Abstract
Ensuring optimum preoperative and postoperative pain management should always be a priority in children.
Keywords: Pediatrics, Postsurgical pain, Surgery
Key Points
Persistent postsurgical pain (PPSP) is a recognized complication following surgery in children with an estimated median prevalence of 20% at 12 months after surgery.
Presurgical factors predictive of PPSP include presurgical pain intensity, child anxiety, child pain coping efficacy, and parental pain catastrophizing.
Treatment of PPSP involves the creation of an individualized management plan informed by the biopsychosocial formulation and using multidisciplinary interventions.
1. Introduction
Persistent postsurgical pain (PPSP) or chronic postsurgical pain (CPSP) is a recognized complication following surgical interventions in adults. There is no universally agreed definition of PPSP; however, the working definition proposed by Macrae32 and subsequently refined by Werner45 is commonly used ie, PPSP is pain persisting at least 3 months after surgery (various authors propose differing thresholds from 2 to 6 months), not present or substantially different in character and intensity to any preoperative pain, localized to the surgical site or referred area and that cannot be attributed to other causes (eg, cancer recurrence, infection). Rates of between 10% and 80% have been reported in adults for a wide variety of surgical procedures.34 The ongoing pain can lead to significant suffering and functional disability for the individual and place a burden on health care and economic resources.
Although the mechanisms for the development of PPSP in adults are not fully elucidated, a number of risk factors have been identified. These include preoperative pain, severe postoperative pain, multiple surgeries, younger age, female gender, site of surgery, risk of nerve damage, plus genetic, and psychological factors.34
In pediatric populations, there is a growing awareness of the potential for PPSP and its implications. There has been an increase in academic and clinical interest in recent years with a number of studies published investigating incidence, potential risk factors, outcomes, and pain trajectories, although there is less data to guide the clinician on the specific management of PPSP in pediatric populations.
2. Incidence
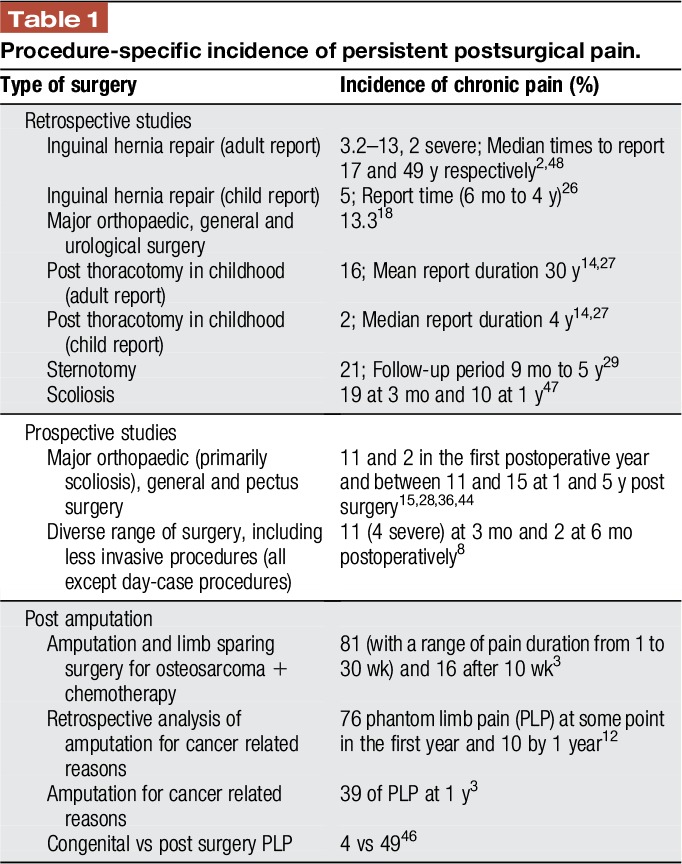
Data are not yet available on the precise incidence of PPSP after specific surgical interventions as reported in adult studies; investigations in children often involve a range of procedures and are further complicated by differing definitions of PPSP and pain threshold, high dropout rates and long durations between time of surgery and report (Table 1). A recent meta-analysis, based on 4 studies with a total of 628 participants across all surgery types, documented a median prevalence of PPSP across studies to be 20% at 12 months after surgery.40
Table 1.
