Abstract
Anthropogenic warming is projected to trigger positive feedbacks to climate by enhancing carbon losses from the soil1. While such losses are, in part, owing to increased decomposition of organic matter by invertebrate detritivores, it is unknown how detritivore feeding activity will change with warming2, especially under drought conditions. Here, using four year manipulation experiments in two North American boreal forests, we investigate how temperature (ambient, +1.7 °C, +3.4 °C) and rainfall (ambient, -40% summer precipitation) perturbations influence detritivore feeding activity. In contrast to general expectations1,3, warming had negligible net effects on detritivore feeding activity at ambient precipitation. However, when combined with precipitation reductions, warming decreased feeding activity by ~14%. As across all plots and dates, detritivore feeding activity was positively associated to bulk soil microbial respiration, our results suggest slower rates of decomposition of soil organic matter, and thus reduced positive feedbacks to climate under anthropogenic climate change.
Soil invertebrate detritivores are crucial drivers of the decomposition of soil organic matter4,5, and decomposition of soil organic matter is key to carbon sequestration in the soil1,6–8. Invertebrate detritivores contribute to about 30-40% of decomposition of soil organic matter via their feeding activities on detritus and soil microorganisms9,10. Recent studies have predicted that on-going climate warming increases soil carbon losses globally by increasing decomposition rates in the soil3,11. However, these predictions rely mostly on enhanced soil microbial activities in response to climate warming12, without considering the feeding responses of soil invertebrate detritivores (‘soil detritivores’ hereafter for brevity) to climate warming.
Ectothermic organisms, such as soil microorganisms and soil detritivores, generally exhibit greater activity with warming because of elevated metabolic demands13. However, there are at least two key constraints for higher metabolism of ectotherms at higher temperature that could minimize (or even reverse) greater activity in response to warming14. First, process rates, such as feeding or respiration at higher temperature, often acclimate to warmer temperature by returning to rates exhibited at ambient temperature15. Soil microorganisms occasionally show thermal acclimation after a given period of time16–18 possibly dependent upon on the rate of warming19, and mostly owing to adjustments in their substrate-use efficiency at higher temperature14,15. However, the relation between substrate-use efficiency of soil microorganisms and soil carbon dynamics remains unclear due to the lack of a general pattern20–22. Furthermore, our understanding of soil detritivore acclimation to warming is extremely limited as most of the evidence of their greater activity at higher temperature comes from short-term laboratory incubation studies23,24, which may not be of sufficient duration to allow thermal acclimation in detritivores. The second constraint involves potential resource limitation for ectothermic organisms in warmed conditions. Warming could reduce the activity of ectothermic detritivore organisms when warming increases resource or substrate depletion in soil25–27, such as warming-induced reduction in soil water. In fact, climate warming seems to increase soil microbial activity only in soils with sufficiently moist conditions1,28. Increasing frequency of summer drought in terrestrial biomes29 thus could also reduce the expected increase of detritivore feeding activity at higher temperatures, as demonstrated for soil microorganisms28,30.
In this study, we report the feeding activity of soil detritivores in response to climate warming using a highly resolved temporal data set (more than 40 time points) across four years. Further, we investigate the interactive effects of climate warming and summer drought (via reduced summer precipitation) on the feeding activity of soil detritivores. Our results are based on two independent southern boreal forest field experiments with three levels of climate warming (ambient, ambient +1.7°C, and ambient +3.4°C) crossed with ambient precipitation and reduced summer precipitation. The warming levels were chosen to meet the two target levels of simultaneous plant and soil warming as per the predictions of the climate models for approximately 75–100 years from now31,32. We measured the feeding activity of soil detritivores using a rapid ecosystem assessment technique (bait lamina strips33) every two weeks in the growing season from 2012 to 2015 (usually from April to November). We hypothesize that climate warming will enhance the feeding activity of soil detritivores, however, only at ambient precipitation.
