Summary
It is estimated that fungal infections, caused most commonly by Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans, result in more deaths annually than malaria or tuberculosis. It has long been hypothesized the fungal metabolism plays a critical role in virulence though specific nutrient sources utilized by human pathogenic fungi in vivo has remained enigmatic. However, the metabolic utilisation of preferred carbon and nitrogen sources, encountered in a host niche-dependent manner, is known as carbon catabolite and nitrogen catabolite repression (CCR, NCR), and has been shown to be important for virulence. Several sensory and uptake systems exist, including carbon and nitrogen source-specific sensors and transporters, that allow scavenging of preferred nutrient sources. Subsequent metabolic utilisation is governed by transcription factors, whose functions and essentiality differ between fungal species. Furthermore, additional factors exist that contribute to the implementation of CCR and NCR. The role of the CCR and NCR-related factors in virulence varies greatly between fungal species and a substantial gap in knowledge exists regarding specific pathways. Further elucidation of carbon and nitrogen metabolism mechanisms is therefore required in a fungal species- and animal model-specific manner in order to screen for targets that are potential candidates for anti-fungal drug development.
Introduction
It is estimated that infections caused by the fungal pathogens Candida albicans, Aspergillus fumigatus and Cryptococcus neoformans, kill more people annually than malaria and tuberculosis (Denning and Bromley, 2015; Meyer et al., 2016). These fungi cause a spectrum of diseases, ranging from superficial to systemic and invasive, depending on the underlying disturbance in host defence responses and physiology (Mayer et al., 2013; Abad et al., 2010; Lee et al., 2013).
Pathogenicity, the ability to cause disease, is a multifactorial trait which encompasses a variety of survival and fitness-enhancing factors and pathways that determine the virulence of a pathogen in a given host. Among these, nutrient acquisition and subsequent metabolic processes are a critical virulence determinant as they are essential for promoting fungal fitness, survival, and virulence within the host. Essential nutrients include minerals such as iron and zinc which are required in small amounts, while carbon and nitrogen, the main energy sources for sustaining biosynthetic processes, must be obtained in large quantities from the environment (Ramachandra et al., 2014). Fungi have preference for certain carbon and nitrogen sources that are rapidly metabolised and therefore provide quick energy for growth and niche colonisation (Ruijter and Visser, 1997; Schure et al., 2000). In the presence of these favoured energy sources, the utilisation of alternative, less preferred carbon and nitrogen sources may be repressed, a process known as carbon and nitrogen catabolite repression (CCR, NCR). This review presents an overview of the current literature on CCR and NCR, including sensing and uptake of preferred carbon and nitrogen sources, whilst emphasising the importance of these regulatory mechanisms for the pathogenicity and virulence of C. albicans, A. fumigatus and C. neoformans.
In vivo carbon and nitrogen source availability
In the context of infection, the main carbon sources available to pathogenic fungi are glucose, lactate and acetate, whose availability largely depends on the host niche. In addition, potential nitrogen sources can also serve as carbon sources and are available throughout the human host mainly in the form of proteins. The primary site of infection for A. fumigatus and C. neoformans is the respiratory tract and occurs through inhalation of fungal spores. The airway surface liquid (ASL) of the lung and nasal secretions typically contain less than 0.5 mM glucose in healthy individuals, about 10-fold less than serum (Philips et al., 2003, Baker et al., 2007). In addition, lactate and amino acids have been identified in the ASL of mice and concentrations of these metabolites increase with inflammation (Grahl et al., 2011, Hu et al., 2008). Furthermore, ammonium, a preferred nitrogen source that enters the human body either as dietary free amino acids or is produced locally by bacterial microbiomes of the gastrointestinal (GI) tract (Richardson et al., 2013) and lungs, can also be found in the lung airways. Underlying respiratory diseases, such as cystic fibrosis, significantly increase local ammonium concentrations (Gaston et al., 2002). The lung mucous layer is composed of different glycoproteins such as mucin, which likely present good carbon/nitrogen sources for fungi. Fungal-induced damage to the mucosal barriers allows access to the underlying connective tissue which mainly consists of the structural proteins collagen and elastin (Farnell et al., 2012; Kronstad et al., 2012). A similar situation can be envisaged for C. albicans when switching from a gastrointestinal (GI) and vaginal commensal organism to a pathogenic organism, inducing penetration of the gut and vaginal mucosal membranes and gaining access to the underlying tissue.
Further tissue-induced damage would subsequently result in all three fungi reaching the bloodstream, which typically contains glucose and lactate levels of 4–5.5 mM and 0.5–2mM, respectively, depending on diet, and medical conditions such as diabetes and cystic fibrosis, which increase serum glucose levels (Baker et al., 2006, Ament et al., 1997). Furthermore, interorgan transport ensures the distribution of amino acids throughout the body which are required for protein synthesis and specific metabolic functions (Brosnan, 2003). The concentration of plasma amino acids is around 2.5 mM with glutamine being the most abundant amino acid in the blood (Brosnan, 2003) and in the intracellular amino acid pool of most tissues (Stumvoll et al., 1999). Dissemination via the bloodstream enables organ colonisation; in the case of C. neoformans, access to the cerebrospinal fluid (CSF), crossing of the blood brain barrier (BBB) and invasion of the brain. The organic acids lactate and acetate are produced by bacteria of the human gut or in a tissue-dependent manner, especially during inflammation where infiltrating immune cells primarily produce lactate for ATP generation from glucose (Jiménez-López et al., 2013; Schug et al., 2016) (Borregaard & Herlin, 1982). In the CSF, glucose levels are typically about 60–70% of serum glucose levels (Seehusen et al., 2003). Additionally, the levels of carbon and nitrogen sources in vivo change with corticosteroid treatment, a risk factor for many fungal infections, and upon inoculation with fungal conidia (Beattie et al., 2017). An important area for further research is how medical treatments that pre-dispose individuals to fungal infections alter the nutrient environment of specific organ systems. Thus, in addition to being able to utilize available carbon sources, pathogens must also be able to adapt to a rapidly changing nutrient environment within the host. Therefore, when considering human pathogenic fungi, we must examine metabolism of both glucose and alternative, non-preferred carbon and nitrogen sources. Figures 1 and 2 give an overview of known factors involved in carbon and nitrogen metabolic utilization.
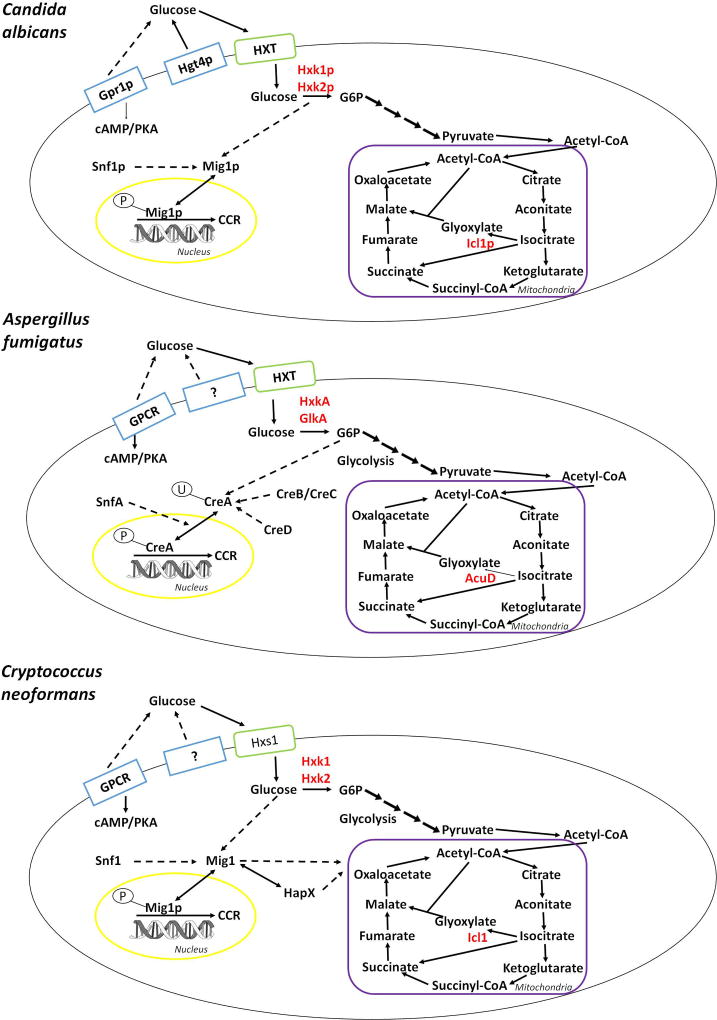
Figure 1.
Glucose sensing, uptake and metabolic pathways in C. albicans, A. fumigatus and C. neoformans (blue boxes: confirmed and putative sensors; green boxes: confirmed and putative transporters; red = metabolic enzymes, solid arrows = confirmed cellular processes, dashed arrows = cellular processes that are not elucidated; yellow = nucleus; purple = mitochondria). Glucose is sensed and taken up into the cell by specific hexose transporters (HXT) and is subsequently phosphorylated by the carbohydrate kinases hexokinase (HXK) or glucokinase (GLK). Further metabolic utilisation occurs via glycolysis and the TCA (tricarboxylic acid) cycle. Glucose uptake and subsequent phosphorylation serve as signals for the carbon catabolite repressor (CCR) Mig1p/CreA/Mig1 to translocate to the nucleus and repress target genes. Several factors, such as the protein kinase SNF, the de-ubiquitinylation complex CreB/CreC or the transcriptional regulator of iron metabolism HapX, have been shown to be involved in CCR by either interacting directly or indirectly, with Mig1p/CreA/Mig1 in a species-dependent manner.
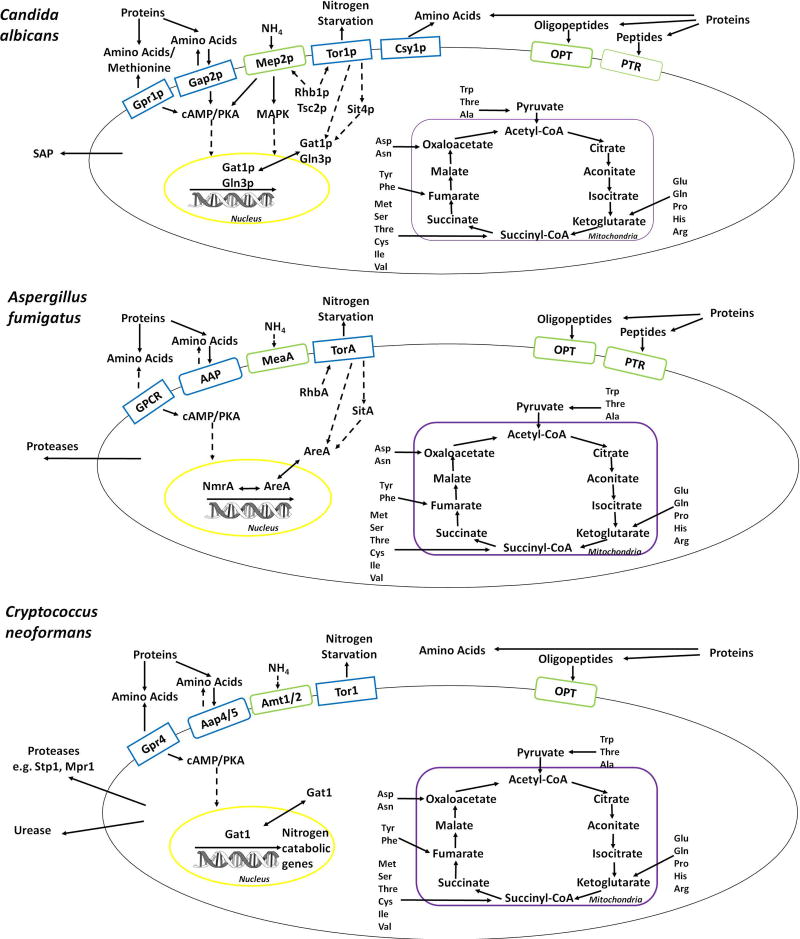
Figure 2.
