The current study shows that blockade of opioid receptors systemically in the periaqueductal gray and the rostral ventromedial medulla prevents analgesia by 8 weeks of wheel running in a chronic muscle pain model. We further show increases in serotonin transporter expression and reversal of hyperalgesia with a selective reuptake inhibitor in the rostral ventromedial medulla in the chronic muscle pain model, and exercise normalizes serotonin transporter expression.
Keywords: Pain, Analgesia, Exercise, Physical activity, Serotonin, Opioid, Periaqueductal gray, Rostral ventromedial medulla, Serotonin transporter, SERT, Supraspinal
Abstract
Introduction:
It is generally believed that exercise produces its effects by activating central opioid receptors; there are little data that support this claim. The periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) are key nuclei in opioid-induced analgesia, and opioids interact with serotonin to produce analgesia.
Objectives:
The purpose was to examine central inhibitory mechanisms involved in analgesia produced by wheel running.
Methods:
C57/Black6 mice were given access to running wheels in their home cages before induction of chronic muscle hyperalgesia and compared with those without running wheels. Systemic, intra-PAG, and intra-RVM naloxone tested the role of central opioid receptors in the antinociceptive effects of wheel running in animals with muscle insult. Immunohistochemistry for the serotonin transporter (SERT) in the spinal cord and RVM, and pharmacological blockade of SERT, tested whether the serotonin system was modulated by muscle insult and wheel running.
Results:
Wheel running prevented the development of muscle hyperalgesia. Systemic naloxone, intra-PAG naloxone, and intra-RVM naloxone reversed the antinociceptive effect of wheel running in animals that had received muscle insult. Induction of chronic muscle hyperalgesia increased SERT in the RVM, and blockade of SERT reversed the hyperalgesia in sedentary animals. Wheel running reduced SERT expression in animals with muscle insult. The serotonin transporter in the superficial dorsal horn of the spinal cord was unchanged after muscle insult, but increased after wheel running.
Conclusion:
These data support the hypothesis that wheel running produced analgesia through central inhibitory mechanisms involving opioidergic and serotonergic systems.
1. Introduction
People who are physically active are less likely to develop chronic musculoskeletal pain.48,49,111 Similarly, previous studies by us and others show that prior physical activity prevents development of hyperalgesia in animal models of muscle and neuropathic pain.6,29,77,94 Thus, these data suggest that the underlying mechanisms by which physical activity prevents development of pain are key factors for the transition from acute to chronic pain.
Although it is generally believed that exercise produces its effects by activating central opioid receptors, there are little data that support this claim. In healthy human subjects, a single exercise task increases beta-endorphin and produces analgesia that is blocked by naloxone.37,82 In uninjured animals, regular exercise produces tolerance at mu-opioid receptors, and analgesia is blocked by opioid receptor antagonists.37,44,60,62,97,98 In animals with neuropathic pain, treadmill exercise started after injury, increases endogenous opioids in the periaqueductal gray (PAG) (β-endorphin) and the rostral ventromedial medulla (RVM) (met-enkephalin), and both systemic and intracerebroventricular naloxone reverses the antinociception.101 In animals with chronic muscle pain, treadmill running starting after development of hyperalgesia produces antinociception that is reversed by systemic naloxone.5 Furthermore, in animals with nerve injury, the serotonin transporter (SERT) is increased, and serotonin is decreased in the RVM, both of which are normalized by treadmill running starting after injury,6 and systemic serotonergic depletion prevents this exercise-induced analgesia.6,63These studies suggest that opioids and serotonin play a role in analgesia produced by exercise in human subject and animals with and without pain, and suggest that central pain inhibitory pathways underlie this analgesia.
The RVM mediates hyperalgesia after tissue insult, as well as opioid-induced analgesia, and PAG-mediated opioid analgesia.9,16,25,31,36,71,74,102–106,108 For example, systemic morphine analgesia is reduced by lesions or opioid receptor blockade in the PAG,20,22 and analgesia produced by activation of the PAG is prevented by opioid receptor blockade or lesions of the RVM.4,25,27,46,79,100,110 Further opioid receptors and opioid peptides are located in the RVM, and activation of opioid receptors in the RVM produces analgesia.1,24,36,39,43,72,73 Furthermore, serotonergic RVM neurons receive input from endogenous opioid peptides and project to the spinal cord to produce analgesia, or hyperalgesia, depending on the receptor activated.63 In the RVM, microinjection of 5-HT produces analgesia, and mice lacking central 5-HT neurons show greater inflammatory hyperalgesia.56,57,113 Furthermore, systemic serotonergic depletion, or blockade of serotonin receptors in the RVM, prevents analgesia from morphine injected systemically or into the PAG.11,45,81 These data show a critical role for the PAG and RVM in endogenous inhibition, that this, analgesia involves opioids and serotonin.
The purpose of the current study was to examine the central opioid sites involved in the prevention of chronic muscle pain by regular physical activity, if SERT expression in the RVM and spinal cord is modulated by regular physical activity, and if SERT mediates the hyperalgesia in animals with chronic muscle pain.
2. Material and methods
2.1. Sedentary vs physically active mice
All experiments were approved by the Animal Care and Use Committee at the University of Iowa. Male (n = 42) and female (n = 30) C57BL/6 mice were used for these experiments. Sedentary and naive mice were housed in their home cage with food and water. Physically active mice had free access to running wheels in their home cages for 8 weeks, and activity, in km/d, was recorded. Mice were housed individually. Running wheels were removed from home cages after 8 weeks, before induction of the chronic muscle pain model. We previously showed that 8 weeks of running wheel activity prevents development of hyperalgesia in this model that lasts for 8 days after stopping the running wheel activity.93
2.2. Noninflammatory chronic muscle pain
The model of chronic muscle pain was induced by 2 injections of unbuffered pH 4.0 saline (20 μL) into the left gastrocnemius muscle 5 days apart while the mouse was anesthetized with 4% isoflurane, as previously published.93 This insult produces enhanced mechanical sensitivity of the paw and muscle bilaterally without tissue damage.18,93
2.3. Behavior testing
Mice were acclimated for 2×/day for 2 days prior to behaviour testing procedures. This included acclimation to a gardener's glove used to immobilize the animals, acclimation to the wire mesh in small Lucite cubicles, and acclimation to the grip force test. All experiments were performed with the experimenter blinded to the group.
Muscle withdrawal thresholds were tested by applying a pair of calibrated forceps to the gastrocnemius muscle as previously described and validated.89,107 Mice were placed in a gardener's glove, and one hindlimb was extended. A pair of forceps was applied perpendicularly to the middle of the gastrocnemius muscle belly until the animal withdrew the hindlimb. Tests were performed bilaterally and 3 trials averaged.
The response frequency to mechanical stimulation of the paw was tested as previously described.18 The number of withdrawals to a von Frey filament (2.44, 0.4 mN) applied to the paw 5 times was assessed, followed by a 5-minute waiting period. This was repeated 10 times, averaged, and presented as percent response.
Grip strength of the hindpaw and forepaw was tested as previously described.10 Mice were pulled by the tail until a good grip was felt to read grip force for either the hindpaw or the forepaw. An average of 5 trials was recorded.
