Abstract
Objective
The present study aimed to compare a range of cooling methods possibly utilised by occupational workers, focusing on their effect on body temperature, perception and manual dexterity.
Methods
Ten male participants completed eight trials involving 30 min of seated rest followed by 30 min of cooling or control of no cooling (CON) (34°C, 58% relative humidity). The cooling methods utilised were: ice cooling vest (CV0), phase change cooling vest melting at 14°C (CV14), evaporative cooling vest (CVEV), arm immersion in 10°C water (AI), portable water-perfused suit (WPS), heliox inhalation (HE) and ice slushy ingestion (SL). Immediately before and after cooling, participants were assessed for fine (Purdue pegboard task) and gross (grip and pinch strength) manual dexterity. Rectal and skin temperature, as well as thermal sensation and comfort, were monitored throughout.
Results
Compared with CON, SL was the only method to reduce rectal temperature (P = 0.012). All externally applied cooling methods reduced skin temperature (P<0.05), though CV0 resulted in the lowest skin temperature versus other cooling methods. Participants felt cooler with CV0, CV14, WPS, AI and SL (P<0.05). AI significantly impaired Purdue pegboard performance (P = 0.001), but did not affect grip or pinch strength (P>0.05).
Conclusion
The present study observed that ice ingestion or ice applied to the skin produced the greatest effect on rectal and skin temperature, respectively. AI should not be utilised if workers require subsequent fine manual dexterity. These results will help inform future studies investigating appropriate pre-cooling methods for the occupational worker.
Introduction
There is a fine balance between maintaining productivity and safety of individuals working in environmental extremes. While high environmental temperatures will result in body heat gain at rest [1], the additional heat production from physical activity further hastens heat storage in these environments [2]. The resultant increase in body temperature is associated with reductions in work capacity [3–5]. Further, continuation of physical activity in these environments may result in serious heat-related injury or even death [6].
Alleviating thermal strain during work in hot conditions may be possible with some form of cooling before work. Broadly, an individual may utilise external or internal cooling methods [7,8]. External cooling involves the application of a cooling medium (e.g. cold water, ice vest) to an individual’s skin. The cooled skin may subsequently cool the cutaneous circulating blood and abate the rise in deep body temperature during work [9,10]. Internal cooling involves an individual ingesting (e.g. ice slushy) or inhaling (e.g. cold air) a medium capable of cooling. Both internal cooling methods may result in a decrease in deep body temperature, with little change in skin temperature [7].
Using internal or external cooling strategies before work (i.e. pre-cooling) may improve performance or reduce thermal strain during fixed exercise intensity [11–16]. Specific to occupational workers, Tokizawa [17] was able to reduce participants’ thermal strain during walking in the heat (37°C, 40% relative humidity) dressed in a chemical protective garment with the use of 30 min of pre-cooling using a fan and water sprayed over the entire body. Similarly, a reduction in thermal strain during work was achieved with ice slushy ingestion before walking in the heat (39°C, 18% relative humidity) dressed in wildland firefighting garments [18].
Deciding on the cooling method to utilise involves consideration of the effectiveness (of cooling), access, transport, time and cost [14–16]. Within occupational settings, particularly emergency response teams, the unknown location, resources and time available are all factors that may influence the choice of pre-cooling method. At present, there are no clear guides and limited data in an occupational context on the optimal length of time a cooling method should be applied for before work. This may result in individuals undertaking a cooling protocol for a longer period than necessary. Additionally, individuals should also consider any detrimental impact the type of pre-cooling method may have on subsequent occupational task requirements [19]. For example, while arm immersion may be successful in reducing thermal strain [20], cooling the arms may subsequently negatively impact manual dexterity [21].
Unfortunately, many investigations assessing the effect of cooling on body temperature are limited by the number of pre-cooling methods compared. Investigations often compare a single pre-cooling method against a control of no cooling [22,23], where others have compared only two [12,24,25] or three [15,26] pre-cooling methods. Therefore, the aim of the present study is to compare a broad range of pre-cooling methods as a repeated measures design, focusing on their effect on body temperature and manual dexterity. As the present study focuses on those who may utilise cooling before work in an occupational setting, the opportunity to assess a potentially novel internal cooling method, Heliox inhalation, is investigated. It is hypothesised that external cooling methods will lower skin temperature, while ice slushy ingestion will lower deep body temperature. Additionally, it is hypothesised manual dexterity will be negatively impacted by arm immersion only.
Methods
This study was approved by the Queensland University of Technology’s Human Research Ethics Committee and complied with standards set in the Declaration of Helsinki. The participants were made aware of the purpose, procedures and risks of the study before giving their informed written consent. Ten male participants volunteered; their physical characteristics were as follows (mean [SD]): 23 (3) years of age, height of 180 (6) cm, body mass of 86 (7) kg and body fat of 26 (8) %. All participants were non-smokers and free from any vascular, blood and respiratory conditions. Participants were instructed to refrain from alcohol, caffeine and strenuous exercise in the 24 h preceding each visit to the laboratory.
Experimental sessions
Following familiarisation, participants attended the laboratory for eight experimental sessions at the same time of day, separated by a minimum of 24 h. Within each visit participants remained seated in a climate controlled chamber (dry bulb temperature 34.4 [0.5] °C, wet bulb temperature 27.6 [0.8] °C, relative humidity 58 [4] %) for a baseline period of 30 min followed by 30 min of cooling application or control (CON) of no cooling (detailed below). Environmental temperature and humidity were recorded throughout and measured using a wet bulb globe thermometer (QUESTemp 36, 3M, Minnesota, USA). The order of testing was randomised using a random number generator (Research Randomiser, v4, Social Psychology Network). Participants wore the same t-shirt, shorts and shoes for each trial. Where applicable, the cooling garment was applied over participant’s clothing.