Procedure-specific incidence of persistent postsurgical pain.
3. Nature of pediatric persistent postsurgical pain
As with adults, the precise mechanisms underlying the development PPSP in pediatric populations have not been elucidated. Nevertheless, the determinants of the transition from acute postsurgical pain to PPSP are likely to be complex reflecting the interplay of biological, psychological and socioenvironmental factors.13 To date, data evaluating the nature of PPSP in studies have relied on the use of verbal descriptors of pain by patients and quantitative sensory testing (QST) in a small number of study participants. Persistent postsurgical pain is typically thought to involve both nociceptive and neuropathic components.
3.1. Pain descriptors
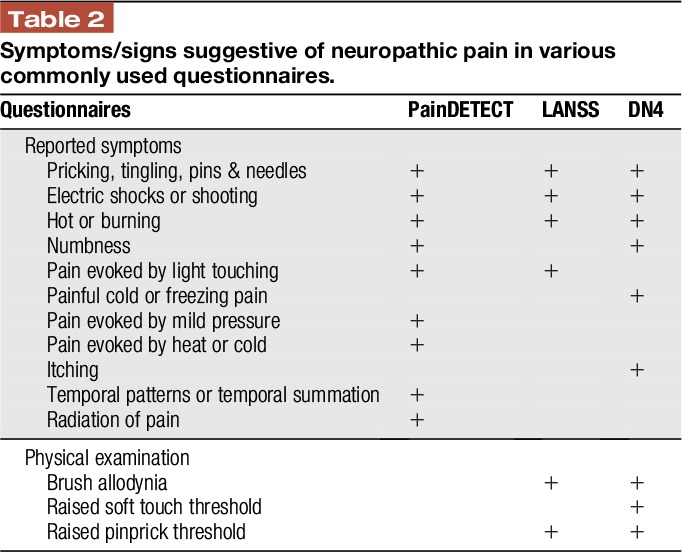
Over recent years, a number of screening tools for distinguishing neuropathic from nociceptive pain have been validated in adults and shown to have high sensitivity and specificity,1 but their evaluation and use in children have been limited. Some of them, ie, the Neuropathic Pain Questionnaire (NPQ),6,25 ID Pain,39 and PainDETECT,19 rely only on interview questions. The Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) scale9 and Douleur Neuropathique en 4 Questions questionnaire11 use both interview questions and physical tests and therefore achieve higher sensitivity and specificity than the screening tools that use only interview questions. Table 2 provides a comparison between commonly used tools and the symptoms and signs they assess.
Table 2.
Symptoms/signs suggestive of neuropathic pain in various commonly used questionnaires.
Batoz et al8 used an adult neuropathic pain questionnaire (DN4) and demonstrated a score ≥4 in 64% of patients. Conversely, in the study by Chou et al14 looking at thoracotomy, there was a 2% incidence of PPSP, but 6% scored ≥12 on the LANNS score.
In other studies neuropathic descriptors of pain (such as burning/hot, stabbing, sharp, tingling, and shooting) were commonly reported when patients were asked either to describe their pain or if these descriptors accurately described their pain. Other descriptors such as achy, dull, cramping, stiff, and sore were also commonly used, which potentially do not fit with a neuropathic origin for pain.26,29,36,47
In the clinical setting, it can be difficult to precisely determine the nature of pain from history and examination. Patients may have difficulty describing and locating their pain and differentiating between different types of pain. Pain is also often present preoperatively and may be due to any combination of that associated with the underlying medical and/or surgical problem, musculoskeletal, or neuropathic. If pain persists postoperatively, it can be problematic to decide what is and is not PPSP and its nature and mechanism.
