Genotyping-based treatment decisions optimize care and reduce costs through lower medication utilization and fewer adverse events. Genotyping should be considered for patients with chronic pain.
Keywords: Genotyping, Chronic pain
Abstract
Introduction:
Genotyping-based treatment decisions may optimize treatment response and minimize adverse drug events (ADEs) in patients with chronic pain.
Objectives:
To estimate the financial impact of genotyping-based treatment decisions in patients with moderate to severe chronic pain in a managed care setting.
Methods:
A budget impact model was built with a 1-year time horizon to estimate costs of genotyping-based treatment decisions in a 1000-patient cohort. The model includes drug costs, type and cost of ADEs, distribution of treatments used, and genotyping costs. Event rates and health care costs were derived from primary literature. Three patient cohorts were assessed with and without genotyping-based treatment decisions: no genetic testing; 50% genetic testing; and 100% genetic testing. Sensitivity analysis was performed varying costs, adherence, and the percentage of patients treated according to genotyping results.
Results:
Medical and ADE costs varied by patient severity and genotyping rates. Without genotyping, drug and ADE costs ranged from $1,544,377 to $24,313,844. With genotyping-based treatment, total costs ranged from $1,780,922 to $18,868,032. Sensitivity analysis, varying costs, adherence, and genotyping rates suggested genotyping improves outcomes and is cost saving in patients with chronic pain.
Conclusion:
Genotyping-based treatment costs are offset by reduced medication utilization and adverse event costs. Genotyping should be considered for patients with chronic pain in clinical practice and within clinical trials.
1. Introduction
Chronic noncancer pain is associated with a wide range of injury and disease and is a leading cause of health care utilization in the United States.1,19,23 Conservatively, chronic pain affects nearly 40 percent more people than diabetes, heart disease, and cancer combined.1,6,23 Chronic pain is the most common cause of long-term disability and associated with impaired physical and physiological well-being with significant use of health services.6,27,28,36 In a managed care setting, patients with chronic pain have been estimated to incur nearly $32,000 in direct total costs per year.25
Several large studies have demonstrated an increasing trend in opioid use among noncancer patients with opioids becoming one of the most prescribed classes of medication for chronic noncancer pain in the United States.6,15,30 Increased opioid use brings potential therapeutic benefit and a higher rate of adverse drug events (ADEs). Association between genetic polymorphisms and the analgesic efficacy and clinical outcome of opioid analgesics for noncancer pain provide the potential for personalized treatment approaches leading to lower ADEs and better pain management.19
Greater than half of the variance in pain response to morphine is hypothesized to be related to genetic variation.30 Although there is a strong rationale for expecting benefits from gene-based therapy, the actual impact has not been well-studied.26 The objective of this study was to estimate the potential financial impact of genotyping-based treatment decisions in patients with moderate to severe chronic noncancer pain in a managed care setting.
2. Methods
A budget impact analysis was conducted over a 1-year time horizon using overall and average cost per patient modeling based on a theoretical 1000-patient cohort. This model assumed that all patients suffered chronic noncancer pain. Three 1000-patient cohorts were assessed under varying genotyping-based testing scenarios. Patient cohorts were 100% mild cases; 50% mild and 50% severe cases; and 100% severe cases. The genotyping-based treatment decisions scenarios assessed were no genetic testing (standard of care), 50% genetic testing, and 100% genetic testing. The total annual costs assessed included medical, pharmacy, and genetic testing costs. Sensitivity analysis was performed to assess the robustness of outcomes by varying the model input parameters. The base case value for each parameter was varied from the default value ±50%.
An established model structure reflecting current clinical practice in the treatment of chronic noncancer pain was used.4,11,12,14,16,21,22 The model was built in Microsoft Excel (Microsoft Corporation, Redmond, Washington) with a 1-year time horizon and included direct health care costs. The model was modified (Fig. 1) to include ADE rates, ADE costs, and the potential impact of genetic testing. Event rates, health care costs, and genetic testing impacts were derived from primary literature.5,13,18,21 All costs were reported in 2016 US dollars.
Figure 1.
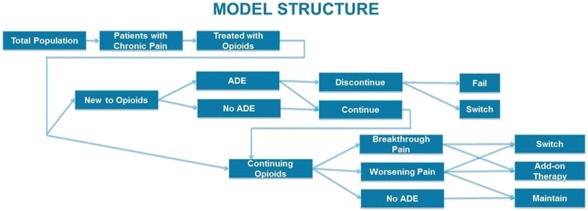
Model structure. ADE, adverse drug event.
