Abstract
Background/Objectives
Overall and long-term opioid use among older adults have increased since 1999. Less is known about opioid use in older adults in nursing homes (NH).
Design
Cross-sectional.
Setting
13,522 U.S. NHs
Participants
315,949 long-stay NH resident Medicare beneficiaries with a Minimum Data Set 3.0 (MDS) assessment between 4/1/2012 – 6/30/2012 and 120 days of follow-up.
Measurements
We used Medicare Part D claims to measure length of opioid use in the 120 days from the index assessment (short-term: ≤30 days, medium-term: >30–89 days, long-term: ≥90 days), adjuvants (e.g., anticonvulsants), and other pain medications (e.g., corticosteroids). MDS assessments in the follow-up period were used to measure nonpharmacologic pain management use. Modified Poisson models were used to estimate adjusted prevalence ratios (aPR) and 95% confidence intervals (CI) for age, gender, race/ethnicity, cognitive and physical impairment and long-term opioid use.
Results
Among all long-stay residents, 32.4% were prescribed any opioid and 15.5% were prescribed long-term. Pain adjuvants (32.9% vs. 14.9% in non-users), other pain medications (25.5% vs. 11.0%) and nonpharmacologic approaches (24.5% vs. 9.3%) were more common among opioid users versus non-users. Long-term opioid use was higher in women (vs. men, aPR: 1.21, 95% CI: 1.18–1.23) and lower in racial/ethnic minorities (non-Hispanic blacks vs. whites, aPR: 0.93, 95% CI: 0.90–0.94) and those with severe cognitive impairment (vs. no/mild impairment, aPR: 0.82, 95% CI: 0.79–0.83).
Conclusion
One in 7 NH residents were prescribed opioids long-term. National concerns of opioid safety highlight reducing long-term opioid use, but this is challenging in NHs because residents may not benefit from nonpharmacologic and nonopioid interventions. Studies to address concerns of opioid safety and effectiveness (e.g., on pain and functional status) in NHs are needed.
Keywords: opioids, pain management, nursing homes, pain adjuvants
INTRODUCTION
In the United States, prescription opioid use quadrupled to >240 million prescriptions annually from 1999–2010.1 At the same time, rates of opioid misuse, abuse, addiction, and fatal and non-fatal overdoses increased for both younger and older adults.2–5 In response to this epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines for managing chronic pain that caution against opioid use and warn that the benefits for improving pain and function must outweigh the risks when prescribing opioids.6 The short-term effectiveness of opioids for pain management has been documented.7,8 No study has demonstrated that long-term opioid use (≥3 months) is effective while many studies document risks (e.g. falls, fractures, overdoses).9 Despite this, use of opioids long-term has increased in community-dwelling older adults.10,11 To our knowledge, no studies have described long-term opioid use in older adults living in nursing homes.
Managing pain in nursing homes is challenging, and this care setting has a documented history of undertreating pain.12–15 Prescribers must balance the risks associated with untreated/undertreated pain (e.g., dependence in activities of daily living, anxiety, depression)16–18 with potential risks of opioids. Opioids are prescribed to 60% of nursing home residents in persistent pain.14,19 Elderly nursing home residents may be uniquely vulnerable to the sedating side effects of opioids (even at therapeutic doses) and adverse drug events due to their older age, frailty, and high burden of comorbidities and polypharmacy in comparison to community-dwelling elders.20–23 Yet, little is known about how opioids and concurrent pharmacologic/nonpharmacologic therapies for pain are currently being used in nursing homes despite the potential harms associated with long-term opioid use.6,9
To date, opioid prescribing guidelines and national campaigns have largely focused on younger adults and community-dwelling elders and may not be applicable to nursing home residents despite the burden of pain and extensive analgesic use in this population.6,24–26 We conducted a study to: 1) estimate the prevalence of overall and long-term opioid use; 2) describe patterns of opioid and other pharmacologic/nonpharmacologic pain management by length of opioid use; and 3) describe variation in long-term opioid use by key resident factors.