2.4. Implantation of guide cannula
Intracerebral guide cannulae were placed in the vlPAG and RVM 2 days before the first intramuscular injection of 4.0 as previously described.21,92 Mice were anesthetized with ketamine/xylazine and positioned in a stereotaxic head holder. The skull was exposed, and a small hole drilled for placement of guide cannulae. For the vlPAG, cannula were placed −4.1 mm from bregma, 0.4 mm from midline, and −2.6 mm from surface; for the RVM, cannula were placed −5.6 mm from bregma, 0.0 mm from midline, and −5.7 mm from surface. Cannulae were secured to the skull with 2 screws and dental cement. Mice were allowed to recover 3 to 7 days before testing. To examine the placement of the cannula, 0.2 μL methylene blue dye was injected through the cannula at the end of the experiment. After euthanasia, the brain was removed and postfixed in 10% formalin. The day before cutting, brains were transferred to 30% sucrose. Forty micrometer sections were cryosectioned through the injection site and examined under light microscopy for the placement of the cannula. Sites were verified by 2 individuals and recorded in a spreadsheet. In additional mice, guide cannula were misplaced outside the RVM, into the gigantocellularis nucleus (n = 8), and outside the vlPAG into the dorsolateral PAG (n = 2), lateral PAG (n = 1), or deep mesencephalic nucleus (n = 1).
2.5. Drugs
Naloxone hydrochloride and naloxone methiodide (Sigma-Aldrich, St. Louis, MO; 10 mg/kg, i.p.) were dissolved in 0.9% sterile saline for systemic delivery, and dosing was based on previously published data in mice.67,68,70,109 For microinjection, naloxone hydrochloride was dissolved in saline at a concentration of 10 nmol/0.2 μL; dosing was based on previously published data in mice.12,14 Fluoxetine doses (Sigma-Aldrich) were extrapolated from previous reports and tested in preliminary studies.13,58 For fluoxetine, 2 nmol/0.2 μL was dissolved in 0.9% sterile saline and 20 nmol/0.2 μL in 10% 2-hydroxypropyl-B-cyclodextrin (HBC) in sterile saline. Vehicles for all experiments were 0.9% sterile saline, except for the 20 nmol/0.2 μL, which used 10% HBC in sterile saline.
2.6. Immunohistochemistry
Animals were deeply anesthetized with 150 mg/kg sodium pentobarbital and transcardially perfused with heparinized saline followed by 4% paraformaldehyde. The brainstem and lumbar spinal cord was removed, stored in 30% sucrose overnight, and frozen at −20°C. Sections were cut on a cryostat at 20 μm on to slides. All sections were stained using an antibody to SERT (rabbit Anti-5HT Transporter; 1:1000) using standard immunofluorescent techniques as previously described.6 Preliminary data show specificity of the antibody to SERT because removal of the primary antibody and SERT−/− mice showed no immunoreactivity.54 On day 1, sections were blocked in 3% normal goat serum, avidin, and biotin and incubated in the primary antibody overnight at room temperature. On day 2, sections were rinsed, blocked in 3% normal goat serum, and incubated in biotinylated immunoglobulin G (IgG) for 1 hour at room temperature (1:1,000; Invitrogen, Eugene, OR). These sections were then reacted with streptavidin conjugated to Alexa Fluor 568 (Goat Anti-rabbit IgG; 1:1000) for 1 hour at room temperature. Sections were coverslipped with Vectashield (Vector Labs, Burlingame, CA) and stored until analysis. Brain and spinal cord sections for each group were stained simultaneously to avoid variability between stains across days.
Images of stained sections were taken in the Central Microscopy Facility at the University of Iowa on an Olympus BX-51 light microscope equipped with a SPOT camera (RT Slider; Diagnostic Instruments, Sterling Heights, MI). Rostral ventromedial medulla sections were identified at Bregma −6.00 mm from the Mouse Brain Atlas.69 All sections were taken under the same condition and stored for later analysis. Using Image J software, optical density was analyzed as previously described.38 Each picture was converted to 8-bit gray scale, calibrated independently using the “uncalibrated OD” function, and density values were analyzed per area. Ten sections of the RVM and superficial dorsal horn were quantified.
2.7. Experimental design
Experiment 1 tested whether a regular physical activity prevented development of chronic muscle hyperalgesia using the following groups: (1) sedentary with muscle insult (n = 3 M, 3 F), (2) wheel running with muscle insult (n = 4 M, 4 F), and (3) naive (n = 3 M, 3 F). Mice were tested before wheel running, and before and 24 hours after muscle insult for muscle withdrawal thresholds, paw response frequency, and grip strength. Naive mice did not receive muscle insult and did not have access to running wheels. Running wheels were stopped at the time of the first intramuscular injection.
Experiment 2 tested the role of peripheral and central opioid systems on prevention of hyperalgesia by wheel running using the following groups: (1) vehicle (n = 3 M, 2 F), (2) naloxone hydrochloride (10 mg/kg, i.p.; n = 3 M, 2 F), and (3) naloxone methiodide (10 mg/kg, i.p.; n = 2 M, 3 F). Muscle withdrawal thresholds were assessed before and 24 hours after muscle insult; and 30 and 120 minutes after injection. Running wheels were stopped at the time of the first intramuscular injection. Naloxone or vehicle was administered 24 hours after the second intramuscular injection.
Experiment 3 tested whether the PAG and RVM, central opioid sites involved in analgesia, were involved in the prevention of hyperalgesia by wheel running. For PAG, active mice with muscle insult were divided in 2 groups: (1) vehicle (saline, n = 3 M, 3 F) and (2) naloxone hydrochloride (10 nmol/0.2 μL, i.c.; n = 4 M, 3 F). For RVM, active animals with the muscle insult were divided into 2 groups: (1) vehicle (n = 3 M, 2 F) and (2) naloxone hydrochloride (10 nmol/0.2 μL, i.c.; n = 3 M, 3 F). Muscle withdrawal thresholds were assessed before and 24 hours after muscle insult, and 30 and 120 minutes after microinjection. Running wheels were stopped at the time of cannulae placement. Two days after the placement of cannulae, the first intramuscular injection of acidic saline was given. Naloxone or vehicle was administered 24 hours after the second intramuscular injection.
Experiment 4 tested the involvement of the serotonin system in the chronic muscle pain model and modulation by wheel running. Using animals from experiment 1, SERT, expression in the RVM, and superficial dorsal horn using immunohistochemistry in sedentary, physically active, and naive mice was assessed.
Experiment 5 tested whether pharmacological blockade of SERT in the RVM, in animals with muscle insult, mimicked the behavioural effects of exercise. Sedentary mice were implanted with guide cannula in RVM, induced with chronic muscle pain model, and randomly divided as follows: (1) Saline (n = 5 M, 1 F), (2) Saline with 10% HBC (n = 3 M, 2 F), (3) Fluoxetine (2 nmol/0.2 μL) (n = 4 M, 2 F), and (4) Fluoxetine (20 nmol/0.2 μL) (n = 3 M, 3 F). Muscle withdrawal thresholds were tested before and 24 hours after muscle insult; and 30 and 120 minutes after microinjection.