2.1. Model inputs
2.1.1. Target population
The analysis focused on patients with mild (treated up to 180 days) and severe (treated more than 120 days) chronic pain treated with opioids. Mild cases were added each quarter over the course of the year and treated for 180 days.21 Mild cases from the previous quarter continued as prevalent patients for the duration of their treatment. Treatment for severe cases started after 120 days of therapy and continued for 1 year.17
2.1.2. Drug treatments
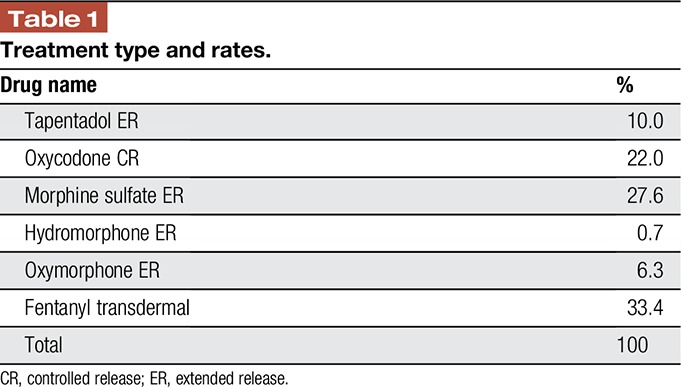
Six treatment options and their rate of use were identified (Table 1).21 The formulary allocation percentages, unit costs, daily average consumption, and copays for each treatment were obtained from the IMS National Prescription Audit reported in Merchant et al,21 adjusted to 2016 dollars. These values were based on a weighted average by using a blended rate for the available strengths for each drug.
Table 1.
Treatment type and rates.
2.1.3. Treatment adverse drug events
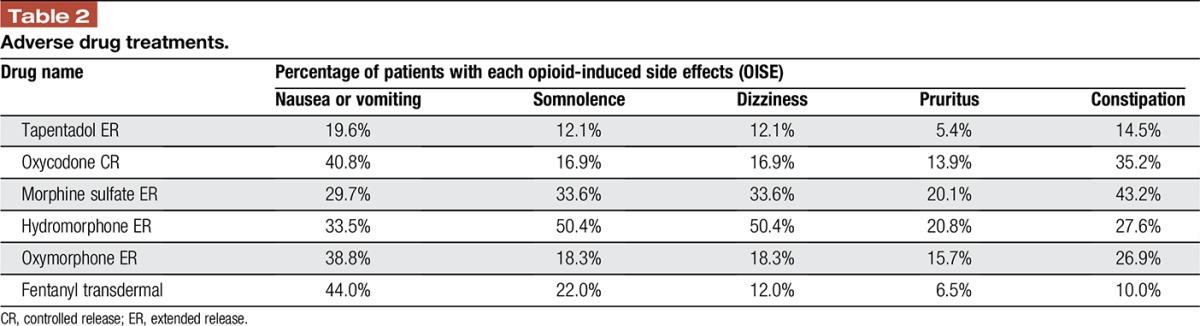
The model accounted for the costs of ADEs associated with each treatment (Table 2).21 The ADE cost was based on Medicare payments for physician office visits, emergency department visits, hospitalization, surgery, diagnostic tests, and other interventions associated with treating opioid-induced side effects (OISEs) and pain management.5 As not all patients seek medical care for treating OISEs or for inadequate pain management (some patients may self-manage), these costs were only assigned to the proportion of patients who received treatment because of an OISE. The model assumed that all patients who switched to another treatment or failed treatment would have an office visit, and that a smaller percentage of patients who failed opioid therapy would undergo other tests and procedures.
Table 2.
Adverse drug treatments.
2.1.4. Genetic testing
The cost of genetic testing was set at $600 per test. Although some literature indicates that genetic testing may improve outcomes by as much as 75%, our model conservatively assessed the possibility of only a 25% improvement expressed increased efficacy, a reduction in ADEs, and reduced resource utilization.2,30,33 The overall budget impact of using genetic testing to optimize treatment was calculated over a 1-year period.