Immediately before and after cooling, participants were asked to perform a battery of tests to assess their manual dexterity. These tests included: 1) Purdue Pegboard test, 2) grip strength and 3) pinch strength. The Purdue Pegboard test (Model 32020, Lafayette Instrument, Lafayette, USA) was utilised to assess fine manual dexterity where participants are required to place pins into the right- or left-hand row of vertical holes in the board for 30 s. Separated by 30 s, participants started with their dominant hand first, followed by their non-dominant hand and then each hand simultaneously. All three scores were summed. Following this, grip and then pinch strength was assessed using a dynamometer (Digital Multi-Myometer, MIE Medical Research Ltd., Leeds, UK). For grip strength, participants were measured with the shoulder at 0° flexion, elbow at 90° and their wrists in a neutral position. For pinch strength, the shoulder and elbow were positioned the same as for grip strength, whereas the wrist was pronated with participants pinching the dynamometer between the thumb and index finger only. Peak force (N) of grip and pinch strength were averaged over three attempts that were held for 3 s, each separated by 60 s.
Cooling methods
Cooling vests
Three different cooling vests were tested: 1) an ice-based cooling vest (CV0), stored in a -20°C freezer (ICEEPAK Australia, Mooloolaba, Australia); 2) a non-ice-based cooling vest with a melting temperature of 14°C (CV14), stored in a 4°C fridge (KewlFit, Model 6626-PEV, TechNiche, Vista, USA); 3) an evaporative cooling vest, immersed in water (~17°C) for at least 2 min immediately before the commencement of cooling (CVEV) (KewlShirt, Model 6201, TechNiche, Vista, USA).
Arm immersion (AI)
Participants sat in a collapsible chair (Kore Kooler Rehab Chair, DQE, Indianapolis, USA) with disposable bags placed in the troughs built into the armrests and filled with cold water (10.4 [0.7] °C) immediately before arm immersion. Participants were instructed to fully immerse their hands and forearms to approximately 5 cm above the medial epicondyle of the humerus. No attempt was made to maintain the water temperature which rose to 19.9 (0.8) °C by the end of cooling.
Water-perfused suit (WPS)
Participants donned a three-piece portable battery-operated water-perfused suit (WPS; BCS4 Cooling System, Med-Eng, Ottawa, Canada) that covered the entire body, except the face, hands and feet. The WPS consists of tubing sewn into a stretchable pullover, trousers and hood. Water was circulated at ~375 mL·min–1 from an integrated portable pump (Delta Wing Pump, Med-Eng, Ottawa, Canada) connected to a specifically designed bottle which initially contained 90% ice and 10% water. This resulted in ~10°C water entering the WPS when first turned on. Like the AI protocol, no attempt was made to maintain water temperature.
Heliox (HE)
Participants breathed a mixture of 21% O2 and 79% He (BOC Limited, Ipswich, Australia) from a Douglas bag attached to a two-way T-shape non-rebreathing valve (Model 2700, Hans-Rudolph, Shawnee, USA) and head support (Model 2726, Hans-Rudolph, Shawnee, USA). The temperature of the heliox mixture was matched to the ambient temperature (i.e. 35°C).
Ice slushy (SL)
Participants ingested 7.5 g·kg–1 of ice slushy (-2.2 [0.4] °C) at a rate of 1.25 g·kg–1 every 5 min to standardise the ingestion rate [11]. Each drink was prepared using a slushy machine (Model SSM-180, ICETRO, Incheon, South Korea) with the same flavouring used for each participant (Fruchilla Natural 99% Fruit Juice, The Slushie Specialists, Bentleigh East, Australia).
Measurements and calculations
Body composition was measured using dual-energy X-ray absorptiometry (Lunar Prodigy, GE Healthcare Lunar, Madison, USA) and analysed using dedicated software (enCORE, version 9, GE Healthcare Lunar, Madison, USA). Pre-trial hydration status was confirmed by urine specific gravity (PAL 10s, ATAGO, Tokyo, Japan) of <1.020 [27]. If participants provided a sample >1.020 they were given an additional 500 mL of tap water, which was consumed 30 min before the commencement of the trial.
The experiments followed the termination criteria set in ISO 12894 [28]; however, no participants terminated early. Deep body temperature was estimated from rectal temperature (Trec) using a thermistor (YSI 400, DeRoyal, Knox, USA) self-inserted 12 cm beyond the anal sphincter and recorded using a data logger (Squirrel 2020 series, Grant Instruments, Cambridge, UK). Mean skin temperature () was estimated using wireless iButton thermocrons (DS1922L-F50 iButtons, Maxim Integrated, San Jose, USA) attached to eight sites using a single piece of adhesive tape (Premium Sports Tape, AllCare, Kumeu, New Zealand) and calculated as (ISO 9886 [29]):
Mean body temperature () was calculated as [30]:
Both Trec and were recorded at 2 s intervals, averaged per min and analysed every 5 min. The temperature of SL and water during AI were measured using a calibrated thermometer (TL-1W, ThermoProbe, Pearl, USA). Thermal sensation was assessed using a modified scale [31], where 1 had the anchor of ‘unbearably cold’, 7 ‘neutral’ and 13 ‘unbearably hot’. Similarly, thermal comfort was assessed using a modified scale [31], where 1 had the anchor of ‘comfortable’ and 5 ‘extremely uncomfortable’. Both thermal sensation and thermal comfort were recorded every 5 min.
