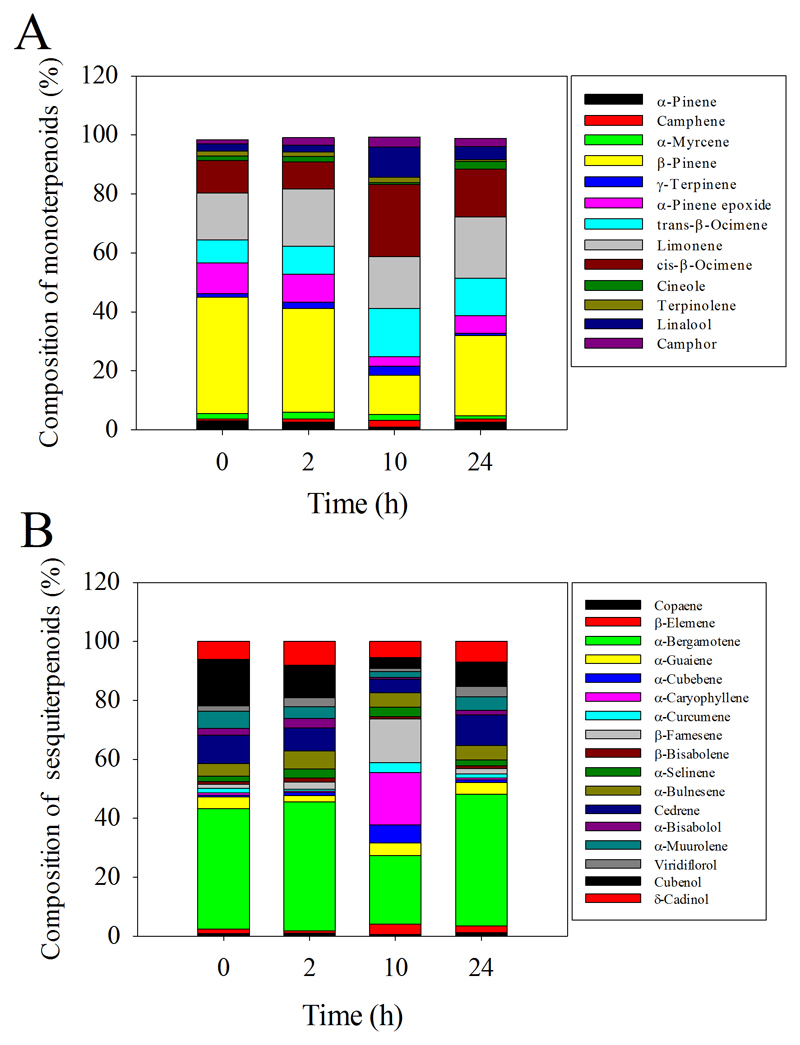
Among the individual compounds, nonanal (33.7%) and decanal (28.5%) were the dominant fatty acid derivatives exhibiting significantly enhanced emissions in 20 min since the start of the MeJA treatment. β-pinene (39.5%), α-pinene epoxide (10.4%), limonene (15.9%), and cis-β-ocimene (11.0%) were the dominant monoterpenoids in 20 min after MeJA spraying (Fig. 6A), while α-bergamotene (41.0%) and cubenol (15.7%) were the dominant sesquiterpenoids in 20 min since the start of treatment (Fig. 6B). At this early stage of the induction response, the composition of emitted mono- and sesquiterpenes was similar among treated and non-treated plants (Fig. 6A, B). However, in 10 h, the compositions of mono- and sesquiterpenoids in treated plants differed significantly from those in the controls, with β-pinene (13.3%), trans-β-ocimene (16.5%), limonene (17.6%), cis-β-ocimene (24.5%), and linalool (10.2%) being the dominant monoterpenoids (Fig. 6A) and trans-α-bergamotene (23.3%), α-caryophyllene (17.8%), β-farnesene (14.8%) being the dominant sesquiterpenoids (Fig. 6B) in MeJA-treated plants. This evidence suggests that the first burst of the volatile emission including fatty acid derivatives, monoterpenoids and sesquiterpenoids reflected an enhancement of constitutive emissions without changes in the composition, while the second emission burst of terpenoids with significant variation of the composition reflected MeJA effects at gene expression and protein synthesis levels.