Amino acid and ammonium sensing, uptake and metabolism in C. albicans, A. fumigatus and C. neoformans (blue boxes: confirmed and putative sensors; green boxes: confirmed and putative transporters; solid arrows = confirmed cellular processes, dashed arrows = cellular processes that are not elucidated; yellow = nucleus; purple = mitochondria). Proteins are degraded into peptides or proteins by secreted proteases (SAP = secreted aspartyl proteases). Peptides are internalised via oligopeptide (OPT) or peptide (PTR) transporters. Amino acids and ammonium are sensed and taken up by respective transporters and signals are relayed via several predicted pathways including cAMP and protein kinase A (PKA). Amino acids serve as precursors for TCA (tricarboxylic acid) cycle intermediates to generate ATP. Transcription factors (Gat1p, Gln3p/AreA, NmrA/Gat1) are activated and translocate to the nucleus where they regulate the catabolic and anabolic utilisation of different nitrogen sources.
Carbon catabolite metabolism in human pathogenic fungi
Glucose sensing and uptake
Given the importance of glucose in fungal metabolism, fungi have evolved sensitive systems for the sensing and uptake of glucose. In the well-studied model organism, S. cerevisiae, there are two major types of glucose sensing mechanisms: the G-Protein Coupled Receptor (GPCR) system, with the receptor Gpr1p and G-protein Gpa2p, and the hexose transporter gene family members Snf3p and Rgt2p [reviewed in (Peeters and Thevelein, 2014)]. Here we use this well-studied system as a basis for discussion but note that unlike the human pathogenic fungi discussed herein, S. cerevisiae is a Crabtree positive organism, i.e., it produces ethanol in aerobic conditions and high external glucose concentrations rather than accumulating biomass via the tricarboxylic acid (TCA) cycle. Thus, additional opportunities exist to discover glucose sensing and uptake mechanisms in the most commonly encountered human pathogenic fungi. In C. albicans, Gpr1p/Gpa2p regulate hyphal morphogenesis through activation of the cAMP-PKA (protein kinase A) pathway (Miwa et al., 2004, Maidan et al., 2005a). Whether this receptor is directly sensing glucose is unclear, however evidence suggests that this receptor also responds to methionine to stimulate hyphal formation in response to carbon source (Maidan et al., 2005b). The role of the Gpr1p sensing system in virulence was assessed in two studies, where Maidan and colleagues (2005a) reported reduced hyphal formation, tissue invasion and virulence of a gpr1−/− strain when injected intravenously in an inbred mouse model of systemic candidiasis, whereas Miwa et al. (2004) observed wild type virulence of intravenously-injected hetero- and homozygous mutants of GPR1 and GPA2 using an outbred mouse strain in a systemic model of candidiasis. Additionally, each study used a different cell density (optical density) inoculum, thus this signalling pathway may be important for virulence in a host-specific or dose-dependent context. In C. neoformans, Gpa2 functions upstream of cAMP-PKA signalling, and is required for cAMP signalling in response to glucose, however, the upstream sensor associated with this G protein remains unknown (Xue et al., 2006) and deletion of GPA2 did not affect virulence in an inhalation model of murine infection using both immune competent and immune deficient (severe combined immunodeficiency, SCID) mice (Li et al., 2007).
The genome of A. fumigatus encodes at least 15 GPCRs, of which only GprC, GprD and GprK, have been characterised (Grice et al., 2013, Jung et al., 2016). GprC and GprD show amino-acid sequence similarity to ScGpr1p, and deletion of either of these receptors results in a severe, temperature-dependent growth defect independent of carbon source (Gehrke et al., 2010, Han et al., 2004). Although no carbon-dependent growth defect of GprD was observed, metabolomic studies demonstrated that loss of the homologue receptor in A. nidulans altered glucose and central carbon metabolism (de Souza et al., 2013). Deletion of gprC resulted in delayed mortality whereas deletion of gprD attenuated virulence, when administered intranasally, in a neutropenic mouse model of infection, although the mechanism behind this reduction in virulence is unknown (Gehrke et al., 2010). GprK plays a role in carbon sensing, with the null mutant displaying increased germination under carbon starvation conditions and decreased growth on pentose sugars. Although this mutant showed significantly less invasion into A549 cells in vitro, there was no significant difference in survival using a wax moth larvae model, thus the role of this receptor in pathogenesis remains unclear (Jung et al., 2016).
The second major glucose sensing mechanism in S. cerevisiae uses high and low affinity hexose transporters (HXT), Snf3p and Rgt2p, respectively. These two proteins do not actively transport glucose but rather act as sensors for glucose concentration and transduce signals that regulate the expression of HXT genes appropriate for the glucose environment (Forsberg and Ljungdahl, 2001, Ozcan et al., 1998). In C. albicans, there are 20 predicted hexose transporters (HGT1-20), one of which encodes an Snf3p/Rgt2p homologue, Hgt4p, which senses glucose and subsequently regulates the expression of 6 HGT genes and is partially responsible for filamentation in response to this stimulus (Brown et al., 2006, Fan et al., 2002). Loss of HGT4 results in an increased median survival of mice when injected intravenously into an immunocompetent systemic model of candidiasis, supporting the hypothesis that sugar sensing is an important aspect of C. albicans virulence (Brown et al., 2006). Furthermore, HGT6 and HGT12 were highly expressed in a mouse model of oral candidiasis (OPC) when compared to the in vitro control condition (Fanning et al., 2012), HGT7 was significantly expressed in a mouse model of intra-abdominal candidiasis (Cheng et al., 2013) and HGT2, 4, 7, 12 and 17 were induced during an infection time course experiment in mice with systemic candidiasis (Wenjie et al., 2015). These results indicate that sugar scavenging, sensing and/or transport are important during early and late infection at various sites of the mammalian host. The exact role played by these HGT genes in C. albicans virulence remains to be determined.
The most closely related proteins to Snf3p/Rgt2p/Hgt4p in C. neoformans are Hxs1 and Hxs2. Transcriptome analysis of C. neoformans strains isolated from the cerebrospinal fluid (CSF) of 2 AIDS patients, showed high induction of Hxs1 when compared to the in vitro control condition (Chen et al., 2014). Hxs1 was shown to be a high affinity glucose transporter that did not complement the glucose sensing defect of an S. cerevisiae SNF3/RGT2 mutant, nor did loss of HXS1 affect expression of hexose transporters, suggesting that it may not act as a glucose sensor (Liu et al., 2013). Deletion of HXS2 was not possible but a role in glucose sensing is questioned given the low expression of this gene in high and low glucose (Liu et al., 2013). Sugar sensing and uptake systems and their relationship with virulence have not been characterized in A. fumigatus, however 17 putative hexose transporter genes have been identified in A. nidulans (Wei et al., 2004). Among these 17 hexose transporters, 15 predicted homologues are found in A. fumigatus. In summary, significant opportunities exist to better define the roles of sugar sensing and uptake in human fungal virulence.
Carbon catabolite repression (CCR)
In many fungi, carbon source utilization is regulated via a finely-tuned system that allows preferential utilization of glucose and preferred sugars before other available carbon sources. This system is called Carbon Catabolite Repression (CCR) and is regulated in part by the C2H2 zinc-finger transcription factor Mig1p/CreA/Mig1, which represses the expression of genes required for catabolism of less preferable carbon sources, as well as gluconeogenic genes, and nutrient acquisition genes in response to carbon starvation (Ruijter and Visser, 1997; Zaragoza et al., 2000; Caza et al., 2016). CCR in S. cerevisiae, regulated by the transcriptional repressor Mig1p and the corepressors Tup1p and Ssn6p, and CreA in A. nidulans and Aspergillus niger, has been studied exhaustively and is reviewed in (Kim et al., 2013, Gancedo, 1998, Klein et al., 1998).
Transcriptional studies of Mig1p and Tup1p in C. albicans, indicate that each transcription factor regulates unique sets of genes, in addition to shared genes between both repressors (Murad et al., 2001). Unique Mig1p targets and those shared by Mig1p and Tup1p were largely annotated as carbohydrate uptake and catabolism factors, implicating these repressors in CCR. Furthermore, microarray studies of Mig1p identified this regulator to have predominantly repressing functions, with only 0.8% of measured genes down-regulated upon loss of Mig1p, versus ~12.5% of measured genes whose mRNA levels increased in response to Mig1p loss (Murad et al., 2001).
In C. neoformans, Mig1 regulates the expression of amino acid, heme and carboxylic acid biosynthesis genes, TCA cycle and electron transport genes. The loss of Mig1 results in increased sensitivity to oxidative stress, respiratory inhibitors and antifungal drugs. Interestingly, a large number of genes are regulated by Mig1 under iron limitation, suggesting a role for this protein in iron homeostasis or metabolic adaptation to low iron (Caza et al., 2016). Indeed, a relationship of Mig1 with HapX, a bZIP transcription factor required for adaptation to iron limiting and iron excess conditions and virulence in several fungal pathogens (Jung et al., 2010, Schrettl et al., 2010, Gsaller et al., 2014, Chen et al., 2011, Lopez-Berges et al., 2012), was identified in C. neoformans (Caza et al., 2016). Although current studies on Tup1 provide little evidence as to whether Tup1 is involved in CCR (Lee et al., 2007, Lee et al., 2005, Lee et al., 2009), a role for Tup1 in positive regulation of iron homeostasis genes was identified, including CIG1, a gene encoding the putative heme binding protein (Lee et al., 2009), that was also identified to be positively regulated by Mig1 (Caza et al., 2016). Although interactions have yet to be identified between Mig1 and Tup1 in C. neoformans, a potential mechanistic link between these regulatory proteins in low iron conditions is an intriguing possibility.
In filamentous fungi, the Mig1p homologue, CreA, has been well studied in A. nidulans where the presence of glucose results in CreA translocation to the nucleus and subsequent repression of genes encoding enzymes required for the utilization of alternative carbon sources (Kulmburg et al., 1993, Tamayo et al., 2008, Garcia et al., 2004; Brown et al., 2013; Ries et al., 2016). Unlike CCR in yeasts, the Tup1p homologe, RcoA does not play a role in CCR in A. nidulans at the level of gene expression, however, loss of RcoA results in changes in nucleosome positioning at some CreA regulated promoters (Hicks et al., 2001, Garcia et al., 2008). Notably, loss of CreA in A. nidulans results in a leaky lethal phenotype (Dowzer and Kelly, 1991), resulting in an extremely sick strain. Full lethality of this gene deletion has been reported in several other filamentous fungi including Fusarium oxysporum (Jonkers and Rep, 2009), Penicillium chrysogenum (Cepeda-García et al., 2014) and Colletotrichum gloeosporoides (Bi et al., 2015), whereas in Neurospora crassa (Sun and Glass, 2011), Trichoderma reesei, and A. fumigatus (Beattie et al., 2017), generation of a creA-genetic null mutant is viable, underscoring divergence of the role of this transcription factor across fungal species. In A. fumigatus, the loss of creA results in a carbon and nitrogen-independent growth defect on solid media, with carbon-dependent growth defects in liquid culture. Similar to C. neoformans, loss of creA also results in increased sensitivity to mitochondrial inhibitors and the antifungal drug, voriconazole. It is unclear if the increased susceptibility to these inhibitors is a direct effect, or a result of increased drug permeability due to altered cell wall composition in the creA null mutant. Interestingly, in contrast to C. albicans, where the predominant changes in gene expression of MIG1 indicated a repressor only function for Mig1p, RNA-sequencing of the A. fumigatus creA genetic null mutant revealed nearly equal numbers of genes whose transcript levels decreased and increased in glucose medium compared to the wild-type parent (Beattie et al., 2017). While work to characterize the direct targets of CreA is ongoing, these results suggest that CreA may play a role in transcriptional activation in addition to repression in A. fumigatus as previously observed in Trichoderma reesei (Portnoy et al., 2011) and A. nidulans (Mogensen et al., 2006).
The variable essentiality of Mig1/CreA homologues across fungal species suggests functional divergence of this transcription factor. Indeed, primary amino acid sequence alignments of Mig1/CreA between yeasts and filamentous fungi show that only the C2H2-zinc finger domain is conserved (Figure 3). Thus, understanding the role of these transcription factors and their interacting partners in each species is a promising approach to better understand and define the metabolic similarities and differences of between fungal species and how these differences impact metabolism and growth in vivo.