Statistical analyses
Statistical analyses were conducted using SPSS version 23 for Windows (IBM Corporation, New York, USA). An α of 0.05 was used to determine statistical significance. Data were assessed for normality with a Shapiro-Wilk test and visual inspection of data (e.g. boxplots). Baseline values between cooling methods were compared using a one-way repeated measures analysis of variance. As baseline physiological responses were similar between cooling methods (see Results), delta (Δ) Trec, and thermal sensation and comfort were compared between cooling methods and CON (i.e. trial) across time using a two-way repeated measures analysis of variance.
Additionally, ΔTrec, and were analysed within trial over time, with time points analysed sequentially. That is, the first comparison was always made between baseline and subsequent time points (e.g. 0 vs 5, 0 vs 10). When a significant difference was observed (e.g. 10th min), that time point was compared to the next time point (e.g. 10 vs 15). These steps were repeated until all pairwise comparisons were conducted (i.e. six for each trial). Hand Tsk and manual dexterity variables between cooling methods and CON were analysed before and after cooling using a two-way repeated measures analysis of variance. When a main effect or significant interaction was achieved, paired samples t-tests were conducted with Bonferroni adjustments applied for multiple comparisons.
Effect sizes were calculated for pairwise comparisons using Cohen’s dav [32] and interpreted as small (0.2–0.4), moderate (0.5–0.7) or large (≥0.8) [33,34]. All data in text, figures and tables are presented as mean and SD.
Results
Physiological responses
All baseline physiological variables were similar between trials (Table 1; P > 0.05).
Table 1. Baseline rectal, mean skin and mean body temperature.
| Trec (°C) | (°C) | (°C) | |
|---|---|---|---|
| CON | 37.2 (0.1) | 34.8 (0.4) | 36.7 (0.1) |
| CV0 | 37.3 (0.2) | 34.7 (0.6) | 36.8 (0.3) |
| CV14 | 37.4 (0.2) | 35.0 (0.4) | 36.9 (0.2) |
| CVEV | 37.3 (0.2) | 34.8 (0.3) | 36.8 (0.1) |
| AI | 37.3 (0.2) | 34.9 (0.6) | 36.8 (0.2) |
| WPS | 37.3 (0.2) | 34.7 (0.4) | 36.7 (0.2) |
| SL | 37.3 (0.2) | 34.8 (0.6) | 36.8 (0.3) |
| HE | 37.3 (0.2) | 35.1 (0.5) | 36.8 (0.2) |
Delta rectal temperature
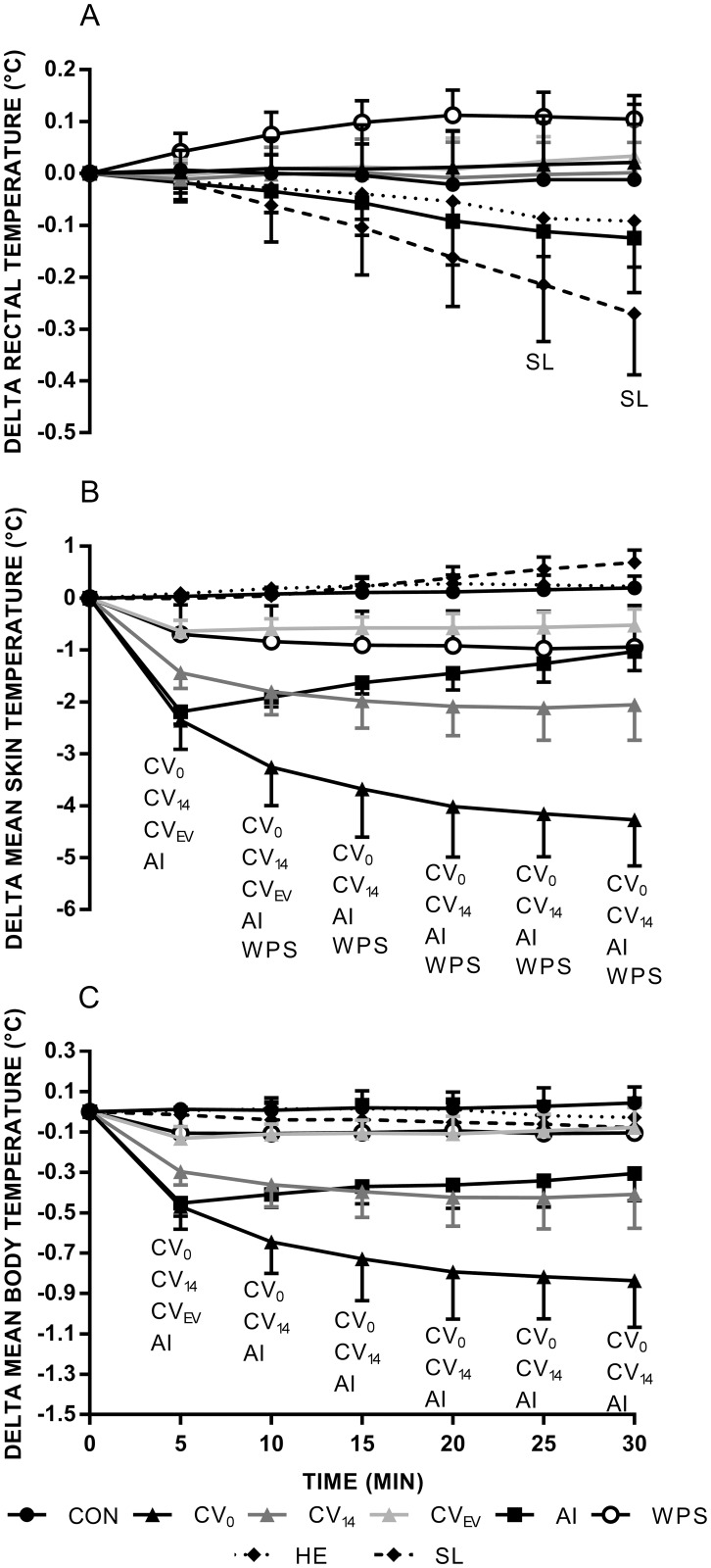
There was a significant main effect for trial (P < 0.001), time (P = 0.001) and interaction (P < 0.001). Pairwise comparisons of the main effect for trial revealed ΔTrec for WPS was significantly different versus CVEV, AI, HE and SL. Within trial analyses revealed Trec was lowered throughout cooling in SL (0 > 20 > 25 > 30 min), while it increased in WPS (0 < 10 < 15 min). Pairwise comparisons of the interaction revealed CON ΔTrec significantly differed from SL at minute 25 (Fig 1A; P = 0.048, dav = 1.8) and 30 (P = 0.012, dav = 2.3).