3. Results
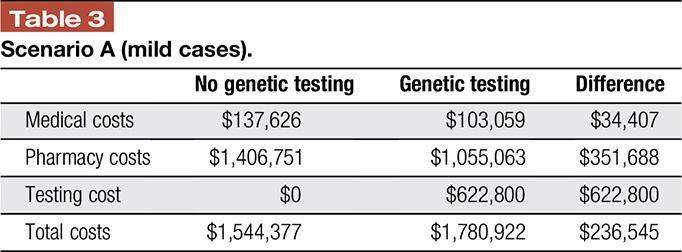
In the hypothetical cohort of 1000 patients with mild chronic noncancer pain, the health system incurs $1,544,377 in health care costs per year. With genetic testing guiding treatment decisions, medical and pharmacy costs decrease for these patients by $34,407 and $351,688, respectively. However, total costs increase over $230,000, as the medical and pharmacy savings do not fully offset the cost of testing in this cohort of patients (Table 3).
Table 3.
Scenario A (mild cases).
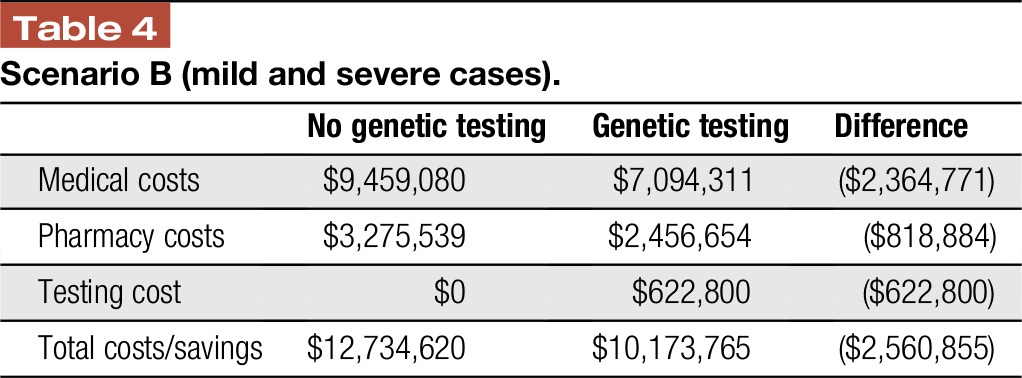
In the cohort with patients with 50% mild and 50% severe chronic noncancer pain, the total cost to the health system is over $12 million. With genetic testing guiding treatment decisions, medical and pharmacy costs decrease by $2,364,771 million and $818,884, respectively. Total costs are reduced by over $2.5 million, as the medical and pharmacy savings are more than offset by the cost of testing in this cohort of patients (Table 4).
Table 4.
Scenario B (mild and severe cases).
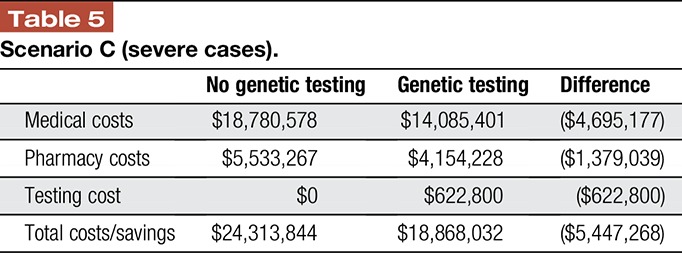
In the cohort with patients with 100% severe chronic noncancer pain, the total cost to the health system is over $24 million. With genetic testing guiding treatment decisions, medical and pharmacy costs decrease for these patients by $4,695,177 million and $1,379,039, respectively. Total costs are reduced by over $5 million, as the medical and pharmacy savings are more than offset by the cost of testing in this cohort of patients (Table 5).
Table 5.
Scenario C (severe cases).
Sensitivity analysis–varying benefits of genotype-guided treatment and efficacy assumptions by ±10% made little difference to any of the scenarios assessed model results.