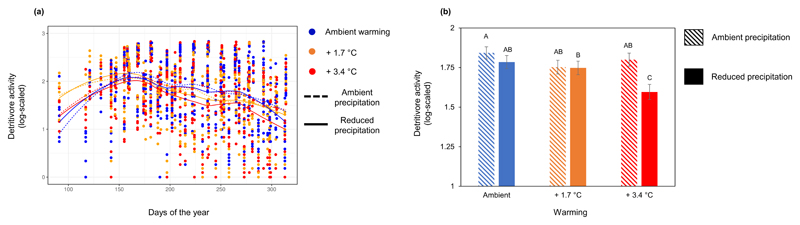
The main effects of experimental warming and reduced precipitation on the feeding activity of soil detritivores were not significant (Table 1). However, warming reduced the feeding activity of soil detritivores under the low precipitation treatment (Fig. 1a and 1b, Table 1, significant Warming x Precipitation interaction), especially from the middle (e.g. July) through the late periods (e.g. November) of the growing season (Fig. 1a). Overall, the feeding activity was ~14% lower in the +3.4°C treatment under low precipitation compared to either the +3.4°C treatment under ambient precipitation or the ambient temperature and ambient precipitation treatment (which were similar) (Fig. 1b).
Table 1.
Results from linear mixed-effects models for treatment effects on soil detritivore feeding activity. The denominator degrees of freedom are based on the Satterthwaite approximation. Variance and standard deviations (in brackets) are given for the random effects used in models (see methods for details). Bold F-values are statistically significant (***: p<0.001, **: p<0.01, *: p<0.05.)
| Treatments | Soil detritivore feeding activity |
|---|---|
| Experimental warming (W) | F1, 1680 = 1.79 |
| Reduced precipitation (P) | F1, 1680 = 0.28 |
| W × P | F1, 1680 = 10.37** |
| Random effects | |
| Year | 0.29 (0.53) |
| Day of the year/Site | 0.21 (0.46) |
| Day of the year/Site/Block | <0.001 (<0.001) |
Figure 1. Soil detritivore feeding activity in response to experiment warming and reduced precipitation.
a, Temporal pattern of detritivore feeding activity in response to experimental warming and reduced precipitation. Data shown here are from four years (2012 to 2015) of detritivore feeding activity measurements at two independent boreal forest sites. The curves are based on “loess” smoothing function with lambda=0.5 from the “ggplot2” package in R. For each time point, we assessed 18 plots per site (see methods for details). b, Mean (±SE) detritivore feeding activity in response to warming and precipitation treatments. The bar diagram is based on average detritivore feeding activity (log-scaled) from four years of in-situ measurements and from two independent sites. The letters on top of the bar are from post-hoc Tukey HSD tests run on mixed-effects models. In both panels, blue, orange, and red refer to ambient, +1.7°C, and +3.4°C warming, respectively. Dashed and solid lines/bars refer to ambient and reduced precipitation, respectively.
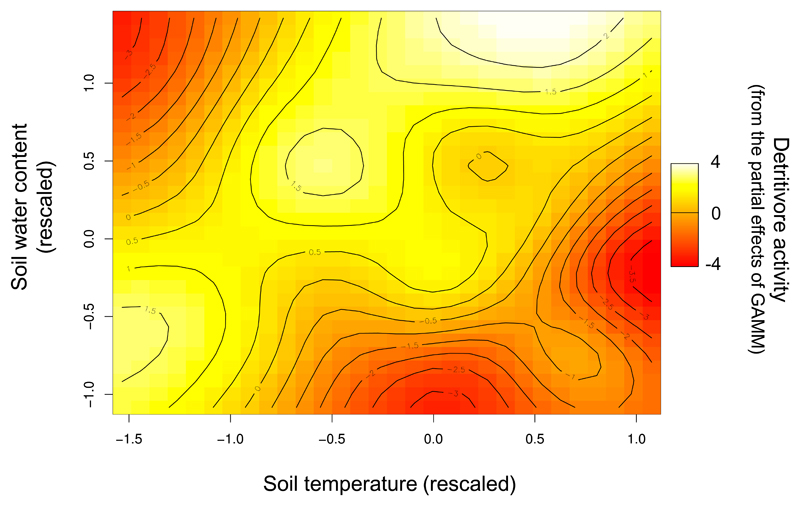
To test whether the treatment effects on detritivore feeding activity can be explained by changes in the microenvironment, we also analyzed the results using models that included data on continuously measured soil water content and soil temperature in the plots (more than 40 time points). Because of seasonal changes in soil temperature and soil water content, we ran generalized additive regression models with soil temperature and soil water as smooth terms. These models also revealed an interactive effect of soil temperature and soil water content on detritivore feeding activity (Table 2), agreeing with our experimental treatment results (Table 1). In general, the feeding activity of soil detritivores was low when soils were cold and wet or warm and dry, and high when soils were warm and wet or cold and dry (Fig. 2).
