Abstract
Hybridization probes have been intensively used for nucleic acid analysis in medicine, forensics and fundamental research. Instantaneous hybridization probes (IHPs) enable signalling immediately after binding to a targeted DNA or RNA sequences without the need to isolate the probe-target complex (e. g. by gel electrophoresis). The two most common strategies for IHP design are conformational switches and split approach. A conformational switch changes its conformation and produces signal upon hybridization to a target. Split approach uses two (or more) strands that independently or semi independently bind the target and produce an output signal only if all components associate. Here, we compared the performance of split vs switch designs for deoxyribozyme (Dz) hybridization probes under optimal conditions for each of them. The split design was represented by binary Dz (BiDz) probes; while catalytic molecular beacon (CMB) probes represented the switch design. It was found that BiDz were significantly more selective than CMBs in recognition of single base substitution. CMBs produced high background signal when operated at 55°C. An important advantage of BiDz over CMB is more straightforward design and simplicity of assay optimization.
Keywords: Deoxyribozyme sensors, split hybridization probes, Catalytic molecular beacons, single nucleotide polymorphisms, instantaneous hybridization probes, fluorescent sensors
Hybridization probes for nucleic acid analysis have been used since 1961.[1] The approach takes advantage of selective recognition of a targeted nucleic acid fragment of known sequence by short complementary DNA or RNA probes. The resulting hybrid can be detected by a variety of techniques including blot, fluorescent in situ hybridization or gel electrophoresis. One major step in the evolution of hybridization probes is the development of instantaneous hybridization probes (IHPs) that can produce a signal (e. g. fluorescence) immediately upon hybridization to the target.[2] IHPs eliminate the need for time-consuming downstream analysis of hybridization mixtures, like gel-based separation of probe-analyte complex from the excess amount of the unbound probe. Most common designs for IHPs are conformational switches or split (multicomponent design).[3] Switch IHPs (e. g. molecular beacon probes[4] and their variations[4b,5]) change conformations upon hybridization to the target sequence and produce a signal. Split probes (e. g. adjacent hybridization probes[6]) consist of several parts, which produce output signal when all the components and the analyzed sequence form an associate.[7] In this study, we directly compare the performance of the two designs.
One of the most promising IHPs are deoxyribozyme (DZ)-based probes, for which both the switch[11] and the split[12] designs were explored earlier. DZs are short catalytic DNA sequences derived by in vitro selection.[13] Most of the DZs used in analytical applications catalyze cleavage of RNA phosphodiester bonds,[13] which can be conveniently converted to a fluorescent response, as shown in Scheme 1A: DZ binds a fluorophore- and a quencher-labeled DNA/RNA chimera substrate (F_sub) and cleaves the internal RNA phosphodiester bond (Scheme 1A), thus separating the fluorophore from the quencher. We and others take advantage of RNA-cleaving DZ in developing IHPs.[14] One major advantage of DZ probes over other IHP (e. g. MB probes) is improved sensitivity and reduced limits of detection (LOD) due to the catalytic amplification of the output signal: one analyte bound to a DZ probe triggers cleavage of multiple F_sub molecules (Scheme 1A). Another advantage is that expensive F_sub can be used for multiple analytes, given that DZa and DZb are adjusted to each new analyte sequence, which reduces the cost if multiple sequences need to be analyzed.
Scheme 1.

Design of deoxyribozyme (DZ) probes that produce fluorescent signal upon hybridization to specific nucleic acid analytes. A) Parent RNA-cleaving DZ can cleave a fluorophore- and quencher-labelled substrate (F_ sub), thus producing high fluorescence. B) Switch design for DZ hybridization probes: the catalytic core and/or substrate binding arms of DZ are inactivated by binding to the ‘inhibitory fragment’; hybridization of the analyte to the analyte binding domain releases the substrate binding arms of the DZ construct and enables cleavage of F_sub; C) Split design: two DNA strands DZa and DZb hybridize to the analyte sequence and form catalytically active DZ, which cleaves F_sub.