3.2. Quantitative sensory testing
Quantitative sensory testing analyses perception in response to external stimuli of controlled intensity. Quantitative sensory testing is being increasingly used in both the clinical and research settings in children to help diagnose the nature of the pain that is being experienced, and standardized protocols and reference values are now available.10 In a study looking at post sternotomy pain, 13 of the children experiencing scar pain underwent testing: In 10 of these, pinprick hyperalgesia and brush and cold allodynia were demonstrated.29 Three patients with pain after inguinal herniotomy showed pinprick hyperalgesia and reduced pain pressure thresholds in another study.26
In 88 patients tested as adults after thoracotomy as children 55% and 67% demonstrated hypoesthesia to touch and pinprick, respectively, and 26% demonstrated hyperesthesia to pinprick. Of the 3 patients still with ongoing pain, all had hypoesthesia and hyperesthesia. Allodynia was only seen in 1 patient, who reported no ongoing pain. Tactile and pressure pain thresholds were significantly higher on the operated side.27 Increased use and study of QST is required in children with PPSP will help to further elucidate the results of the investigation and how they relate to the nature and degree of ongoing pain.
4. Risk factors
Nikolajsen et al suggested a number of risk factors that could potentially be associated with the development of PPSP in children35 and a recent systematic review identified presurgical pain intensity, child anxiety, child pain coping efficacy, and parental pain catastrophizing as the only presurgical factors predictive of CPSP. Biological and medical factors assessed were not associated with CPSP in any of the reviewed studies.40
4.1. Age
It has been thought that younger age is protective against the development of PPSP.35 In general, the incidences reported in the pediatric literature are lower than those seen in adults, although there is a mixed picture from the effect of age within pediatric study groups.
The studies looking at pain after inguinal herniotomy were using subjects mainly less than 5 years at the time of surgery. Rates of PPSP were low and in those where it was present, it was manly seen in those ≥5 years. It was not seen at all below the age of 3 months, although a negative effect of recall bias on this finding cannot be ruled out.2,26
Data from adults following thoracotomy in childhood showed an overall rate of PPSP of 16%, although for those aged 0–6 years at the time of surgery it was 3.2%, 7–12 years 19.4% and 13–25 years 28.5%. The 3 patients still in pain at the time of study were 11, 11, and 18 years old at the time of surgery.27 The finding that PSPP is uncommon at lower age groups is similar to other investigations of neuropathic pain in pediatric populations.22
Following scoliosis surgery, the incidence of delayed pain postoperatively (at 5 years) was higher in older patients.44 An effect of age was not seen in other prospective studies reported above, although these investigations tended to use children from older age groups.
4.2. Gender
To date, no study has found an effect of gender on the incidence of PPSP, although in other areas of pediatric chronic pain female sex has been shown to be more common.42 In a number of the studies, the majority of the patients were female (72%–82%), but further study is required to investigate whether sex plays a role in the development of PPSP.7,15,44
4.3. Preoperative pain
Pain prior to surgery often forms part of the formulation driving the need for surgery. It is also well recognized as a risk factor for PPSP in adults. To date, such a clear link has not been established for children although in some studies there is evidence of its effect.