Figure 3.
ClustalW alignment of CreA/Mig1p/Mig1 amino acid sequences from A. fumigatus (AfCreA), A. nidulans (AnCreA), S. cerevisiae (ScMig1p), C. albicans (CaMig1p) and C. neoformans (CnMig1p). Percent identity is shown with shading, where darker shading indicates higher percent identity. Zinc-finger domains are outlined in black, based on SMART domain prediction of ScMig1p.
Additional factors involved in CCR
In S. cerevisiae, glucose repression is dependent on the hexokinase, Hxk2p, which is required for phosphorylation of Mig1p by Snf1p (Herrero et al., 1998, Ahuatzi et al., 2007). In A. fumigatus, the hexokinases, hxkA and to a larger extent glkA, appear to also play a role in CCR, as loss of these proteins increase isocitrate lyase (ICL, glyoxylate cycle) activity in the presence of glucose and ethanol (Fleck and Brock, 2010). The expression of C. albicans HXK2 is regulated in response to glucose, however, null mutants of this gene have yet to be characterized and a role in CCR remains undefined. In C. neoformans, the deletion of both hexokinases, HXK1 and HXK2, results in the inability to utilize glucose, however their role in CCR has not yet been investigated. The loss of both hexokinases together, but not single deletions, results in reduced virulence when inoculated intranasally in an immunocompetent mouse model (Price et al., 2011).
The AMP-activated protein kinase Snf1p plays an important role in CCR in S. cerevisiae through regulating the cellular localization of Mig1p (De Vit et al., 1997, Ostling and Ronne, 1998). Snf1p belongs to a highly conserved family of serine/threonine protein kinases, with an essential role in mediating the response to glucose abundance/depletion thus regulating cellular energy homeostasis (Mizuno et al., 2015). Regulation of Mig1p by Snf1p has not yet been characterized in C. albicans, but a homozygous mutant of Snf1p is lethal (Petter et al., 1997). Characterization of a heterozygous SNF1 mutant revealed a growth defect, however carbon source utilization was not altered, and the mutant maintained wild-type virulence in an immunocompetent systemic model, despite a hyper-filamentous phenotype in vitro (Petter et al., 1997). Similar to S. cerevisiae, re-localization of A. nidulans CreA to the cytoplasm following transfer to glucose starvation conditions, as well as expression of cellulase and hemicellulase-encoding genes is dependent on SnfA (Brown et al., 2013). However, the role of SnfA in CCR has yet to be fully defined in filamentous human pathogens. In C. neoformans, generation of a SNF1-null mutant is viable and experimental evidence implicates this kinase in the regulation of CCR (Hu et al., 2008a). Interestingly, Snf1 appears to have divergent roles across serotypes within the species (Hu et al., 2008a) but in all strains, Snf1 is essential for virulence in both pulmonary and systemic immunocompetent models of cryptococcosis (Hu et al., 2008a, Yang et al., 2010).
Studies of CCR in A. nidulans also identified three additional factors which regulate glucose repression in this organism: CreB, CreC and CreD (Hynes and Kelly, 1977, Kelly and Hynes, 1977). CreB, a deubiquitination enzyme (Lockington and Kelly, 2001), and CreC, a WD-40 repeat protein (Todd et al., 2000), form a complex that is required for proper glucose repression (Lockington and Kelly, 2002). Mutation of either of these proteins yields strains that have growth defects in both repressing and de-repressing conditions (Hynes and Kelly, 1977, Lockington and Kelly, 2001, Ries et al., 2016). Although CreB has deubiquitination activity, the role of ubiquitin in the regulation of CreA is unclear. Ries and colleagues (Ries et al., 2016) identified an ubiquitin smear on a CreA immunoprecipitation Western blot, however, mass spectrometry to identify post-translational modifications on CreA found several phosphorylated peptides, but no ubiquitinylated peptides in repressing or de-repressing conditions (Alam et al., 2016). CreD, on the other hand, is an arrestin and PY motif containing protein that appears to regulate CCR through opposing the action of the CreB/CreC complex. Yeast two-hybrid studies of CreD identified a physical interaction between CreD and the ubiquitin ligase, HulA, suggesting that this protein may be involved in ubiquitination of targets, potentially sharing targets with the CreB/CreC complex (Boase and Kelly, 2004). Homologues of CreB, CreC and CreD are all present in A. fumigatus but a role of these proteins in virulence has not yet been determined. Homologues of these three proteins are also present in C. albicans and include the ubiquitin hydrolase Upb13p (CreB orthologue) with a predicted role in biofilm formation (Nett et al., 2009; Bonhomme et al., 2011); the alpha subunit of the COPI (coat protein) vesicle coatomer complex (CreC orthologue) which functions in vesicle and retrograde vesicle-mediated transport between the ER (endoplasmic reticulum) and the Golgi (Bonhomme et al., 2011) and lastly, the membrane protein Rod1p (CreD orthologue) which has a role in drug tolerance and is under the transcriptional control of Rgt1p, which is also involved in the regulation glucose transporter-encoding genes (Sexton et al., 2007). A recent study described the involvement of A. oryzae CreD in glucose-induced endocytosis of a maltose transporter, whose expression is regulated by CCR, through working as an adaptor protein for HulA, required for the ubiquitination of target plasma membrane transporters and subsequent endocytosis (Tanaka et al., 2017). It is therefore an intriguing possibility that the role of CreB, CreC and CreD may be one in CCR-related vesicle transport, responding to a change in available nutrients, rather than directly targeting CreA. No homologues of these three proteins have been identified in C. neoformans.
Role of CCR in virulence
Progress towards understanding the utilization of potential carbon sources during an infection has been made using transcriptional studies of C. albicans, A. fumigatus and C. neoformans exposed to immune cells or in infection models (Fradin et al., 2005, Lorenz et al., 2004, Barelle et al., 2006, Chen et al., 2014). Similarities between the response of these fungi to phagocytosis include the induction of metabolic pathways required during carbon starvation, including gluconeogenesis, fatty acid metabolism and the glyoxylate shunt, many of which are targets of the CCR system in these fungi. However, phagocytosis represents only one host niche, and during infection, occupation of several niches is likely required for full pathogenesis. This is demonstrated by reporter fusion studies, where Barelle and colleagues (2006) reported the upregulation of the glyoxylate cycle genes in phagocytosed cells, however, the population of cells in the kidney during systemic infection were largely expressing glycolytic genes and some fungal cells even expressed gluconeogenic and glyoxylate cycle genes. The aforementioned studies indicate that fungal cells experience a variety of micro-environmental conditions in vivo, and the ability to adapt to and thrive under several carbon conditions is critical for virulence (Barelle et al., 2006). Furthermore, characterisation of gene expression in fungal strains isolated directly from human subjects, as has recently be done for 2 C. neoformans isolates extracted from the CSF of two AIDS patients (Chen et al., 2014), can also provide novel insights into infection-related processes. Gene expression of C. neoformans in human CSF was more similar to the in vitro YPD control condition than in ex vivo CSF, an observation that was predicted to be due to varying nutrient availability, pH and epigenetic changes that occur specifically in the human host (Chen et al., 2014). Such studies are valuable as they characterise the genetic response of fungal pathogens that are metabolically active during human host infection, and do not rely on animal models or in vitro conditions that simulate mammalian environments, therefore providing insight into in vivo infection processes in the human host.
These studies highlight the potential for differential metabolic requirements across fungal pathogens, likely dependent on the host niche occupied by each species and the models used to test each mutant. Further support for niche specific metabolic requirements comes from Hu and colleagues (Hu et al., 2008a), who reported differential expression of several core carbon metabolism genes in C. neoformans, including the high affinity glucose transporter HXT1 and phosphoenolpyruvate carboxykinase PCK1, which were highly expressed early in pulmonary infection, but not in CSF infection, suggesting low glucose availability in the lungs early in infection. The same study identified the upregulation of carbon starvation and alternative carbon utilization pathways, such as beta-oxidation, the glyoxylate shunt and the utilization and production of acetyl-coA (Hu et al., 2008a, Steen et al., 2003). Furthermore, deletion of the high-affinity glucose transporter HXS1, required for growth in low glucose, results in attenuated virulence of C. neoformans in a pulmonary model of cryptococcosis (Liu et al., 2013).
Although these clues to in vivo carbon source utilization have identified expression of targets of carbon catabolite repression, Mig1p/Mig1 is dispensable for virulence (as measured by murine mortality) in an immunocompetent systemic model of C. albicans and an immunocompetent pulmonary model of C. neoformans, suggesting that at least in the models tested, the global de-repression of carbon catabolite genes is not detrimental to growth within the host or the expression of required virulence factors (Zaragoza et al., 2000, Caza et al., 2016). Interestingly, although MIG1 deletion had no effect on virulence in a murine inhalation model of cryptococcosis, fungal burden in the blood of MIG1 deletion inoculated animals was significantly higher than wild type inoculated animals at the time of death (Caza et al., 2016). This suggests that although mitochondrial metabolism in this mutant is perturbed, the metabolic state provides a growth or survival advantage in this niche (Caza et al., 2016).
In contrast to the pathogenic yeasts, A. fumigatus CreA plays a role in virulence in a host-specific manner. The absence of CreA results in a significant reduction in virulence of A. fumigatus in a triamcinolone model of IPA highlighted by qualitative differences in immune effector cell function, however, in a neutropenic model of IPA highlighted by quantitative differences in leukocyte populations, the creA-null mutant causes 100% mortality with a one-day increase in median survival compared to wild type. Notably, the defect in virulence in the triamcinolone model is not due to a defect in initiation of infection, as both wild type and the creA null mutant germinate and grow to the same extent over the first 48 hours of infection. Thus, it is fitness in the established infection site microenvironment where the creA null mutant fails (Beattie et al., 2017). In support of this, established biofilms of the creA null mutant are unable to proliferate in low oxygen conditions, a feature of fungal lesions that has been characterized in the triamcinolone model of IPA (Grahl et al., 2011, Beattie et al., 2017). Thus the role of this transcription factor in virulence depends on the context of the host immune status and microenvironmental conditions, and is not required for in vivo growth per se, but for adaptation to the established infection site microenvironment. CreA was thus termed a disease progression factor (DPF) for genes dispensable for infection initiation but critical for infection persistence and progression. It is hypothesized that other key regulators of fungal and host metabolism are likely DPFs given the profound changes that occur in nutrient availability during the course of microbial infections.
In addition, however, the implication of several CCR regulated pathways in vivo and the apparent lack of a role for yeast Mig1p during infection using standard genetic null mutants raises an interesting question as to whether a genetic null mutant is the best way to assess the role of a transcriptional repressor in vivo. If de-repression of alternative carbon source utilization in vivo is required for growth, perhaps a constitutively repressing Mig1p/CreA mutant may yield additional insights to the role of CCR in vivo. Studies in S. cerevisiae have identified a phosphorylation site on serine 311, that when mutated, results in constitutive repression of target genes (Ahuatzi et al., 2007). While it is unclear whether regulatory mechanism of Mig1p homologues in pathogenic fungi are conserved, creation of these types of mutants may give more insight to the role of CCR and the importance of metabolism of alternative carbon sources in various infection models.