Table 2.
Results from generalized additive mixed-effects models (GAMMs) with soil temperature and soil water as smooth terms. Variance and standard deviations (in brackets) are given for the random effects used in models. Bold F-values are statistically significant (***: p<0.001, **: p<0.01, *: p<0.05.)
| Soil abiotics | Soil detritivore feeding activity |
|---|---|
| Soil temperature | F1, 1695.79 = 6.72** |
| Soil water | F4.57,1695.79 = 0.55 |
| Soil temperature × Soil water | F16.63, 1695.79 = 0.86*** |
| Random effects | |
| Year | 8.46 (2.91) |
| Site | 2.62 (1.62) |
| Site/Block | 0.01 (0.13) |
Figure 2. Interactive effects of soil temperature and soil water content on the feeding activity of soil detritivores.
The heat map is based on the partial residuals of the smooth terms (soil temperature and soil water) used in generalized additive models. Both soil temperature and soil water were rescaled using the “arm” package46.
In order to compare our results of detritivore feeding activity with other commonly reported ecosystem processes, we further examined the patterns of total soil respiration and bulk soil microbial respiration (with a similar temporal resolution to that of detritivore feeding activity; that is, more than 40 time points across four years) in warmed and reduced precipitation treatments. We found that total soil respiration increased with experimental warming only in the reduced precipitation treatments, whereas it did not change with experimental warming in the ambient precipitation treatments (Supplementary figure 1a, Supplementary table 1). These responses of total soil respiration thus contradicted the observed decline of detritivore feeding activity in response to warming and reduced precipitation (Fig. 1b).
Bulk soil microbial respiration decreased in the ambient as well as warmest temperature treatments under reduced precipitation compared to the ambient warming and ambient precipitation treatments (Supplementary figure 1b, Supplementary table 1). The significant decline of soil microbial respiration in the warmest temperature and reduced precipitation treatments resembled with the decline of detritivore feeding activity under the same treatment combination (Fig. 1b). Accordingly, we found a significantly positive correlation between detritivore feeding activity and soil microbial respiration across all treatments and sampling dates (Supplementary figure 2).
Climate warming and reduced precipitation are two of the main global change factors determining the effect of soil organisms on the decomposition of soil organic matter. To our knowledge, this is the first study investigating the feeding activity of soil detritivores - a key determinant of decomposition - in response to climate warming and summer drought with such high temporal resolution across multiple years and two sites. Overall, our results show that climate warming of +3.4°C, in combination with reduced precipitation, reduced the feeding activity of soil detritivores. This was mainly due to warmer and drier soils, which occurred, as expected, more frequently in this treatment combination. In contrast, warming had only negligible net effects on soil detritivore feeding activity in the ambient precipitation treatment, because warming modestly enhanced activity in cool wet periods (e.g. spring), which offset warming-induced lower activity later in the growing season (Fig.1 a, Supplementary figures 3 and 4). These results contradict the assumption of greater feeding activity of invertebrates (within their thermal limits) at higher temperature24,34.
Drier habitats imply drier substrates for detritivore species, which are difficult to ingest and digest4. As a consequence, mortality among detritivore species could peak in drier soil due to reduction in both foraging and reproductive success35–37. Further, when warming increases the feeding requirements of ectothermic detritivores38,39, failure to forage in drier soil conditions will either enhance the mortality of detritivores or they will disperse to more favourable habitats. We speculate these two possibilities as plausible scenarios in this study leading to reduced feeding activity of soil detritivores in warm and dry soil (Fig. 1b, Fig. 2). The lack of an overall warming-induced increase in the net feeding activity over the experimental period (Fig. 1b) was due to shorter durations of peak activities at elevated temperatures across the vegetation growing period (Fig. 1a). Surprisingly, warming treatments were wetter at the start of the growing season (Supplementary figure 4), which may have affected the strength of the net effects of warming on the detritivore feeding activity. This was, however, driven by the year 2015 when the growing season began earlier than the previous years, and thus causing a slower thawing of snow in the ambient warming plots than in the warmer plots (Supplementary figure 5).