In the study by Batoz et al, pain present greater than 1 month prior to surgery was associated with an increased risk of PSPP although pain intensity at time at surgery was not.8
Following scoliosis surgery, the majority of patients with pain preoperatively had reduced pain postoperatively associated with surgical recovery, although in some patients, increased baseline pain took longer than expected to recover and high pain scores preoperatively predicted worse pain scores at 2 to 5 years. Decreased functional ability alongside pain preoperatively also predicted increased pain long term.15,28,44
4.4. Postoperative pain
Again although recognized as a risk factor for PPSP in adults the data for children is less comprehensive. Following a mixed group of major surgical procedures, the relative risk of PPSP at 6 and 12 months was increased by higher reported pain intensity at 2 weeks postoperatively (3.3 and 2.5 respectively).37
High pain scores in the first 24 postoperative hours were associated with an increased risk of PPSP following thoracotomy and orthopedic surgery with an odds ratio of 4.8 Following scoliosis and pectus surgery, higher pain scores during the first 3 postoperative days and at 2 weeks predicted slower recovery and higher pain scores at 4 and 12 months.41
4.5. Psychological factors
The role of child and parent psychological factors in the development and maintenance of PPSP have been investigated. For scoliosis surgery, increased levels of preoperative child anxiety predicted slower pain recovery. Similar results were not seen for mood or general measures of mental health.15,44 Parental pain catastrophizing and anxiety preoperatively and at 2 to 3 days postoperatively also predicted increased pain intensity at 1 year.36,41
The cohort of patients used by Page et al showed high parent and child anxiety at 2 to 3 days postoperatively predicted the transition from high acute postoperative pain to PPSP. The maintenance of PPSP, however, was predicted by anxiety sensitivity (anxiety related symptoms interpreted as harmful outcomes), leading the authors to suggest the drivers for the development and maintenance of PPSP may be different.36,37
Body image may also have a role. Lower preoperative scores for perception of deformity and desire to change appearance predicted improved postoperative pain recovery.28,44 Maladaptive coping styles may also predispose to longer pain recovery and increased functional disability.30
4.6. Others
There are sparse data for other factors and their role in the development of PPSP—genetic, site of surgery, potential for nerve damage, analgesic regimens, or anesthetic techniques. In the studies above preoperative medical and surgical factors (eg, degree of spinal curvature), when looked at, did not predict subsequent pain. In general, studies investigating more major surgical interventions showed higher rates of PPSP but there are significant limitations to the completeness of this data.
5. Pain trajectories
Understanding the course of pain following surgical interventions may aid in elucidating the natural process for a particular procedure, and also understand interindividual difference in pain experiences. Two recent studies in children undergoing major surgery revealed distinct pain recovery trajectories within the groups. Rabbitts et al showed early (82% of patients) and late (18%) recovery groups. Pain intensity was measured preoperatively, in hospital and at 2 weeks, 4 months, and 1 year. Although both groups showed recovery over 1 year, the profiles of the recovery was delayed in the late group compared with the early group. The late group had increased pain in the acute phase post surgery (in hospital and at 2 weeks) and also increased pain intensity and frequency at 1 year. The late group also showed poorer health-related quality of life and functional ability at 1 year.41
A study looking at scoliosis surgery and pain up to 5 years post surgery identified 5 different pain trajectories within their cohort of patients.44 The majority (54%) showed “pain improvement” with time in line with surgical recovery and 12% had little or no pain throughout the perioperative and recovery course. Eighteen percent had increased pain through the first year but improvement from then on. A further 11% showed with no preoperative pain had mild pain in the first year, but high pain scores at 5 years. Six percent had high pain scores preoperatively, some improvement initially, but high pain scores at 2 and 5 years.44
6. Management
The severity and functional disability associated with PPSP can vary widely. Data from the studies cited above would suggest a proportion of PPSP in children is of low intensity and intermittent frequency with limited impact on activities of daily living; however, some patients do require the services of a Chronic Pain Clinic.23 In our institution, around 10% of children attending the Chronic Pain Clinic have a diagnosis of PPSP (unpublished personal data).
For the clinician treating PPSP in pediatric populations, there are few specific data on which to base management decisions. Treatment plans are often extrapolated from those used to treat other chronic pain diagnoses in children and those used in adult practice.
6.1. Biopsychosocial formulation
Chronic pain presentations in childhood are often best understood by adopting a biospychosocial model that is developmentally appropriate.20,31 Initial assessment of pain-specific symptoms, signs, and behaviors will help identify possible mechanisms for the pain, the impact on function and potential treatment targets.
If feasible, the use of age appropriate assessments of physiological and psychological function alongside diagnostic and/or health screening questionnaires may be helpful. Also pain evaluation and management should be initiated alongside any ongoing investigation or diagnostic clarification.
Once in place, the formulation is shared with the patient and family and can help establish a constructive therapeutic relationship to act as a platform to provide context and explain interventions.31,42
6.2. Management pathway
The biopsychosocial formulation constructed for a patient will allow the development of an individualized treatment plan. This will include pain education, relevant therapeutic interventions, and setting functional outcome goals, which are acceptable, appropriate and meaningful to both patient and family.