Fig. 6.
Variation in the composition of monoterpenoids (A) and sesquiterpenoids (B) detected in the floral emissions of O. basilicum for 24 h after application of MeJA (Fig. 4 for absolute differences in the emission rates).
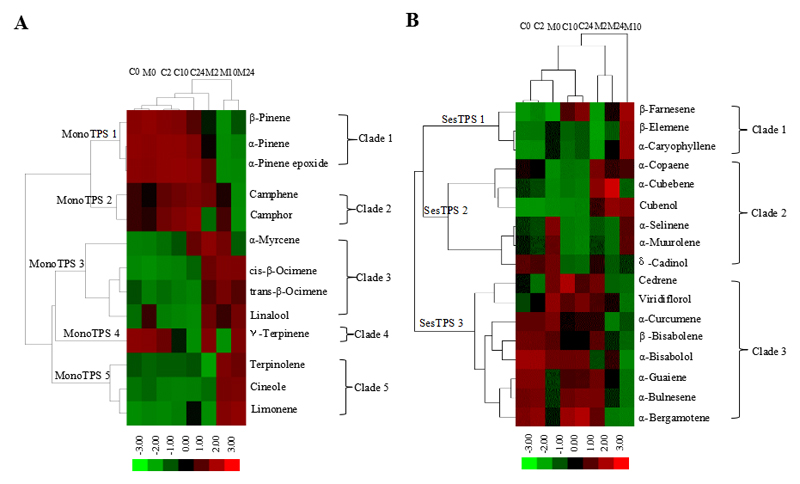
Cluster analysis of putative terpenoid biosynthesis pathways based on floral volatile emissions
Our cluster analysis based on time-dependent changes in emission profiles using 13 monoterpenoid compounds (8 monoterpene and 5 monoterpene oxides) divided the monoterpenoids into 5 clades (Fig. 7A), suggesting that at least 5 monoterpene synthase genes are involved in the biosynthesis of monoterpenoids emitted from the flowers of O. basilicum. The second cluster analysis with 17 sesquiterpenoid compounds (13 sesquiterpene and 4 sesquiterpene oxides) divided the emitted sesquiterpenes into 3 clades (Fig. 7B), suggesting that at least 3 sesquiterpene synthase genes are involved in the production of sesquiterpenoids that constitute the floral emission blend in O. basilicum.
Fig. 7.
Cluster analysis of 13 monoterpenoids (A) and 17 sesquiterpenoids (B) emitted from flowers of O. basilicum. Hierarchical K-means cluster analysis was performed based on the emission rates of monoterpenoids and sesquiterpenoids from the flowers of O. basilicum under control and MeJA treatments at four time steps (20 min, 2 h, 10 h, 24 h), and putative terpene synthases (TPS) were defined. The colour codes denote the changes in the emission rate due to MeJA treatment. Red represents the up-regulation of the emission rate in response to MeJA treatment, while green represent down-regulation.
Discussion
General characteristics of O. basilicum volatile emissions
Because of its distinctively pungent and sweet aroma and flavor, sweet basil is a highly popular spice all over the world. Many studies have investigated the composition of O. basilicum essential oil mainly focusing on leaves (Abdollah and others 2013; Carovič-Stanko and others 2010; Kwee and Niemeyer 2011; Ameneh and others 2013; Lee and others 2005). However, as discussed in the Introduction, the essential oil composition can be strongly driven by plant material preparation and essential oil extraction methods, e.g. steam distillation vs. high temperature extraction with hydrophobic solvents. Due to the methodological issues, as well as due to compound-to-compound differences in solubility of volatile compounds in leaf liquid and lipid phases and specialized storage structures (Niinemets and others 2004), one should not expect a direct correlation between volatile content and emission profiles. We argue that the aerial volatiles collected from the headspace provide a more accurate indicator of the odor of O. basilicum sensed by the human olfactory system. In this study, we systematically investigated the emission of volatiles from leaves, intact flowers and different flower parts. Headspace collection and identification by GC-MS demonstrated that flowers emit volatiles with a higher rate than leaves (Fig. 1). We have further demonstrated that the sesquiterpenoids were the main compounds in the emission blend in O. basilicum (Table 2), which is not consistent with the previous reports that suggested that the essential oil of O. basilicum leaves and flowers is primarily dominated by oxygenated benzenoids and monoterpenes (eugenol, methyl chavicol, geraniol, linalool, 1,8-cineole). On the other hand, several compounds reported to be present in the essential oil could not be detected in the headspace of leaves and flowers. For example, eugenol was the only benzenoid compound identified in the volatile emissions of O. basilicum, while several other benzenoids, especially some methyl esters (methyl eugenol, methyl chavicol and methyl cinnamate) are commonly identified in the essential oil. In addition, the fraction of oxygenated monoterpenes is characteristically greater in the essential oil than in the headspace collection. This could indicate a greater share of compounds from specialized storage structures detected in the essential oils as well as possible chemical transformations during essential oil extraction.