The allosteric switch DZ probe named in the original publication ‘catalytic molecular beacon’[11] (CMB) is shown in Scheme 1B. The CMB design uses a monolith construct, in which the DZ catalytic site is inhibited by hybridization within the complementary fragment: the strategy akin to the intrasteric regulation in proteins.[15] Binding of the complementary DNA/RNA analyte reduces the inhibition and releases the catalytic core, which cleaves fluorogenic substrate (F_sub) and produces the detectable signal.[11] In the split strategy, the parent DZ is divided into two parts with analyte binding arms appended to each part (DZa and DZb in Scheme 1C).[12] In the presence of a complementary analyte, the two strands assemble the catalytic site, which cleaves F_sub followed by increase in fluorescence. In this study, we compared the two designs in terms of selectivity (the ability to differentiate analytes with a single nucleotide substitution (SNS)), limit of detection (LOD) and optimization simplicity. We assume that any two sensors can be compered using the aforementioned parameters demonstrated by each sensor under near optimal conditions. Up to the best of our knowledge, this is the first direct experimental comparison of the two designs.
The following analytes were used in this study (Table S1): T1–21 and T1–86 represented 21 and 86 nucleotide-long fragments of the TWIST1 gene coding for a transcription factor involved in cancer development.[16] For testing selectivity of DZ sensors, SNS sites were introduced into the analytes to make T2–21 and T2–86. The second target, A1–70 represents the sequence from amelogenin gene and is used as a genetic marker for sex determination.[17] A2–70 analyte contained a SNS for selectivity study. The choice of the two practically significant but otherwise random targets would ensure more general applicability of the conclusions made in this study. We designed two variants of CMB and BiDz probes based on the most catalytically efficent deoxyribozyme 10–23[18,14f] - one designed to recognize the analyte at 30°C, another optimal at 55°C, indiacted by numblers 1 and 2, respectively. Lower temperature was chosen to compare our results with the earlier reported data collected under similar conditions[4,5] and because of the importance of point-of-care diagnostic assays operating at ambient temperature.[19] Both switch and split designs were accomplished according to the strategies reported earlier for optimal performance of each design under given conditions.[11,12–14]
For detecting TWIST1 gene-related analytes at 30°C, we tested three CMB constructs (CMB_T1, CMB_T1_a, and CMB_ T1_b see Table S1) with diffrent lengths of the inhibitory fragments, which was progressively elongated to suppress CMB response in the absence of T1 analyte. The three CMB probes were also HPLC purified prior testing to reduce the undesired backgroud response. The initially designed CMB_T1_a, and CMB_T1_b with short inhibitory fragments demostrated high bacgkground response in the absence of analyte (Figures S1 and S2). Finally, CMB_T1 produced low background fluorescence and signal-to-backgroung ratio (S/B) of ~ 3 after 1 hr of incubation with 5 nM analyte (Figure 1 A). Importantly, the differentiation of single base mismatched analyte T2 was only moderate (Figure 1 A, bar graphs), which was quantified using the selectivity factor (SF, Table 1). Importenty, CMB_T1 recognized short T1–21 analyte with greater selectivity than the 40 nucleotide-long fragment of T1–86, which is in agreement with the affinity-specificity dilemma described by Demidov and Frank-Kmenetskii.[19] This data also correlates well with earlier observations that CMB constructs can unambiguously discriminate only analytes with two mismatched bases, but not with SNS.[11a,14a] In contrast, BiDZ probe demostrated near perfect selectivity (Figure 1B). Importantly, no HPLC purification of DZa_ T1 and DZb_T1 was needed, since contamiantion with shorter DNA products arising form incomplete oligonucleotide synthesis do not contribute to the elevated background as in the case of CMB design. This further reduces the sythetic cost of BiDz constructs. Furthermore, the low background for BiDZ can be achieved by optimizing the concentrations of DZa and DZb strands: we achieved near-optimal performance of the initially designed DZa_T1 and DZb_T1 simply by reducing their concentrations to 5 nM. Similar probe design and optimizations were performed for CMB and BiDZ probes that recognize A1–70 and A2–70 analyes. The obtained results demostrate similar trend in terms of probe selectivity and LODs (Table 1 and Figures S9, S10).