Once in place, this treatment plan requires assessment at predetermined intervals. Figure 1 is a suggested pathway for chronic pain management in children irrespective of etiology. Depending on the setting in which the patient is being seen failure to progress or nonresolution of symptoms may require reformulation or referral to a more specialist center.
Figure 1.
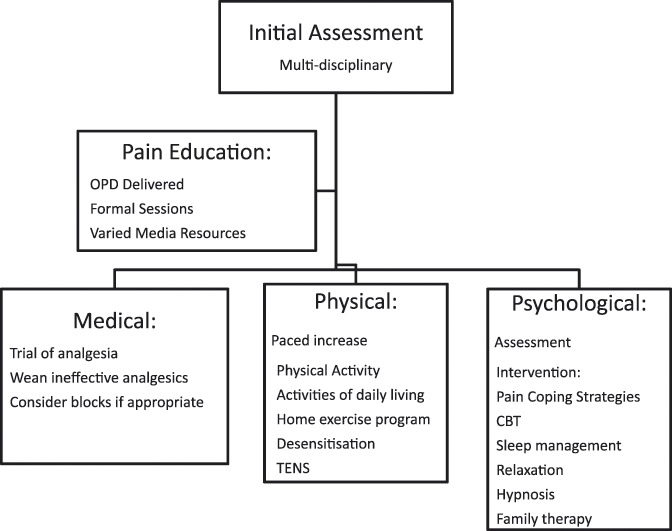
Suggested initial management pathway for child with persistent postsurgical pain. Which strategies and specific interventions used with vary depending on the individual patient. Once the plan is in place it will need regular assessment and potential modification depending on effect.
6.3. Pain education
Education requires the development of a narrative that allows patients and families to understand the scientific nature of their pain alongside their subjective experience of it and thus promote compliance with management strategies. Many media have potential uses and a range should be available to allow use at all developmental ages.
6.4. Pharmacological intervention
Despite the widespread use of pharmacological interventions for treating chronic pain, including PPSP, in children, there are no data on which evidence-based decisions can be made. In light of this, new analgesic agents should be introduced on a trial basis and stopped if not effective or in the presence of unacceptable side effects.
The choice of analgesic for PPSP in children usually depends on the presumed underlying mechanism of the pain, previous medication use, adult data, and clinician experience. In some cases combination therapy may be appropriate.
Simple analgesics such as paracetamol or non-steroidal anti-inflammatory drugs have often been taken by patients prior to presentation and are usually ineffective for PPSP. If effective, however, they can be used singly or in combination, on an “as required” or regular basis, but must be monitored over time.
Antineuropathic agents can be prescribed if neuropathic pain is part of the formulation. Tricyclic antidepressants and the gabapentinoids are most commonly used in children.22 Low-dose amitriptyline is often chosen where there is accompanying sleep disturbance. As with adults the efficacy of these agents would appear to be low in children, and significant side effects can limit their tolerability.
Topical Lidocaine and Capsaicin applications are commonly used for neuropathic pain in adults. There is anecdotal evidence for their efficacy in some children, but the pain associated with application of high concentration preparations of capsaicin may not be tolerated in children.
Melatonin can help with sleep disturbances, but has also been found to have analgesic, anti-inflammatory, and anxiolytic effects.33
Opioids are not usually effective for PPSP and are generally not indicated for chronic pain in children because of the side effects and other pharmacodynamic issues associated with their long-term use. Thus they are rarely used for PPSP in children. Often at presentation children are found to have been using opioids for some time and gradual withdrawal is required as part of the treatment plan.
6.5. Interventional strategies
The use of interventional strategies in the treatment of PPSP is not common in childhood and the evidence supporting their use in other chronic pain conditions is weak.49 In some presentations, however, they may prove beneficial. If the nature and location of the pain is favorable, the authors have found that local anesthetic blockade (± steroid) can have analgesic effect significantly beyond the duration of action of the analgesic agents for some patients. Often, repeated blocks are required and appropriate patient selection alongside a robust risk/benefit analysis is required (personal communication).