Spatial and temporal controls on the floral emissions
To understand the spatial variation in the release of floral volatiles, different flower parts were separately measured. Commonly, petals and/or pistils and stamina constitute the main source of flower volatiles (Dudareva and others 2003; Dudareva and others 2005). Unexpectedly, in O. basilicum, sepals rather than petals or pistils and stamina were found to be the main emitter of volatiles (Fig. 1; Table 2). Given that the biological function of sepals is mainly defense, we argue that the volatile release of sepals might also be chiefly associated with defense. Furthermore, we found important differences in the emission spectrum among sepals and the other flower parts (Fig. 1; Table 2). In particular, myrcene and 1,8-cineole were emitted from petals, pistils and stamina as the dominant compounds. Despite being mainly a self-pollinating species (Pereira and others 2015), such differences suggest the potential role of these compounds in attracting the pollinators for cross-pollination.
The release of floral volatiles, regulated by both internal and external factors can strongly vary during the day (Farré-Armengol and others 2014; Farré-Armengol and others 2015). Such diurnal patterns could reflect an endogenous rhythm or light-dependent changes in substrate availability for the synthesis of these compounds or both. In our study, real-time flower emission kinetics were tracked over two photoperiods (48 h) using a state-of-the-art proton-transfer reaction mass spectrometer (PTR-MS). We found a clear rhythmic pattern in the emissions of LOX and monoterpenoids with the second emission maximum observed at about 4:00 in the morning. Under dark conditions, this second emission maximum was missing, although the emissions in darkness still occurred at a moderate level. This evidence suggests that LOX and monoterpene emissions can be regulated by changes in the activity of rate-controlling enzymes, and/or by changes in substrate availability. Especially in the case of monoterpenes that are synthesized in the plastids, two different emission pathways have been demonstrated in several plant species: emissions from storage structures and emissions of de novo synthesized monoterpenes from plastids (Grote and others 2013). Emissions from storage structures are typically independent of light, while emissions from photosynthesizing plastids, chloroplasts, are strongly light-dependent due to light effects on the substrate pool size (Komenda and Koppmann 2002; Tarvainen and others 2005; Grote and others 2013). Given that green sepals had the highest emission rates in O. basilicum, contribution of de novo light-dependent monoterpene emissions to diurnal emission dynamics is likely.
MeJA-dependent elicitation of floral emissions
To our knowledge, very few studies have investigated the effects of MeJA on the emission of floral volatiles (Kessler and others 2011). In our study, we found evidence of both an immediate stress response and a longer-term acclimation response in MeJA-treated flowers of O. basilicum. The immediate stress response in 20 min after MeJA treatment was associated with enhanced emissions of fatty acid derivatives and terpenoids (Fig 5A, B), but the composition of individual compounds was similar in MeJA treated and control leaves. We suggest that the first synchronous enhancement of emissions of fatty acid derivatives and terpenoids is caused by rapid damage at membrane level as observed upon severe stress when LOX-dependent stress-sensing pathways are activated (Liavonchanka and Feussner 2006; Wang and others 2008; Andreou and Feussner 2009). Upon membrane damage, free fatty acids are released, leading to formation of volatile LOX products. On the other hand, membrane damage leads to the leakage and burst of terpenoid volatiles constitutively present in cytosolic plastoglobuli as well as in the oil glands (Flinn and others 1993; Brilli and others 2011). Although such a rapid response is characteristically observed upon physical wounding (Hudgins and others 2004), no study has yet reported such an instantaneous terpenoid emission pattern in response to MeJA treatment. The second, induced response, is commonly observed, but it typically starts in several hours after MeJA application (Noge and others 2011). It is likely that due to low time resolution in volatile detection in most previous studies, this initial stress response can remain often undetected.