Fig 1. Delta rectal (A), mean skin (B) and mean body temperature (C) throughout cooling.
Note: Abbreviations denote significant (P < 0.05) difference from CON.
Delta mean skin temperature
There was a significant main effect for trial (P < 0.001), time (P = 0.036) and interaction (P < 0.001). Pairwise comparisons of the main effect for trial revealed Δ differed between cooling methods (CV0 < CV14, AI < CVEV < CON, HE, SL). WPS produced a lower compared with HE and SL but was greater than CV0. Within trial analyses revealed increased over time for CON (0 < 30 min), SL (0 < 20 < 25 < 30 min) and HE (0 < 15 min), and lowered over time for CV0 (0 > 5 > 10 > 20 min), CV14 (0 > 5 > 10 > 20 min) and CVEV (0 > 5 min). Initially AI lowered but subsequently rose (0 > 5 < 10 < 15 < 25 < 30 min). Pairwise comparisons of the interaction revealed CON differed from CV0 (Fig 1B; P < 0.001, dav = 7.1–9.1), CV14 (P < 0.001, dav = 5.8–8.1), CVEV (P < 0.001, dav = 3.4–5.0), AI (P < 0.001, dav = 5.3–14.7) and WPS (P = 0.032–0.039, dav = 2.5–2.7) throughout cooling.
Delta mean body temperature
There was a significant main effect for trial (P < 0.001), time (P = 0.010) and interaction (P < 0.001). Pairwise comparisons of the main effect for trial revealed was significantly lower in CV0 than all other trials (P < 0.05) except CV14 (P > 0.05). was lower in CV14 and AI compared with CVEV, HE, SL and CON (P < 0.004). WPS did not differ from CON (P > 0.05). Within trial analyses revealed was lowered over time for CV0 (0 > 5 > 10 > 25 min), CV14 (0 > 5 min) and CVEV (0 > 5 min). AI initially lowered but was soon followed by a subsequent increase (0 > 5 < 10 min). Pairwise comparisons of the interaction revealed CON differed from CV0 (Fig 1C; P < 0.001, dav = 5.1–6.8), CV14 (P < 0.001, dav = 3.7–6.5), CVEV (P = 0.001, dav = 3.3) and AI (P = 0.001–0.002, dav = 3.2–9.9) throughout cooling.
Hand skin temperature
There was a significant main effect for trial (P < 0.001), time (P = 0.010) and interaction (P < 0.001). Hand Tsk was similar between cooling methods before cooling (CON: 35.0 [0.5] °C). Pairwise comparisons revealed at the end of cooling hand Tsk was cooler following AI (19.6 [1.0] °C) compared with CON (35.3 [0.3] °C; P < 0.001, dav = 24.1).
Manual dexterity
Purdue pegboard
There was a significant main effect for trial (P = 0.031), time (P = 0.004) and interaction (P < 0.001). Pairwise comparisons revealed participants achieved a lower score following AI compared with CON (Table 2; P = 0.001, dav = 1.7).
Table 2. Purdue pegboard, grip strength and pinch strength before and after cooling.
| Purdue (score) | Grip Strength (N) | Pinch Strength (N) | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| CON | 67 (5) | 64 (8) | 522 (61) | 511 (55) | 81 (17) | 70 (16) |
| CV0 | 62 (4) | 65 (3) | 517 (61) | 478 (45) | 76 (7) | 74 (11) |
| CV14 | 64 (11) | 66 (9) | 498 (52) | 477 (46) | 78 (13) | 74 (13) |
| CVEV | 62 (6) | 62 (5) | 493 (49) | 476 (58) | 78 (14) | 74 (12) |
| AI | 66 (4) | 49 (5) a | 500 (57) | 485 (58) | 84 (17) | 82 (19) |
| WPS | 67 (5) | 66 (5) | 525 (66) | 509 (48) | 74 (17) | 70 (18) |
| SL | 66 (5) | 64 (4) | 521 (66) | 500 (52) | 73 (15) | 71 (12) |
| HE | 65 (6) | 66 (5) | 518 (51) | 525 (51) | 78 (17) | 74 (19) |
aSignificant difference from CON after cooling (P < 0.05).
N, Newtons.
Grip and pinch strength
For grip strength, there was a significant main effect for trial (P = 0.048) and time (P = 0.004), but no interaction (P = 0.272). Pairwise comparisons revealed participants achieved greater scores before versus after cooling (Table 2; P = 0.028, dav = 0.4).
For pinch strength, there was a significant main effect for trial (P = 0.034) and time (P = 0.001), but no interaction (P = 0.063). Like grip strength, pairwise comparisons revealed participants achieved greater scores before versus after cooling (Table 2; P = 0.001, dav = 0.3).