Figure 1.
Predicted structures of the probe-analyte complexes and selectivity data for CMB (A) and BiDZ (B) recognizing TWIST1 gene-related analytes at 30°C. For structures: catalytic core nucleotides are in green; single base substitution site is red underlined; ribonucleotides are in low case. For right panels: all samples contained 200 nM F_ sub and either 5 nM CMB_T1 or 5 nM each DZa_T1 and DZb_T1 in the reaction buffer: 50 mM HEPES, 50 mM MgCl2, 20 mM KCl, 120 mM NaCl, 0.03% Triton X-100, 1 % DMSO. Samples T1 and T2 contained 5 nM of fully complementary T1–21, T–86 or 5 nM single base mismatched T2–21 or T2–86 analytes, respectively (for full sequences see Table S1). Fluorescence intensity at 517 nm (emission at 485 nm) was measured after 1 hr of incubation. The data is average values of 3 independent experiments.
Table 1.
Selectivity factor (SF) and limits of detection (LOD) for DZ probe used in this study
| Analytes | T°C | Assay time, hr | SF | LOD, pM | |
|---|---|---|---|---|---|
| CMB | T1–86/T2–86 | 30 | 1 | 0.31 | 89 |
| 3 | 80 | ||||
| T1–86/T2–86 | 55 | 1 | 0.38 | 31 | |
| 3 | ND[c] | ||||
| A1–80/A2–80 | 30 | 1 | 0.18 | 74 | |
| 3 | 59 | ||||
| A1–80/A2–80 | 55 | 1 | 0.07 | ND | |
| 3 | ND | ||||
| BiDZ | T1–86/T2–86 | 30 | 1 | 0.83 | 164 |
| 3 | 26 | ||||
| T1–86/T2–86 | 55 | 1 | 0.65 | 133 | |
| 3 | 98 | ||||
| A1–80/A2–80 | 30 | 1 | 0.84 | 47 | |
| 3 | 15 | ||||
| A1–80/A2–80 | 55 | 1 | 0.89 | 11 | |
| 3 | 8 |
SF was calculated according to the formula: SF=1−Fmm/Fm, where Fmm and Fm are average fluorescence of samples in the presence of mismatched and fully matched analytes, respectively, using data presented in Figure S1–S5).
LOD was determined by 3 individual trials after at 1 and 3 hrs or assay (Figure S6–S9).
ND - the LOD was not determined due to the high background signal.
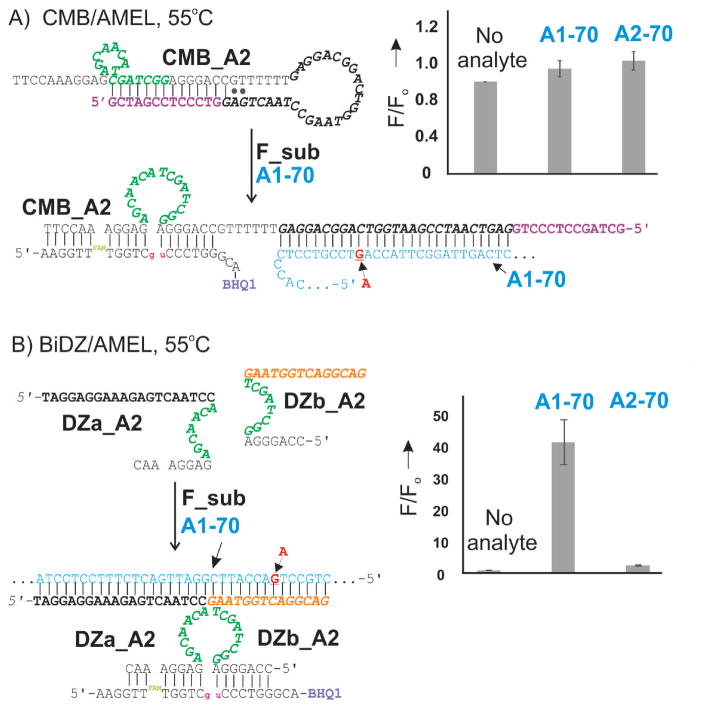
It was shown earlier that BiDZ based on deoxyribozyme 10–23 has higher catalytic rates and lower LODs at elevated tempartures.[14f] We, therefore, designed CMB and BiDZ probes for the detection of T1–86 and A1–70 analytes at 55°C. For both BiDZ and CMB designs, substrate-binding arms were elongated by 3 nucleotides each to enable binding of F_sub by the DZ constructs even at 55°C. Figure 2 A demonstrates the design and the selectivity data for one of the CMB construct, CMB_A2. Importantly, the probe experienced leakage, as reflected by the high background (Figure 2B, ‘no analyte’ bar). We attempted to redesign the CMB with increased length of the inhibitory fragments to increase the stability of the folded structure in the absence of the