In 10h after MeJA application, terpenoid emissions were enhanced again, but the composition of emitted terpenoids in MeJA-treated flowers at that moment of time was significantly different from the controls (Fig. 6). Previously, the content of terpenoids in O. basilicum leaves was reported to be significantly increased by MeJA treatment over a longer time period (4 days; Li and others 2007). Thus, we suggest that the second peak of floral emission of monoterpenoids and sesquiterpenoids primarily reflected a gene expression level response, regulated by the overexpression or silencing of specific terpene synthases.
This evidence collectively suggests that flowers have a large capacity to respond to jasmonic acid signaling pathway elicitors similarly to foliage tissues, overall suggesting that biotic stress can importantly modify floral terpenoid biosynthesis and emission in O. basilicum.
Possible molecular basis of changes in the emission blends
In this study, terpenoids were found as the main and most diverse component in floral emissions of O. basilicum. What could be the molecular basis for the overall abundance and diversity of terpenoids detected in floral emissions in this species? To gain insight into the genetic complexity of terpene biosynthesis in O. basilicum, we analyzed the emission profiles in control and MeJA-treated plants through the experimental treatments. Important variations in the blend of emitted terpenoids was observed in this experiment, reflecting changes in regulation of expression of terpene synthase (TPS) genes. Although terpenoid synthases typically catalyze formation of multiple products (Chen and others 2011; Rajabi Memari and others 2013), proportions of terpenes synthesized by different terpenoid synthases are typically different (Chen and others 2009). On the other hand, multiple products of a single terpene synthase often share a similar type of cyclization (Chen and others 2011; Keeling and others 2011). Thus, differences in product profiles of different synthases and differences in expression regulation could be used to identify the minimum number of synthases responsible for the overall emission spectrum. Based on a hierarchical cluster analysis, we separated eight clades (Fig. 7), each characterizing a similar pattern of terpene accumulation through the MeJA application treatment, suggesting that the terpenes in each clade are synthesized by at least one different TPS enzyme. In our study, each of the clades showed a specific type of cyclization, supporting the assumption that the terpenoid products of each clade are formed by one terpene synthase, although we cannot rule out that several similar co-expressed terpene synthases are involved. Based on this reasoning, at least five TPS enzymes are involved in the production of monoterpenes and at least three TPS enzymes are involved in the production of sesquiterpenes in O. basilicum under control and MeJA-elicited conditions.
In previous studies in O. basilicum, transcript abundance of genes related to terpene synthesis, including the key genes involved in mevalonate (MVA) / non- mevalonate (MEP) biosynthetic pathways and putative terpene synthases (TPS), have been characterized by read mapping and transcript abundance measurements and Real-time PCR analyses (Rastogi and others 2014; Iijima and others 2004b; Xie and others 2008). Several TPS genes expressed in leaves have also been functionally characterized (Iijima and others 2004a; Iijima and others 2004b), and facilitate gaining an insight into the production of terpenoids compounds from flowers, especially under stresses. By comparison of our cluster analysis of floral terpenoids with the terpene products of TPS identified in the study of Iijima and others (2004a, b), we found that several TPS inferred to be involved in the production of floral volatiles in our study correspond well to the TPS isolated from O. basilicum leaves (Iijima and others 2004b). For example, the postulated MonoTPS3 and MonoTPS5 genes in our study correspond to MYS (β-myrcene synthase) and TES (terpinolene synthase) genes, respectively, while SesTPS3 gene in our study correspond to ZIS (α-zingiberene synthase) gene in the Iijima et al. (2004a) study. This evidence supports the use of cluster analysis as a tool to identify putative TPS responsible for the terpene blend in flower emissions.