6.6. Psychological interventions
Psychological interventions that are currently widely used for the management of pediatric chronic pain of any etiology include multicomponent cognitive behavioral therapy (CBT) augmented by strategies such as relaxation, hypnosis, biofeedback, sleep management, and operant strategies aimed at parents.
A systematic review found psychological treatments to be effective in reducing pain intensity for headache and also improved pain and disability in non-headache pain (including JIA and sickle cell disease).16 Evidence is limited to estimate the effects of psychological therapies on mood and also for effects on disability in children with headache. The inclusion of parents significantly improves child symptoms for painful conditions immediately post-treatment.17 Studies directly comparing self-administered vs therapist-administered interventions have found similar effects on pain reduction.38
Interdisciplinary outpatient and intensive inpatient treatment have been shown to improve pain intensity and disability in children with chronic pain, and the effects are maintained at the 1-year follow-up.21
6.7. Physical interventions
The use of physical interventions as part of a multidisciplinary management for chronic pain in children is well established.5 Which therapy or therapies are most appropriate will depend on the formulation for the individual patient. These may include physiotherapy, graded increase in muscle strength, local heat or cold application, transcutaneous electrical nerve stimulation, massage therapy, and desensitisation techniques, graded motor imagery and complimentary medicine interventions such as acupuncture, though evidence for any intervention alone is weak.42
Exercise has a beneficial effect on tissue healing, function, and mood. These can all benefit the patient with PPSP. Alongside this, promoting activity is also thought to prevent deconditioning that could promote a negative cycle of decreasing function and increasing pain.4
7. Prevention
Again, there are no data for strategies that will successfully reduce or stop the development of PPSP in pediatric populations. Although careful patient selection, effective postoperative pain management, and refinement of surgical intervention may all have their place, further study is required. The studies above suggest a number of possible targets for intervention and point the way for further trials.
7.1. Acute pain management
Effectively treating acute pain may decrease the incidence of PPSP. Though which strategies and regimens are effective in which procedures and for which patients needs further elucidation. The study by Batoz et al found that regional anesthesia was not protective against the development of PPSP, but nearly all the patients involved received regional anesthesia perioperatively so this finding is difficult to interpret.8
7.2. Targeting mechanisms for persistent postsurgical pain
Understanding the mechanisms that promote PPSP could lead to analgesic interventions at the time of surgery that specifically target its development. For example, repeated doses of gabapentin after spinal fusion reduces postoperative opioid requirements, but its effects on PPSP are unknown.43
7.3. Patient screening and intervention
Use of preoperative assessment tools for pain, function, and psychological factors may allow identification of patients at risk for PPSP and the use of appropriate interventions in these individuals and their families.
7.4. Acute pain clinics
Involvement of pain services to follow patients post discharge using multidisciplinary assessment and treatment models has the potential to monitor and refine analgesic management to individual need, identify patients at risk of PPSP early and allow timely intervention. There are reports of the use of these clinics in adults and similar services are beginning to be used in specialist pediatric centers.24
8. Conclusion
Persistent postsurgical pain in being increasingly recognized in pediatric populations and scientific and clinical interest in the field is growing. Current evidence suggests that it has a lower incidence than in adults and the pain intensity for many patients is short lived and associated with limited functional disability. For some children, however, it is severe with significant impact on day-to-day life that requires specialist multidisciplinary pain management, assessment and intervention. We are also beginning to elucidate the risk factors for its development and these may provide us with effective targets for prevention, early intervention, and management. Currently, based on the limited evidence available, we recommend that ensuring optimum preoperative and postoperative pain management should always be a priority in children.