METHODS
Study Design and Data Sources
This cross-sectional study (approved by the University of Massachusetts School Internal Review Board) used routinely-collected, federally-required administrative data from 2012 of all nursing home residents in Medicare- and/or Medicaid-certified nursing homes (the Minimum Data Set [MDS] 3.0; covering ~96% of US nursing homes) merged to facility characteristics data (Certification and Survey Provider Enhanced Reporting) and pharmacy claims (Medicare Part D). The MDS 3.0 is a standardized assessment conducted by trained, registered nurses with 400+ items including medical conditions, cognitive/physical functioning, and pain/pain management.27–29 Based on medical record review and interviews with staff and direct caregivers, assessments are conducted at admission and quarterly thereafter. Measures have demonstrated validity and reliability (K ≥0.78 for pain management measures).27
Study Sample
Our cohort included Medicare beneficiaries who were long-stay residents (>100 consecutive days in nursing home) and had a MDS assessment between 4/1/2012–6/30/2012 (n=602,122). The first eligible assessment was selected. Long-stay residents were included because they generally require extensive, long-term assistance from nursing homes to manage their chronic disabilities.30 After restricting to those ≥65 years of age without a cancer diagnosis or receiving hospice care, 315,949 residents met inclusion/exclusion criteria applied for practical purposes (e.g., missing data;see Supplementary Figure S1).
Opioid Use
We were conceptually interested in opioid use during 120 days of follow-up (starting at index date), which we operationalized using Medicare Part D claims. Part D claims provided information on the generic drug name (used to identify opioids), prescription fill date, days’ supply, dosage form, and dosage strength. Opioids were classified by their duration of action (short- vs. long-acting). The number of prescribed opioids during the 120 days of follow-up was calculated. Dosage form was categorized as oral, injection, transdermal, or other.
We estimated cumulative days of opioid use during the 120 day study period based on opioid prescription fill dates plus days’ supply, assuming that the opioid was used on the fill date and daily for as long as the medication was prescribed.31 We assumed that residents with overlapping opioid prescriptions (e.g., filling a second opioid prescription with ≥1 day of opioid use still remaining from the previous prescription) used both medications simultaneously as prescribed. We categorized opioid use as long-term (≥90 days cumulative use during the 120 days)32,33, medium-term(31–89 cumulative days), and short-term (1–30 days). We categorized the average daily dose in oral morphine equivalents (OME) using recent CDC guidelines as <50 mg, 50–89 mg, and ≥90 mg OME/day.6,34
Part D claims provide no information on the administration of pain medications. Although not specific to opioids, MDS assessments during follow-up (items J0100A and J0100B) were used to broadly describe pain management regimens in the preceding five days as scheduled and/or pro re nata (PRN).
Pain Management and Other Medications
Part D claims provided information on the total number of nonopioid medications and alternative analgesics / pain adjuvants prescribed during the 120 day follow-up. Nonopioid pharmacotherapies included prescribed nonsteroidal anti-inflammatory agents (NSAIDS; excluding aspirin). The American Geriatrics Society (AGS) 2009 guidelines26 were used to identify pain adjuvants and other medications used for pain.
MDS assessments during the follow-up provided information on potentially contraindicated psychopharmacologic medication use in the 7 days preceding the MDS (anxiolytics, hypnotics).6 We used the MDS because benzodiazepines were not covered by Part D in 2012. We also measured the percentage of residents receiving ≥2 antipsychotics, anxiolytics, and/or hypnotics because concurrent use of ≥2 central nervous system-active medications with opioids can increase the risk of falls/fractures beyond opioid use alone.9,35
Guidelines recommend that persons receiving opioids receive nonpharmacological interventions.6 MDS 3.0 item J0100C documented receipt of nonpharmacologic pain management in the 5 days before the assessment.27
Resident Characteristics
Age, gender, race/ethnicity, and cognitive/physical impairment have been documented to influence opioid use.12–16,36 Age (65–74 years, 76–84 years, ≥85 years), gender, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latino, Asian, other), physical impairment, and cognitive impairment came from the MDS. Physical impairment was measured using the MDS-ADL scale.37 Cognitive impairment was classified using CMS definitions.38 We also evaluated persistent pain (defined as pain with a duration ≥3 months),26 and intermittent pain.15 We considered resident characteristics that may be potential confounders including length of nursing home stay (<1 year, 1–2 years, 2–3 years, ≥3 years), marital status (married vs. other), comorbidities known to cause pain (e.g., arthritis, fractures), and total comorbidity burden (based on MDS 3.0 Section I; categorized into quartiles).