analyte, but without success (Figure S3 and S4). As a result of the high background, the signal for both the matched and mismatched analytes was only slightly above the background; the differentiation between fully matched A1–70 and SNS containing A2–70 was not observed (Figure 2 A, bar graph, Table 1). The BiDZ probe, on the other hand, demonstrated high analyte-dependent turn-on ratio (S/B), as well as impressive selectivity (see SF in Table 1). The LOD of 8 pM was achieved after 3 hrs of incubation, which is consistent with the previously reported data.[14f,j] The lowest LOD for CMB was about 7-fold higher than the best value for BDZ. Similar design, optimization experiments and results were obtained for TWIST1 gene-related analytes and their corresponding sensors (Table 1 and Figure S11, S12).
Figure 2.
Predicted structures of probe-analyte complexes and selectivity data for CMB_A2 (A) and BiDZ_A2 (B) recognizing A1–70 analyte at 55°C. For right panels: all samples contained 200 nM F_ sub and either 5 nM CMB or 5 nM DZa_T and 5 nM DZb_T in the reaction buffer. Samples A1 and A2 contained 1 nM complementary A1 and 1 nM mismatched A2 analyte, respectively (for full sequences see Table S1). Fluorescence intensity at 517 nm (emission at 485 nm) was measured after 1 hr of incubation. The data are average values of 3 independent experiments.
Among the enzyme-free IHP approaches, switch probes have received the most attention for the last 20 years and now dominate in majority of applications and the original developments.[2,4,5] For example, allosteric switch probes based on spinach aptamer have been reported recently.[21] In parallel, binary and multicomponent probes have been introduced.[6,7,22] In this study, we directly compared the two designs using one of the most promising IHPs, DZ probes as a representative example. Our main conclusions are the following. (i) The detection limits for both design are comparable and consistent with the data published by different research groups. (ii) Split design is simpler and less expensive to optimize. (iii) Switch probes fail to work at 55°C. (iv) Split probes have significantly high selectivity toward single base substitutions at both 30°C and 55°C. Our results taken together with the prior observations strongly suggest that split is advantageous over switch design especially if SNS differentiation is required. At the same time, switch probes can still be useful if the formation of multistranded associates is hampered by a crowded environment e. g. found inside living cells. In this case the monolith design can facilitate sensor response.