Our results contradict several short-term studies, which have shown warming-induced greater feeding activity of soil invertebrates24,40,41. We argue that such positive warming effects could be a transient response of soil invertebrates to warming and may disappear as warming-induced reductions in soil water content intensify with time as has been suggested for the decline in soil respiration with reductions in soil water content in response to warming26,42. Our total respiration results, however, also contradict this assumption as they were still higher in warmed and reduced precipitation treatments (Supplementary figures 1 a and b). Although these results are based on a relatively long-term warming experiment (7 years of warming and 4 years of detritivore feeding measurements), we believe that decadal-scale experiments are needed to provide crucial insights to understand the varying patterns of detritivore feeding and soil respiration in response to warming and drought. The general trend of total soil respiration, which includes autotrophic respiration (plant roots) and heterotrophic respiration, showed resemblance to the widely observed greater soil carbon loss at warmer temperatures43, i.e., greater respiration rates in response to soil warming. This significant relationship was, surprisingly, observed only in the reduced precipitation treatment. Moreover, the observed mismatch between total soil respiration and detritivore feeding activity at higher temperature indicates complex shifts in the contributions of plants, soil microorganisms, and soil detritivores to soil carbon dynamics in warmer boreal forests, potentially due to their varying levels of temperature sensitivities.
Detritivore communities may also acclimate to warmer climate when sufficient amounts of resources are available15. Thermal acclimation might explain the negligible increase in detritivore feeding in the warmed ambient precipitation treatments in our study (Fig. 1b); given that our method of feeding measurements included regularly added substrate (see methods). Future studies are required for directly assessing the potential of thermal acclimation of soil detritivores. For instance, this could potentially be done by assessing the feeding rates and assimilation efficiencies of soil detritivores from field experiments by exposing them to different temperatures in long-term laboratory trials.
The feeding activity of soil detritivores is a crucial first step in the decomposition of soil organic matter4, and their reduced activity in response to joint warming and reduced precipitation can potentially slow down decomposition of soil organic matter. Lower feeding rates of detritivores can also directly affect the decomposition of organic matter by microbial communities. For instance, fragmentation of litter would be lower at reduced feeding activity of detritivores leading to slower decomposition by microbial communities4.
Our results provide novel insights into the climate-dependent dynamics of detritivore feeding activity in boreal forests, which are predicted to get warmer and drier in the future44. We though recommend that future studies should investigate warming effects on soil biological activity in winter months (which were not feasible in our study) for improving our understanding of complex relationships between snow cover dynamics and soil biological processes45. Nevertheless, our findings encourage next-generation earth system models to include soil detritivore responses together with plant and soil microbial responses for improving the predictions of soil carbon dynamics under anthropogenic climate change.
Methods
Site description and experimental design
The experiment is located in the temperate-boreal ecotone region of northern Minnesota in North America at two field stations of the University of Minnesota: the Cloquet Forestry Center at Cloquet (46°40’ 46” N, 92° 31’ 12” W) and the Hubachek Wilderness Research Center at Ely (47°56’ 46” N, 91° 45’ 29” W). These two study sites are ~150 km apart from each other32.
The overall design of the experiment comprises two sites, two habitats (closed and open forest canopy), and three warming treatments (ambient, +1.7°C and +3.4°C), replicated six times. In 2008, warming treatments were established at both sites using infrared heaters for aboveground heating, and belowground cables for belowground heating. Warming treatments were set up in both closed (40-60 year old mixed aspen-birch-fir) and relatively open (cleared in 2007) canopy conditions32. The plots were circular with a diameter of 3 m. Further, the experiment has a randomized block design (each block consists of six plots) nested within sites. Warming treatments are applied from spring to fall (usually from April to November) every year from the start of the experiment, and do not run during the winter months (From December to February or March) due to logistic constraints (e.g. difficulties in maintaining the desired treatment temperature) and uncertainty related to simulating future winter snow depths.
At the start of the experiment, 11 tree species were planted intermixed with the naturally occurring herbaceous and low shrub species. These tree species belong either to the northern temperate climate or the southern boreal climate47. The reduced precipitation treatments were applied at the beginning of the year 2012 only in the open canopy plots during summer months (from June to September) causing a reduction of summer precipitation by about 40% (Supplementary figure 6). The open canopy plots represent a common forest canopy disturbance, and, we expected summer drought effects to be more pronounced in these plots. Moreover, owing to logistic constraints, reduced precipitation treatments were only established in the open canopy. Thus, to assess interactions of warming and reduced precipitation, soil invertebrate feeding activity and soil water measurements were carried out only in the open canopy plots at both experimental sites (Cloquet and Ely).
