A critical “waiting period” of ~3 months is generally accepted in patients with newly-diagnosed heart failure with reduced ejection fraction (HFrEF) outside the context of an acute myocardial infarction prior to reassessing left ventricular (LV) ejection fraction and considering implantable cardioverter-defibrillator (ICD) therapy. This time window is offered to allow optimization of guideline-directed medical therapy (GMDT) to promote LV reverse remodeling, which if above a certain threshold, would render the need for an ICD unnecessary. Consideration for an ICD after this time-frame is endorsed by major professional groups,1 serves as a key quality and performance measure, and is deemed “appropriate” by the Appropriate Use Criteria for ICD therapy.2 This duration also guides reimbursement schema, e.g. the Centers for Medicare & Medicaid Services limit coverage for ICDs in non-ischemic dilated cardiomyopathy to after this 3-month waiting period.
Perhaps it is time to lengthen this time-frame prior to ICD decision-making in newly-diagnosed patients with HFrEF. In many cases, 3 months is not sufficient to truly optimize GDMT and allow adequate chance for LV recovery. Evolving risks of sudden cardiac death (SCD), recent expansion of the HF therapeutic armamentarium, and greater focus on shared decision-making, all support extension of this time window. We summarize these converging lines of evidence and critically appraise the merits of extending this traditional “waiting period.” We contend that consideration for ICD implantation should only occur once GDMT has been achieved at target doses and may be deferred up to 1 year after diagnosis in appropriately-selected patients. As the Centers for Medicare & Medicaid Services plan to update the national coverage determination regarding ICD implantation over the next year, we believe this issue is timely and topical to address.
Landscape of Sudden Cardiac Death
Epidemiological studies3 and clinical trials4 over the last several decades have demonstrated that overall rates of SCD have declined, potentially reflecting greater uptake of GDMT, more complete coronary revascularization, and improved overall processes of care, including for comorbidities. After hospital discharge, patients face low-to-modest risks of SCD. Based on trials enrolling patients hospitalized for HFrEF with infrequent baseline utilization of ICDs (<15%), the estimated risks of SCD at 30 days is <1%, at 6 months is 2–4%, and at 1 year is 7% (Figure 1).5,6
Figure 1. Risks of Sudden and Non-Sudden Death after Hospitalization for Heart Failure with Reduced Ejection Fraction.
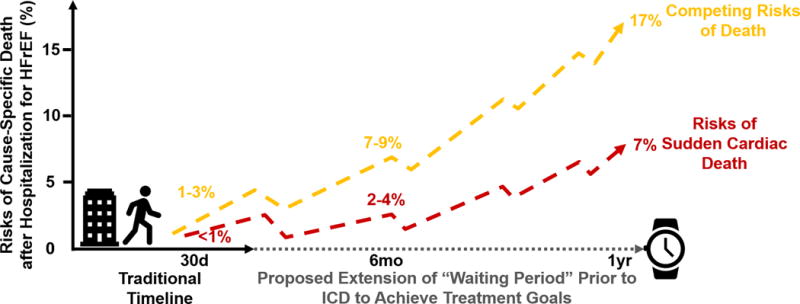
Cause-specific death risk estimates were derived from the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure), EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan),5 and RELAX-AHF (Relaxin in Acute Heart Failure) trials.6 Abbreviations: HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator.
This risk appears to accrue gradually and there does not appear to be a heightened period of SCD risk during the “vulnerable phase” after hospital discharge. This has been corroborated by data from over 30,000 patients enrolled in contemporary chronic HF trials.4 Although the absolute risks of SCD increase with the duration of HF diagnosis, patients recently diagnosed with HF face low absolute rates of SCD.4 “Real-world” experiences show that patients who receive wearable cardioverter-defibrillators experience rates of sustained ventricular tachyarrhythmias in 1% and 3% in non-ischemic and ischemic cardiomyopathies, respectively, at 3 months.7
Competing Risks of Death
The HF population in general is elderly with multi-morbidity burden. While SCD is an important consideration and is the most common mode of death in patients with chronic, stable HFrEF, progressive pump failure death and non-cardiovascular deaths predominate and account for twice the SCD event rate at each follow-up time-point after hospital discharge (Figure 1). This highlights the need for an individualized approach to determine the optimal timeline prior to ICD consideration based on age, functional status, hospitalization burden, and comorbidities.