The importance of understanding carbon source utilization in vivo is exemplified by studies that show the carbon source and nutrient environment influences virulence determinants and efficacy of drug treatments. Ene and colleagues showed that growth on the physiologically relevant carbon source, lactate, changes fungal cell wall architecture and structure, which affects the physical properties and results in increased resistance to osmotic stress, cell wall perturbing agents and antifungal drugs, a phenomenon that occurs across Candida species (Ene et al., 2012). Further, cells grown on lactate or amino acids were more virulent in an intravenous model of candidiasis (Ene et al., 2012), and lactate-grown cells were phagocytosed less efficiently than glucose-grown cells, and increased IL-10 and IL-17 immune response from PBMCs (Ene et al., 2013). Similar observations in A. fumigatus have revealed altered cell wall composition based on growth medium, where growth on yeast extract resulted in reduced beta- and alpha-glucan levels and increased chitin when compared to cells grown in RPMI. These differences in cell wall composition translate to differences to an increased minimal effective concentration (MEC) of caspofungin in RPMI when compared to yeast extract, and increased efficacy of combinatorial treatment with caspofungin and nikkomycin Z in RPMI versus yeast extract (Clavaud et al., 2012). Furthermore, for C. albicans, glucose, as well as galactose, fructose and sucrose, stimulate hyphal growth, a morphological feature that is considered to be a major virulence factor of this organism (Hudson et al., 2004, Maidan et al., 2005b). These changes in virulence attributes and drug resistance are important to understand, especially from the perspective of treatment, given that lab tests for drug susceptibility are performed in RPMI, a glucose-rich media, which given the above results, may not be an accurate reflection of the susceptibilities of strains within a patient. Therefore, understanding the metabolic state of cells within a host not only opens up the possibility of novel metabolic drug targets, but may also help in developing drug susceptibility testing that is more reflective of the in vivo status of the fungus. Table 1 lists all aforementioned CCR-related factors and their importance in virulence.
Table 1.
Factors involved in glucose sensing and metabolism and their role in virulence, when compared to the respective wild-type (WT) strain, in the human pathogenic fungi Aspergillus fumigatus, Cryptococcus neoformans and Candida albicans (NC = not characterized).
| S.c protein | Role/function | A. fumigatus | C. neoformans | C. albicans | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Homolog | Virulence | Homolog | Virulence | Homolog | Virulence | ||
|
| |||||||
| Glucose sensing and uptake | |||||||
|
| |||||||
| Gpr1p | GPCR | GprC | Delayed (Gehrke et al., 2010) | Gpr1 (NC) | NC | Gpr1p | Context dependent (Miwa et al., 2004; Maidan et al., 2005b) |
|
| |||||||
| Gpr1p | GPCR | GprD | Attenuated (Gehrke et al., 2010) | - | - | - | - |
|
| |||||||
| - | GPCR | GprK | WT (wax moth larvae model; Jung et al., 2016) | - | - | - | - |
|
| |||||||
| Gpa2p | G protein | NC | NC | Gpa2 | WT (Li et al., 2007) | Gpa2p | WT (Miwa et al., 2004) |
|
| |||||||
| Snf3p | Glucose sensor | NC | NC | Hsx1 | Delayed (Liu et al., 2013) | Hgt4p | Delayed (Brown et al., 2006) |
|
|
|
||||||
| Rgt2p | Glucose sensor | NC | NC | Hsx2 (essential) | NC | ||
|
| |||||||
| Carbon catabolite repression | |||||||
|
| |||||||
| Mig1p | Transcriptional repressor of CCR | CreA | Attenuated (Beattie et al., 2017) | Mig1 | WT (Caza et al., 2016) | Mig1p | WT (Zaragoza et al., 2000) |
|
| |||||||
| Tup1p | Co-repressor of CCR | Rco1 | NC | Tup1 | Delayed (Lee et al., 2009) | Tup1p | Avirulent (Murad et al., 2001) |
|
| |||||||
| Additional factors involved in CCR | |||||||
|
| |||||||
| Snf1p | Protein kinase | SnfA | NC | SnfA | Avirulent/delayed (Hu et al., 2008a; Yang et al., 2010) | Snf1p (Essential) | NC/Heterozygous mutant - WT (Petter et al., 1997) |
|
| |||||||
| Hxk2p | Hexokinase | HxkA/GlkA | NC | Hxk1/Hxk2 | Avirulent -double mutant only (Price et al., 2011) | Hxk2p | NC |
|
| |||||||
| - | De-ubiquitination enzyme | CreB | NC | - | - | Ubp13p | NC |
|
| |||||||
| - | WD-40 repeat protein | CreC | NC | - | - | Cop1p (C3_01720C_A) | NC |
|
| |||||||
| - | Arrestin | CreD | NC | - | - | Rod1p | NC |
Nitrogen catabolite metabolism in human pathogenic fungi
Ammonium and glutamine sensing and uptake
The preferred nitrogen sources for most microorganisms are ammonium and glutamine as they are easily assimilated and readily used for protein synthesis (Wong et al., 2008). Ammonium and glutamine metabolism are intrinsically linked, with glutamine being synthesised from ammonium and glutamate by the enzyme glutamine synthetase (Margelis et al., 2001). Fungi have evolved several sophisticated nitrogen sensory and uptake systems which allow the cell to scavenge the preferred nitrogen sources, even when present in limiting concentrations. The genome of C. albicans encodes 2 ammonium permeases MEP1 and MEP2, that allow growth under low ammonium concentrations, with Mep2p shown to be crucial for pseudohyphal growth induction through activating downstream mitogen activated protein (MAP) kinase and cAMP-dependent pathways (Biswas and Morschhäuser, 2005; Dabas et al., 2009). Homologues of Mep1p and Mep2p are encoded in the genome of A. fumigatus (MeaA, Afu1g10930) but have not been characterised to date in this fungus. Similar to C. albicans, low concentrations of extracellular ammonium induce pseudohyphal growth in C. neoformans, a process that relies on the two ammonium permeases Amt1 and Amt2 (Lee et al., 2012). Neither Amt1 nor Amt2 were required for C. neoformans pathogenicity when immunocompetent mice were infected by nasal inhalation (Rutherford et al., 2008).
In C. albicans, the amino acid sensor Csy1p was shown to sense and induce the transport of glutamine (Brega et al., 2004). Csy1p plays an important role in the induction of pseudohyphal growth in serum-based media (Brega et al., 2004) and together with the general amino acid permease (AAP) and major nitrogen source sensor Gap2p (Kraidlova et al., 2011) activates the PKA (protein kinase A) pathway which is important for virulence in pathogenic fungi (Donaton et al., 2003; Kraidlova et al., 2016). A direct role of the homozygous CSY1 deletion strain in virulence has not been published (Brega et al., 2004). The genome of A. fumigatus encodes several AAPs, including the orthologue of Gap2p (Afu7g04290), but none have been characterized so far and their role in virulence remains unknown (Sugui et al., 2008; Morton et al., 2011; Gehrke et al., 2010; Oosthuizen et al., 2011). Similar to A. fumigatus, the genome of C. neoformans encodes 10 AAPs whose expression is temperature- and nitrogen source-dependent, with Aap4 and Aap5 (both transporting glutamine) being important for growth during high temperature, oxidative stress and in vivo virulence in an immunocompetent murine model of pulmonary cryptococcosis (Fernandes et al., 2015; Cruz Martho et al., 2016).
The main nitrogen nutrient sensor, and regulator of downstream signalling pathways is the target of rapamycin (TOR) pathway (extensively reviewed in Rohde et al., 2008). The TOR pathway consists of one or two (depending on the fungal species) Tor protein kinases which are activated by amino acid-, including glutamine, derived signals and regulate a wide variety of cellular processes (Crespo et al., 2002; De Virgilio et al., 2006; Su et al., 2012; Staschke et al., 2010). The genomes of C. albicans, A. fumigatus and C. neoformans encode one TOR kinase which is essential for viability. In C. albicans, Tor1p controls NCR, the expression of Gcn4p, morphogenesis, cell adhesion, and condition-specific biofilm formation, whereas in A. fumigatus, amino acid transport and metabolism are under the regulatory control of TorA (Bastidas et al., 2009; Baldin et al., 2015; Cruz et al., 1999; Lee et al., 2012). Deletion of the C. albicans gene SIT4, homologue of the S. cerevisiae PP2A-like protein phosphatase Sit4p which mediates NCR repression by TORC1, significantly affected yeast and hyphal growth and resulted in a strain with attenuated virulence in an immunocompetent mouse model of systemic candidiasis (Lee et al., 2004). In C. albicans, Tor1p-mediated signalling is controlled by the small GTPase Rhb1p and the GTPase-activating protein Tsc2p, both of which are thought to control the expression of the ammonium permease Mep2p (Tsao et al., 2009). Rhb1p homozygous deletion mutants are viable although a role in infection of this strain has not been investigated yet. Similar to C. albicans, the A. fumigatus PP2A-like protein phosphatase SitA, was shown to be important for the cell wall integrity (CWI) pathway, adhesion and the deletion of the corresponding gene resulted in an avirulent strain when administered by nasal instillation in a neutropenic mouse model (Bom et al., 2015). Similarly, deletion of the GTPase RhbA, homologue of C. albicans Rhb1p, resulted in a strain with attenuated virulence when in a neutropenic mouse of pulmonary aspergillosis (Panepinto et al., 2003). In C. neoformans, Sin1, the orthologue of the S. cerevisiae TORC2 complex component Avo1p, and Ypk1, which is involved in the TOR-dependent phosphorylation of ribosomal proteins in S. cerevisiae, were shown to be important for virulence in an immunocompetent murine model of infection when administered via tail vein injection (Lee et al., 2012). Additional TOR-mediated, nitrogen metabolism-related signalling processes have not been studied in C. neoformans.
Nitrogen catabolite repression (NCR)
The preferential utilisation of easily assimilated nitrogen sources, such as ammonium and glutamine, is known as NCR. In A. nidulans, NCR is mediated by the co-repressor NmrA, with the deletion of the corresponding gene resulting in the partial de-repression of genes encoding enzymes required for the utilisation of alternative nitrogen sources such as asparagine and urea in the presence of favoured nitrogen sources (Andrianopoulos et al., 1998). The genome of A. fumigatus encodes a homologue of A. nidulans NmrA (Afu5g02920) although it has not been characterised and a role in virulence has not been studied to date. Homologues of NmrA do not appear to present in the genomes of C. albicans and C. neoformans.
Upon the depletion of the preferred nitrogen sources ammonium and glutamine, the fungus switches from nitrogen anabolism to catabolism by inducing the expression of catabolic genes required for the scavenging and utilisation of energetically less favoured nitrogen sources (Tudzynski, 2014). The GATA-type transcription factor Gat1p/AreA/Gat1 induces the expression of catabolic genes in C. albicans, A. fumigatus and C. neoformans. The functions as well as the role in virulence of Gat1p/AreA/Gat1 diverge in all three fungal species. Deletion of GAT1 in C. albicans still allowed the use of a number of nitrogen sources except for isoleucine, tyrosine and tryptophan (Limjindaporn et al., 2003). The genome of C. albicans encodes a second GATA-type transcription factor Gln3p, which shares overlapping functions with Gat1p, regulating the expression of genes encoding enzymes involved in nitrogen metabolism and ammonium or amino acid uptake (Liao et al., 2008). Both transcription factors have been shown to be crucial for virulence in an immunocompetent murine model of systemic disseminated candidiasis (Limjindaporn et al., 2003; Liao et al., 2008), which may be due to the inability of the homozygous deletion mutants to induce filamentation in a nitrogen source-dependent manner (Liao et al., 2008). Deletion of areA in A. nidulans and A. fumigatus abolishes growth on a wide range of nitrogen sources except for ammonium and glutamine (Langdon et al., 1995; Hensel et al., 1998). AreA was shown to contribute to, but not be essential for A. fumigatus virulence in a neutropenic murine model of pulmonary aspergillosis, with the deletion mutants presenting a delayed growth phenotype during infection (Hensel et al., 1998). In C. neoformans, deletion of GAT1 resulted in severely reduced growth on most amino acids (except arginine and proline), urea and ammonium (Kmetzsch et al., 2011). Knockout of GAT1 in C. neoformans was shown to be important for the production of capsular glucuronoxylomannan, but did not affect virulence in an immunocompetent murine model of pulmonary cryptococcosis (Kmetzsch et al., 2011; McClelland et al., 2005).