2.8. Statistical analysis
A repeated-measures analysis of variance was performed to test for changes in withdrawal thresholds of the muscle, the response frequency to mechanical stimulation of the paw, and grip force to examine for differences between groups and across time. This was followed by post hoc testing with a Tukey's test for differences between groups. Experiment 1 had 3 groups (sedentary, wheel running, and naive) and 3 time periods (baseline, before insult, and after insult). Experiment 2 had 3 groups (vehicle, naloxone hydrochloride, and naloxone methiodide) and 4 time periods (before and after muscle insult, 30 minutes and 1 hour after drug). Experiment 3 had 2 groups per site (vehicle and naloxone hydrochloride) and 4 time periods (before and after muscle insult, 30 minutes and 1 hour after drug). Experiment 5 had 5 groups (saline, saline with 10% HBC, fluoxetine 2 nmol, fluoxetine 20 nmol, missed sites) and 4 time periods before and after muscle insult, 30 minutes and 1 hour after drug). We report within-subjects effects, within-subjects contrasts, and between-subjects effects for group for each experiment in Table 1. Post hoc analysis examined for individual differences between groups with Tukey's test. For immunohistochemistry in experiment 4, a 1-way analysis of variance tested for differences between groups for the changes in SERT expression which had 3 groups (sedentary, wheel running, and naive) and 1 time period followed by post hoc analysis for group differences with Tukey's test. All data are mean ± SEM, and P ≤ 0.05 is considered significant.
Table 1.
Statistical results for repeated-measures analysis of variance for experiments 1, 2, 3, and 5.
3. Results
3.1. Physical activity prevents development of chronic muscle hyperalgesia
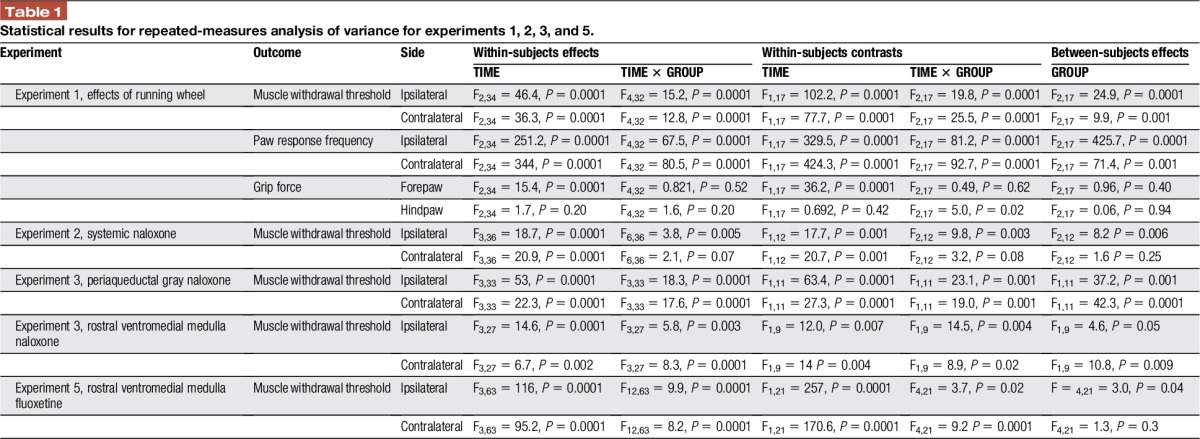
On average, mice ran 2.46 ± 0.87 km/d over the 8-week time period with a range from 0.86 km/d to 9.48 km/d. There was a significant overall difference in muscle withdrawal thresholds for time, an interaction between time and group, and a significant difference between groups (Table 1). Specifically, 24 hours after induction of the chronic muscle pain model, the sedentary mice showed significantly lower muscle withdrawal thresholds when compared with the physically active mice (ipsilateral: P = 0.0001, contralateral: P = 0.02) and the naive mice (ipsilateral: P = 0.0001; contralateral: P = 0.0001) (Fig. 1A, B).
Figure 1.
Long-term running wheel activity prevents development of hyperalgesia produced by repeated acid injections. Graphs show the muscle withdrawal threshold (A and B), paw response frequency (C and D), and grip force (E and F) before (B1) and after 8 weeks of running wheel (B2), and 24 hours after the second injection of acidic saline for naive (blue), sedentary (red), and running wheel (active, green) groups. The withdrawal threshold and paw response frequency were significantly lower in sedentary animals after the second acid injection when compared with the naive or physically active groups bilaterally (*P < 0.05). The hind paw grip force was significantly greater in the active group compared with the naive group for the forepaw and significantly less after the second acidic saline injection for the active group (*, P < 0.05) compared with the time after running wheel (+, P < 0.05). Data are the mean ± SEM.
For response frequency of the paw, there was a significant overall difference for time, an interaction between time and group, and a significant difference between groups (Table 1). Specifically, 24 hours after induction of the chronic muscle pain model, the sedentary mice showed a greater frequency of response to repeated mechanical stimulation of the paw bilaterally when compared with the physically active mice (ipsilateral, contralateral: P = 0.0001) and naive mice (ipsilateral, contralateral: P = 0.0001) (Fig. 1C, D).
The forepaw showed a significant difference in grip force across time, but no interaction between time and group, and no group differences (Table 1). In the active group, there were significant increases in grip force after 8 weeks of wheel running (P = 0.02, paired to-test) that were maintained 24 hours after induction of the chronic muscle pain model (P = 0.0001). The hindpaw grip force showed a significant interaction between time and group, but no difference for time (Table 1). In the active group, hindpaws showed significant decreases in force after induction of the chronic pain model (P = 0.001) when compared with the value 8 weeks after wheel running (Fig. 1E, F).
3.2. Central opioids mediate analgesia produced by wheel running
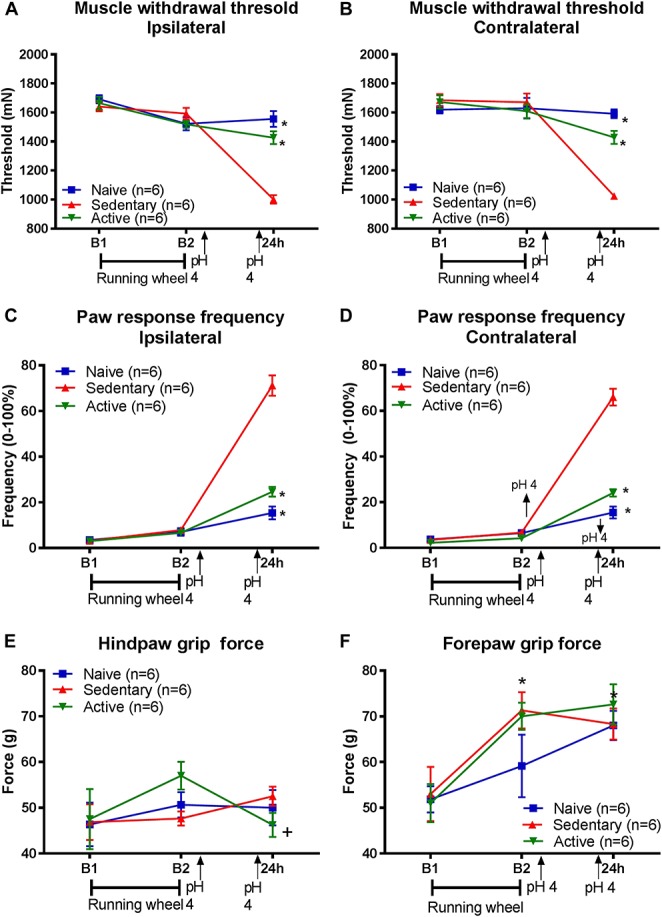
As opioids have been reported to mediate the analgesic effects of exercise,5,101 we tested if systemic naloxone reversed the analgesic effects of wheel running 24 hours after muscle insult. We compared this to the peripherally restricted opioid antagonist, naloxone methiodide, to examine contributions of peripheral opioid receptors. There was a significant overall difference for time, an interaction between time and group, and a significant difference between groups (Table 1). The naloxone-treated group was significantly lower than the saline-treated group (ipsilateral: P = 0.01; contralateral P = 0.001). Naloxone methiodide had no effect on the analgesia and was not different from the saline-treated group (ipsilateral: P = 0.94; contralateral: P = 0.50) (Fig. 2A, B).