Disclosures
The authors have no conflict of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].CADTH Rapid Response Reports. Diagnostic methods for neuropathic pain: a review of diagnostic accuracy. Ottawa: Canadian Agency for Drugs and Technologies in Health Copyright (c) 2015 Canadian Agency for Drugs and Technologies in Health, 2015. [PubMed] [Google Scholar]
- [2].Aasvang EK, Kehlet H. Chronic pain after childhood groin hernia repair. J Pediatr Surg 2007;42:1403–8. [DOI] [PubMed] [Google Scholar]
- [3].Anghelescu DL, Kelly CN, Steen BD, Wu J, Wu H, DeFeo BM, Scobey K, Burgoyne L. Mirror therapy for phantom limb pain at a pediatric oncology institution. Rehabil Oncol 2016;34:104–10. [PMC free article] [PubMed] [Google Scholar]
- [4].Asmundson GJ, Noel M, Petter M, Parkerson HA. Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res Manag 2012;17:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ayling Campos A, Amaria K, Campbell F, McGrath PA. Clinical impact and evidence base for physiotherapy in treating childhood chronic pain. Physiother Can 2011;63:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Backonja MM, Krause SJ. Neuropathic pain questionnaire–short form. Clin J Pain 2003;19:315–16. [DOI] [PubMed] [Google Scholar]
- [7].Bastrom TP, Marks MC, Yaszay B, Newton PO. Prevalence of postoperative pain in adolescent idiopathic scoliosis and the association with preoperative pain. Spine 2013;38:1848–52. [DOI] [PubMed] [Google Scholar]
- [8].Batoz H, Semjen F, Bordes-Demolis M, Benard A, Nouette-Gaulain K. Chronic postsurgical pain in children: prevalence and risk factors. A prospective observational study. Br J Anaesth 2016;117:489–96. [DOI] [PubMed] [Google Scholar]
- [9].Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. PAIN 2001;92:147–57. [DOI] [PubMed] [Google Scholar]
- [10].Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. PAIN 2010;149:76–88. [DOI] [PubMed] [Google Scholar]
- [11].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [12].Burgoyne LL, Billups CA, Jirón JL, Kaddoum RN, Wright BB, Bikhazi GB, Parish ME, Pereiras LA. Phantom limb pain in young cancer-related amputees: recent experience at St. Jude Children's Research Hospital. Clin J Pain 2012;28:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017;18:359.e351–359.e338. [DOI] [PubMed] [Google Scholar]
- [14].Chou J, Chan CW, Chalkiadis GA. Post-thoracotomy pain in children and adolescence: a retrospective cross-sectional study. Pain Med 2014;15:452–9. [DOI] [PubMed] [Google Scholar]
- [15].Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, Ariagno JE, Price N, Schwend R. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine 2014;39:E174–81. [DOI] [PubMed] [Google Scholar]
- [16].Eccleston C, Palermo TM, de C Williams AC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2012;12:CD003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev 2012;8:CD009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fortier MA, Kain ZN. Pain after pediatric surgery. PAIN 2111;156:2111–12. [DOI] [PubMed] [Google Scholar]
- [19].Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [20].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- [21].Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, Zernikow B. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics 2015;136:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Howard RF, Wiener S, Walker SM. Neuropathic pain in children. Arch Dis Child 2014;99:84–9. [DOI] [PubMed] [Google Scholar]
- [23].Kachko L, Ben Ami S, Lieberman A, Shor R, Tzeitlin E, Efrat R. Neuropathic pain other than CRPS in children and adolescents: incidence, referral, clinical characteristics, management, and clinical outcomes. Pediatr Anesth 2014;24:608–13. [DOI] [PubMed] [Google Scholar]
- [24].Katz J, Weinrib A, Fashler SR, Katznelzon R, Shah BR, Ladak SS, Jiang J, Li Q, McMillan K, Mina DS, Wentlandt K, McRae K, Tamir D, Lyn S, de Perrot M, Rao V, Grant D, Roche-Nagle G, Cleary SP, Hofer SO, Gilbert R, Wijeysundera D, Ritvo P, Janmohamed T, O'Leary G, Clarke H. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015;8:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain 2003;19:306–14. [DOI] [PubMed] [Google Scholar]