NCR is subject to negative regulation and in S. cerevisiae, this is mediated by the two GATA transcription factors Dal80p and Gzf3p. Both regulators are predicted to antagonise the functions of Gln3p and Gat1p by competing for the same binding site in NCR-regulated genes (Coffman et al., 1997; Svetlov and Cooper, 1998; for an extensive review please refer to Magasanik and Kaiser, 2002). The genome of C. albicans encodes only one homologue of S. cerevisiae Gzf3p and Dal80p, termed GZF3, whose deletion resulted in altered colony morphology at 30°C and 37°C (Homann et al., 2009). A role for this transcription factor in virulence has not been investigated yet. In A. nidulans, the homologue of Dal80p is the GATA zinc finger transcription factor AreB, which was predicted to antagonise the activation of AreA under nitrogen-limiting or – starvation conditions (Wong et al., 2009). The mechanistic nature underlying AreB-mediated regulation of AreA is currently unknown. In addition, in the presence of ammonium and glutamine, NmrA interacts with AreA, preventing the induction of genes required for the utilisation of non-preferred nitrogen sources; whereas in the absence of ammonium and glutamine, NmrA dissociates from AreA, allowing nitrogen catabolite gene activation (Andrianopoulos et al., 1998; Han et al., 2016). Transcription of nmrA and areA is inversely regulated, with both genes responding to available nitrogen sources in an opposite manner, whereas overexpression of nmrA prevented AreA function irrespective of the nitrogen source (Wong et al., 2007). Furthermore, nmrA transcription was shown to be dependent on MeaB, a bZIP DNA-binding protein, with transcriptional expression patterns of both nmrA and meaB mirroring each other (Wong et al., 2007). The expression of meaB was repressed under nitrogen starvation conditions and induced in the presence of preferred and alternative carbon sources (Wong et al., 2007). In A. fumigatus, the homologue of MeaB (Afu3g10930) has not been characterised. In C. neoformans, additional regulatory proteins involved in NCR have not been described and protein BLAST analysis of the aforementioned S. cerevisiae, C. albicans and A. nidulans factors against the C. neoformans genome database did not generate any significant hits.
Similar to CCR discussed above, the aforementioned studies highlight substantial differences in the regulation of NCR and the role it plays in establishing and maintaining an infection in all three human fungal pathogens. In Aspergillus spp., there appears to be a clear division between the utilisation of preferred and non-preferred nitrogen sources, with both processes being regulated by two independent factors which also control the function of each other (Adrianopolous et al., 1998). In C. albicans, two independent transcription factors also regulate nitrogen metabolism, although a clear division between NCR and NC induction, as seen in Aspergillus spp., is not observed. Both transcription factors rather have overlapping and specific functions regulating genes encoding enzymes required for the utilisation of a wide range of nitrogen sources, thereby creating a complex regulatory network underlying nitrogen metabolism in this fungus (Limjindaporn et al., 2003; Liao et al., 2008). In C. neoformans, only one transcription factor involved in NCR has been characterised that is required for growth on both preferred and non-preferred (with the exception of proline and arginine) nitrogen sources (Kmetzsch et al., 2011). It remains to be determined how Gat1 regulates the utilisation of different nitrogen sources and whether a second NCR transcription factor exists that could be involved in these processes.
Additional factors involved in ammonium and glutamine acquisition
In C. neoformans, the enzyme urease, which converts urea into ammonium, is an important virulence factor, that is thought to contribute, together with additional factors, to the transmigration of the blood-brain-barrier (BBB) (Shi et al., 2010). Indeed, disruption of the C. neoformans urease-encoding gene resulted in attenuated virulence in both an immunocompetent murine inhalation and intravenous infection model of cryptococcosis (Cox et al., 2000). The exact mechanism by which urease production enables dissemination into the brain is not known but ammonia production could increase adherence or toxicity to capillary endothelial cells (Olszewski et al., 2004; Shi et al., 2010). The genomes of both C. albicans and A. fumigatus also encode ureases but a role for these enzymes in infection has not been determined.
A key feature and major virulence factor of pathogenic fungi is their ability to secrete different classes of proteases which degrade proteins, therefore releasing peptides and/or amino acids which can subsequently be taken up and metabolised (Monod et al., 2002) when extracellular nitrogen sources are scarce. In C. albicans, ten aspartic proteinases (Sap1p-Sap10p) are secreted in an infection stage-dependent manner and have been shown to mediate various virulence attributes such as adhesion, biofilm formation and host tissue penetration (Naglik et al., 2003; 2004; 2008). Furthermore, they present various immunomodulatory properties, thus allowing evasion of the host immune responses (Gropp et al., 2009; Bras et al., 2012; Wu et al., 2013). Single deletions of SAP1, SAP2 and SAP3 resulted in attenuated virulence when injected intravenously into immunocompetent mice (Hube et al., 1997) whereas the simultaneous deletion of SAP4, SAP5 and SAP6, led to reduced growth in protein-rich medium and attenuated virulence in immunocompetent mouse and guinea pig models of systemic candidiasis (Sanglard et al., 1997). Sap8p is essential for regulating CWI and biofilm formation through the proteolytic cleavage of Msb2p, a sensor of the Cek1p-MAPK pathway, a factor that is important for virulence (Puri et al., 2012).
The genome of A. fumigatus is predicted to encode at least 99 secreted proteases (Machida et al., 2005; Nierman et al., 2005) of which different mixtures are secreted in a nitrogen source-dependent manner (Farnell et al., 2012). Protease secretion is under the control of NCR mediated by the transcriptional regulator, PrtT, which was shown to not be essential for virulence when neutropenic and cortisone acetate-treated mice models of pulmonary aspergillosis (Bergmann et al., 2009; Sharon et al., 2009). Similar to C. albicans, proteases modulate host immune responses (Behnsen et al., 2010) and are thought to contribute to A. fumigatus virulence by facilitating host tissue degradation and invasion (Gautam et al., 2006; Balenga et al., 2015; Kogan et al., 2004). Deletion of the alkaline serine protease alp1/aspf13 led to significantly reduced virulence in nasally infected neutropenic mice (Kolatukuddy et al., 1993), although a more recent study showed that disruption of alp1 had no significant effect on virulence, when the strain was administered intranasally, in a cortisone acetate mouse model (Behnsen et al., 2010). This discrepancy may be due to the use of different mouse models or fungal strains. A high degree of redundancy is likely to exist amongst these secreted proteases as no other secreted protease has been shown to play an important role in virulence in A. fumigatus.
Proteases secreted by C. neoformans also have immunomodulatory properties (Chen et al., 1996) and some have been found to play a role in modulating cell wall integrity (CWI) pathway components that are important for virulence (Bien et al., 2009). The metalloproteinase Mpr1 was shown to be required for establishing an infection in the central nervous system (CNS) by promoting migration of C. neoformans across the brain endothelium (Vu et al., 2014). Deletion of MPR1 resulted in attenuated virulence due to reduced brain fungal burden in an immunocompetent mouse model of pulmonary and systemic cryptococcosis (Vu et al., 2014). Similarly, C. neoformans serine proteases are thought to increase the permeability of the blood brain barrier (BBB) therefore facilitating CNS invasion (Xu et al., 2014).
Role of NCR in virulence
The utilisation of preferred nitrogen sources affects various virulence determinants in human pathogenic fungi. In C. albicans, limiting ammonium levels induce filamentous growth, a prominent virulence factor (Biswas and Morschhäuser, 2005). Furthermore, several amino acids, including glutamine, promote alkalinization of the extracellular environment, triggering hyphal morphogenesis and ensuring fungal survival in environments with a range of different pHs which is predicted to be important during infection establishment (Vylkova et al., 2011). In C. neoformans, growth in the presence of ammonium results in reduced capsule size but increases cellular melanin content (Kronstad et al., 2011) that has been determined to be an important virulence factor (Jacobson, 2000). In A. fumigatus however, a relationship between the utilisation of preferred and non-preferred nitrogen sources and the elaboration of virulence factors has not been extensively studied.
In general, carbon metabolism has received more attention than nitrogen metabolism, although several studies investigating gene expression in pathogenic fungi during early pulmonary infection showed induction of genes required for amino acid biosynthesis and transport, thereby suggesting nitrogen starvation is an important stress response faced by fungi during infection establishment (McDonagh et al., 2008; Hu et al., 2008). Further characterisation of nitrogen metabolism in C. albicans, A. fumigatus and C. neoformans during later infection time points and in different host niches is therefore required in order to further dissect the role of nitrogen acquisition during in vivo host infection and its relevance for fungal virulence determinants. NCR is intrinsically embedded in a complex regulatory network that finely tunes nitrogen metabolism, adapting the fungal cells to different types and concentrations of nitrogen sources, influencing virulence factor and potentially disease outcome. Table 2 summarises all aforemention factors that are important for NCR as well as their role in virulence in all three human pathogenic fungi.
Table 2.
Factors involved in ammonium and amino acid sensing and metabolism and their role in virulence, when compared to the respective wild-type (WT) strain, in the human pathogenic fungi Aspergillus fumigatus, Cryptococcus neoformans and Candida albicans (NC = not characterized).
| Homolog | Virulence | Homolog | Virulence | Homolog | Virulence | ||
|
| |||||||
| Nitrogen sensing and uptake | |||||||
|
| |||||||
| Mep3p | Ammonium permease | MeaA | NC | Amt1 | WT (Rutherford et al., 2008) | Mep1p | NC |
|
| |||||||
| Mep2p | Ammonium permease | Afu1g10930 | NC | Amt2 | WT (Rutherford et al., 2008) | Mep2p | NC |
|
| |||||||
| Ssy1p | Amino acid sensor | - | - | - | - | Csy1p | NC |
|
| |||||||
| Gap1p | Nitrogen sensor and amino acid permease | Afu7g04290 | NC | Aap4 | Avirulent (Cruz Martho et al., 2016) | Gap2p | NC |
| Aap5 | |||||||
|
| |||||||
| Tor1p, Tor2p | Protein kinase, major nitrogen sensor | TorA | NC | Tor1 | NC | Tor1p | NC |
|
| |||||||
| Sit4p | Protein phosphatase | SitA | Avirulent (Bom et al., 2015) | NC | NC | Sit4p | Attenuated (Lee et al., 2004) |
|
| |||||||
| Rhb1p | GTPase | RhbA | Attenuated (Panepinto et al., 2003) | NC | NC | Rhb1p | NC |
|
| |||||||
| Nitrogen catabolite repression (NCR) | |||||||
|
| |||||||
| - | Co-repressor | NmrA | NC | - | - | - | - |
|
| |||||||
| Gat1p | GATA-type transcription factor | AreA | WT (Hensel et al., 1998) | Gat1 | WT (McClelland et al., 2005) | Gat1p | Avirulent (Limjindaporn et al., 2003) |
|
| |||||||
| Gln3p | GATA-type transcription factor | - | - | - | - | Gln3p | Attenuated (Liao et al., 2008) |
|
| |||||||
| Gzf3p | GATA-type transcription factor | AreB | NC | - | - | Gzf3p | NC |
|
| |||||||
| - | Transcription factor | MeaB | NC | - | - | - | - |
|
| |||||||
| Additional factors involved in NCR | |||||||
|
| |||||||
| Dur1p | Urease | Afu1g04560 (UreB) | NC | Ure1 | Attenuated (Cox et al., 2000) | Dur1p | NC |
| Dur2p | Afu2g12900 (UreD) | Dur2p | |||||
|
| |||||||
| Yps3p | Aspartic proteinase | OpsB | NC | - | - | Sap1p | Attenuated (Hube et al., 1997) |
|
| |||||||
| Yps3p | Aspartic proteinase | OpsB | NC | - | - | Sap2p | Attenuated (Hube et al., 1997) |
|
| |||||||
| Yps3p | Aspartic proteinase | OpsB | NC | - | - | Sap3p | Attenuated (Hube et al., 1997) |
|
| |||||||
| Yps3p, Bar1p | Aspartic proteinases | OpsB | NC | - | - | Sap4p, Sap5p, Sap6p | Attenuated/Triple mutant only (Hube et al., 1997) |
|
| |||||||
| - | Metalloproteinase | Mep/Aspf5 | NC | Mpr1 | Attenuated (Vu et al., 2014) | - | - |
|
| |||||||
| Urc2p | Transcription factor | PrtT | WT (Bergmann et al., 2009; Sharon et al., 2009) | NC | NC | Zcf19p | NC |
Future perspectives
The aforementioned studies highlight the importance of carbon and nitrogen metabolism mechanisms in fungal pathogenicity and virulence as they are required for infection establishment and progression and modulating production and activity of virulence factors such as fungal cell wall structure/content and melanin, or in the case of yeast, phenotypic dimorphism. The current understanding on when and where specific pathways are required during an infection and their mechanistic nature, presents a major knowledge gap and detailed characterisation of these pathways in in vivo-relevant conditions is expected to lead to the identification of unique targets or strategies for anti-fungal drug development. In this respect, fungal species-specific niches and mechanisms need to be studied, preferably in multiple clinically relevant animal models, as the severity and degree of an infection varies with the underlying immune system disturbance. In contrast to A. fumigatus, C. albicans is often isolated from blood cultures, supporting crucial differences in infection exerted by different fungal pathogens. The role of CCR and NCR in this process remains unclear as insufficient data exists, but systemic dissemination is likely to rely on additional factors such as fungal morphology and the progression and/or the development of the immune status of the patient. A. fumigatus rapidly germinates in the lung airways into hyphae that may be more difficult to disseminate via the blood stream when compared to yeast cells. Systemic dissemination of A. fumigatus is a rare event, that is not seen in mice models and typically happens very late in affected human patients. Nevertheless, comparative studies with so called non-pathogens may be revealing as shown for differences in metabolic flexibility between S. cerevisiae and C. albicans due to the presence/absence of ubiquitination sites on enzymes required for the utilisation of alternative carbon sources (Sandai et al., 2012).