4. Discussion
Both patients and physicians want efficient, effective pain relief without ADEs. Currently, management of chronic, noncancer pain often takes an empirical, step-wise approach, starting with nonopioid analgesics and progressing to opioids. In addition to their addiction potential, opioids are fraught with a myriad of other side effects including life-threatening ones. Thus, identification of patients who might not respond appropriately to usual doses of an opioid, or who may be at an increased risk of an adverse drug reaction, would be helpful to both the clinician and patient. Much of the variability in opioid pharmacokinetics and pharmacodynamics is due to variations in the genotypes of individuals, especially about variations in the cytochrome P4502D6 and P4503A, UDP-glucuronosyltransferase 2B7, opioid receptor mu–1 (OPRM1), opioid receptor kappa–1, opioid delta receptor–1, drug transporter, and catechol-O-methyltransferase genes.3 Therefore, knowledge of a patient's pharmacogenetic profile should provide a more rational approach to opioid management. Indeed, the results of this analysis suggest that treatment decisions based on genotyping results in fewer ADEs and lower medical and pharmacy costs in patients with moderate or severe pain.
For many drugs, the major groups of enzymes that metabolize them belong to the cytochrome P450 family, which are encoded by about 57 genes in humans. This family has been divided into 2 classes: class I composed of CYP1A1, CYP1A2, CYP2E1, and CYP3A4, which do not have important functional polymorphisms and are active in metabolizing procarcinogens and drugs; and class II composed of CYP2A6, CYP2B6, CYP2C9, CYP2C19, and CYP2D6, which are highly polymorphic and are important for the metabolism of many drugs.29 Based on genetic analysis, patients may be broadly classified into poor metabolizers (PMs) if they carry 2 defective alleles resulting in an inactive enzyme, intermediate metabolizers with either 1 or 2 defective alleles leading to an enzyme with reduced activity, extensive metabolizers with 2 functional alleles and normal enzyme activity, and ultrarapid metabolizers (UMs) with more than 2 active gene copies and increased enzyme activity. With a drug such as codeine that is a prodrug and must be metabolized to morphine for full analgesic effect, a PM will have decreased efficacy because of low biotransformation to morphine, whereas an UM may develop a morphine overdose due to excessive biotransformation. Indeed, 2 case reports illustrate this issue: a 73-year-old man who had a UM developed life-threatening respiratory depression after 3 days of treatment with codeine; and an infant died of morphine overdose after breast feeding from a mother who was a CYP2D6 UM and was taking codeine.10,20 CYP2D6 variants are also major determinants of the metabolism of hydrocodone, oxycodone, and tramadol, with PM demonstrating decreased efficacy and UM increased toxicity.7,9,24,31,32,34 Methadone is primarily metabolized by CYP2B6 and UM that are at increased risk for cardiotoxicity.8,35 Although fentanyl is metabolized by CYP34A, it is the opioid receptor's polymorphisms that affect efficacy. Some patients who are homozygous for the G allele of the OPRM1 rs1799971 marker have decreased efficacy relative to patients homozygous for the A allele.37
Ideally, for pharmacogenomic information to have its optimal effect, the testing should be performed on all adults before they require a drug that is susceptible to metabolic variation due to genetic polymorphisms. The results should be available in the electronic medical record in the order entry section, so when a physician orders a medication that, based on the patient's pharmacogenomic profile, may be toxic or nonefficacious at usual doses, an alert would appear with suggestions for alternative medications, much as drug–drug and drug–allergy alerts appear in today's electronic medical records. This “just-in-time” information is most likely to result in more appropriate drug prescribing, enhanced patient safety, and health care cost savings.
4.1. Limitations
Despite using a 25% improvement from genetic testing, the lack of published point estimates makes it difficult to estimate the full benefits of genotype-based treatment (eg, clinical improvement, reduction in ADEs, and total reduction in costs). Another limitation is that the model underestimates the full benefits of genotyping, as it does not take into account societal costs such as quality of life, work productivity, suicide, and caregiver burden nor the indirect costs associated with the clinical interpretation of the tests. The model assessed a limited set of therapy options; a future study that identifies potential drug–gene interactions and drug–drug–gene interactions may lead to a more comprehensive and effective method for predicting which patients are most likely to experience adverse drug reactions. Although no specific genetic tests or testing companies are recommended in this study, research identifying the benefits of genetic testing may benefit companies like Pathway Genomics, the sponsor of this study. Finally, the model is based on the established literature and may underestimate or overestimate the cost and outcomes if practice patterns have changed since the underlying model was developed.
5. Conclusion
The results of this budget impact model suggest genotyping-based treatment reduces medical and pharmacy costs for chronic pain sufferers. For mild pain sufferers, the reduction in medical and pharmacy costs do not fully offset the cost of testing. In populations with 50% or 100% patients with severe noncancer chronic pain, genetic testing costs are more than offset by reduced medical and pharmacy costs resulting in a net savings to the health system.