Perceptual responses
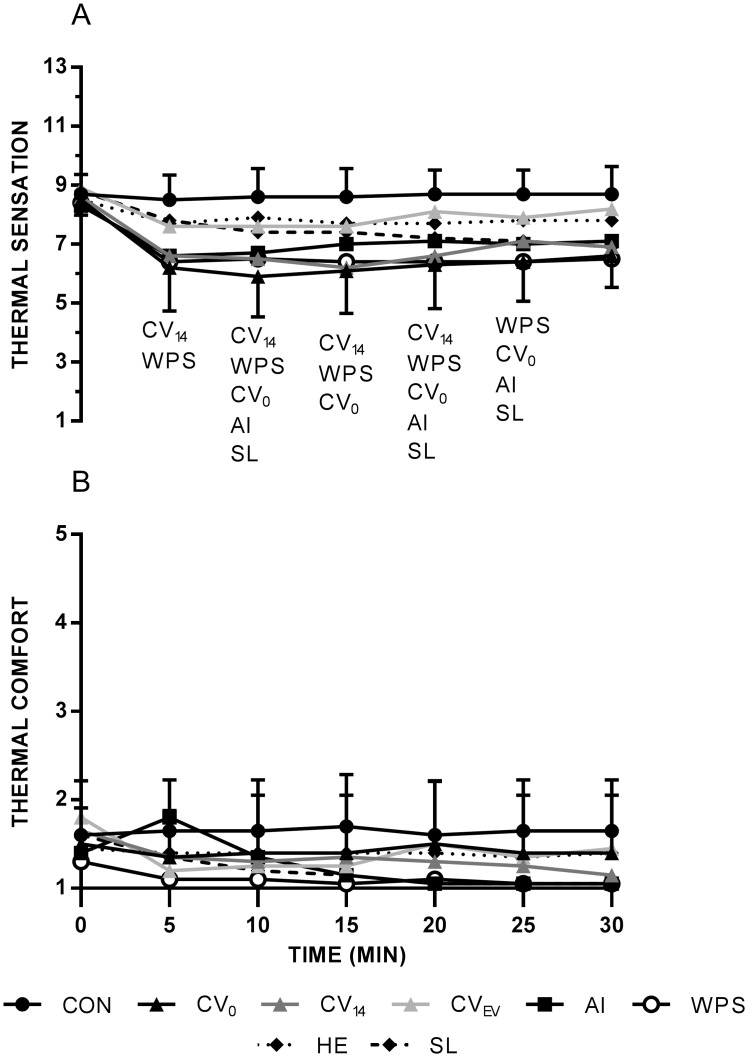
For thermal sensation, there was a significant main effect for trial (P < 0.001), time (P = 0.010) and interaction (P < 0.001). Pairwise comparisons of the main effect for trial revealed compared with CON thermal sensation was lower in CV0, CV14, AI, SL and WPS (P < 0.05). Further, thermal sensation was lower during the WPS trial compared with CVEV and HE (P < 0.05). Pairwise comparisons for the interaction revealed CON differed from CV0 (Fig 2A; P = 0.027–0.047, dav = 2.1–2.3), CV14 (P = 0.09–0.018, dav = 2.3–2.5), AI (P = 0.020–0.033, dav = 2.0–2.8), SL (P = 0.017–0.047, dav = 1.6–2.3) and WPS (P = 0.007–0.045, dav = 2.3–2.5).
Fig 2. Thermal sensation (A) and comfort (B) throughout cooling.
Note: For reader clarity, SD is shown for CON and CV0 only. Abbreviations denote significant (P < 0.05) difference from CON.
For thermal comfort, there was a significant main effect for trial (P = 0.001), time (P = 0.001) and interaction (P < 0.001). However, no significant pairwise comparisons were revealed for the main effect of cooling method or interaction (Fig 2B; P > 0.05).
Discussion
This study adds to the current literature by providing data from seven methods that could be utilised for cooling before work and their effect on body temperature, dexterity and thermal perception. The primary findings were that SL was the only cooling method to reduce deep body temperature (Fig 1A) and all external cooling methods reduced (Fig 1B). AI reduced Purdue pegboard performance by 23%, with no effect on grip or pinch strength (Table 2).
Normothermic individuals who have ingested SL have experienced a decrease in Trec between 0.3°C to 0.66°C [11,24,25,35,36]. The magnitude of Trec reduction is similar to previous studies when an ice slushy was consumed in a warm environment (>30°C) [24,25,36]. In contrast, Siegel et al. [11] asked participants to consume an ice slushy in an ambient temperature of 24°C and reported a 0.66°C reduction in Trec. It is likely that when an ice slushy is consumed in a warm environment there appears to be a more modest reduction in Trec due to a reduced dry heat loss.
Occupational settings, such as firefighting, may sometimes require breathing apparatus filled with compressed air, which could be replaced with other gas mixtures. The effect of inhaled HE on deep body temperature in animal models is equivocal [37–39]. Data from human studies are limited, with one study reporting a reduction in deep body temperature when inhaling heliox within a hyperbaric environment [40]. However, HE did not reduce deep body temperature in the present study (Fig 1A). The reason for this may relate to the gas characteristics. Thermal conductivity of helium is six times that of nitrogen [41], however, respiratory heat loss is dependent on thermal capacity [42,43]. Thus, despite a greater specific heat capacity for helium versus nitrogen, helium has a lower density [41] and therefore a lower thermal capacity (i.e. thermal capacity = specific heat × density).
As hypothesised, all external cooling methods reduced but did not reduce Trec. Previous research has demonstrated that application of an ice vest to normothermic individuals does not reduce deep body temperature [9,12,26]. Similarly, non-ice based cooling vests consistently shows no reduction in deep body temperature [12,44,45], aligning with the present study’s findings.