Analysis
Descriptive statistics summarized 1) variation in resident characteristics by age group; 2) medication use / characteristics of use by length of opioid use during follow-up; and 3) length of opioid use by resident characteristics. Modified Poisson models with robust variance estimation (using generalized estimating equations and exchangeable correlation structure) were used to estimate crude and adjusted prevalence ratios (aPR) with 95% confidence intervals (CI) for key resident characteristics and long-term opioid use.39 Adjusted analyses included nursing home state and all resident characteristics. We conducted analyses restricted to those in persistent pain to provide further information on this vulnerable subgroup and to compare our results to prior studies.12,14,15
Supplemental analyses to examine potential selection bias due to loss to follow-up by estimating any opioid use in those who were censored (excluding those who died or received hospice care).
RESULTS
The mean age of long-stay residents was 84.4 years (standard deviation [SD]: 8.7); the majority were women (76.2%), non-Hispanic white (80.6%), with a median length of stay of 2.1 years (interquartile range [IQR]: 1.3–3.6; see Table 1). Most residents were moderately or severely physically and/or cognitively impaired, with higher prevalence of severe cognitive impairment and dementia in older age groups. More than 40% of residents had ≥7 comorbidities, and painful comorbidities including arthritis (32.8%; more prevalent in older groups), anxiety (25.8%), depression (54.5%), and diabetes (31.6%; more prevalent in younger groups) were common. Persistent pain and intermittent pain occurred in 15.5% and 16.1% of residents, respectively.
Table 1.
Characteristics of long-stay nursing home residents who were Medicare beneficiaries in 2012, overall and stratified by age (N=315,949).
| Characteristic, %a | Overall (N=315,949) | Stratified by age | ||
|---|---|---|---|---|
|
| ||||
| 65–74 years (n= 50,005) | 75–84 years (n=95,297) | ≥85 years (n=170,647) | ||
| Women | 76.2 | 55.5 | 70.8 | 85.3 |
| Race/ethnicity | ||||
| Non-Hispanic white | 80.6 | 73.2 | 77.3 | 84.6 |
| Non-Hispanic black | 12.6 | 19.4 | 14.5 | 9.5 |
| Hispanic / Latino | 4.7 | 5.5 | 5.8 | 3.9 |
| Asian | 1.5 | 1.2 | 1.6 | 1.5 |
| Other | 0.6 | 0.8 | 0.8 | 0.5 |
| Married | 15.7 | 18.6 | 21.1 | 11.8 |
| Length of nursing home stay | ||||
| <1 year | 17.2 | 17.9 | 18.6 | 16.2 |
| 1–2 years | 31.1 | 31.7 | 32.5 | 30.1 |
| 2–3 years | 19.4 | 18.2 | 19.4 | 19.9 |
| >3 years | 32.3 | 32.3 | 29.6 | 33.8 |
| Physical impairmentb | ||||
| Moderate | 50.9 | 46.6 | 49.2 | 53.2 |
| Severe | 25.5 | 24.3 | 26.4 | 25.4 |
| Cognitive impairmentc | ||||
| Moderate | 29.4 | 29.2 | 29.8 | 29.3 |
| Severe | 44.7 | 29.8 | 42.1 | 50.6 |
| Comorbidities | ||||
| Arthritis | 30.5 | 20.6 | 27.6 | 35.0 |
| Osteoporosis | 19.5 | 11.6 | 16.6 | 23.4 |
| Hip fracture | 1.1 | 0.5 | 1.0 | 1.3 |
| Other fracture | 1.6 | 1.1 | 1.4 | 1.7 |
| Diabetes | 31.6 | 42.6 | 37.7 | 24.9 |
| Dementia | 64.5 | 45.7 | 64.5 | 70.0 |
| Parkinson’s | 7.2 | 7.7 | 9.4 | 5.9 |
| Pressure ulcers | 3.0 | 3.2 | 3.0 | 2.9 |
| Anxiety | 25.8 | 26.9 | 27.0 | 24.7 |
| Depression | 54.5 | 57.2 | 56.9 | 52.5 |
| Asthma, COPD, chronic lung failure | 18.3 | 22.6 | 19.8 | 16.2 |
| Respiratory failure | 0.6 | 1.3 | 0.6 | 0.3 |
| Renal failure | 7.8 | 8.0 | 7.8 | 7.8 |
| >8 total comorbiditiesd | 19.1 | 21.0 | 20.7 | 17.8 |
| Pain duratione | ||||
| Intermittent pain | 16.1 | 15.7 | 16.0 | 16.2 |
| Persistent pain | 15.5 | 18.4 | 16.4 | 14.1 |
Abbreviations: BIMS: Brief Interview for Mental Status; COPD: chronic obstructive pulmonary disease; CPS: Cognitive Performance Scale; MDS: Minimum Data Set.