Figure 2.
Central opioid receptors in periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) mediate the analgesia produced by wheel running. Muscle withdrawal thresholds for animals treated with naloxone systemically (A and B), into the PAG (C and D) and into the RVM (E and F) for both the ipsilateral and contralateral sides. All animals were given running wheels for 8 weeks before induction of the chronic muscle pain model. Thirty minutes after injection of naloxone systemically, or into the PAG or RVM, there was a significant decrease in the withdrawal thresholds of the muscle (*, P < 0.05) when compared with animals that received vehicle. Missed injection sites at the level of the PAG or RVM show significantly greater withdrawal thresholds compared with those injected with naloxone into the PAG or RVM (+, P < 0.05). Data are the mean ± SEM.
Because systemic naloxone, but not naloxone methiodide, reversed the analgesic effects of regular physical activity, we tested whether central sites were involved in the antinociception. Because the PAG also uses opioids to produce analgesia,25 we tested whether blockade of opioid receptors in the PAG reversed the antinociceptive effects of wheel running. Microinjection of naloxone hydrochloride into the vlPAG 24 hours after muscle insult reversed the antinociceptive effects of wheel running. There was a significant overall difference for time, an interaction between time and group, and a significant difference between groups (Table 1). Specifically, the withdrawal thresholds of the muscle were significantly lower in the physically active mice that received naloxone in the PAG when compared with the saline group (ipsilateral, contralateral: P = 0.0001) 30 minutes after injection (Fig. 2C, D). This effect was temporary, and withdrawal thresholds were not significantly different from the saline group 2 hours after induction of the hyperalgesia (ipsilateral: P = 0.7; contralateral: P = 0.52) (Fig. 2C, D). Furthermore, 4 animals had cannulae misplaced outside the vlPAG into the dlPAG, lPAG and deep mesencephalic nucleus. In these animals, the withdrawal threshold remained unchanged after microinjection of naloxone hydrochloride, and was significantly greater than in those injected with naloxone into the vlPAG (Fig. 2C, D) (P < 0.05).
Because the RVM plays a significant role in analgesia, receives projections from the PAG, and uses opioid receptors to produce analgesia,25 we tested whether opioid receptors in the RVM were involved in the analgesic effect of wheel running after muscle insult. Microinjection of naloxone into the RVM 24 hours after induction of the chronic muscle pain model reversed the antinociceptive effects of regular physical activity. There was a significant overall difference for time, an interaction between time and group, and a significant difference between groups (Table 1). Specifically, after microinjection of naloxone into the RVM, muscle withdrawal thresholds were significantly lower than those that received saline (ipsilateral: P = 0.002; contralateral: P = 0.001) (Fig. 2E, F). The effect was temporary, and antinociception returned 2 hours after naloxone injection (vs vehicle; ipsilateral: P = 0.54; contralateral: P = 0.36) (Fig. 2E, F). In 8 mice, naloxone hydrochloride was injected into the gigantocellularis nucleus (Gi). In these animals, there was no change in withdrawal thresholds after microinjection, and these were significantly different than withdrawal thresholds from animals injected with naloxone into the RVM (Fig. 2E, F) (P < 0.05).
3.3. Physical activity modulates serotonin transporter expression
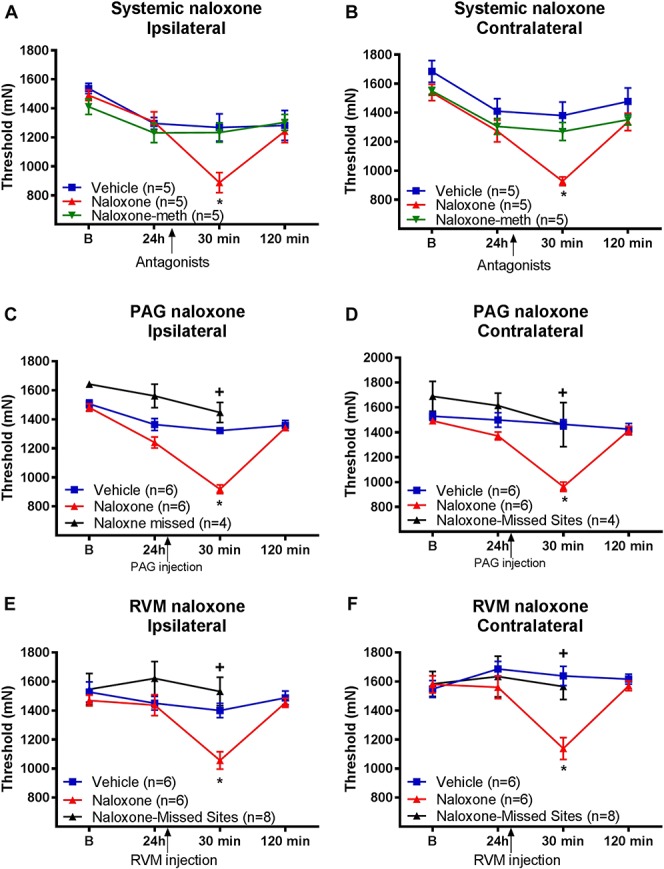
Because there is an interaction between the serotonergic and opioidergic systems in the RVM,11,45 we tested whether there were changes in SERT in the RVM. Figures 3A and D shows SERT staining density in the nucleus raphe magnus (NRM) 24 hours after muscle insult in sedentary animals compared with those that had performed wheel running and naive controls. After muscle insult, there were significant differences in the density of SERT immunoreactivity in the NRM (F2,18 = 13.1, P = 0.0001), with sedentary mice showing an increase in SERT density 24 hours after muscle insult when compared with naive controls (P = 0.009). Furthermore, SERT density in the physically active mice was significantly lower than that in sedentary mice 24 hours after muscle insult (P = 0.0001), but not different from naive mice (P = 0.35).
Figure 3.
Wheel running modulates expression of serotonin transporter (SERT) in the RVM and spinal cord. Representative photomicrographs of the nucleus raphe magnus (NRM, A-C) and spinal cord dorsal horn (ipsilateral: E-G; contralateral: I-K) from naive, sedentary, and active animals immunohistochemically stained for SERT. In the NRM, there was an increase in staining density in the sedentary animals (B and D) when compared with the naive (A and D) that was reduced in the exercised group (C and D). Quantitation of the density of staining showed a significant increase in staining density in the sedentary group in the NRM (*, P < 0.05) that was significantly reduced in the physically active animals (#, P < 0.05). For the dorsal horn, there was minimal immunohistochemical staining for SERT in the superficial dorsal horn in naive and sedentary mice. In the physically active animals, there was an increase in staining density in the superficial dorsal horn bilaterally. Quantification showed a significant increase in staining density in the active group when compared with sedentary or naive mice (*, P < 0.05). Data are the mean ± SEM.