Considering work capability (performance or capacity) is limited, in part, by high deep body temperatures [46], the pre-cooling method that has the greatest effect on deep body temperature would appear the most optimal choice. Despite no Trec reduction within 30 min of external cooling in the present study, these methods may abate the rise in body temperature during subsequent work. This is supported by research that has shown external cooling to reduce thermal strain when applied before work in the heat in protective clothing [17]. Thus, considering SL and CV0 provided the greatest cooling (physiologically and perceptually) and are relatively accessible, future work should focus on utilising these cooling methods before work in the heat and protective clothing.
Pre-cooling protocols are varied in their length of application, ranging from 20 min [26,47] to 45 min [12] with little rationale. The present study demonstrated CV0 and CV14 resulted in changes within 5 min and further reductions were observed up until the 20th min. However, CVEV only produced a reduction in within 5 min, with AI initially lowering and then subsequently increasing. Practically, if an individual has only 5 min for pre-cooling then these methods will result in lower , but CVEV and AI will not result in further benefits past this. As no further reductions in were observed following 20 min of cooling using the ice or non-ice cooling vest it is suggested this is an optimal cooling duration.
Colder hand and finger Tsk are associated with reductions in manual dexterity [48]. Previous research has demonstrated extremity cooling results in a reduction in both fine (e.g. Purdue pegboard) [49,50] and gross task performance (e.g. grip strength) [51,52]. In the present study, however, AI lowered hand Tsk and adversely affected Purdue pegboard performance, but did not affect grip or pinch strength (Table 2). The reason for the lack of effect of cold extremities on grip and pinch strength in the present study is unclear, however, it is obvious that fine tasks are most affected by cold extremities.
While the present study reports the responses to a broad range of pre-cooling methods, the results may not accurately translate to all individuals. Factors such as age, sex, body mass, surface area-to-mass ratio and body composition may influence responses to cooling [53]. Despite this, while the magnitude of responses may differ between distinctive groups, the pre-cooling methods that are most effective may not.
It is concluded that while SL will lower deep body temperature, the external cooling methods used in this study will lower , with no reductions in deep body temperature. The user should be mindful that using external cooling methods similar to the present study provides no further cooling past 20 min. Finally, cooling the extremities may compromise manual dexterity, and therefore individuals requiring fine manual dexterity should opt for an alternative pre-cooling method.
Acknowledgments
The authors thank the participants for their time.
Data Availability
The data are publicly available in the Dryad Digital Repository and can be located on the web page (https://datadryad.org/) using the DOI number: 10.5061/dryad.np58q.
Funding Statement
This project is financially supported by the US Government through the Technical Support Working Group within the Combating Terrorism Technical Support Office. This support does not represent an endorsement of the contents or conclusions of the project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hardy JD, Du Bois EF. Basal metabolism, radiation, convection and vaporization at temperatures of 22 to 35°C. Journal of Nutrition. 1938;15: 477–497. [Google Scholar]
- 2.Gagge AP, Gonzalez RR. Mechanisms of heat exchange: biophysics and physiology In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4: Environmental Physiology. New York: Oxford University Press; 1996. pp. 45–84. doi: 10.1002/cphy.cp040104 [Google Scholar]
- 3.Dill DB, Edwards HT, Bauer PS, Levenson EJ. Physical performance in relation to external temperature. Arbeitsphysiologie. 1931;4: 508–518. doi: 10.1007/BF02010116 [Google Scholar]
- 4.Costello JT, Stewart KL, Stewart IB. The effects of metabolic work rate and ambient environment on physiological tolerance times while wearing explosive and chemical personal protective equipment. BioMed Research International. 2015;857536: 7 pages. doi: 10.1155/2015/857536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart IB, Stewart KL, Worringham CJ, Costello JT. Physiological tolerance times while wearing explosive ordnance disposal protective clothing in simulated environmental extremes. PLoS ONE. 2014;9: e83740 doi: 10.1371/journal.pone.0083740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang J, Hansen A, Pisaniello D, Bi P. Extreme heat and occupational heat illnesses in South Australia, 2001–2010. Occupational and environmental medicine. 2015;72: 580–586. doi: 10.1136/oemed-2014-102706 [DOI] [PubMed] [Google Scholar]
- 7.Ross M, Abbiss C, Laursen P, Martin D, Burke L. Precooling methods and their effects on athletic performance: A systematic review and practical applications. Sports Medicine. 2013;43: 207–225. doi: 10.1007/s40279-012-0014-9 [DOI] [PubMed] [Google Scholar]