However, mono- and sesquiterpene oxides, that were also included in the cluster analyses, are formed from corresponding mono- or sesquiterpenes by enzymes from another gene family, typically by cytochrome P450-dependent oxidases (a superfamily of terminal oxidase enzymes in electron transfer chains) (Dixon 1999; Li and others 2002; Rastogi and others 2014). Nevertheless, the production of mono- and sesquiterpene oxides occurs almost synchronously with their corresponding monoterpene or sesquiterpene substrates (Bell and others 2001), explaining classification of these compounds with their corresponding substrate molecules (e.g. α-pinene oxide with α-pinene in clade 1, Fig. 7A).Combining the results of variable compositions of monoterpenoids and sesquiterpenoids after 10 h of MeJA treatment with cluster analysis, we hypothesize that the down-regulated expression of monoterpene synthase (Mono TPS 1), producing cyclic α-pinene and up-regulated expression of monoterpene synthase (Mono TPS 3), producing acyclic linalool and β-ocimene mainly contribute to the variation in the composition of monoterpenoid compounds. Typically, linalool and β-ocimene are the key stress-elicited monoterpenes (Crowell and others 2002; Martin and others 2003; Li and others 2007). Similarly, down-regulated expression of sesquiterpene synthases (SesTPS 2 and SesTPS 3) with trans-α-bergamotene and cubenol as the dominant products and up-regulated expression of sesquiterpene synthase (SesTPS 1) with dominant products of characteristic stress-sesquiterpenes α-caryophyllene and β-farnesene may contribute to the changes in the composition of sesquiterpenoid compounds in floral volatile blend after 10 h of MeJA treatment.
Conclusion
Studies on the regulation of emission and biosynthesis of floral terpenoids are important for selection of optimum time periods and tissue fractions for collection of floral volatiles. We have used O. basilicumas a model to elucidate the profile, spatial distribution, kinetics and MeJA elicitation of emissions of floral volatiles by dynamic headspace collection and identification using gas chromatography mass spectrometry (GC-MS) and proton-transfer reaction mass spectrometry (PTR-MS). We separated the sources of flower volatile production among different flower parts and demonstrated important diurnal and MeJA-dependent regulations. Based on hierarchical cluster analyses, a putative genetic structure of floral terpenoid biosynthetic pathway was proposed to explain the diversity and complexity of floral volatiles in O. basilicum. Future molecular studies isolating and functionally characterizing key TPS genes expressed in flowers of O. basilicum are needed to confirm the postulated genetic basis of regulation of emission profiles upon stress. Such studies would facilitate modification of the volatile composition by genetic approaches and create novel cultivars with milder or stronger scents.
Acknowledgements
This study has been possible with the funds provided by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation), the European Research Council (advanced grant 322603, SIP-VOL+).
References
- Abdollah GP, Seyed ES, Keykavous P. A review (Research and Patents) on jasmonic acid and its derivatives. Arch Pharm Chem Life Sci. 2014;347:229–239. doi: 10.1002/ardp.201300287. [DOI] [PubMed] [Google Scholar]
- Abdollah GP, Elahe M, Lyle C. Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem. 2013;141:2440–2449. doi: 10.1016/j.foodchem.2013.05.098. [DOI] [PubMed] [Google Scholar]
- Ameneh A, Mahdi M, Bohloul A, Shouresh A, Ali K. Comparison of essential oil from leaves and inflorescence of three basil (Ocimum basilicum L.) populations under drought stress. Int J Plant Prod. 2013;4(10):2764–2767. [Google Scholar]
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou A, Feussner I. Lipoxygenases-structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Andrews ES, Theis N, Adler LS. Pollinator and herbivore attraction to Cucurbita floral volatiles. J Chem Ecol. 2007;33:1682–1691. doi: 10.1007/s10886-007-9337-7. [DOI] [PubMed] [Google Scholar]
- Baritaux O, Richard H, Touche J, Derbesy M. Effects of drying and storage of herbs and spices on the essential oil. Part I. Basil, Ocimum basilicum L. Flavour Frag J. 1992;7:267–271. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant Cell Environ. 2005;28:1334–1343. [Google Scholar]