Time to Optimize Guideline-Directed Medical Therapy
Neurohormonal modulation significantly reduces both HF-related death and SCD. There, however, remain many missed opportunities to modify the risk of SCD in patients with HFrEF. At the time of hospitalization for de novo HFrEF, prescription of GDMT is relatively low, even in clinical trial populations.8 With progresses in GDMT, clinicians will require more time to optimize multi-drug regimens (Figure 2). The step-wise initiation of 3 or more agents safely with simultaneous ambulatory monitoring of laboratory parameters, hemodynamics, and symptoms, requires frequent medical contact. As suggested by recent guidelines,9 most therapies require 1 to 3 dosing changes prior to achieving target doses, with titration intervals between 2 to 8 weeks. Even structured programs employing a nurse facilitator to guide aggressive titration to target doses of GDMT require ~6 months.10 Merged data from the National Cardiovascular Data Registry (NCDR) ICD Registry and Medicare administrative data show that only ~60% of patients filled any neurohormonal antagonist prescription prior to primary prevention ICD implant.11 The variable interpretation of the timeline (as time from initial diagnosis of HFrEF or time on optimal GDMT) may contribute to this observed poor utilization of GDMT. There are dose-dependent effects of HF therapies on improvements in LV ejection fraction that may occur beyond the 3-month time window. Thus, taking time to achieve recommended doses is important and may take up to 1 year to not only optimize therapy, but to see the effect of optimal therapy after it has been achieved.
Figure 2. How Long Does It Take to Optimize Guideline-Directed Medical Therapy in Newly-Diagnosed Heart Failure with Reduced Ejection Fraction?
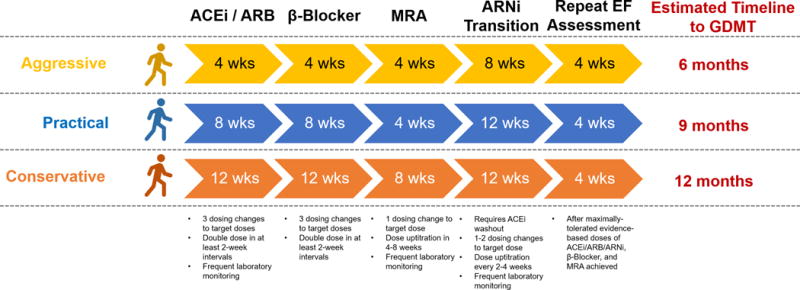
We have developed a theoretical timeline for the step-wise initiation and uptitration of 3–4 drugs in contemporary regimens for newly-diagnosed heart failure with reduced ejection fraction. Starting doses, target doses, titration schedule, and necessary laboratory monitoring were based on suggestions from the 2016 European Society of Cardiology Guidelines.9 This generic timeline does not account for pre-existing therapies, specific order of initiation of therapies, or simultaneous drug uptitration. Abbreviations: ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNi = angiotensin receptor neprilysin inhibitor; EF = ejection fraction; GDMT = guideline-directed medical therapy; MRA = mineralocorticoid receptor antagonist.
Reappraisal of Benefits with ICD Therapy
The recent DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality) trial in patients with non-ischemic cardiomyopathy and LV ejection fraction ≤35% reaffirm the uncertainty of ICD benefit in certain cohorts,12 especially those with high uptake of GDMT or who face greater competing risks of mortality. Indeed, ICD therapy is of uncertain value in patients who experience recurrent hospitalizations or have severe comorbidities (class IIb, level of evidence B). Device implantation is met with procedural risks, costs, require routine follow-up, and may malfunction. As such, decisions to pursue ICD therapy should be well-informed, calculated, guided by individualized risk estimates of SCD vs. non-SCD death, and only occur after an adequate trial of GDMT.