Soil detritivore feeding activity and soil abiotic measurements
Soil detritivores comprise several large groups of invertebrates residing in the soil that feed on dead organic matter that enters soil as well as on soil microorganisms. Earthworms, isopods, millipedes, enchytraeids, Collembola, oribatid mites, and nematodes are usually considered as the major soil detritivores4 (Supplementary figure 7). The feeding activity of soil detritivores was measured by inserting bait lamina strips33 in the upper 10 cm of the soil. Bait lamina strips are commonly used as a rapid assessment of feeding activity of soil detritivores41,48,49,50 (Supplementary figure 8) and do not represent microbial activity in the soil41. Previous studies have reported no loss of substrates used in bait lamina strips in defaunated soil (but with soil microorganisms)41 and soil invertebrates as the main drivers of substrate loss from bait lamina strips51. Each strip contained 16 holes of 2 mm diameter, which were filled with an artificially prepared organic substrate. The substrate was made of 65% cellulose (micro granular), 15% agar (pulverized), 10% loess, and 10% wheat bran (finely ground and sieved) by weight. Loess was obtained from the soil adjacent to field sites. The substrate paste was prepared by adding water (about 15 ml in 2050 g of solid substrate) and organic fluorescent dye (10 ml) to increase the accuracy of the method48. Six strips filled with the substrate material were inserted in each plot and placed at least 2 cm apart from each other. Strips were removed every two weeks, and all 16 holes were thoroughly checked for detritivore feeding activity. Empty holes were scored as 1 (indicator of high feeding activity), whereas holes filled with substrate were scored 0 (indicator of no feeding activity)33,48. Some holes were also partially empty and scored as 0.5, which indicates intermediate feeding activity of soil detritivores. Hence, detritivore feeding activity could vary between 0 to 16 per strip with 0 meaning no feeding activity and 16 indicating maximum feeding activity. Scores from six strips from each plot were averaged for statistical analyses. Feeding activity was measured every two weeks from May to November in 2012, 2013, 2014, and 2015. In 2015, we also measured feeding activity in March and April as the growing season for vegetation started earlier in this year, however, this varied between the two sites (Supplementary figure 9). In total, 172,800 bait lamina holes were assessed which resulted in 1,770 data points. Bait lamina assessments were performed only in the growing seasons as soil biological activities peak with vegetation growing periods.
Soil water content and soil temperature were measured at each time point at which detritivore feeding activity was determined. Volumetric soil water content (cm3 H2O/cm3 soil) was measured using a 30 cm Campbell Scientific CS-616 probe inserted at an angle of 45°32. Soil water content was measured at the depth of 20 cm from the soil surface. We compared the measurement of soil water content at the depth of 20 cm to less frequently measured (three times across three years) soil water content of the top 10 cm of the soil (where the detritivore feeding activities were recorded). We found a significant positive correlation (Pearson’s r = 0.43; Supplementary figure 10), justifying the use of soil water content at the depth of 20 cm. Although this correlation was significantly positive, we encourage future studies to measure soil water content at multiple soil depths at regular intervals. Soil temperature (°C) was measured using two sealed thermocouples (type T) installed at a depth of 10 cm and at a distance of about 6.6 cm from the nearest heating cable in the soil32. Other details can be found in Rich et al.32.
Total soil respiration (bulk soil and root respiration) and bulk soil microbial respiration (excluding root respiration) were measured with three PVC collars (10.2 cm diameter) installed in every plot. Two of these collars per plot extended about 2 cm into the soil enabling the measurement of total soil respiration. The third collar in every plot was inserted extending 40 cm deeper (~48 cm) into the soil to capture only the bulk soil microbial respiration. The visual inspections from soil cores confirmed negligible amount of plant roots beyond 40 cm soil depth in our experimental plots. All collars for the measurement of soil respiration were kept free of plant cover. Soil CO2 flux (either total or microbial respiration) was measured using a LICOR 6400 infrared gas analyzer (IRGA) with attached soil chambers (LI-COR Biosciences, Inc., Lincoln, NE, USA). Soil respiration measurements were carried out every two weeks in the vegetation growing period.