- 8.Bongers CCWG, Hopman MTE, Eijsvogels TMH. Cooling interventions for athletes: an overview of effectiveness, physiological mechanisms, and practical considerations. Temperature. 2017;4: 60–78. doi: 10.1080/23328940.2016.1277003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price MJ, Boyd C, Goosey-Tolfrey VL. The physiological effects of pre-event and midevent cooling during intermittent running in the heat in elite female soccer players. Applied physiology, nutrition, and metabolism. 2009;34: 942–949. doi: 10.1139/H09-078 [DOI] [PubMed] [Google Scholar]
- 10.Kay D, Taaffe DR, Marino FE. Whole-body pre-cooling and heat storage during self-paced cycling performance in warm humid conditions. Journal of Sports Sciences. 1999;17: 937–944. doi: 10.1080/026404199365326 [DOI] [PubMed] [Google Scholar]
- 11.Siegel R, Maté J, Brearley MB, Watson G, Nosaka K, Laursen PB. Ice slurry ingestion increases core temperature capacity and running time in the heat. Medicine and Science in Sports and Exercise. 2010;42: 717–725. doi: 10.1249/MSS.0b013e3181bf257a [DOI] [PubMed] [Google Scholar]
- 12.Bogerd N, Perret C, Bogerd CP, Rossi RM, Daanen HAM. The effect of pre-cooling intensity on cooling efficiency and exercise performance. Journal of Sports Sciences. 2010;28: 771–779. doi: 10.1080/02640411003716942 [DOI] [PubMed] [Google Scholar]
- 13.Duffield R, Green R, Castle P, Maxwell N. Precooling can prevent the reduction of self-paced exercise intensity in the heat. Medicine and Science in Sports and Exercise. 2010;42: 577–584. doi: 10.1249/MSS.0b013e3181b675da [DOI] [PubMed] [Google Scholar]
- 14.Minett GM, Duffield R, Marino FE, Portus M. Duration-dependant response of mixed-method pre-cooling for intermittent-sprint exercise in the heat. European Journal of Applied Physiology. 2012;112: 3655–3666. doi: 10.1007/s00421-012-2348-2 [DOI] [PubMed] [Google Scholar]
- 15.Minett GM, Duffield R, Marino FE, Portus M. Volume-dependent response of precooling for intermittent-sprint exercise in the heat. Medicine and Science in Sports and Exercise. 2011;43: 1760–1769. doi: 10.1249/MSS.0b013e318211be3e [DOI] [PubMed] [Google Scholar]
- 16.Minett GM, Duffield R, Kellett A, Portus M. Mixed-method pre-cooling reduces physiological demand without improving performance of medium-fast bowling in the heat. Journal of Sports Sciences. 2012;30: 907–915. doi: 10.1080/02640414.2012.679677 [DOI] [PubMed] [Google Scholar]
- 17.Tokizawa K, Sawada S, Oka T, Yasuda A, Tai T, Ida H, et al. Fan-precooling effect on heat strain while wearing protective clothing. International journal of biometeorology. 2014;58: 1919–1925. doi: 10.1007/s00484-014-0794-8 [DOI] [PubMed] [Google Scholar]
- 18.Pryor RR, Suyama J, Guyette FX, Reis SE, Hostler D. The effects of ice slurry ingestion before exertion in Wildland firefighting gear. Prehospital emergency care. 2015;19: 241–246. doi: 10.3109/10903127.2014.959221 [DOI] [PubMed] [Google Scholar]
- 19.Chan APC, Song W, Yang Y. Meta-analysis of the effects of microclimate cooling systems on human performance under thermal stressful environments: Potential applications to occupational workers. Journal of Thermal Biology. Elsevier; 2015;49–50: 16–32. doi: 10.1016/j.jtherbio.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 20.DeGroot DW, Gallimore RP, Thompson SM, Kenefick RW. Extremity cooling for heat stress mitigation in military and occupational settings. Journal of Thermal Biology. 2013;38: 305–310. doi: 10.1016/j.jtherbio.2013.03.010 [Google Scholar]
- 21.Heus R, Daanen HAM, Havenith G. Physiological criteria for functioning of hands in the cold: a review. Applied Ergonomics. 1995;26: 5–13. doi: 10.1016/0003-6870(94)00004-I [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann M, Landers G, Wallman KE, Saldaris J. The effects of crushed ice ingestion prior to steady state exercise in the heat. International Journal of Sport Nutrition and Exercise Metabolism. 2017;27: 120–127. doi: 10.1123/ijsnem.2015-0215 [DOI] [PubMed] [Google Scholar]
- 23.Taylor L, Mauger AR, Watkins SL, Fitch N, Brewer J, Maxwell NS, et al. Precooling does not improve 2,000-m rowing performance of females in hot, humid conditions. Journal of Strength and Conditioning Research. 2014;28: 3416–3424. doi: 10.1519/JSC.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 24.James CA, Richardson AJ, Watt PW, Gibson OR, Maxwell NS. Physiological responses to incremental exercise in the heat following internal and external precooling. Scandinavian Journal of Medicine and Science in Sports. 2015;25: 190–199. doi: 10.1111/sms.12376 [DOI] [PubMed] [Google Scholar]
- 25.Siegel R, Maté J, Watson G, Nosaka K, Laursen PB. Pre-cooling with ice slurry ingestion leads to similar run times to exhaustion in the heat as cold water immersion. Journal of Sports Sciences. 2012;30: 155–165. doi: 10.1080/02640414.2011.625968 [DOI] [PubMed] [Google Scholar]
- 26.Castle PC, Macdonald AL, Philp A, Webborn A, Watt PW, Maxwell NS. Precooling leg muscle improves intermittent sprint exercise performance in hot, humid conditions. Journal of applied physiology. 2006;100: 1377–1384. doi: 10.1152/japplphysiol.00822.2005 [DOI] [PubMed] [Google Scholar]
- 27.Armstrong LE. Hydration assessment techniques. Nutrition reviews. 2005;63: S40–S54. [DOI] [PubMed] [Google Scholar]
- 28.International Organisation for Standardisation. ISO 12894: Ergonomics of the thermal environment—Medical supervision of individuals exposed to extreme hot or cold environments. Geneva: International Organisation for Standardisation; 2001.