Because the RVM projects to the spinal cord, and serotonin plays a role in pain inhibition in the spinal cord,3,20,25 we tested whether there were differences in SERT in the spinal cord. Figures 3E and L shows the SERT staining density in the spinal cord 24 hours after muscle insult in sedentary animals and those that performed 8 weeks of wheel running compared with naive controls. There was a significant difference in the density of immunoreactivity for SERT bilaterally in the spinal cord dorsal horn (ipsilateral: F2,18 = 9.9, P = 0.002; contralateral: F2,18 = 7.4.1, P = 0.005). There was no significant difference between sedentary mice in SERT density in the spinal cord 24 hours after muscle insult when compared with naive controls (ipsilateral: P = 0.97; contralateral: P = 0.88). However, in animals that performed wheel running and received muscle insult, SERT density was significantly greater bilaterally than naive (ipsilateral: P = 0.006; contralateral: P = 0.007) or sedentary mice 24 hours after muscle insult (ipsilateral: P = 0.003; contralateral: P = 0.02).
3.4. Blockade of serotonin transporter reverses hyperalgesia
Because there were increases in SERT in the RVM after muscle insult and regular physical activity decreases these SERT increases, we tested whether blockade of SERT in the RVM of sedentary animals with muscle insult mimicked the effects of wheel running. Fluoxetine was microinjected into the RVM 24 hours after induction of the model. For these experiments, there was a significant difference in withdrawal thresholds of the muscle, a significant overall difference of time, an interaction between time and group, and a significant difference between groups (Table 1). Muscle withdrawal thresholds from the group that received 20 nmol fluoxetine were significantly greater than those from the group that received saline (ipsilateral: P = 0.017; contralateral: P = 0.001), 2 nmol fluoxetine (ipsilateral: P = 0.0001), and HBC-vehicle (ipsilateral: P = 0.0001; contralateral: P = 0.004) 15 minutes after injection (Fig. 4). Fluoxetine effects were temporary, and by 2 hours, there were no significant differences between groups. In 3 animals that received 2 nmol dose of fluoxetine, the cannula were misplaced and withdrawal thresholds were decreased bilaterally (ipsilateral: 1172 + 43 mN; contralateral: 1246 + 15.6 mN).
Figure 4.
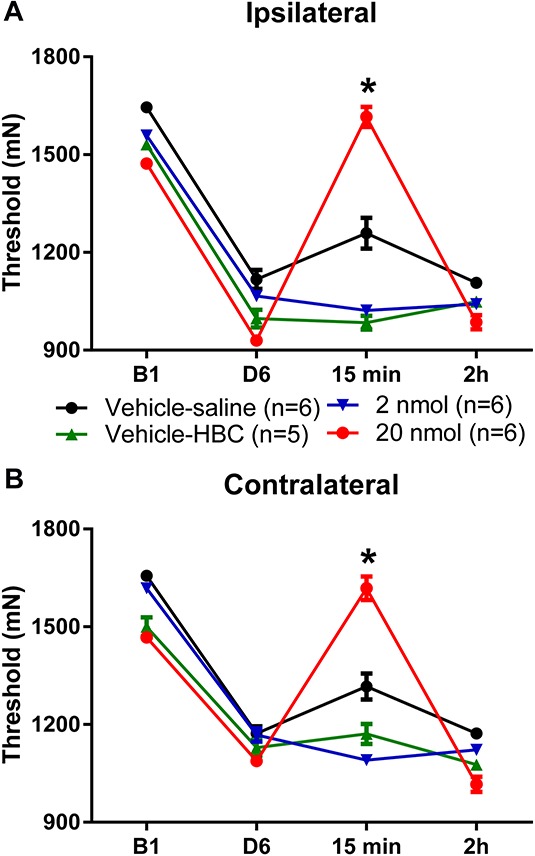
Blockade of serotonin transporter reverses hyperalgesia produced by repeated acid injections. Graphs represent muscle withdrawal thresholds before and 24 hours after the second acid injection in sedentary animals. The decrease in withdrawal threshold of the paw induced by repeated acid injection was reversed 15 minutes after microinjection of 20 nmol/0.2 μL of fluoxetine (*, P < 0.05) for both the ipsilateral (A) and contralateral (B) hindlimbs. By 2 hours after injection, the hyperalgesia returned to preinjection thresholds. Data are the mean ± SEM.
4. Discussion
The current study showed that blockade of opioid receptors in the RVM and PAG reversed the antinociception produced by long-term wheel running before muscle insult. These data are in agreement with prior studies in healthy human subjects showing an increase in systemic β-endorphin, and blockade of analgesia with systemic naloxone, after high-intensity exercise.7,15,33,82 In animals without tissue injury, systemic naloxone reduces analgesia produced by strength training, treadmill exercise, and long-term running wheel activity.26,53,86,101 Furthermore, long-term wheel running also decreased analgesia to exogenously applied mu-opioid agonists,44,60,61 suggesting that opioid tolerance had developed. Thus, prior studies in human subjects and animal without tissue injury, clearly show a role for opioids in analgesia using not only high-intensity exercise, but also lower-intensity tasks like wheel running.
More recent studies evaluated effects of opioid antagonism on exercise-induced analgesia in animal models of pain. In these studies, systemic naloxone reversed analgesia produced by moderate- to low-intensity exercise started after development of hyperalgesia in animals with neuropathic pain, chronic muscle pain, and in those that exercised before induction of acetic acid–induced pain.5,59,62,83,101 In parallel, animals with neuropathic pain that perform moderate intensity treadmill exercise, after nerve injury, show increases β-endorphin in the PAG and met-enkephalin in the RVM, and supraspinal blockade of opioids (intracerebroventricularly) prevents the exercise-induced analgesia.101 On the other hand, blockade of peripheral opioid receptors has no effect on exercise-induced analgesia in animals with neuropathic pain101; the current study shows similar results.
The current study also showed that naloxone in either the PAG or the RVM reversed the antinociception produced by regular physical activity, and this effect was temporary. This would suggest that exercise does not prevent central sensitization that occurs after repeated acid injections, but that endogenous opioids produced by exercise decrease nociceptive sensitivity. While the PAG and RVM are involved in pain inhibition, they are also involved in pain facilitation, particularly in chronic musculoskeletal pain models.21,102 It is therefore also possible that the increases in endogenous opioids in PAG and RVM are part of a parallel and independent pathway masking the ongoing central sensitization. Thus, increases in central opioids, within the PAG and RVM, mediate the analgesia by regular exercise through a modifiable mechanism.