Disclosures
The authors have no conflict of interest to declare.
Support provided by Pathway Genomics, San Diego, CA.
R. Morlock is a scientific consultant to Pathway Genomics, San Diego, CA. G. D. Braunstein is the Chief Medical Officer at Pathway Genomics.
All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].American Academy of Pain Medicine. AAPM facts and figures on pain. Available at: http://www.painmed.org/patientcenter/facts_on_pain.aspx#refer. Accessed October 17, 2016.
- [2].Agarwal D, Udoji MA, Trescot A. Genetic testing for opiod pain management: a primer. Pain Ther 2017. Doi: 10.1007/s40122-017-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Branford R, Droney J, Ross JR. Opioid genetics: the key to personalized pain control? Clin Genet 2012;82:301–10. [DOI] [PubMed] [Google Scholar]
- [4].Buynak R, Shapiro DY, Okamoto A, Van Hove I, Rauschkolb C, Steup A, Lange B, Lange C, Etropolski M. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert Opin Pharmacother 2010;11:1787–804. [DOI] [PubMed] [Google Scholar]
- [5].Centers for Medicare and Medicaid Services, Department of Health and Human Services. Medicare program; revisions to payment policies under the physician fee schedule, and other part B payment policies for CY 2008; revisions to the payment policies of ambulance services under the ambulance fee schedule for CY 2008; and the amendment of the e-prescribing exemption for computer generated facsimile transmissions (Addendum B.-Relative value units and related information used in determining medicare payments for 2008). Fed Regist 2007;72:66222–578. [PubMed] [Google Scholar]
- [6].Chapman CR. Opioid pharmacotherapy for chronic non-cancer pain: the American experience. Korean J Pain 2013;26:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Leon J, Dinsmore L, Wedlund P. Adverse drug reactions to oxycodone and hydrocodone in CYP2D6 ultrarapid metabolizers. J Clin Psychopharmacol 2003;23:420–1. [DOI] [PubMed] [Google Scholar]
- [8].Eap CB, Crettol S, Rougier JS, Schläpfer J, Sintra Grilo L, Déglon JJ, Besson J, Croquette-Krokar M, Carrupt PA, Abriel H. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther 2007;81:719–28. [DOI] [PubMed] [Google Scholar]
- [9].Elkalioubie A, Allorge D, Robriquet L, Wiart JF, Garat A, Broly F, Fourrier F. Near-fatal tramadol cardiotoxicity in a CYP2D6 ultrarapid metabolizer. Europ J Clin Pharmacol 2011;67:855–8. [DOI] [PubMed] [Google Scholar]
- [10].Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, Desmeules J. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med 2004;351:2827–31. [DOI] [PubMed] [Google Scholar]
- [11].Gregorian RJ, Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain 2010;11:1095–108. [DOI] [PubMed] [Google Scholar]
- [12].Grünenthal GmbH. A study to evaluate the efficacy and safety of CG5503 prolonged release (PR) in subjects with moderate to severe chronic pain due to osteoarthritis of the knee. In: ClinicalTrials.gov [internet]. Bethesda: National Library of Medicine (US), 2007–2012. Available at: https://clinicaltrials.gov/ct2/show/NCT00486811. NLM Identifier: NCT00486811. [Google Scholar]
- [13].Healthcare blue book. Available at: http://www.healthcarebluebook.com. Accessed October 21, 2016. [Google Scholar]
- [14].Janssen Pharmaceuticals, Inc. Duragesic [internet]. Titusville: 2012. Available at: https://www.janssenmd.com/pdf/duragesic/DURAGESIC_PI.pdf. Accessed October 17, 2016. [Google Scholar]
- [15].Kemp J, Bossarte R. Suicide data report, 2012. Department of Veterans Affairs, Mental Health Services Suicide Prevention Program; Available at: https://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed October 17, 2016. [Google Scholar]
- [16].Langford R, McKenna F, Ratcliffe S, Vojtassák J, Richarz U. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: a randomized, placebo-controlled trial. Arthritis Rheum 2006;54:1829–37. [DOI] [PubMed] [Google Scholar]
- [17].Leider HL, Dhaliwal J, Davis EJ, Kulakodlu M, Buikema AR. Healthcare costs and nonadherence among chronic opioid users. Am J Manag Care 2011;17:32–40. [PubMed] [Google Scholar]