- 29.International Organisation for Standardisation. ISO 9886: Ergonomics—Evaluation of thermal strain by physiological measurements. Geneva: International Organisation for Standardisation; 2004. 10.1016/B978-075067555-0/50157-2
- 30.Hardy JD, Du Bois EF. The technic of measuring radiation and convection. Journal of Nutrition. 1938;15: 461–475. [Google Scholar]
- 31.Gagge AP, Stolwijk JAJ, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environmental Research. 1967;1: 1–20. [DOI] [PubMed] [Google Scholar]
- 32.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4: 863 doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 34.Cohen J. A power primer. Psychological bulletin. 1992;112: 155–159. [DOI] [PubMed] [Google Scholar]
- 35.Stevens CJ, Thoseby B, Sculley D V., Callister R, Taylor L, Dascombe BJ. Running performance and thermal sensation in the heat are improved with menthol mouth rinse but not ice slurry ingestion. Scandinavian Journal of Medicine and Science in Sports. 2016;26: 1209–1216. doi: 10.1111/sms.12555 [DOI] [PubMed] [Google Scholar]
- 36.Naito T, Ogaki T. Pre-cooling with intermittent ice ingestion lowers the core temperature in a hot environment as compared with the ingestion of a single bolus. Journal of Thermal Biology. Elsevier; 2016;59: 13–17. doi: 10.1016/j.jtherbio.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 37.Harrison MR, Hysing ES, Bo G. Control of body temperature: use of the respiratory tract as a heat exchanger. Journal of pediatric surgery. 1977;12: 821–828. [DOI] [PubMed] [Google Scholar]
- 38.Tapper D, Arensman R, Johnson C, Folkman J. The effect of helium-oxygen mixtures on body temperature. Journal of Pediatric Surgery. 1974;9: 597–603. doi: 10.1016/0022-3468(74)90094-3 [DOI] [PubMed] [Google Scholar]
- 39.David HN, Haelewyn B, Chazalviel L, Lecocq M, Degoulet M, Risso J-J, et al. Post-ischemic helium provides neuroprotection in rats subjected to middle cerebral artery occlusion-induced ischemia by producing hypothermia. Journal of Cerebral Blood Flow & Metabolism. 2009;29: 1159–1165. doi: 10.1038/jcbfm.2009.40 [DOI] [PubMed] [Google Scholar]
- 40.Piantadosi CA, Thalmann ED, Spaur WH. Metabolic response to respiratory heat loss-induced core cooling. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;50: 829–834. [DOI] [PubMed] [Google Scholar]
- 41.Gagge AP, Nishi Y. Heat exchange between human skin surface and thermal environment In: Lee DH., Falk HL, Murphy SD, editors. Handbook of Physiology: Reactions to Environmental Agents. Bethesda, MD: American Physiological Society; 1977. pp. 69–92. doi: 10.1002/cphy.cp090105 [Google Scholar]
- 42.Cain JB, Livingstone SD, Nolan RW, Keefe AA. Respiratory heat loss during work at various ambient temperatures. Respiration Physiology. 1990;79: 145–150. doi: 10.1016/0034-5687(90)90014-P [DOI] [PubMed] [Google Scholar]
- 43.Cramer MN, Jay O. Biophysical aspects of human thermoregulation during heat stress. Autonomic Neuroscience: Basic and Clinical. Elsevier B.V.; 2016;196: 3–13. doi: 10.1016/j.autneu.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 44.Quod MJ, Martin DT, Laursen PB, Gardner AS, Halson SL, Marino FE, et al. Practical precooling: Effect on cycling time trial performance in warm conditions. Journal of Sports Sciences. 2008;26: 1477–1487. doi: 10.1080/02640410802298268 [DOI] [PubMed] [Google Scholar]
- 45.Filingeri D, Fournet D, Hodder S, Havenith G. Mild evaporative cooling applied to the torso provides thermoregulatory benefits during running in the heat. Scandinavian Journal of Medicine and Science in Sports. 2015;25: 200–210. doi: 10.1111/sms.12322 [DOI] [PubMed] [Google Scholar]
- 46.Nybo L, Rasmussen P, Sawka MN. Performance in the heat-physiological factors of importance for hyperthermia-induced fatigue. Comprehensive Physiology. 2014;4: 657–689. doi: 10.1002/cphy.c130012 [DOI] [PubMed] [Google Scholar]
- 47.Price M, Maley MJ. The effects of ice vest pre-cooling on skin blood flow at rest and during exercise in the heat. Extreme Physiology & Medicine. BioMed Central Ltd; 2015;4: A127 doi: 10.1186/2046-7648-4-S1-A127 [Google Scholar]
- 48.Havenith G, Heus R, Daanen HAM. The hand in the cold, performance and risk. Artic Medical Research. 1995;54: 37–47. [PubMed] [Google Scholar]
- 49.Lockhart JM, Kiess HO, Clegg TJ. Effect of rate and level of lowered finger surface temperature on manual performance. Journal of applied physiology. 1975;60: 106–113. [DOI] [PubMed] [Google Scholar]
- 50.Cheung SS, Montie DL, White MD, Behm D. Changes in manual dexterity following short-term hand and forearm immersion in 10°C water. Aviation Space and Environmental Medicine. 2003;74: 990–993. [PubMed] [Google Scholar]
- 51.Vincent MJ, Tipton MJ. The effects of cold immersion and hand protection on grip strength. Aviation Space and Environmental Medicine. 1988;59: 738–741. [PubMed] [Google Scholar]
- 52.Chi CF, Shih YC, Chen WL. Effect of cold immersion on grip force, EMG, and thermal discomfort. International Journal of Industrial Ergonomics. Elsevier Ltd; 2012;42: 113–121. doi: 10.1016/j.ergon.2011.08.008 [Google Scholar]
- 53.Stocks JM, Taylor NAS, Tipton MJ, Greenleaf JE. Human physiological responses to cold exposure. Aviation, Space and Environmental Medicine. 2004;75: 444–457. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are publicly available in the Dryad Digital Repository and can be located on the web page (https://datadryad.org/) using the DOI number: 10.5061/dryad.np58q.