Serotonin is a major neurotransmitter found in endogenous inhibitory pathways including the RVM and spinal cord and plays a significant role in analgesia. Classical studies show that increasing 5-HT in the NRM, by direct microinjection of 5-HT or a reuptake inhibitor, produces analgesia.40,56,57 By contrast, blockade of 5-HT2B receptors in the NRM prevents fear-induced analgesia,19 and morphine-induced analgesia.45,56 These data suggest that, in the RVM, increases in serotonin are analgesic, decreases in serotonin are hyperalgesic, and serotonergic analgesia involves the opioidergic system. Serotonin transporters control extracellular levels of 5-HT, thereby regulating serotonergic transmission.51 The current study showed that increases in SERT in the RVM were observed 24 hours after induction of the chronic muscle pain model, suggesting increased removal of released serotonin. These studies are in agreement with our prior study showing decreased 5-HT, and increases in SERT, in the NRM in animals with neuropathic pain.6 We extended these prior studies by showing that pharmacological blockade of SERT in the RVM reverses hyperalgesia in this model, providing a physiological role for SERT in the hyperalgesia that develops in chronic muscle pain. Depletion of serotonin with p-chlorophenylalanine prevents exercise-induced analgesia in healthy animals and in those that exercised after the development of with neuropathic pain,6,62 supporting the proposal that an increase in serotonin mediates the analgesia by exercise. Indeed, both our current and prior studies6 show decreases in SERT expression in the RVM in exercised animals, whether exercising before or after injury, suggesting regulation of SERT expression by regular exercise. It is unknown, whether the decreases in SERT are specific to animals with hyperalgesia, or whether these changes are observed in uninjured animals that exercise. Serotonin transporter surface expression is dynamically regulated by intracellular signalling pathways. Protein kinase C phosphorylation increases SERT internalization, and p-38 phosphorylation increases surface expression.50,51,76,78 Thus, SERT expression in chronic pain conditions may be dynamically modulated by regular physical activity through the release of endogenous opioids.
Serotonin fibers in the spinal cord originate in the RVM,8 and stimulation of the PAG or RVM increases 5-HT spinally.34,99 The current study shows no change in SERT immunoreactivity in the spinal cord after the induction of the chronic muscle pain model, suggesting similar levels of serotonin in the chronic muscle pain model. However, physically active mice show increased SERT in the superficial dorsal horn, suggesting that there is less serotonin available in the spinal cord in exercised animals. The literature on spinal serotonin is varied, with some studies showing a role for serotonin in inhibition and others in facilitation of nociception.28,30,32,41,84 Whether serotonin is inhibitory or excitatory in the spinal cord depends on the serotonin receptor activated, the animal model used, or the analgesic modality. Illustrating the variability, intrathecal injection of methysergide, a 5-HT2 antagonist, reduces hyperalgesia in nerve injured mice80 and prevents analgesia by electrical stimulation of the RVM, morphine injected into the RVM or PAG, and transcutaneous electrical nerve stimulation.2,35,41,75 Furthermore, spinal blockade of 5-HT1 receptors reverses hyperalgesia produced by inflammation111 and prevents analgesia produced by electrical stimulation of the RVM or by joint manipulation.23,88 Thus, future studies will need to investigate whether the increases in SERT in the spinal cord play a functional role in exercise-induced analgesia.
The chronic muscle pain model used in the current study produces bilateral hyperalgesia of the muscle and paw, visceral hyperalgesia, and anxiety-like and depressive-like behaviors.55,65,84,93 Once it develops, hyperalgesia is maintained by central mechanisms93 that include sensitization of spinal dorsal horn neurons manifested by bilateral expansion of receptive fields,95 and activation of central facilitatory pathways in the PAG, RVM, and spinal cord.17,38,87,102 The chronic muscle pain model has a similar pharmacological profile to fibromyalgia, a centrally mediated clinical pain condition, with analgesic effects using centrally acting drugs, eg, reuptake inhibitors, but not peripherally acting drugs, eg, nonsteroidal anti-inflammatory drugs.47,64,66,96 Thus, this animal model produces widespread pain similar to that observed in fibromyalgia that involves sensitization of central nervous system pathways.91
In conclusion, long-term voluntary wheel running produces analgesia through activation of endogenous inhibitory mechanisms that include opioids and serotonin, confirming several long-standing theories for how exercise produces analgesia. We propose that exercise resets the nervous system, so that subsequently painful stimuli normally perceived as painful in sedentary individuals, are not perceived as painful in active individuals. We suggest that there is a balance between inhibition and excitation in the central nervous system that favours excitation in sedentary individuals resulting in development of pain, and favours inhibition in physically active individuals preventing development of chronic pain. Exercise has the capacity to increase endogenous inhibition, which is reduced in individuals with chronic pain,42,52,90 and thus may be a therapy that can alter central processing of nociception.
Disclosures
The authors have no conflict of interest to declare.
This work was supported by the National Institutes of Health grant AR061371.
Acknowledgments
The authors thank Lucas Lima for assistance with photomicrographs.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 1995;15:3328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res 1985;343:223–9. [DOI] [PubMed] [Google Scholar]
- [3].Basbaum AI. Descending control of pain transmission: possible serotonergic-enkephalinergic interactions. Adv Exp Med Biol 1981;133:177–89. [DOI] [PubMed] [Google Scholar]
- [4].Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci U S A 1976;73:4685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil 2005;86:1736–40. [DOI] [PubMed] [Google Scholar]
- [6].Bobinski F, Ferreira TA, Cordova MM, Dombrowski PA, da CC, Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. PAIN 2015;156:2595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bortz WM, Angwin P, Mefford IN, Boarder MR, Noyce N, Barchas JD. Catecholamines, dopamine, and endorphin levels during extreme exercise. N Engl J Med 1981;305:466–7. [PubMed] [Google Scholar]
- [8].Bowker RM, Westlund KN, Sullivan MC, Wilber JF, Coulter JD. Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: a multiple transmitter complex. Brain Res 1983;288:33–48. [DOI] [PubMed] [Google Scholar]
- [9].Burgess SE, Gardell LR, Ossipov MH, Malan TP, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 2002;22:5129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3-/- mice. Am J Physiol Regul Integr Comp Physiol 2008;294:R1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carruba MO, Nisoli E, Garosi V, Sacerdote P, Panerai AE, da PM. Catecholamine and serotonin depletion from rat spinal cord: effects on morphine and footshock induced analgesia. Pharmacol Res 1992;25:187–94. [DOI] [PubMed] [Google Scholar]
- [12].Cavun S, Resch GE, Evec AD, Rapacon-Baker MM, Millington WR. Blockade of delta opioid receptors in the ventrolateral periaqueductal gray region inhibits the fall in arterial pressure evoked by hemorrhage. J Pharmacol Exp Ther 2001;297:612–19. [PubMed] [Google Scholar]
- [13].Cervantes-Duran C, Rocha-Gonzalez HI, Granados-Soto V. Peripheral and spinal 5-HT receptors participate in the pronociceptive and antinociceptive effects of fluoxetine in rats. Neuroscience 2013;252:396–409. [DOI] [PubMed] [Google Scholar]
- [14].Chu X, Xu N, Li P, Mao L, Wang JQ. Inhibition of cardiovascular activity following microinjection of novel opioid-like neuropeptide nociceptin (orphanin FQ) into the rat rostral ventrolateral medulla. Brain Res 1999;829:134–42. [DOI] [PubMed] [Google Scholar]