Columns may not add up to 100% due to rounding
Defined using the MDS ADL Self-Performance Hierarchy Scale (range 0–7): None/mild (0–2), moderate (3–4), severe (5–6)
Defined using the BIMS (range 0–15) or CPS (range 0–7): no/mild impairment (BIMS 13–15 or CPS 0–2), moderate (BIMS 8–12 or CPS 3–4), severe (BIMS 0–7 or CPS 5–6)
Total comorbidity burden was defined by summing all comorbidities in MDS 3.0 section I on index assessment and categorizing into quartiles. Only top quartile is displayed.
Defined as any self-reported or staff-assessed pain on both the index MDS assessment and a preceding MDS assessment (90 +/− 20 days before the index assessment)
Thirty-two percent were prescribed any opioids during the 120 day follow-up period, with 10.4%, 6.5%, and 15.5% of all participants prescribed opioids for short-, medium-, and long-term (Table 2). The most common opioids were hydrocodone (52.6%; see Supplementary Table S1 for further detail), tramadol (31.8%), fentanyl (12.5%) and oxycodone (11.8%). The majority of short-term (99.0%), medium-term (94.5%) and long-term opioid users (65.7%) were prescribed short-acting opioids only. Long-term opioid users were prescribed more long-acting opioids (34.1% of long-term vs. 1.0% of short-term users) and had higher average daily doses (16.0% of long-term had average daily dose ≥90 mg OME/day vs. 3.3% of short-term). The majority of opioid prescriptions were oral formulations, though nearly one-quarter of long-term users received transdermal prescriptions (fentanyl). The majority of long-term users received scheduled analgesics (97.0%) with 29.5% receiving PRN analgesics. Scheduled analgesic use was lower for short-term (scheduled: 43.5%, PRN: 42.0%) and medium-term users (scheduled: 77.6%, PRN: 47.6%).
Table 2.
Characteristics of opioids, nonopioid pharmacologic alternatives, and potentially contraindicated psychopharmacologic medications prescribed during 120 days of follow-up in long-stay nursing home residents in 2012 (N=315,949).
| Medication use during follow-upa | No opioid use (n=213,652) | Length of opioid useb | ||
|---|---|---|---|---|
|
| ||||
| Short-term (n=32,841) | Medium-term (n=20,615) | Long-term (n=48,841) | ||
| Opioid usec | ||||
| Median number opioid claims, (IQR) | - | 1 (1–2) | 5 (3–7) | 6 (5–10) |
| Duration of action | ||||
| Short-acting only | - | 99.0 | 94.5 | 65.7 |
| Long-acting only | - | 0.6 | 1.8 | 12.5 |
| Short- and long-acting | - | 0.4 | 3.6 | 21.8 |
| Average daily dose (in oral morphine equivalents)d | ||||
| <50 mg/day | - | 78.4 | 77.2 | 68.1 |
| 50–89 mg/day | - | 18.4 | 19.0 | 15.9 |
| ≥90 mg/day | - | 3.3 | 3.8 | 16.0 |
| Dosage forme | ||||
| Oral | - | 99.5 | 98.8 | 91.5 |
| Injection | - | 0.2 | 0.1 | 0.1 |
| Transdermal | - | 0.7 | 3.6 | 24.3 |
| Other | - | 0.01 | 0.0 | 0.0 |
| Nonopioid pharmacologic alternatives | ||||
| Standalone prescription NSAIDS | 8.4 | 15.3 | 17.5 | 16.0 |
| Any pain adjuvants and/or other medications used for painf | 23.4 | 41.4 | 50.3 | 50.3 |
| Pain adjuvants | 14.9 | 27.6 | 34.7 | 35.7 |
| Anticonvulsants | 9.7 | 19.7 | 25.5 | 25.4 |
| Antidepressants | 6.4 | 11.6 | 15.6 | 17.1 |
| Other medications used for pain | 11.0 | 21.8 | 27.4 | 27.2 |
| Corticosteroids | 6.5 | 11.2 | 13.1 | 12.2 |
| Muscle relaxants | 2.7 | 6.6 | 9.1 | 9.6 |
| Transdermal Lidocaine | 2.4 | 6.2 | 9.2 | 9.5 |
| Potentially contraindicated medication useg | ||||
| Any anxiolytic or hypnotic use | 17.5 | 27.6 | 35.5 | 32.7 |
| ≥2 antipsychotic, anxiolytic and/or hypnoticg | 8.0 | 11.5 | 14.8 | 13.5 |
Abbreviations: ER: extended release; IQR: interquartile range; PRN: pro re nata; NSAIDS: nonsteroidal anti-inflammatory agents
Numbers are percentages unless otherwise noted. Percentages may not add up to 100% due to rounding
Based on MDS assessments during follow-up, prevalence of scheduled and PRN analgesics use varied by short- (scheduled: 43.5%, PRN: 42.0%), medium- (scheduled: 77.6%, PRN: 47.6%) and long-term users (scheduled:97.0%, PRN: 29.5%).