- Bell GS, Sowden JR, Wong LL. Engineering the haem monooxygenase cytochrome P450 cam for monoterpene oxidation. Chem Commun. 2001;7:635–636. [Google Scholar]
- Bourtsoukidis E, Williams J, Kesselmeier J, Jacobi S, Bonn B. From emissions to ambient mixing ratios: online seasonal field measurements of volatile organic compounds over a Norway spruce-dominated forest in central Germany. Atmos Chem Phys. 2014;14:6495–6510. [Google Scholar]
- Brilli F, Hörtnagl L, Bamberger I, Schnitzhofer R, Ruuskanen TM, Hansel A, Loreto F, Wohlfahrt G. Qualitative and quantitative characterization of volatile organic compound emissions from cut grass. Environ Sci Technol. 2012;46:3859–3665. doi: 10.1021/es204025y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction ‘time-of-flight’ mass spectrometry (PTR-TOF) PLoS ONE. 2011;6(5):e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calín-Sánchez Á, Lech K, Szumny A, Figiel A, Carbonell-Barrachina ÁA. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res Int. 2012;48:217–225. [Google Scholar]
- Calogirou A, Larsen BR, Brussol C, Duane M, Kotzias D. Decomposition of terpenes by ozone during sampling on Tenax. Anal Chem. 1996;68:1499–1506. doi: 10.1021/ac950803i. [DOI] [PubMed] [Google Scholar]
- Carovič-Stanko K, Orlič S, Politeo O, Strikič F, Kolak I, Milos M, Zlatko S. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010;119(1):196–201. [Google Scholar]
- Chalchat JC, Özcan MM. Comparative essential oil composition of flowers, leaves and stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008;110(2):501–503. doi: 10.1016/j.foodchem.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signaling compounds in three tree species with differing waterlogging tolerance. Plant Cell and Environ. 2010;33:582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Al-Ahmad H, Joyce B, Zhao N, Kollner TG, Degenhardt J, Stewart CN. Within-plant distribution and emission of sesquiterpenes from Copaifera officinalis. Plant Physiol Biochem. 2009;47:1017–1023. doi: 10.1016/j.plaphy.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Crowell AL, Williams DC, Davis EM, Wildung MR, Croteau R. Molecular cloning and characterization of a new linalool synthase. Arch Biochem and Biophys. 2002;405:112–121. doi: 10.1016/s0003-9861(02)00348-x. [DOI] [PubMed] [Google Scholar]
- Darmais S, Dutaur L, Larsen B, Cieslik S, Luchetta L, Simon V, Torres L. Emission fluxes of VOC by orange trees determined by both relaxed eddy accumulation and vertical gradient approaches. Chemosphere: Global Change Science. 2000;2:47–56. [Google Scholar]
- Degenhardt DC, Lincoln DE. Volatile emissions from an odorous plant in response to herbivory and methyl jasmonate exposure. J Chem Ecol. 2006;32:725–743. doi: 10.1007/s10886-006-9030-2. [DOI] [PubMed] [Google Scholar]
- Díaz-Maroto CM, Palomo SE, Castro L, Viñas GMA, Pérez-Coello SM. Changes produced in the aroma compounds and structural integrity of basil (Ocimum basilicum L.) during drying. J Sci Food Agr. 2004;84(15):2070–2076. [Google Scholar]
- Dinh ST, Baldwin IT, Galis I. The HERBIVORE ELICITOR-REGULATED1 (HER1) gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol. 2013;162:2106–2124. doi: 10.1104/pp.113.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA. Plant natural products: the molecular genetic basis of biosynthetic diversity. Curr Opin Biotech. 1999;10:192–197. doi: 10.1016/s0958-1669(99)80034-2. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell. 2000;12:949–961. doi: 10.1105/tpc.12.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol1. 2000;22:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J, Croteau RB. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA. 2005;102:933–938. doi: 10.1073/pnas.0407360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Faldt J, Miller B, Bohlmann J. (E)-β-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell. 2003;15:1227–1241. doi: 10.1105/tpc.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré-Armengol G, Filella I, Llusià J, Niinemets Ü, Peñuelas J. Changes in floral bouquets from compound-specific responses to increasing temperatures. Global Change Biol. 2014;20:3660–3669. doi: 10.1111/gcb.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré-Armengol G, Filella I, Llusià J, Niinemets Ü, Peñuelas J. Optimum temperature for floral terpene emissions tracks the mean temperature of the flowering season. Funct Plant Biol. 2015 doi: 10.1071/FP14279. (In Press) [DOI] [PubMed] [Google Scholar]