- [15].Colt EW, Wardlaw SL, Frantz AG. The effect of running on plasma beta-endorphin. Life Sci 1981;28:1637–40. [DOI] [PubMed] [Google Scholar]
- [16].Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. PAIN 1998;78:59–69. [DOI] [PubMed] [Google Scholar]
- [17].da Silva LFS, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. PAIN 2010;11:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulates pain behaviors. PAIN 2010;151:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de OR, de Oliveira RC, Falconi-Sobrinho LL, da Silva SR, Jr, Coimbra NC. 5-Hydroxytryptamine2A/2C receptors of nucleus raphe magnus and gigantocellularis/paragigantocellularis pars alpha reticular nuclei modulate the unconditioned fear-induced antinociception evoked by electrical stimulation of deep layers of the superior colliculus and dorsal periaqueductal grey matter. Behav Brain Res 2017;316:294–304. [DOI] [PubMed] [Google Scholar]
- [20].Deakin JF, Dostrovsky JO. Involvement of the periaqueductal grey matter and spinal 5-hydroxytryptaminergic pathways in morphine analgesia: effects of lesions and 5-hydroxytryptamine depletion. Br J Pharmacol 1978;63:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeSantana JM, da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 2009;163:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dostrovsky JO, Deakin JF. Periaqueductal grey lesions reduce morphine analgesia in the rat. Neurosci Lett 1977;4:99–103. [DOI] [PubMed] [Google Scholar]
- [23].El-Yassir N, Fleetwood-Walker SM. A 5-HT1 receptor mediates the antinociceptive effect of nucleus raphe magnus stimulation in the rat. Brain Res 1990;523:92–9. [DOI] [PubMed] [Google Scholar]
- [24].Fang FG, Haws CM, Drasner K, Williamson A, Fields HL. Opioid peptides (DAGO-enkephalin, dynorphin A(1-13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res 1989;501:116–28. [DOI] [PubMed] [Google Scholar]
- [25].Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Textbook of pain. Philadelphia: Elsevier, 2006. pp. 125–42. [Google Scholar]
- [26].Galdino GS, Duarte ID, Perez AC. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res 2010;43:906–9. [DOI] [PubMed] [Google Scholar]
- [27].Gebhart GF, Sandkuhler J, Thalhammer JG, Zimmermann M. Inhibition of spinal nociceptive information by stimulation in midbrain of the cat is blocked by lidocaine microinjected in nucleus raphe magnus and medullary reticular formation. J Neurophysiol 1983;50:1446–59. [DOI] [PubMed] [Google Scholar]
- [28].Goodchild CS, Guo Z, Freeman J, Gent JP. 5-HT spinal antinociception involves mu opioid receptors: cross tolerance and antagonist studies. Br J Anaesth 1997;78:563–9. [DOI] [PubMed] [Google Scholar]
- [29].Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. PAIN 2016;157:2012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gravitt K, Marson L. Effect of the destruction of cells containing the serotonin reuptake transporter on urethrogenital reflexes. J Sex Med 2007;4:322–30. [DOI] [PubMed] [Google Scholar]
- [31].Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett 2004;366:201–5. [DOI] [PubMed] [Google Scholar]
- [32].Guo W, Miyoshi K, Dubner R, Gu M, Li M, Liu J, Yang J, Zou S, Ren K, Noguchi K, Wei F. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol Pain 2014;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Haier RJ, Quaid K, Mills JC. Naloxone alters pain perception after jogging. Psychiatry Res 1981;5:231–2. [DOI] [PubMed] [Google Scholar]
- [34].Hammond DL, Tyce GM, Yaksh TL. Efflux of 5-hydroxytryptamine and noradrenaline into spinal cord superfusates during stimulation of the rat medulla. J Physiol (London) 1985;359:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res 1984;298:329–37. [DOI] [PubMed] [Google Scholar]
- [36].Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. PAIN 2001;92:129–38. [DOI] [PubMed] [Google Scholar]
- [37].Hoeger Bement MK, Sluka KA. Exercise-induced hypoalgesia: an Evidence-based review. In: Sluka KA, editor. Pain mechanisms and management for the physical therapist. Philadelphia: Wolters Kluwer, 2016. pp. 177–202. [Google Scholar]
- [38].Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 2003;23:5437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci 2000;20:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Inase M, Nakahama H, Otsuki T, Fang JZ. Analgesic effects of serotonin microinjection into nucleus raphe magnus and nucleus raphe dorsalis evaluated by the monosodium urate (MSU) tonic pain model in the rat. Brain Res 1987;426:205–11. [DOI] [PubMed] [Google Scholar]
- [41].Jensen TS, Yaksh TL. Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res 1986;363:114–27. [DOI] [PubMed] [Google Scholar]
- [42].Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. PAIN 2005;114:295–302. [DOI] [PubMed] [Google Scholar]
- [43].Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 1998;392:528–47. [PubMed] [Google Scholar]
- [44].Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D'Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav 1998;61:19–27. [DOI] [PubMed] [Google Scholar]
- [45].Kiefel JM, Cooper ML, Bodnar RJ. Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res 1992;597:331–8. [DOI] [PubMed] [Google Scholar]
- [46].Kiefel JM, Rossi GC, Bodnar RJ. Medullary mu and delta opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res 1993;624:151–61. [DOI] [PubMed] [Google Scholar]
- [47].Kim SH, Song J, Mun H, Park KU. Effect of the combined use of tramadol and milnacipran on pain threshold in an animal model of fibromyalgia. Korean J Intern Med 2009;24:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. PAIN 2011;152:2241–7. [DOI] [PubMed] [Google Scholar]
- [49].Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal associations between exercise and pain in the general population–the HUNT pain study. PLoS One 2013;8:e65279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lau T, Horschitz S, Bartsch D, Schloss P. Monitoring mouse serotonin transporter internalization in stem cell-derived serotonergic neurons by confocal laser scanning microscopy. Neurochem Int 2009;54:271–6. [DOI] [PubMed] [Google Scholar]
- [51].Lau T, Schloss P. Differential regulation of serotonin transporter cell surface expression. Wires Membr Transp Signal 2012;1:259–68. [Google Scholar]
- [52].Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 1997;13:189–96. [DOI] [PubMed] [Google Scholar]
- [53].Li G, Rhodes JS, Girard I, Gammie SC, Garland T., Jr Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol Behav 2004;83:515–24. [DOI] [PubMed] [Google Scholar]
- [54].Lima LV, DeSantana JM, Rasmussen LA, Sluka KA. Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms. PAIN 2017 Jun 8 10.1097/j.pain.0000000000000967 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu YT, Shao YW, Yen CT, Shaw FZ. Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiol Behav 2014;131:105–10. [DOI] [PubMed] [Google Scholar]
- [56].Llewelyn MB, Azami J, Roberts MH. The effect of modification of 5-hydroxytryptamine function in nucleus raphe magnus on nociceptive threshold. Brain Res 1984;306:165–70. [DOI] [PubMed] [Google Scholar]
- [57].Llewelyn MB, Azami J, Roberts MHT. Effects of 5-hydroxytryptamine applied into the nucleus raphe magnus on nociceptive thresholds and neuronal firing rate. Brain Res 1983;258:59–68. [DOI] [PubMed] [Google Scholar]
- [58].Manna SS, Umathe SN. A possible participation of transient receptor potential vanilloid type 1 channels in the antidepressant effect of fluoxetine. Eur J Pharmacol 2012;685:81–90. [DOI] [PubMed] [Google Scholar]
- [59].Martins DF, Mazzardo-Martins L, Soldi F, Stramosk J, Piovezan AP, Santos AR. High-intensity swimming exercise reduces neuropathic pain in an animal model of complex regional pain syndrome type I: evidence for a role of the adenosinergic system. Neuroscience 2013;234:69–76. [DOI] [PubMed] [Google Scholar]
- [60].Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav 2001;74:245–51. [DOI] [PubMed] [Google Scholar]