Statistical analyses
Soil detritivore feeding activity comprised of 44 time points at Cloquet, and 41 time points at Ely over the four years of measurements. Accordingly, we used a repeated measures mixed-effects ANOVA with experimental warming (linear term) and reduced precipitation as two fixed effects and day of the year as the random effect. Further, experimental blocks and the two sites were nested within the day of the year. Finally, we also used year as a separate random intercept term. The model structure was Soil detritivore feeding activity ~ Warming × Precipitation + (1|Day of the year/Site/Experimental block) + (1|Year). Site- and year-specific differences in the detritivore feeding activity are graphically shown in the Supplementary figures 9 and 11, respectively. As the ambient soil temperature and soil water varied in the measurement years in both experimental sites (Supplementary figures 5 and 12), we included year as a random intercept in all our models. The “lme4” package in R was used to run the repeated measures mixed-effects ANOVA models52, and the lmerTest package was used to obtain F- and p- values with Satterthwaite approximation for degrees of freedom. The feeding activity of soil detritivores was log-transformed (log(x+1)) to improve the model-fit. Mixed-effect models were run using Gaussian error term with linear model assumptions being met (e.g. no correlation between fitted vs. residual values). Post-hoc Tukey tests were run with the interaction term using the “multcomp” package53. We ran mixed-effects models to investigate the responses of soil respiration (separately for total soil respiration and soil microbial respiration) with the same model structure as for the responses of detritivore feeding activity to warming and reduced precipitation. The analyses on both respiration data were based on 56 time points in Cloquet, whereas 49 time points in Ely over the four years of measurements (2012-2015).
In order to compare the results of treatments effects (warming and reduced precipitation) with soil temperature and soil water measurements in the plots, we used these measurements (with the same temporal resolution) to explain the variance in the feeding activity of soil detritivores. Since both soil temperature and soil water fluctuate non-linearly within a year (Supplementary figures 3 and 4), we used generalized additive mixed-effects models (GAMMs) to test their interactive effects on the feeding activity of soil detritivores. Although GAMMs contain linear predictors as linear mixed-effects models do, they include smooth functions of covariates allowing flexible specifications of relationships without restricting relationships to be linear, quadratic, or cubic54. The model structure for GAMM in our analysis was Soil detritivore feeding activity ~ s(Soil temperature) × s(Soil water) + (1| Site/Experimental block) + (1|Year), where “s” indicates smooth function for GAMM. Please note that we did not use “day of the year” as a random effect in our GAMM as both soil temperature and soil water changed with the day of the year (Supplementary figures 3 and 4). We ran GAMMs using the “gamm4” package55. The test-statistics from GAMMs were obtained from the “itsadug” package56. All statistical analyses were run with the R statistical software57.
Data availability
Soil detritivore feeding activity, soil abiotic data and R codes for the statistical analysis are available by direct request to the corresponding author.
Supplementary Material
Acknowledgements
We gratefully acknowledge several interns who spent innumerable hours in the field assessing bait lamina strips. We are thankful to Sarah Zieger and Julia Siebert for providing the images of detritivores and bait lamina strips, respectively. MPT and NE acknowledge funding by the DFG in the frame of the Emmy Noether research group (Ei 862/2). This project also received support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no 677232). Further support came from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118). The B4WarmED project is funded by the US Department of Energy (Grant No. DE-FG02-07ER64456), and the College of Food, Agricultural and Natural Resource Sciences (CFANS) at the University of Minnesota.
Footnotes
Author contributions: PBR and SEH conceived the B4WarmED experiment. NE conceived the study of soil detritivore feeding activity. AS, RR, KR, WCE collected the data. MPT developed the ideas for this manuscript, analyzed the data and wrote the manuscript with substantial input from NE and PBR. All authors contributed to revisions.