- Flinn CL, Murray R, Simon JE. Anatomical investigation of essential of glands and their distribution in Ocimum basilicum varieties. HortScience. 1993;28(5):531. [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Hudgins JW, Christiansen E, Franceschi VR. Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic perspective. Tree Physiol. 2004;24:251–264. doi: 10.1093/treephys/24.3.251. [DOI] [PubMed] [Google Scholar]
- Iijima Y, Gang RD, Fridman E, Lewinsohn E, Pichersky E. Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol. 2004a;134:370–379. doi: 10.1104/pp.103.032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y, Davidovich RR, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E. The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol. 2004b;136:3724–3736. doi: 10.1104/pp.104.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant isoprenoids: methods and protocols. Humana Press; New York: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Keeling CI, Weisshaar S, Ralph SG, Jancsik S, Hamberger B, Dullat HK, Bohlmann J. Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.) BMC Plant Biol. 2011;11:43. doi: 10.1186/1471-2229-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenda M, Koppmann R. Monoterpene emissions from Scots pine (Pinus sylvestris): field studies of emission rate variabilities. J Geophys Res. 2002;107:4161. [Google Scholar]
- Li XC, Schuler MA, Berenbaum MR. Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature. 2002;419:712–715. doi: 10.1038/nature01003. [DOI] [PubMed] [Google Scholar]
- Li ZG, Wang X, Chen F, Kim HJ. Chemical changes and overexpressed genes in sweet basil (Ocimum basilicum L.) upon methyl jasmonate treatment. J Agr Food Chem. 2007;55:706–713. doi: 10.1021/jf062481x. [DOI] [PubMed] [Google Scholar]
- Liavonchanka A, Feussner N. Lipoxygenases: occurrence, functions and catalysis. Journal Plant Physiol. 2006;163:348–357. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Jiang YF, Zhao N, Wang F, Chen F. Emission and regulation of volatile chemicals from globe amaranth flowers. J Amer Soc Hort Sci. 2011a;136:16–22. [Google Scholar]
- Jiang YF, Chen XL, Lin H, Wang F, Chen F. Floral scent in Wisteria: chemical composition, emission pattern, and regulation. J Amer Soc Hort Sci. 2011b;136(5):307–314. [Google Scholar]
- Kessler A, Halitschke R, Poveda K. Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant–pollinator interactions. Ecology. 2011;92(9):1769–1780. doi: 10.1890/10-1945.1. [DOI] [PubMed] [Google Scholar]
- Kong Y, Sun M, Pan HT, Zhang QX. Composition and emission rhythm of floral scent volatiles from eight lily cut flowers. J Amer Soc Hort Sci. 2012;137(6):376–382. [Google Scholar]
- Kwee EM, Niemeyer ED. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011;128(4):1044–1050. [Google Scholar]
- Lee SJ, Umano K, Shibamoto T, Lee KG. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. [Google Scholar]
- Martin DM, Gershenzon J, Bohlmann J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003;132(3):1586–1599. doi: 10.1104/pp.103.021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010;7:2203–2223. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noge K, Abe M, Tamogami S. Phenylacetonitrile from the giant knotweed, Fallopiasachalinensis, infested by the Japanese beetle, Popillia japonica, is induced by exogenous methyl jasmonate. Molecules. 2011;16:6481–6488. doi: 10.3390/molecules16086481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormeño E, Fernandez C, Mévy JP. Plant coexistence alters terpene emission and content of Mediterranean species. Phytochemistry. 2007;68:840–852. doi: 10.1016/j.phytochem.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Ormeño E, Goldstein A, Niinemets Ü. Extracting and trapping biogenic volatile organic compounds stored in plant species. Trend Anal Chem. 2011;30:978–989. [Google Scholar]