Acknowledgments
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing financial support. SRB was supported, in part, by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers T32GM008704 and T32AI007519. RAC holds an Investigator in the Pathogenesis of Infectious Diseases Award supported by the Burroughs Wellcome Fund (BWF) and is also supported by a National Institute of Allergy and Infectious Diseases (NIAID) award 2R01AI081838, National Institute of General Medicine Sciences (NIGMS) award P30GM106394 (Bruce Stanton, principal investigator), and a Cystic Fibrosis Foundation award (Bruce Stanton, principal investigator). The authors declare that no conflict of interest exists in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
GHG, RAC, SRB and LNAR contributed to the conception and design as well as the analysis and interpretation of the data of this manuscript. LNAR and SRB wrote the manuscript.
Conflict of interest
The authors declare that no conflict of interest exists.
References
- Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol. 2010;27:155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Ahuatzi D, Riera A, Palaez R, Herrero P, Moreno F. Hxk2 regulates the phosphorylation state of Mig1 and therefore its nucleocytoplasmic distribution. J Biol Chem. 2007;282:4485–4493. doi: 10.1074/jbc.M606854200. [DOI] [PubMed] [Google Scholar]
- Alam MA, Kamlangdee N, Kelly JM. The CreB deubiquitinating enzyme does not directly target the CreA repressor protein in Aspergillus nidulans. Curr Genet. 2016;63:647–667. doi: 10.1007/s00294-016-0666-3. [DOI] [PubMed] [Google Scholar]
- Andrianopoulos A, Kourambas S, Sharp JA, Davis MA, Hynes MJ. Characterization of the Aspergillus nidulans nmrA gene involved in nitrogen metabolite repression. J Bacteriol. 1998;180:1973–1977. doi: 10.1128/jb.180.7.1973-1977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament W, Huizenga JR, Mook GA, Gips CH, Verkerke GJ. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int J Sports Med. 1997;18:35–39. doi: 10.1055/s-2007-972592. [DOI] [PubMed] [Google Scholar]
- Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Philips BJ. Hyperglycaemia and pulmonary infection. Proc Nutr Soc. 2006;65:227–235. doi: 10.1079/pns2006499. [DOI] [PubMed] [Google Scholar]
- Baldin C, Valiante V, Krüger T, Schafferer L, Haas H, Kniemeyer O, Brakhage AA. Comparative proteomics of a tor inducible Aspergillus fumigatus mutant reveals involvement of the Tor kinase in iron regulation. Proteomics. 2015;15:2230–2243. doi: 10.1002/pmic.201400584. [DOI] [PubMed] [Google Scholar]
- Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyperresponsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas RJ, Heitman J, Cardenas ME. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 2009;5:e1000294. doi: 10.1371/journal.ppat.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie SR, Mark KMK, Thammahong A, Ries LNA, Dhingra S, Caffrey-Carr AK, et al. Filamentous fungal carbon catabolite repression supports metabolic plasticity and stress responses essential for disease progression. PLoS Pathog. 2017;13:e1006340. doi: 10.1371/journal.ppat.1006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, Zipfel PF, Brakhage AA. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun. 2010;78:3585–3594. doi: 10.1128/IAI.01353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A, Hartmann T, Cairns T, Bignell EM, Krappmann S. A regulator of Aspergillus fumigatus extracellular proteolytic activity is dispensable for virulence. Infect Immun. 2009;77:4041–4050. doi: 10.1128/IAI.00425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi F, Barad S, Ment D, Luria N, Dubey A, Casado V, et al. Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi. Molecular Plant Pathology. 2015 doi: 10.1111/mpp.12355. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CM, Chang YC, Nes WD, Kwon-Chung KJ, Espenshade PJ. Cryptococcus neoformans Site-2 protease is required for virulence and survival in the presence of azole drugs. Mol Microbiol. 2009;74:672–690. doi: 10.1111/j.1365-2958.2009.06895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Morschhäuser J. The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol Microbiol. 2005;56:649–669. doi: 10.1111/j.1365-2958.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Boase NA, Kelly JM. A role for creD, a carbon catabolite repression gene from Aspergillus nidulans, in ubiquitination. Mol Microbiol. 2004;53:929–940. doi: 10.1111/j.1365-2958.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- Bom VL, de Castro PA, Winkelströter LK, Marine M, Hori JI, Ramalho LN, et al. The Aspergillus fumigatus sitA Phosphatase Homologue Is Important for Adhesion, Cell Wall Integrity, Biofilm Formation, and Virulence. Eukaryot Cell. 2015;14:728–744. doi: 10.1128/EC.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme J, Chauvel M, Goyard S, Roux P, Rossignol T, d’Enfert C. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol Microbiol. 2011;80:995–1013. doi: 10.1111/j.1365-2958.2011.07626.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras G, Bochenska O, Rapala-Kozik M, Guevara-Lora I, Faussner A, Kozik A. Extracellular aspartic protease SAP2 of Candida albicans yeast cleaves human kininogens and releases proinflammatory peptides, Met-Lys-bradykinin and des-Arg(9)-Met-Lys-bradykinin. Biol Chem. 2012;393:829–839. doi: 10.1515/hsz-2012-0157. [DOI] [PubMed] [Google Scholar]
- Brega E, Zufferey R, Mamoun CB. Candida albicans Csy1p is a nutrient sensor important for activation of amino acid uptake and hyphal morphogenesis. Eukaryot Cell. 2004;3:135–143. doi: 10.1128/EC.3.1.135-143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr. 2003;133:2068S–2072S. doi: 10.1093/jn/133.6.2068S. [DOI] [PubMed] [Google Scholar]
- Brown NA, de Gouvea PF, Krohn NG, Savoldi M, Goldman GH. Functional characterisation of the non-essential protein kinases and phosphatases regulating Aspergillus nidulans hydrolytic enzyme production. Biotechnol Biofuels. 2013;6:91. doi: 10.1186/1754-6834-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Sexton JA, Johnston M. A glucose sensor in Candida albicans. Eukaryot Cell. 2006;5:1726–1737. doi: 10.1128/EC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza M, Hu G, Price M, Perfect JR, Kronstad JW. The Zinc Finger Protein Mig1 Regulates Mitochondrial Function and Azole Drug Susceptibility in the Pathogenic Fungus Cryptococcus neoformans. mSphere. 2016;1 doi: 10.1128/mSphere.00080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda-García C, Domínguez-Santos R, García-Rico RO, García-Estrada C, Cajiao A, Fierro F, et al. Direct involvement of the CreA transcription factor in penicillin biosynthesis and expression of the pcbAB gene in Penicillium chrysogenum. Applied Microbiology and Biotechnology. 2014;98:7113–7124. doi: 10.1007/s00253-014-5760-1. [DOI] [PubMed] [Google Scholar]
- Chen LC, Blank ES, Casadevall A. Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol. 1996;3:570–574. doi: 10.1128/cdli.3.5.570-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Pande K, French SD, Tuch BB, Noble SM. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10:118–135. doi: 10.1016/j.chom.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Toffaletti DL, Tenor JL, Litvintseva AP, Fang C, Mitchel TG, et al. The Cryptococcus neoformans transcriptome at the site of human meningitis. MBio. 2014;5:e01087–13. doi: 10.1128/mBio.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Clancy CJ, Wenjie X, Schneider F, Hao B, Mitchell AP, et al. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J Infect Dis. 2013;208:1529–1537. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaud C, Beauvais A, Barbin L, Munier-Lehmann H, Latge JP. The composition of the culture medium influences the beta-1,3-glucan metabolism of Aspergillus fumigatus and the antifungal activity of inhibitors of beta-1,3-glucan synthesis. Antimicrob Agents Chemother. 2012;56:3428–3431. doi: 10.1128/AAC.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman JA, Rai R, Loprete DM, Cunningham T, Svetlov V, Cooper TG. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3416–3429. doi: 10.1128/jb.179.11.3416-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–448. doi: 10.1128/iai.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JL, Powers T, Fowler B, Hall MN. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Scie USA. 2002;99:6784–6789. doi: 10.1073/pnas.102687599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Cavallo LM, Görlach JM, Cox G, Perfect JR, Cardenas ME, et al. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol Cell Biol. 1999;19:4101–4112. doi: 10.1128/mcb.19.6.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Martho KF, de Melo AT, Takahashi JP, Guerra JM, Santos DC, Purisco SU, et al. Amino acid permeases and virulence in Cryptococcus neoformans. PLoS One. 2016;11:e0163919. doi: 10.1371/journal.pone.0163919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabas N, Schneider S, Morschhäuser J. Mutational analysis of the Candida albicans ammonium permease Mep2p reveals residues required for ammonium transport and signalling. Eukaryot Cell. 2009;8:147–160. doi: 10.1128/EC.00229-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science. 2015;27:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- De Souza WR, Morais ER, Krohn NG, Savoldi M, Goldman MH, Rodrigues F, et al. Identification of metabolic pathways influences by the G-protein coupled receptors GprB and GprD in Aspergillus nidulans. PLoS One. 2013;8:e62088. doi: 10.1371/journal.pone.0062088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C, Loewith R. The TOR signalling network from yeast to man. Int J Biochem Cell Biol. 2006;38:1476–1481. doi: 10.1016/j.biocel.2006.02.013. [DOI] [PubMed] [Google Scholar]
- De Vit MJ, Waddle JA, Johnston M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaton MC, Holsbeeks I, Lagatie O, Van Zeebroeck G, Crauwels M, Winderickx J, Thevelein JM. The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2003;50:911–929. doi: 10.1046/j.1365-2958.2003.03732.x. [DOI] [PubMed] [Google Scholar]
- Dowzer CE, Kelly JM. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol Cell Biol. 1991;11:5701–5709. doi: 10.1128/mcb.11.11.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Cheng SC, Netea MG, Brown AJ. Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect Immun. 2013;81:238–248. doi: 10.1128/IAI.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Chaturvedi V, Shen SH. Identification and phylogenetic analysis of a glucose transporter gene family from the human pathogenic yeast Candida albicans. J Mol Evol. 2002;55:336–346. doi: 10.1007/s00239-002-2330-4. [DOI] [PubMed] [Google Scholar]
- Fanning S, Xu W, Solis N, Woolford CA, Filler SG, Mitchell AP. Divergent targets of Candida albicans biofilm regulator Bcr1 in vitro and in vivo. Eukaryot Cell. 2012;11:896–904. doi: 10.1128/EC.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell E, Rousseau K, Thornton DJ, Bowyer P, Herrick SE. Expression and secretion of Aspergillus fumigatus proteases are regulated in response to different protein substrates. Fungal Biol. 2012;116:1003–1012. doi: 10.1016/j.funbio.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JD, Martho K, Tofik V, Vallim MA, Pascon RC. The role of amino acid permeases and tryptophan biosynthesis in Cryptococcus neoformans survival. PLoS One. 2015;10:e0132369. doi: 10.1371/journal.pone.0132369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck CB, Brock M. Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation. Eukaryot Cell. 2010;9:1120–1135. doi: 10.1128/EC.00362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg H, Ljungdahl PO. Sensors of extracellular nutrients in Saccharomyces cerevisiae. Curr Genet. 2001;40:91–109. doi: 10.1007/s002940100244. [DOI] [PubMed] [Google Scholar]
- Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, et al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I, Gonzalez R, Gómez D, Scazzocchio C. Chromatin rearrangements in the prnD-prnB bidirectional promoter: dependence on transcription factors. Eukaryot Cell. 2004;3:144–156. doi: 10.1128/EC.3.1.144-156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García I, Mathieu M, Nikolaev I, Felenbok B, Scazzocchio C. Roles of the Aspergillus nidulans homologues of Tup1 and Ssn6 in chromatin structure and cell viability. FEMS Microbiol Lett. 2008;289:146–154. doi: 10.1111/j.1574-6968.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- Gaston B, Ratjen F, Vaughan JW, Malhotra NR, Canady RG, Snyder AH, et al. Nitrogen redox balance in the cystic fibrosis airway: effects of antipseudomonal therapy. Am J Respir Crit Care Med. 2002;165:387–390. doi: 10.1164/ajrccm.165.3.2106006. [DOI] [PubMed] [Google Scholar]
- Gautam P, Madan T, Gade WN, Sarma PU. Immunoproteomic analysis of secretory proteins of Aspergillus fumigatus with specific IGE immunoreactivity. Indian J Clin Biochem. 2006;21:12–19. doi: 10.1007/BF02912905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke A, Heinekamp T, Jacobson ID, Brakhage AA. Heptahelical receptors GprC and GprD of Aspergillus fumigatus are essential regulators of colony growth, hyphal morphogenesis, and virulence. Appl Environ Microbiol. 2010;76:3989–3998. doi: 10.1128/AEM.00052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, et al. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice CM, Bertuzzi M, Bignell EM. Receptor-mediated signalling in Aspergillus fumigatus. Front Microbiol. 2013;4 doi: 10.3389/fmicb.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp K, Schild L, Schindler S, Hube B, Zipfel PF, Skerka C. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol Immunol. 2009;47:465–475. doi: 10.1016/j.molimm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Gsaller F, Hortschansky P, Beattie SR, Klammer V, Tuppatsch K, Lechner BE, et al. The Janus transcription factor HapX controls fungal adaptation to both iron starvation and iron excess. EMBO J. 2014;33:2261–2276. doi: 10.15252/embj.201489468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KH, Seo JA, Yu JH. A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol Microbiol. 2004;51:1333–1345. doi: 10.1111/j.1365-2958.2003.03940.x. [DOI] [PubMed] [Google Scholar]
- Han X, Qiu M, Wang B, Yin WB, Nie X, Qin Q, Ren S, et al. Functional analysis of the nitrogen metabolite repression regulator gene nmrA in Aspergillus flavus. Front Microbiol. 2016;7:1794. doi: 10.3389/fmicb.2016.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Arst HN, Jr, Aufauvre-Brown A, Holden DW. The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis. Mol Gen Genet. 1998;258:553–557. doi: 10.1007/s004380050767. [DOI] [PubMed] [Google Scholar]
- Herrero P, Martinez-Campa C, Moreno F. The hexokinase 2 protein participates in regulatory DNA-protein complexes necessary for glucose repression of the SUC2 gene in Saccharomyces cerevisiae. FEBS Lett. 1998;434:71–76. doi: 10.1016/s0014-5793(98)00872-2. [DOI] [PubMed] [Google Scholar]
- Hicks J, Lockington RA, Strauss J, Dieringer D, Kubicek CP, Kelly J, et al. RcoA has pleiotropic effects on Aspergillus nidulans cellular development. Mol Microbiol. 2001;39:1482–1493. doi: 10.1046/j.1365-2958.2001.02332.x. [DOI] [PubMed] [Google Scholar]
- Homann OR, Dea J, Noble SM, Johnson AD. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009;5:e1000783. doi: 10.1371/journal.pgen.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Cheng PY, Sham A, Perfect JR, Kronstad JW. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol Microbiol. 2008a;69:1456–1475. doi: 10.1111/j.1365-2958.2008.06374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JZ, Rommereim DN, Minard KR, Woodstock A, Harrer BJ, Wind RA, et al. Metabolomics in lung inflammation:a high-resolution (1)h NMR study of mice exposed to silica dust. Toxicol Mech Methods. 2008b;18:385–398. doi: 10.1080/15376510701611032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hube B, Sanglard D, Odds FC, Hess D, Monod M, Schäfer W, Brown AJ, Gow NA. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DA, Sciascia QL, Sanders RJ, Norris GE, Edwards PJ, Sullivan PA, et al. Identification of the dialyzable serum inducer of germ-tube formation in Candida albicans. Microbiology. 2004;150:3041–3049. doi: 10.1099/mic.0.27121-0. [DOI] [PubMed] [Google Scholar]
- Hynes MJ, Kelly JM. Pleiotropic mutants of Aspergillus nidulans altered in carbon metabolism. Mol Gen Genet. 1977;150:193–204. doi: 10.1007/BF00695399. [DOI] [PubMed] [Google Scholar]
- Jacobson ES. Pathogenic roles for fungal melanins. Clin Microbiol Rev. 2000;13:708–717. doi: 10.1128/cmr.13.4.708-717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers W, Rep M. Mutation of CRE1 in Fusarium oxysporum reverts the pathogenicity defects of the FRP1 deletion mutant. Mol Microbiol. 2009;74:1100–1113. doi: 10.1111/j.1365-2958.2009.06922.x. [DOI] [PubMed] [Google Scholar]
- Jung WH, Saikia S, Hu G, Wang J, Fung CK, D'Souza C, et al. HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PLoS Pathog. 2010;6:e1001209. doi: 10.1371/journal.ppat.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JM, Hynes MJ. Increased and decreased sensitivity to carbon catabolite repression of enzymes of acetate metabolism in mutants of Aspergillus nidulans. Mol Gen Genet. 1977;156:87–92. doi: 10.1007/BF00272256. [DOI] [PubMed] [Google Scholar]
- Kim JH, Roy A, Jouandot D, Cho KH. The glucose signaling network in yeast. Biochim Biophys Acta. 2013;1830:5204–5210. doi: 10.1016/j.bbagen.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Olsson L, Nielsen J. Glucose control in Saccharomyces cerevisiae: the role of Mig1 in metabolic functions. Microbiology. 1998;144(Pt 1):13–24. doi: 10.1099/00221287-144-1-13. [DOI] [PubMed] [Google Scholar]
- Kmetzsch L, Staats CC, Simon E, Fonseca FL, Oliveira DL, Joffe LS, et al. The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans. Fungal Genet Biol. 2011;48:192–199. doi: 10.1016/j.fgb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N. Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis. 2004;189:1965–1973. doi: 10.1086/420850. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Lee JD, Rogers LM, Zimmerman P, Ceselski S, Fox B, et al. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993;61:2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraidlova L, Van Zeebroeck G, Van Dijck P, Sychrová H. The Candida albicans GAP gene family encodes permeases involved in general and specific amino acid uptake and sensing. Eukaryot Cell. 2011;10:1219–1229. doi: 10.1128/EC.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad JW, Attarian R, Cadieux B, Choi J, D’Souza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, et al. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell. 2012;11:109–118. doi: 10.1128/EC.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmburg P, Mathieu M, Dowzer C, Kelly J, Felenbok B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon catabolite repression in Aspergillus nidulans. Mol Microbiol. 1993;7:847–857. doi: 10.1111/j.1365-2958.1993.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-López C, Lorenz MC. Fungal immune evasion in a model host-pathogen interaction: Candida albicans versus macrophages. PLoS Pathog. 2013;11:e1003741. doi: 10.1371/journal.ppat.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MG, Kim SS, Yu JH, Shin KS. Characterization of gprK encoding a putative hybrid G-protein-coupled receptor in Aspergillus fumigatus. PLoS One. 2016;11:e0161312. doi: 10.1371/journal.pone.0161312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon T, Sheerins A, Ravagnani A, Gielkens M, Caddick MX, Arst HN., Jr Mutational analysis reveals dispensability of the N-terminal region of the Aspergillus transcription factor mediating nitrogen metabolite repression. Mol Microbiol. 1995;17:877–888. doi: 10.1111/j.1365-2958.1995.mmi_17050877.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Chang YC, Kwon-Chung KJ. TUP1 disruption reveals biological differences between MATa and MATalpha strains of Cryptococcus neoformans. Mol Microbiol. 2005;55:1222–1232. doi: 10.1111/j.1365-2958.2004.04458.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Chang YC, Nardone G, Kwon-Chung KJ. TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Mol Microbiol. 2007;64:591–601. doi: 10.1111/j.1365-2958.2007.05666.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Chang YC, Varma A, Kwon-Chung KJ. Regulatory diversity of TUP1 in Cryptococcus neoformans. Eukaryot Cell. 2009;8:1901–1908. doi: 10.1128/EC.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Khanal Lamichhane A, Garraffo HM, Kwon-Chung KJ, Chang YC. Involvement of PDK1, PKC and TOR signalling pathways in basal fluconazole tolerance in Cryptococcus neoformans. Mol Microbiol. 2012;84:130–146. doi: 10.1111/j.1365-2958.2012.08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IR, Lui EY, Chow EW, Arras SD, Morrow CA, Fraser JA. Reactive oxygen species homeostasis and virulence of the fungal pathogen Cryptococcus neoformans requires an intact proline catabolism pathway. Genetics. 2013;194:421–433. doi: 10.1534/genetics.113.150326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IR, Morrow CA, Fraser JA. Nitrogen regulation of virulence in clinically prevalent fungal pathogens. FEMS Microbiol Lett. 2013;345:77–84. doi: 10.1111/1574-6968.12181. [DOI] [PubMed] [Google Scholar]
- Lee CM, Nantel A, Jiang L, Whiteway M, Shen SH. The serine/threonine protein phosphatase SIT4 modulates yeast-to-hypha morphogenesis and virulence in Candida albicans. Mol Microbiol. 2004;51:691–709. doi: 10.1111/j.1365-2958.2003.03879.x. [DOI] [PubMed] [Google Scholar]
- Lee IR, Yang L, Sebetso G, Allen R, Doan TH, Blundell R, et al. Characterization of the complete uric acid degradation pathway in the fungal pathogen Cryptococcus neoformans. PLoS One. 2013;8:e64292. doi: 10.1371/journal.pone.0064292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen G, Zhang ZG, Wang YL, Thompson JK, Wang P. Canonical heterotrimeric G proteins regulating mating and virulence in Cryptococcus neoformans. Mol Biol Cell. 2007;18:4201–4209. doi: 10.1091/mbc.E07-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WL, Ramón AM, Fonzi WA. GLN3 encodes a global regulator of nitrogen metabolism and virulence of C. albicans. Fungal Genet Biol. 2008;45:514–526. doi: 10.1016/j.fgb.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limjindaporn T, Khalaf RA, Fonzi WA. Nitrogen metabolism and virulence of Candida albicans require the GATA-type transcriptional activator encoded by GAT1. Mol Microbiol. 2003;50:993–1004. doi: 10.1046/j.1365-2958.2003.03747.x. [DOI] [PubMed] [Google Scholar]
- Liu TB, Wang Y, Baker GM, Fahmy H, Jiang L, Xue C. The glucose sensor-like protein Hxs1 is a high-affinity glucose transporter and required for virulence in Cryptococcus neoformans. PLoS One. 2013;8:e64239. doi: 10.1371/journal.pone.0064239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockington RA, Kelly JM. The WD40-repeat protein CreC interacts with and stabilizes the deubiquitinating enzyme CreB in vivo in Aspergillus nidulans. Mol Microbiol. 2002;43:1173–1182. doi: 10.1046/j.1365-2958.2002.02811.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Berges MS, Capilla J, Turra D, Schafferer L, Matthijs S, Jochl C, et al. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell. 2012;24:3805–3822. doi: 10.1105/tpc.112.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- Magasanik B, Kaiser CA. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290:1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005a;16:1971–1986. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan MM, Thevelein JM, Van Dijck P. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem Soc Trans. 2005b;33:291–300. doi: 10.1042/BST0330291. [DOI] [PubMed] [Google Scholar]