References
- 1.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–73. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 2.Bradford MA, Wieder WR, Bonan GB, Fierer N, Raymond PA, Crowther TW. Managing uncertainty in soil carbon feedbacks to climate change. Nat Clim Chang. 2016;6:751–758. [Google Scholar]
- 3.Crowther T, et al. Quantifying global soil C losses in response to warming. Nature. 2016;540:104–108. doi: 10.1038/nature20150. [DOI] [PubMed] [Google Scholar]
- 4.Adl S. The ecology of soil decomposition. CABI Publishing; 2003. [Google Scholar]
- 5.Wolters V. Invertebrate control of soil organic matter stability. Biol Fertil Soils. 2000;31:1–19. [Google Scholar]
- 6.Prescott CE. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry. 2010;101:133–149. [Google Scholar]
- 7.Six J, Conant RT, Paul EA, Paustian K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil. 2002;241:155–176. [Google Scholar]
- 8.Jastrow JD, Amonette JE, Bailey VL. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Change. 2007;80:5–23. [Google Scholar]
- 9.Verhoef H, Brussaard L. Decomposition and nitrogen mineralization in natural and agroecosystems: the contribution of soil animals. Biogeochemistry. 1990;11:175–211. [Google Scholar]
- 10.Seastedt T. The Role of Microarthropods in Decomposition and Mineralization Processes. Annu Rev Entomol. 1984;29:25–46. [Google Scholar]
- 11.Pries CEH, Castanha C, Porras R, Torn MS. The whole-soil carbon flux in response to warming. Science. 2017;355:1420–1423. doi: 10.1126/science.aal1319. [DOI] [PubMed] [Google Scholar]
- 12.Allison SD, Wallenstein MD, Bradford MA. Soil-carbon response to warming dependent on microbial physiology. Nat Geosci. 2010;3:336–340. [Google Scholar]
- 13.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–51. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 14.Tucker CL, Bell J, Pendall E, Ogle K. Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Glob Chang Biol. 2013;19:252–263. doi: 10.1111/gcb.12036. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MA. Thermal adaptation of decomposer communities in warming soils. Front Microbiol. 2013;4:1–16. doi: 10.3389/fmicb.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowther TW, Bradford MA. Thermal acclimation in widespread heterotrophic soil microbes. Ecol Lett. 2013;16:469–477. doi: 10.1111/ele.12069. [DOI] [PubMed] [Google Scholar]
- 17.Melillo JM, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Wan S, Hui D, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413:622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- 19.Sihi D, Inglett P, Gerber S, Inglett K. Rate of warming affects temperature sensitivity of anaerobic peat decomposition and greenhouse gas production. Glob Chang Biol. 2017 doi: 10.1111/gcb13839. [DOI] [PubMed] [Google Scholar]
- 20.Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A. Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett. 2013;16:930–9. doi: 10.1111/ele.12113. [DOI] [PubMed] [Google Scholar]
- 21.Hagerty SB, et al. Accelerated microbial turnover but constant growth efficiency with warming in soil. Nat Clim Chang. 2014;4:903–906. [Google Scholar]
- 22.Frey SD, Lee J, Melillo JM, Six J. The temperature response of soil microbial efficiency and its feedback to climate. Nat Clim Chang. 2013;3:395–398. [Google Scholar]
- 23.Lang B, Rall BC, Brose U. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J Anim Ecol. 2012;81:516–23. doi: 10.1111/j.1365-2656.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 24.A’Bear AD, Boddy L, Hefin Jones T. Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing collembola. Glob Chang Biol. 2012;18:1823–1832. [Google Scholar]
- 25.Eliasson PE, et al. The response of heterotrophic CO2 flux to soil warming. Glob Chang Biol. 2005;11:167–181. [Google Scholar]
- 26.Conant RT, et al. Temperature and soil organic matter decomposition rates - synthesis of current knowledge and a way forward. Glob Chang Biol. 2011;17:3392–3404. [Google Scholar]
- 27.Kirschbaum M. Soil respiration under prolonged soil warming: Are rate reductions caused by acclimation or substrate loss? Glob Chang Biol. 2004;10:1870–1877. [Google Scholar]
- 28.Allison SD, Treseder KK. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Chang Biol. 2008;14:2898–2909. [Google Scholar]
- 29.IPCC. Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2014 [Google Scholar]
- 30.Schindlbacher A, et al. Soil respiration under climate change: Prolonged summer drought offsets soil warming effects. Glob Chang Biol. 2012;18:2270–2279. [Google Scholar]
- 31.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2013. [Google Scholar]