- [26].Kristensen AD, Ahlburg P, Lauridsen MC, Jensen TS, Nikolajsen L. Chronic pain after inguinal hernia repair in children. Br J Anaesth 2012;109:603–8. [DOI] [PubMed] [Google Scholar]
- [27].Kristensen AD, Pedersen TA, Hjortdal VE, Jensen TS, Nikolajsen L. Chronic pain in adults after thoracotomy in childhood or youth. Br J Anaesth 2010;104:75–9. [DOI] [PubMed] [Google Scholar]
- [28].Landman Z, Oswald T, Sanders J, Diab M. Prevalence and predictors of pain in surgical treatment of adolescent idiopathic scoliosis. Spine 2011;36:825–9. [DOI] [PubMed] [Google Scholar]
- [29].Lauridsen MH, Kristensen AD, Hjortdal VE, Jensen TS, Nikolajsen L. Chronic pain in children after cardiac surgery via sternotomy. Cardiol Young 2014;24:893–9. [DOI] [PubMed] [Google Scholar]
- [30].Lautenbacher S, Huber C, Schofer D, Kunz M, Parthum A, Weber PG, Roman C, Griessinger N, Sittl R. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: a comparison with other psychological and physiological predictors. PAIN 2010;151:722–31. [DOI] [PubMed] [Google Scholar]
- [31].Liossi C, Howard RF. Pediatric chronic pain: biopsychosocial assessment and formulation. Pediatrics 2016;138. [DOI] [PubMed] [Google Scholar]
- [32].Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [33].Marseglia L, D'Angelo G, Manti S, Aversa S, Arrigo T, Reiter RJ, Gitto E. Analgesic, anxiolytic and anaesthetic effects of melatonin: new potential uses in pediatrics. Int J Mol Sci 2015;16:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martinez V, Baudic S, Fletcher D. Chronic postsurgical pain [in French]. Ann Fr Reanim 2013;32:422–35. [DOI] [PubMed] [Google Scholar]
- [35].Nikolajsen L, Brix LD. Chronic pain after surgery in children. Curr Opin Anaesthesiol 2014;27:507–12. [DOI] [PubMed] [Google Scholar]
- [36].Page MG, Campbell F, Isaac L, Stinson J, Katz J. Parental risk factors for the development of pediatric acute and chronic postsurgical pain: a longitudinal study. J Pain Res 2013;6:727–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Page MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res 2013;6:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. PAIN 2010;148:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin 2006;22:1555–65. [DOI] [PubMed] [Google Scholar]
- [40].Rabbitts JA, Fisher E, Rosenbloom BN, Palermo TM. Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. J Pain 2017;18:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. PAIN 2015;156:2383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rajapakse D, Liossi C, Howard RF. Presentation and management of chronic pain. Arch Dis Child 2014;99:474–80. [DOI] [PubMed] [Google Scholar]
- [43].Rusy LM, Hainsworth KR, Nelson TJ, Czarnecki ML, Tassone JC, Thometz JG, Lyon RM, Berens RJ, Weisman SJ. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg 2010;110:1393–8. [DOI] [PubMed] [Google Scholar]
- [44].Sieberg CB, Simons LE, Edelstein MR, Deangelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain 2013;14:1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Werner MU, Kongsgaard UEI. Defining persistent post-surgical pain: is an update required? Br J Anaesth 2014;113:1–4. [DOI] [PubMed] [Google Scholar]
- [46].Wilkins KL, McGrath PJ, Finley GA, Katz J. Phantom limb sensations and phantom limb pain in child and adolescent amputees. PAIN 1998;78:7–12. [DOI] [PubMed] [Google Scholar]
- [47].Wong GTC, Yuen VMY, Chow BFM, Irwin MG. Persistent pain in patients following scoliosis surgery. Eur Spine J 2007;16:1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zendejas B, Zarroug AE, Erben YM, Holley CT, Farley DR. Impact of childhood inguinal hernia repair in adulthood: 50 years of follow-up. J Am Coll Surg 2010;211:762–8. [DOI] [PubMed] [Google Scholar]
- [49].Zernikow B, Wager J, Brehmer H, Hirschfeld G, Maier C. Invasive treatments for complex regional pain syndrome in children and adolescents: a scoping review. Anesthesiology 2015;122:699–707. [DOI] [PubMed] [Google Scholar]