Short-acting opioids included codeine, dihydrocodeine, hydrocodone, hydromorphone, meperidine, morphine, nalbuphine, oxycodone, oxymorphone, pentazocine, tapentadol, and tramadol. Long-acting opioids included buprenorphine, butorphanol, transdermal fentanyl, hydromorphone extended release (ER), methadone, morphine ER, oxycodone ER, oxymorphone ER, tapentadol ER, and tramadol ER
Calculated by estimating average daily dose of each unique opioid prescription, converting each prescription to oral morphine equivalents, summing the oral morphine equivalents for all prescriptions, and dividing by the estimated cumulative days of opioid use.
Percentages add up to >100% because some participants used multiple opioids with different dosage forms
Antidepressents commonly used as adjuvants included desipramine, nortriptyline, amitriptyline, duloxetine, venlafaxine and milnacipran.26 Anticonvulsants included carbamazepine, gabapentin, lamotrigine, pregabalin. Corticosteroids included dexamethasone, prednisone, prednisolone, and methylprednisolone. Muscle relaxants included baclofen, carisoprodol, chlorzoxazone, cyclobenzaprine, dantrolene, metaxolone, methocarbamol, orphenadrine, and tizanidine.
Defined using the Minimum Data Set during 120-day follow-up (excludes the index MDS assessment).
The median number of unique nonopioid medications prescribed during 120 days was 12 in opioid users (IQR: 8–16) and 9 in non-users (IQR: 6–12). When examining other medications used during follow-up (Table 2), 16.1% of residents prescribed opioids had stand-alone prescription NSAIDS claims versus 8.4% of non-users. Pain adjuvants (32.9% of opioid users) and other medications used for pain (25.5% of opioid users) appeared more than twice as common in opioid users versus non-users. Anxiolytics/hypnotics were more common in opioid users than non-users (31.6% vs. 17.5%), as were ≥2 psychopharmacologics (13.1% vs. 8.0%). See Supplementary Table S2 for specific medications.
Nine percent, 19.8%, 26.0%, and 25.4% of non-, short-term, medium-term, and long-term opioid users received nonpharmacological pain management, respectively.
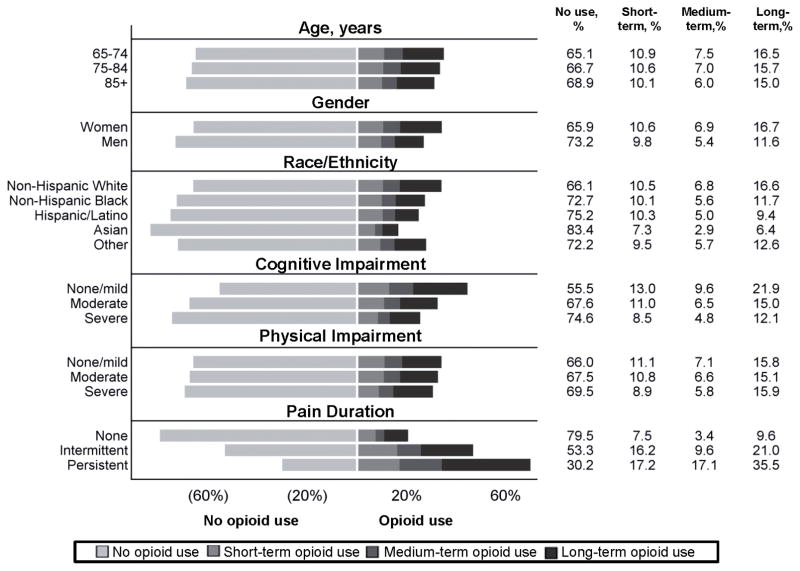
Women (vs. men; overall: 34.1% vs. 26.8%; long-term: 16.7% vs. 11.6%, non-Hispanic whites (vs. non-Hispanic blacks; overall: 33.9% vs. 27.3%; long-term: 16.6% vs. 11.7%), those with no/mild cognitive impairment (vs. severe impairment; overall: 44.5 vs. 25.4%; long-term: 21.9% vs. 12.1%), and those in persistent pain (vs. no pain; overall: 69.8% vs. 20.5%; long-term: 35.6% vs. 9.6%) appeared to have greater overall opioid and long-term opioid use (Figure 1).