- Peng J, Van LJJ, Zheng S, Dicke M. Herbivore-induced volatiles of cabbage (Brassica oleracea) prime defence responses in neighbouring intact plants. Plant Biology. 2011;13:276–284. doi: 10.1111/j.1438-8677.2010.00364.x. [DOI] [PubMed] [Google Scholar]
- Pereira ALC, Taques TC, Valim JOS, Madureira NP, Campos WG. The management of bee communities by intercropping with flowering basil (Ocimum basilicum) enhances pollination and yield of bell pepper (Capsicum annuum) J Insect Conserv. 2015;19:479–486. [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Rastogi S, Meena S, Bhattacharya A, Ghosh S, Shukla KR, Sangwan SN, Lal KR, Gupta MM, Lavania CU, Gupta V, Nagegowda AD, et al. De novo sequencing and comparative analysis of holy and sweet basil transcriptomes. BMC Genomics. 2014;15:588. doi: 10.1186/1471-2164-15-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Crafts-Brander SJ, Pare PW, Henneberry TJ. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J Chem Ecol. 2001;27:679–695. doi: 10.1023/a:1010393700918. [DOI] [PubMed] [Google Scholar]
- Sajjadi SE. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. DARU J Pharml Sci. 2006;14(3):128–130. [Google Scholar]
- Schürmann W, Ziegler H, Kotzias D, Schönwitz R, Steinbrecher R. Emission of biosynthesized monoterpenes from needles of Norway spruce. Naturwissenschaften. 1993;80:276–278. [Google Scholar]
- Semiz G, Blande JD, Heijari J, Işik K, Niinemets Ü, Holopainen JK. Manipulation of VOC emissions with methyl jasmonate and carrageenan in the evergreen conifer Pinus sylvestris and evergreen broadleaf Quercus ilex. Plant Biol. 2012;14:57–65. doi: 10.1111/j.1438-8677.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- Soran ML, Stan M, Niinemets Ü, Copolovici L. Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J Plant Physiol. 2014;171:1436–1443. doi: 10.1016/j.jplph.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher R. Gehalt und Emission von Monoterpenene in oberirdischen Organen von Picea abies (L.) Karst. Dr. rer. Nat. Thesis, Institut für Botanik und Mikrobiologie, Lehrstuhl für Botanik der Technischen Universität München; 1989. [Google Scholar]
- Tarvainen V, Hakola H, Hellén H, Bäck J, Hari P, Kulmala M. Temperature and light dependence of the VOC emissions of Scots pine. Atmos Chem and Phys. 2005;5:989–998. [Google Scholar]
- Theis N. Fragrance of Canada thistle (Cirsium arvense) attracts both floral herbivores and pollinators. J Chemical Ecol. 2006;32:917–927. doi: 10.1007/s10886-006-9051-x. [DOI] [PubMed] [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe MS. Leaf rust induced volatile organic compounds signaling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Veromann E, Toome M, Kännaste A, Kaasik R, Copolovici L, Flink J, Kovács G, Narits L, Luik A, Niinemets Ü. Effects of nitrogen fertilization on insect pests, their parasitoids, plant diseases and volatile organic compounds in Brassica napus. Crop Prot. 2013;43:79–88. [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT. Comparisons of LIPOXYGENASE3 and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiol. 2008;146:904–915. doi: 10.1104/pp.107.109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegge W, Weldegergis BT, Soler R, Vergeer-Van Eijk M, Dicke M, Voesenek LA, Pierik R. Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol. 2013;200:861–874. doi: 10.1111/nph.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZZ, Kapteyn J, Gang DR. A systems biology investigation of the MEP/terpenoid and shikimate/phenylpropanoid pathways points to multiple levels of metabolic control in sweet basil glandular trichomes. Plant J. 2008;54:349–361. doi: 10.1111/j.1365-313X.2008.03429.x. [DOI] [PubMed] [Google Scholar]
- Yousif AN, Scaman CH, Durance TD, Girard B. Flavor volatiles and physical properties of vacuum-microwave- and air-dried sweet basil (Ocimum basilicum L.) J Agr Food Chem. 1999;47(11):4777–4781. doi: 10.1021/jf990484m. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Dicke M. Ecological genomics of plant-insect interactions: from gene to community. Plant Physiol. 2008;146:812–817. doi: 10.1104/pp.107.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang XF, Klingeman WE, Hu J, Chen F. Emission of volatile chemicals from flowering dogwood (Cornus florida L.) flowers. J Agr Food Chem. 2008;56:9570–9574. doi: 10.1021/jf801651v. [DOI] [PubMed] [Google Scholar]