- [61].Mathes WF, Kanarek RB. Chronic running wheel activity attenuates the antinociceptive actions of morphine and morphine-6-glucouronide administration into the periaqueductal gray in rats. Pharmacol Biochem Behav 2006;83:578–84. [DOI] [PubMed] [Google Scholar]
- [62].Mazzardo-Martins L, Martins DF, Marcon R, Dos Santos UD, Speckhann B, Gadotti VM, Sigwalt AR, Guglielmo LG, Soares Santos AR. High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain 2010;11:1393. [DOI] [PubMed] [Google Scholar]
- [63].Millan MJ. Descending control of pain. Prog Neurobiol 2002;66:355–474. [DOI] [PubMed] [Google Scholar]
- [64].Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. PAIN 2006;126:54–63. [DOI] [PubMed] [Google Scholar]
- [65].Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology 2004;126:1082–9. [DOI] [PubMed] [Google Scholar]
- [66].Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol 2004;487:93–103. [DOI] [PubMed] [Google Scholar]
- [67].Park SH, Sim YB, Kang YJ, Kim CH, Kwon MS, Suh HW. The differential profiles of withdrawal symptoms induced by morphine and beta-endorphin administered intracerebroventricularly in mice. Neuroscience 2012;218:216–25. [DOI] [PubMed] [Google Scholar]
- [68].Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, Toll L, McLaughlin JP. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther 2013;346:545–54. [DOI] [PubMed] [Google Scholar]
- [69].Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates academic press. San Diego: Academic Press, 2001. [Google Scholar]
- [70].Pereira MP, Donahue RR, Dahl JB, Werner M, Taylor BK, Werner MU. Endogenous opioid-masked latent pain sensitization: studies from mouse to human. PLoS One 2015;10:e0134441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett 1996;218:127–30. [DOI] [PubMed] [Google Scholar]
- [72].Pierce TL, Wessendorf MW. Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 2000;18:181–207. [DOI] [PubMed] [Google Scholar]
- [73].Pinto M, Sousa M, Lima D, Tavares I. Participation of mu-opioid, GABA(B), and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: implications for descending modulation of nociceptive transmission. J Comp Neurol 2008;510:175–87. [DOI] [PubMed] [Google Scholar]
- [74].Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci 2001;21:5281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Radhakrishnan R, King EW, Dickman J, Richtsmeier C, Schardt N, Spurgin M, Sluka KA. Blockade of spinal 5-HT receptor subtypes prevents low, but not high, frequency TENS-induced antihyperalgesia in rats. PAIN 2003;105:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem 1998;273:2458–66. [DOI] [PubMed] [Google Scholar]
- [77].Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. PAIN 2016;157:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci 2005;25:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation of the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anaesthetised rat. Brain Res 1984;305:77–87. [DOI] [PubMed] [Google Scholar]
- [80].Satoh O, Omote K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res 1996;728:27–36. [PubMed] [Google Scholar]
- [81].Schul R, Frenk H. The role of serotonin in analgesia elicited by morphine in the periaqueductal gray matter (PAG). Brain Res 1991;553:353–7. [DOI] [PubMed] [Google Scholar]
- [82].Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med 1992;13:25–36. [DOI] [PubMed] [Google Scholar]
- [83].Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem 2011;118:224–36. [DOI] [PubMed] [Google Scholar]
- [84].Sharma NK, Ryals JM, Liu H, Liu W, Wright DE. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain 2009;10:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shmauss C, Hammond DL, Ochi JW, Yaksh TL. Pharmacological antagonism of the antinociceptive effects of serotonin in the rat spinal cord. Eur J Pharmacol 1983;90:349–57. [DOI] [PubMed] [Google Scholar]
- [86].Shyu BC, Andersson SA, Thoren P. Endorphin mediated increase in pain threshold induced by long-lasting exercise in rats. Life Sci 1982;30:833–40. [DOI] [PubMed] [Google Scholar]
- [87].Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. PAIN 2002;98:69–78. [DOI] [PubMed] [Google Scholar]
- [88].Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. PAIN 2003;106:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain 2005;6:41–7. [DOI] [PubMed] [Google Scholar]
- [90].Sluka KA, Berkley KJ, O'Connor MJ, Nicolella DP, Enoka RM, Boyan BD, Hart DA, Resnick E, Kwoh CK, Tosi LL, Coutts RD, Kohrt WM. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol Sex Differ 2012;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sluka KA, Danielson J, Rasmussen L, Dasilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc 2012;44:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001;24:37–46. [DOI] [PubMed] [Google Scholar]
- [94].Sluka KA, O'Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol 2013;114:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sluka KA, Price MP, Wemmie JA, Welsh MJ. ASIC3, but not ASIC1, channels are involved in the development of chronic muscle pain. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain. Seattle: IASP Press, 2003. pp. 71–79.
- [96].Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ−, and δ−, but not κ−, opioid receptor agonists. J Pharmacol Exp Ther 2002;302:1146–50. [DOI] [PubMed] [Google Scholar]
- [97].Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav 2004;77:49–57. [DOI] [PubMed] [Google Scholar]
- [98].Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–34. [DOI] [PubMed] [Google Scholar]
- [99].Sorkin LS, Mcadoo DJ, Willis WD. Release of serotonin following brainstem stimulation: correlation with inhibition of nociceptive dorsal horn neurons. In: Besson JM, editor. Serotonin and pain. Amsterdam: Elsevier, 1990. pp. 105–15. [Google Scholar]
- [100].Spinella M, Cooper ML, Bodnar RJ. Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. PAIN 1996;64:545–52. [DOI] [PubMed] [Google Scholar]
- [101].Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip MT., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiol 2011;114:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. PAIN 2008;136:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. J Pharmacol Exp Ther 1999;290:207–13. [PubMed] [Google Scholar]
- [104].Urban MO, Gebhart GF. Characterization of biphasic modulation of spinal nociceptive transmission by neurotensin in the rat rostral ventromedial medulla. J Neurophysiol 1997;78:1550–62. [DOI] [PubMed] [Google Scholar]
- [105].Vanderah TW, Suenaga NMH, Ossipov MH, Malan TP, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci 2001;21:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology 2006;130:2155–64. [DOI] [PubMed] [Google Scholar]
- [107].Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain 2010;11:210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wei H, Pertovaara A. MK-801, an NMDA receptor antagonist, in the rostroventromedial medulla attenuates development of neuropathic symptoms in the rat. Neuroreport 1999;10:2933–7. [DOI] [PubMed] [Google Scholar]
- [109].Wiebelhaus JM, Walentiny DM, Beardsley PM. Effects of acute and repeated administration of Oxycodone and naloxone-Precipitated withdrawal on Intracranial Self-stimulation in rats. J Pharmacol Exp Ther 2016;356:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Young EG, Watkins LR, Mayer DJ. Comparison of the effects of ventral medullary lesions on systemic and microinjection morphine analgesia. Brain Res 1984;290:119–29. [DOI] [PubMed] [Google Scholar]
- [111].Zhang R, Chomistek AK, Dimitrakoff JD, Giovannucci EL, Willett WC, Rosner BA, Wu K. Physical activity and chronic prostatitis/chronic pelvic pain syndrome. Med Sci Sports Exerc 2015;47:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhang Y, Yang Z, Gao X, Wu G. The role of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors in modulating spinal nociceptive transmission in normal and carrageenan-injected rats. PAIN 2001;92:201–11. [DOI] [PubMed] [Google Scholar]
- [113].Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW, Chen ZF. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci 2007;27:6045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