- [18].Machlin S. Expenses for a hospital emergency room visit, 2003. Statistical brief #111. Rockville: Agency for Healthcare Research and Quality; 2006. Available at: http://meps.ahrq.gov/mepsweb/data_files/publications/st111/stat111.pdf. Accessed August 10, 2015. [Google Scholar]
- [19].Mackey S. Future directions for pain management: lessons from the institute of medicine pain report and the national pain strategy. Hand Clin 2016;32:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Madadi P, Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder JS, Teitelbaum R, Karaskov T, Aleksa K. Safety of codeine during breastfeeding: fatal morphine poisoning in the breastfed neonate of a mother prescribed codeine. Can Fam Physician 2007:53;33–5. [PMC free article] [PubMed] [Google Scholar]
- [21].Merchant S, Noe LL, Howe A, Duff S, Gricar J, Ogden K, Mody SH. Budget impact analysis of tapentadol extended Release for the treatment of moderate to severe chronic non-cancer pain. Clin Ther 2013;35:659–72. [DOI] [PubMed] [Google Scholar]
- [22].Nalysnyk L, Kavanagh S, Xu Y, Mercaldi K, Martin A, Merchant S. PSY1 tolerability of oral long-acting opioids in the treatment of chronic pain: a systematic literature review and meta-analysis. Value Health 2011;14:A410. [Google Scholar]
- [23].National Institutes of Health. Pain management fact sheet. Available at: http://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed October 17, 2016. [Google Scholar]
- [24].Otton SV, Schadel M, Cheung SW, Kaplan HL, Busto UE, Sellers EM. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clin Pharmacol Ther 1993;54:463–72. [DOI] [PubMed] [Google Scholar]
- [25].Park PW, Dryer RD, Hegeman-Dingle R, Mardekian J, Zlateva G, Wolff GG, Lamerato LE. Cost burden of chronic pain patients in a large integrated delivery system in the United States. Pain Pract 2015. 10.1111/papr.12357. [DOI] [PubMed] [Google Scholar]
- [26].Peterson K, Dieperink E, Ferguson L, Anderson J, Helfand M. Evidence brief: The comparative effectiveness, harms, and cost-effectiveness of pharmacogenomics-guided antidepressant treatment versus usual care for major depressive disorder. Washington, Department of Veteran Affairs; 2016. [PubMed] [Google Scholar]
- [27].Phillips CJ. Economic burden of chronic pain. Expert Rev Pharmacoecon Outcomes Res 2006;6:591–601. [DOI] [PubMed] [Google Scholar]
- [28].Phillips CJ. The cost and burden of chronic pain. Rev Pain 2009;3:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006;25:1679–91. [DOI] [PubMed] [Google Scholar]
- [30].Ross JR, Rutter D, Welsh K, Joel SP, Goller K, Wells AU, Du Bois R, Riley J. Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J 2005;5:324–36. [DOI] [PubMed] [Google Scholar]
- [31].Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, Rossier MF, Hochstrasser D, Dayer P, Desmeules JA. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol 2010;160:907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, Rossier MF, Hochstrasser D, Dayer P, Desmeules JA. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol 2010;160:919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sharma M, Kantorovich S, Lee C, Anand N, Blanchard J, Fung ET, Meshkin B, Brenton A, Richeimer S. An observational study of the impact of genetic testing for pain perception in the clinical management of chronic non-cancer pain. J Psychiatr Res 2017;89:65–72. [DOI] [PubMed] [Google Scholar]
- [34].Stamer UM, Lehnen K, Höthker F, Bayerer B, Wolf S, Hoeft A, Stuber F. Impact of CYP2D6 genotype on postoperative tramadol analgesia. PAIN 2003;105:231–8. [DOI] [PubMed] [Google Scholar]
- [35].Stringer J, Welsh C, Tommasello A. Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm 2009;66:825–33. [DOI] [PubMed] [Google Scholar]
- [36].Task Force on the Taxonomy of the International Association for the Study of Pain. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed Seattle: IASP Press, 1994. Reprinted 2002. [Google Scholar]
- [37].Walter C, Lötsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. PAIN 2009;146:270–5. [DOI] [PubMed] [Google Scholar]