Supplementary Material
Acknowledgments
Funding from NIH (R15AI10388001 A1) and NSF CCF (1423219) is greatly appreciated.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting information for this article is available on the WWW under https://doi.org/10.1002/slct.201701179
References
- 1.Hall BD, Spiegelman S. Proc Natl Acad Sci U S A. 1961;47:137. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Navarro E, Serrano-Heras G, CastaÇo MJ, Solera J. Clin Chim Acta. 2015;15:231–250. doi: 10.1016/j.cca.2014.10.017. [DOI] [PubMed] [Google Scholar]; b) Armitage BA. Curr Opin Chem Biol. 2011;15:806–812. doi: 10.1016/j.cbpa.2011.10.006. [DOI] [PubMed] [Google Scholar]; c) Østergaard ME, Hrdlicka PJ. Chem Soc Rev. 2011;40:5771–5788. doi: 10.1039/c1cs15014f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Khakshoor O, Kool ET. Chem Commun. 2011;47:7018–7024. doi: 10.1039/c1cc11021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerasimova YV, Kolpashchikov DM. Chem Biol. 2010;17:104–106. doi: 10.1016/j.chembiol.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Tyagi S, Kramer F. R Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]; b) Kolpashchikov DM. Scientifica. 2012;2012:928783. doi: 10.6064/2012/928783. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zheng J, Yang R, Shi M, Wu C, Fang X, Li Y, Li J, Tan W. Chem Soc Rev. 2015;44:3036–3055. doi: 10.1039/c5cs00020c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Wang Q, Chen L, Long Y, Tian H, Wu J. Theranostics. 2013;3:395–408. doi: 10.7150/thno.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Junager NP, Kongsted J, Astakhova K. Sensors (Basel) 2016;16 doi: 10.3390/s16081173. pii: E1173. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kuang T, Chang L, Peng X, Hu X, Gallego-Perez D. Trends Biotechnol. 2016 doi: 10.1016/j.tibtech.2016.09.003. pii: S0167-7799(16)30152-4. [DOI] [PubMed] [Google Scholar]
- 6.a) Cardullo RA, Agrawal S, Flores C, Zamecnik PC, Wolf DE. Proc Natl Acad Sci U S A. 1988;85:8790–8794. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo J, Ju J, Turro NJ. Anal Bioanal Chem. 2012;402:3115–3125. doi: 10.1007/s00216-011-5526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dark P, Blackwood B, Gates S, McAuley D, Perkins GD, McMullan R, Wilson C, Graham D, Timms K, Warhurst G. Intensive Care Med. 2015;41:21–33. doi: 10.1007/s00134-014-3553-8. [DOI] [PubMed] [Google Scholar]; d) Didenko VV. Biotechniques. 2001;31:1106–1116. doi: 10.2144/01315rv02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolpashchikov DM. Chem Rev. 2010;110:4709–4723. doi: 10.1021/cr900323b. [DOI] [PubMed] [Google Scholar]
- 8.a) Breaker RR. Nature. 2004;432:838–845. doi: 10.1038/nature03195. [DOI] [PubMed] [Google Scholar]; b) Schlosser K, Li Y. Chem Biol. 2009;16:311–322. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]; c) Silverman SK. Trends Biochem Sci. 2016;41:595–609. doi: 10.1016/j.tibs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Xiang Y, Wu P, Tan LH, Lu Y. Adv Biochem Eng Biotechnol. 2014;140:93–120. doi: 10.1007/10_2013_242. [DOI] [PubMed] [Google Scholar]; b) Lan T, Lu Y. Met Ions Life Sci. 2012;10:217–248. doi: 10.1007/978-94-007-2172-2_8. [DOI] [PubMed] [Google Scholar]; c) Willner I, Shlyahovsky B, Zayats M, Willner B. Chem Soc Rev. 2008;37:1153–1165. doi: 10.1039/b718428j. [DOI] [PubMed] [Google Scholar]; d) Zhang H, Li F, Dever B, Li XF, Le XC. Chem Rev. 2013;113:2812–2841. doi: 10.1021/cr300340p. [DOI] [PubMed] [Google Scholar]