- 32.Rich R, et al. Design and performance of combined infrared canopy and belowground warming in the B4WarmED (Boreal Forest Warming at an Ecotone in Danger) experiment. Glob Chang Biol. 2015;21:2334–2348. doi: 10.1111/gcb.12855. [DOI] [PubMed] [Google Scholar]
- 33.von Torne E. Assessing feeding activities of soil-living animals. I. Bait-lamina-tests. Pedobiologia (Jena) 1990;34:89–101. [Google Scholar]
- 34.Rall BC, et al. Universal temperature and body-mass scaling of feeding rates. Philos Trans Soc Lond B Biol Sci. 2012;367:2923–34. doi: 10.1098/rstb.2012.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindberg N, Engtsson JB, Persson T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J Appl Ecol. 2002;39:924–936. [Google Scholar]
- 36.Staley JT et al. Effects of summer rainfall manipulations on the abundance and vertical distribution of herbivorous soil macro-invertebrates. Eur J Soil Biol. 2007;43:189–198. [Google Scholar]
- 37.Eisenhauer N, et al. Warming shifts ‘worming’: effects of experimental warming on invasive earthworms in northern North America. Sci Rep. 2014;4:6890. doi: 10.1038/srep06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasseur DA, McCann KS. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am Nat. 2005;166:184–98. doi: 10.1086/431285. [DOI] [PubMed] [Google Scholar]
- 39.Brown J, Gillooly J, Allen A, Savage V. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 40.Lang B, Rall BC, Scheu S, Brose U. Effects of environmental warming and drought on size-structured soil food webs. Oikos. 2013;123:1224–1233. [Google Scholar]
- 41.Gongalsky KB, Persson T, Pokarzhevskii AD. Effects of soil temperature and moisture on the feeding activity of soil animals as determined by the bait-lamina test. Appl Soil Ecol. 2008;39:84–90. [Google Scholar]
- 42.Davidson EA, Trumbore SE, Amundson R. Soil warming and organic carbon content. Nature. 2000;408:789–790. doi: 10.1038/35048672. [DOI] [PubMed] [Google Scholar]
- 43.Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464:579–82. doi: 10.1038/nature08930. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier S, Bernier P, Kuuluvainen T, Shvidenko AZ, Schepaschenko DG. Boreal forest health and global change. Science. 2015;349:819–822. doi: 10.1126/science.aaa9092. [DOI] [PubMed] [Google Scholar]
- 45.Schindlbacher A, Jandl R, Schindlbacher S. Natural variations in snow cover do not affect the annual soil CO2 efflux from a mid-elevation temperate forest. Glob Chang Biol. 2014;20:622–632. doi: 10.1111/gcb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelman A, Yu-Sung S. arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R Packag. 2015 version 1.8-6. [Google Scholar]
- 47.Reich PB, et al. Geographic range predicts photosynthetic and growth response to warming in co-occurring tree species. Nat Clim Chang. 2015;5:148–152. [Google Scholar]
- 48.Eisenhauer N, et al. Organic textile dye improves the visual assessment of the bait-lamina test. Appl Soil Ecol. 2014;82:78–81. [Google Scholar]
- 49.Riutta T, Clack H, Crockatt M, Slade EM. Landscape-Scale Implications of the Edge Effect on Soil Fauna Activity in a Temperate Forest. Ecosystems. 2015 doi: 10.1007/s10021-015-9939-9. [DOI] [Google Scholar]
- 50.Simpson JE, Slade E, Riutta T, Taylor ME. Factors affecting soil fauna feeding activity in a fragmented lowland temperate deciduous woodland. PLoS One. 2012;7:e29616. doi: 10.1371/journal.pone.0029616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birkhofer K, et al. Soil fauna feeding activity in temperate grassland soils increases with legume and grass species richness. Soil Biol Biochem. 2011;43:2200–2207. [Google Scholar]
- 52.Bates D, Maechler M, Bolker BM, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 53.Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biometrical J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 54.Wood SN. Generalized additive models: an introduction with R. Chapman and Hall/CRC; 2006. [DOI] [Google Scholar]
- 55.Wood S, Scheipl F. gamm4: Generalized additive mixed models using mgcv and lme4. R Packag. 2014 version 0.2-3. [Google Scholar]
- 56.van Rij J, Wieling M. itsadug: Interpreting Time Series and Autocorrelated Data Using GAMMs. R Packag. 2016 version 2.2. [Google Scholar]
- 57.R Development Core Team. R: A Language and Environment for Statistical Computing. 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Soil detritivore feeding activity, soil abiotic data and R codes for the statistical analysis are available by direct request to the corresponding author.