A Path Forward
The course after HF diagnosis is riddled with hurdles and opportunities that may modify risks of SCD and non-SCD death (Figure 1). Advances in GDMT have introduced further uncertainty about the benefit of ICD implantation after initial HFrEF diagnosis. Few SCD events occur during the currently acceptable 3-month timeline and GDMT remains poorly optimized prior to many device implantations. In this context, a longer “waiting period” up to 1 year would facilitate several important goals:
Initiation, up-titration, and optimization of multi-drug regimens, and assess their attendant effects on LV recovery
Improve implementation and ensure adherence, especially in underrepresented or low-income populations
Allow time for assessing and managing competing risks (HF-related death or other non-SCD modes of death) and comorbidities
Permit greater opportunity for risk stratification for SCD including application of wearable cardioverter-defibrillators in select patients
Provide sufficient time for patients to understand the HF syndrome, risks of SCD, and harms and benefits of ICD implantation
There will be a small, but significant, and accruing risk of SCD that will be incurred if the proposed extended timeline is routinely undertaken. Younger patients without significant comorbidities should continue to be evaluated for ICD therapy within the traditional timeframe.13 Precision medicine techniques (risk scores, deep phenotyping with biomarkers and imaging, etc.) may be used to identify other subgroups of patients who may benefit from shorter timelines to ICD consideration. The uncertain benefits of early ICD implantation in select patients reinforces the critical role of shared decision-making in guiding timing for individual patents. Cause-specific prognostic risk scores and better valuation of patients’ goals and expectations are needed in clinical practice. Considering the modest early risks of SCD, high cost and other unintended consequences of ICD therapy, and the potential to obviate its need with GDMT, serious consideration should be afforded to extending the time period before proceeding with ICD implantation for primary prevention in appropriately-selected patients with newly-diagnosed HFrEF.
Acknowledgments
None
SOURCES OF FUNDING
None
DISCLOSURES
Dr. Javed Butler has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Relypsa, Vifor Pharma, and ZS Pharma. Dr. Muthiah Vaduganathan is supported by the NHLBI T32 postdoctoral training grant (T32HL007604).
Footnotes
The remaining author reports no conflict of interest.
References
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017 doi: 10.1161/CIR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 2.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013;10:e11–58. doi: 10.1016/j.hrthm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, RAjwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 4.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JG, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJ. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor CM, Miller AB, Blair JEA, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Pina I, Roth S, Sackner-Bernstein J, Traver B, Cook T, Gheorghiade M. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–9.e1. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E, Metra M. Effect of serelaxin on mode of death in acute heart failure: results from the RELAX-AHF study. J Am Coll Cardiol. 2014;64:1591–8. doi: 10.1016/j.jacc.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry) Circulation. 2015;132:1613–9. doi: 10.1161/CIRCULATIONAHA.115.015677. [DOI] [PubMed] [Google Scholar]
- 8.Greene SJ, Hernandez AF, Dunning A, Ambrosy AP, Armstrong PW, Butler J, Cerbin LP, Coles A, Ezekowitz JA, Metra M, Starling RC, Teerlink JR, Voors AA, O’Connor CM, Mentz RJ. Hospitalization for Recently Diagnosed Versus Worsening Chronic Heart Failure: From the ASCEND-HF Trial. J Am Coll Cardiol. 2017;69:3029–39. doi: 10.1016/j.jacc.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzales-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 10.Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, Massie BM. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003;107:2799–804. doi: 10.1161/01.CIR.0000070952.08969.5B. [DOI] [PubMed] [Google Scholar]
- 11.Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of Guideline-Directed Medications for Heart Failure Before Cardioverter-Defibrillator Implantation. J Am Coll Cardiol. 2016;67:1062–9. doi: 10.1016/j.jacc.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen A, Gustaffson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S, DANISH investigators Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 13.Elming MB, Nielsen JC, Haarbo J, Videbaek L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S, Kober L, Thune JJ. Age and Outcomes of Primary Prevention Implantable Cardioverter Defibrillators in Patients with Non-Ischemic Systolic Heart Failure. Circulation. 2017 Sep 6; doi: 10.1161/CIRCULATIONAHA.117.028829. (Epub ahead of print) published online. [DOI] [PubMed] [Google Scholar]