- 10.a) Stojanovic MN, de Prada P, Landry DW. Nucleic Acids Res. 2000;28:2915–2918. doi: 10.1093/nar/28.15.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Singh KK, Parwaresch R, Krupp G. RNA. 1999;5:1348–1356. doi: 10.1017/s1355838299991185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stojanovic MN, de Prada P, Landry DW. Chembiochem. 2001;2:411–415. doi: 10.1002/1439-7633(20010601)2:6<411::AID-CBIC411>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Kolpashchikov DM. Chembiochem. 2007;8:2039–2042. doi: 10.1002/cbic.200700384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Silverman SK. Trends Biochem Sci. 2016;41:595–609. doi: 10.1016/j.tibs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McManus SA, Li Y. Molecules. 2010;15:6269–6284. doi: 10.3390/molecules15096269. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Emilsson GM, Breaker RR. Cell Mol Life Sci. 2002;59:596–607. doi: 10.1007/s00018-002-8452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ward WL, Plakos K, DeRose VJ. Chem Rev. 2014;114:4318–4342. doi: 10.1021/cr400476k. [DOI] [PMC free article] [PubMed] [Google Scholar]; a) Lan T, Lu Y. Met Ions Life Sci. 2012;10:217–248. doi: 10.1007/978-94-007-2172-2_8. [DOI] [PubMed] [Google Scholar]; b) Schlosser K, Li Y. Chem Biol. 2009;16:311–322. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 14.a) Sando S, Sasaki T, Kanatani K, Aoyama Y. J Am Chem Soc. 2003;125:15720–15721. doi: 10.1021/ja0386492. [DOI] [PubMed] [Google Scholar]; b) Tian Y, Mao C. Talanta. 2005;67:532–537. doi: 10.1016/j.talanta.2005.06.044. [DOI] [PubMed] [Google Scholar]; c) Song P, Xiang Y, Xing H, Zhou Z, Tong A, Lu Y. Anal Chem. 2012;84:2916–2922. doi: 10.1021/ac203488p. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Xu J, Li H, Wu ZS, Qian J, Xue C, Jia L. Theranostics. 2016;6:318–327. doi: 10.7150/thno.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fu R, Li T, Lee SS, Park HG. Anal Chem. 2011;83:494–500. doi: 10.1021/ac102719x. [DOI] [PubMed] [Google Scholar]; f) Mokany E, Bone SM, Young PE, Doan TB, Todd AV. J Am Chem Soc. 2010;132:1051–1059. doi: 10.1021/ja9076777. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Gerasimova YV, Cornett E, Kolpashchikov DM. ChemBioChem. 2010;11:811–817. doi: 10.1002/cbic.201000006. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Ruble BK, Richards JL, Cheung-Lau JC, Dmochowski IJ. J Inorg Biochem. 2012;380:386–391. doi: 10.1016/j.ica.2011.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Zagorovsky K, Chan WCW. Angew Chem Int Ed. 2013;52:3168–3171. doi: 10.1002/anie.201208715. [DOI] [PubMed] [Google Scholar]; j) Gerasimova YV, Kolpashchikov DM. Angew Chem Int Ed Engl. 2013;52:10586–10588. doi: 10.1002/anie.201303919. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Gerasimova YV, Cornett EM, Edwards E, Su X, Rohde KH, Kolpashchikov DM. Chembiochem. 2013;14:2087–2090. doi: 10.1002/cbic.201300471. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Bone SM, Hasick NJ, Lima NE, Erskine SM, Mokany E, Todd AV. Anal Chem. 2014;86:9106–9113. doi: 10.1021/ac501811r. [DOI] [PubMed] [Google Scholar]; m) Gerasimova YV, Yakovchuk P, Dedkova LM, Hecht SM, Kolpashchikov DM. RNA. 2015;21:1834–1843. doi: 10.1261/rna.052613.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Zhang L, Zhu J, Li T, Wang E. Anal Chem. 2011;83:8871–8876. doi: 10.1021/ac2006763. [DOI] [PubMed] [Google Scholar]; o) Cox AJ, Bengtson HN, Gerasimova YV, Rohde KH, Kolpashchikov DM. Chembiochem. 2016;21:2038–2041. doi: 10.1002/cbic.201600438. [DOI] [PMC free article] [PubMed] [Google Scholar]; p) Cox AJ, Bengtson HN, Rohde KH, Kolpashchikov DM. Chem Commun. 2016;52:14318–14321. doi: 10.1039/c6cc06889h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobe B, Kemp BE. Nature. 1999;402:373–376. doi: 10.1038/46478. [DOI] [PubMed] [Google Scholar]