- Margelis S, D’Souza C, Small AJ, Hynes MJ, Adams TH, Davis MA. Role of glutamine synthetase in nitrogen metabolite repression in Aspergillus nidulans. J Bacteriol. 2001;183:5826–5833. doi: 10.1128/JB.183.20.5826-5833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland EE, Bernhardt P, Casadevall A. Coping with multiple virulence factors: which is most important? PLoS Pathog. 2005;1:e40. doi: 10.1371/journal.ppat.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A, Fedorova ND, Crabtree J, Yu Y, Kim S, Chen D, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154. doi: 10.1371/journal.ppat.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V, Andersen MR, Brakhage AA, Braus GH, Caddick MX, Cairns TC, et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: a white paper. Fungal Biol Biotechnol. 2016;3 doi: 10.1186/s40694-016-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Takagi Y, Shinozaki M, Yun CW, Schell WA, Perfect JR, et al. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell. 2004;3:919–931. doi: 10.1128/EC.3.4.919-931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Masuda Y, Irie K. The Saccharomyces cerevisiae AMPK, Snf1, negatively regulates the Hog1 MAPK pathway in ER stress response. PLoS Genet. 2015;11:e1005491. doi: 10.1371/journal.pgen.1005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J, Nielsen HB, Hofmann G, Nielsen J. Transcription analysis using high-density micro-arrays of Aspergillus nidulans wild-type and creA mutant during growth on glucose or ethanol. Fungal Genet Biol. 2005;43:593–603. doi: 10.1016/j.fgb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Monod M, Capoccia S, Léchenne B, Zaugg C, Holdom M, Jousson O. Secreted proteases from pathogenic fungi. Int J Med Microbiol. 2002;292:405–419. doi: 10.1078/1438-4221-00223. [DOI] [PubMed] [Google Scholar]
- Morton CO, Varga JJ, Hornbach A, Mezger M, Sennefelder H, Kneitz S, et al. The temporal dynamics of differential gene expression in Aspergillus fumigatus interacting with human immature dendritic cells in vitro. PLoS One. 2011;6:e16016. doi: 10.1371/journal.pone.0016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad AM, d’Enfert C, Gaillardin C, Tournu H, Tekaia F, Talibi D, et al. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol Microbiol. 2001;42:981–993. doi: 10.1046/j.1365-2958.2001.02713.x. [DOI] [PubMed] [Google Scholar]
- Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–926. doi: 10.1111/j.1462-5822.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008;154:3266–3280. doi: 10.1099/mic.0.2008/022293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Lepak AJ, Marchillo K, Andes DR. Tim course global expression analysis of an in vivo Candida biofilm. J Infect Dis. 2009;200:307–313. doi: 10.1086/599838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, et al. Genome sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen JL, Gomez P, Ruan J, Hackett TL, Moore MM, Knight DA, et al. Dual organism transcriptomics of airway epithelial cells interacting with conidia of Aspergillus fumigatus. PLoS One. 2011;6:e20527. doi: 10.1371/journal.pone.0020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostling J, Ronne H. Negative control of the Mig1p repressor by Snf1p-dependent phosphorylation in the absence of glucose. Eur J Biochem. 1998;252:162–168. doi: 10.1046/j.1432-1327.1998.2520162.x. [DOI] [PubMed] [Google Scholar]
- Ozcan S, Dover J, Johnston M. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:2566–2573. doi: 10.1093/emboj/17.9.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto JC, Oliver BG, Fortwendel JR, Smith DL, Askew DS, Rhodes JC. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of Invasive pulmonary aspergillosis. Infect Immun. 2003;71:2819–2826. doi: 10.1128/IAI.71.5.2819-2826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters K, Thevelein JM. Glucose Sensing and Signal Transduction in Saccharomyces cerevisiae. In: Piškur J, Compagno C, editors. Molecular Mechanisms in Yeast Carbon Metabolism. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. pp. 21–56. [Google Scholar]
- Petter R, Chang YC, Kwon-Chung KJ. A gene homologous to Saccharomyces cerevisiae SNF1 appears to be essential for the viability of Candida albicans. Infect Immun. 1997;65:4909–4917. doi: 10.1128/iai.65.12.4909-4917.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips BJ, Meguer JX, Redman J, Baker EH. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- Portnoy T, Margeot A, Linke R, Atanasova L, Fekete E, Sándor E, et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: a master regulator of carbon assimilation. BMC Genomics. 2011;12:269. doi: 10.1186/1471-2164-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MS, Betancourt-Quiroz M, Price JL, Toffaletti DL, Vora H, Hu G, et al. Cryptococcus neoformans requires a functional glycolytic pathway for disease but not persistence in the host. MBio. 2011;2:e00103–00111. doi: 10.1128/mBio.00103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Kumar R, Chadha S, Tati S, Conti HR, Hube B, et al. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLoS One. 2012;7:e46020. doi: 10.1371/journal.pone.0046020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra S, Linde J, Brock M, Guthke R, Hube B, Brunke S. Regulatory networks controlling nitrogen sensing and uptake in Candida albicans. PLoS One. 2014;9:e92734. doi: 10.1371/journal.pone.0092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13:6. doi: 10.1186/1471-2180-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LN, Beattie SR, Espeso EA, Cramer RA, Goldman GH. Diverse Regulation of the CreA Carbon Catabolite Repressor in Aspergillus nidulans. Genetics. 2016;203:335–352. doi: 10.1534/genetics.116.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde JR, Bastidas R, Puria R, Cardenas ME. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 2008;11:153–160. doi: 10.1016/j.mib.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter GJ, Visser J. Carbon repression in Aspergilli. FEMS Microbiol Lett. 1997;151:103–114. doi: 10.1111/j.1574-6968.1997.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Rutherford JC, Lin X, Nielsen K, Heitman J. Amt2 permease is required to induce ammonium-responsive invasive growth and mating in Cryptococcus neoformans. Eukaryot Cell. 2008;7:237–246. doi: 10.1128/EC.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandai D, Zhikang Y, Selway L, Stead D, Walker J, Leach MD, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. MBio. 2012;3:e00495–12. doi: 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Hube B, Monod M, Odds FC, Gow NA. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jochl C, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010;6:e1001124. doi: 10.1371/journal.ppat.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;11:708–717. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schure EG, van Riel NA, Verrips CT. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2000;24:67–83. doi: 10.1111/j.1574-6976.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68:1103–1108. [PubMed] [Google Scholar]
- Sexton JA, Brown V, Johnston M. Regulation of sugar transport and metabolism by the Candida albicans Rgt1 transcriptional repressor. Yeast. 2007;24:847–860. doi: 10.1002/yea.1514. [DOI] [PubMed] [Google Scholar]
- Sharon H, Hagag S, Osherov N. Transcription factor PrtT controls expression of multiple secreted proteases in the human pathogenic mold Aspergillus fumigatus. Infect Immun. 2009;77:4051–4060. doi: 10.1128/IAI.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staschke KA, Dey S, Zaborske JM, Palam LR, McClintick JN, Pan T, et al. Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J Biol Chem. 2010;285:16893–16911. doi: 10.1074/jbc.M110.121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen BR, Zuyderduyn S, Toffaletti DL, Marra M, Jones SJ, Perfect JR, et al. Cryptococcus neoformans gene expression during experimental cryptococcal meningitis. Eukaryot Cell. 2003;2:1336–1349. doi: 10.1128/EC.2.6.1336-1349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M, Perriello G, Meyer C, Gerich J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999;55:778–792. doi: 10.1046/j.1523-1755.1999.055003778.x. [DOI] [PubMed] [Google Scholar]
- Su C, Lu Y, Liu H. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol Biol Cell. 2013;24:385–397. doi: 10.1091/mbc.E12-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui JA, Kim HS, Zarember KA, Chang YC, Gallin JI, Nierman WC, et al. Genes differentially expressed in conidia and hyphae of Aspergillus fumigatus upon exposure to human neutrophils. PLoS One. 2008;3:e2655. doi: 10.1371/journal.pone.0002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Glass NL. Identification of the CRE-1 cellulolytic regulon in Neurospora crassa. PLoS One. 2011;6:e25654. doi: 10.1371/journal.pone.0025654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov VV, Coppe TG. The Saccharomyces cerevisiae GATA factors Dal80p an Deh1p can form homo- and heterodimeric complexes. J Bacteriol. 1998;180:5682–5688. doi: 10.1128/jb.180.21.5682-5688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo EN, Villanueva A, Hasper AA, de Graaff LH, Ramón D, Orejas M. CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes in Aspergillus nidulans. Fungal Genet Biol. 2008;45:984–993. doi: 10.1016/j.fgb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hiramoto T, Tada H, Shintani T, Gomi K. Improved α-amylase production by dephosphorylation mutation of CreD, an arrestin-like protein required for glucose-induced endocytosis of maltose permease and carbon catabolite derepression in Aspergillus oryzae. Appl Environ Microbiol. 2017;83:e00592–17. doi: 10.1128/AEM.00592-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RB, Lockington RA, Kelly JM. The Aspergillus nidulans creC gene involved in carbon catabolite repression encodes a WD40 repeat protein. Mol Gen Genet. 2000;263:561–570. doi: 10.1007/s004380051202. [DOI] [PubMed] [Google Scholar]
- Tsao CC, Chen YT, Lan CY. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet Biol. 2009;46:126–136. doi: 10.1016/j.fgb.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Tudzynski B. Nitrogen regulation of fungal secondary metabolism in fungi. Front Microbiol. 2014;28 doi: 10.3389/fmicb.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Tham R, Uhrig JP, Thompson GR, 3rd, Na Pombeira S, Jamklang M, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5:e01101–e01114. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Lorenz MC. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog. 2014;10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Vienken K, Weber R, Bunting S, Requena N, Fischer R. A putative high affinity hexose transporter, hxtA of Aspergillus nidulans is induced in vegetative hyphae upon starvation and in ascogenous hyphae during cleistothecium formation. Fungal Genet Biol. 2004;41:148–156. doi: 10.1016/j.fgb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Wenjie X, Solis NV, Ehrlich RL, Woolford CA, Filler SG, Mitchell AP. Activation and alliance of regulatory pathways in C. albicans during mammalian infection. PLoS Biol. 2015;13:e1002076. doi: 10.1371/journal.pbio.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Hynes MJ, Davis MA. Recent advances in nitrogen regulation: a comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryot Cell. 2008;7:917–925. doi: 10.1128/EC.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Hynes MJ, Todd RB, Davis MA. Transcriptional control of nmrA by the bZIP transcription factor MeaB reveals a new level of nitrogen regulation in Aspergillus nidulans. Mol Microbiol. 2007;66:534–551. doi: 10.1111/j.1365-2958.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- Wong KH, Hynes MJ, Todd RB, Davis MA. Deletion and overexpression of the Aspergillus nidulans GATA factor AreB reveals unexpected pleiotropy. Microbiology. 2009;155:3868–3880. doi: 10.1099/mic.0.031252-0. [DOI] [PubMed] [Google Scholar]
- Xu CY, Zhu HM, Wu JH, Wen H, Liu CJ. Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J Int Med Res. 2014;42:85–92. doi: 10.1177/0300060513504365. [DOI] [PubMed] [Google Scholar]
- Xue C, Bahn YS, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17:667–679. doi: 10.1091/mbc.E05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li D, Liu X, Pan J, Yan B, Zhu X. Regulation of virulence factors, carbon utilization and virulence by SNF1 in Cryptococcus neoformans JEC21 and divergent actions of SNF1 between cryptococcal strains. Fungal Genet Biol. 2010;47:994–1000. doi: 10.1016/j.fgb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Rodriguez C, Gancedo C. Isolation of the MIG1 gene from Candida albicans and effects of its disruption on catabolite repression. J Bacteriol. 2000;182:320–326. doi: 10.1128/jb.182.2.320-326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]