- 16.Dollfus H, Kumaramanickavel G, Biswas P, Stoetzel C, Quillet R, Denton M, Maw M, Perrin-Schmitt F. J Med Genet. 2001;38:470–472. doi: 10.1136/jmg.38.7.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Nakahori Y, Takenaka O, Nakagome Y. Genomics. 1991;9:264–269. doi: 10.1016/0888-7543(91)90251-9. [DOI] [PubMed] [Google Scholar]; b) Frances O, Portol3s F, GonzQlez JI, Coltell O, Verdú F, Castell+ A, Corella D. Clin Chim Acta. 2007;386:53–56. doi: 10.1016/j.cca.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 18.a) Santoro SW, Joyce GF. Proc Natl Acad Sci U S A. 1997;94:4262–4262. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Santoro SW, Joyce GF. Biochemistry. 1998;37:13330–13342. doi: 10.1021/bi9812221. [DOI] [PubMed] [Google Scholar]
- 19.Demidov VV, Frank-Kamenetskii MD. Trends Biochem Sci. 2004;29:62–71. doi: 10.1016/j.tibs.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 20.a) Henihan G, Schulze H, Corrigan DK, Giraud G, Terry JG, Hardie A, Campbell CJ, Walton AJ, Crain J, Pethig R, Templeton KE, Mount AR, Bachmann TT. Biosens Bioelectron. 2016;81:487–494. doi: 10.1016/j.bios.2016.03.037. [DOI] [PubMed] [Google Scholar]; b) Alladin-MustaN BS, Mitran CJ, Gibbs-Davis JM. Chem Commun. 2015;51:9101–9104. doi: 10.1039/c5cc01548k. [DOI] [PubMed] [Google Scholar]; c) Du TE, Wang Y, Zhang Y, Zhang T, Mao X. Anal Chim Acta. 2015;861:69–73. doi: 10.1016/j.aca.2014.12.044. [DOI] [PubMed] [Google Scholar]; d) Lingam S, Beta M, Dendukuri D, Krishnakumar S. Microrna. 2014;3:18–28. doi: 10.2174/2211536602666131210001511. [DOI] [PubMed] [Google Scholar]
- 21.a) Bhadra S, Ellington AD. RNA. 2014;20:1183–1194. doi: 10.1261/rna.045047.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bhadra S, Ellington AD. Methods Enzymol. 2015;550:215–249. doi: 10.1016/bs.mie.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Akter F, Yokobayashi Y. ACS Synth Biol. 2015;4:655–658. doi: 10.1021/sb500314r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Kolpashchikov DM. J Am Chem Soc. 2005;127:12442–12443. doi: 10.1021/ja0529788. [DOI] [PubMed] [Google Scholar]; b) Kolpashchikov DM. J Am Chem Soc. 2006;128:10625–10628. doi: 10.1021/ja0628093. [DOI] [PubMed] [Google Scholar]; c) Kolpashchikov DM. J Am Chem Soc. 2008;130:2934–2935. doi: 10.1021/ja711192e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kolpashchikov DM, Gerasimova YV, Khan MS. Chembiochem. 2011;12:2564–2567. doi: 10.1002/cbic.201100545. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Cornett EM, O’Steen MR, Kolpashchikov DM. PLoS One. 2013;8:e55919. doi: 10.1371/journal.pone.0055919. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Bengtson HN, Kolpashchikov DM. Chembiochem. 2014;15:228–231. doi: 10.1002/cbic.201300657. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Kikuchi N, Kolpashchikov DM. Chembiochem. 2016;17:1589–1592. doi: 10.1002/cbic.201600323. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Kikuchi N, Kolpashchikov DM. Chem Commun (Camb) 2017;53:4977–4980. doi: 10.1039/c7cc01540b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.