Figure 1.
Crude prevalence of short-, medium-, and long-term opioid use by age, gender, race/ethnicity, cognitive impairment, physical impairment, and pain duration for long stay nursing home resident in 2012 (N=315,949).
Table 3 shows that resident factors associated with increased prevalence of long-term opioid use included being severely physically impaired (vs. no/mild impairment; aPR: 1.25, 95% CI: 1.22–1.28) or a woman (vs. men; aPR: 1.21, 95% CI: 1.18–1.23). Prevalence of long-term use was lower in racial/ethnic minorities (vs. non-Hispanic whites): non-Hispanic blacks (aPR: 0.93 95% CI: 0.90–0.95), Hispanics (aPR: 0.84, 95% CI: 0.80–0.88), Asians (aPR: 0.69, 95% CI: 0.61–0.77), and other (aPR: 0.89, 95% CI: 0.80–0.99). Prevalence of long-term opioid use was lower in those with moderate to severe cognitive impairment (severe vs. no/mild; aPR: 0.82, 95% CI: 0.79–0.83). When restricting to residents in persistent pain, adjusted prevalence ratios were qualitatively similar albeit attenuated for gender physical impairment (Supplementary Table S3).
Table 3.
Association between resident characteristics and long-term opioid use (N=315,949).
| Characteristic | Long-term opioid use, % | Crude PR (95% CI) | Adjusted PRa (95% CI) |
|---|---|---|---|
| Age, years | |||
| 65–74 | 16.5 | Referent | Referent |
| 75–84 | 15.7 | 0.93 (0.91–0.95) | 0.97 (0.95–1.00) |
| ≥85 | 15.0 | 0.88 (0.86–0.90) | 0.94 (0.92–0.97) |
| Gender | |||
| Men | 11.6 | Referent | Referent |
| Women | 16.7 | 1.40 (1.37–1.43) | 1.21 (1.18–1.23) |
| Race/ethnicity | |||
| Non-Hispanic White | 16.6 | Referent | Referent |
| Non-Hispanic Black | 11.7 | 0.77 (0.75–0.80) | 0.93 (0.90–0.95) |
| Hispanic/Latino | 9.4 | 0.69 (0.66–0.73) | 0.84 (0.80–0.88) |
| Asian | 6.4 | 0.51 (0.46–0.57) | 0.69 (0.61–0.77) |
| Other | 12.6 | 0.81 (0.72–0.90) | 0.89 (0.80–0.99) |
| Cognitive Impairment | |||
| No/mild | 21.9 | Referent | Referent |
| Moderate | 15.0 | 0.69 (0.68–0.71) | 0.89 (0.87–0.91) |
| Severe | 12.1 | 0.56 (0.54–0.57) | 0.82 (0.79–0.83) |
| Physical impairment | |||
| No/mild | 15.8 | Referent | Referent |
| Moderate | 15.1 | 0.95 (0.93–0.97) | 1.04 (1.02–1.06) |
| Severe | 15.9 | 1.04 (1.02–1.07) | 1.25 (1.22–1.28) |
Abbreviations: CI: confidence interval; PR: prevalence ratio
In supplemental analyses of opioid use in those excluded due to censoring other than death or hospice, overall opioid use was higher than in our analytic sample (41.8% vs. 32.4%; see Supplementary Text S1).
DISCUSSION
We found that nearly one-third of long-stay residents in 2012 were prescribed opioids during the 120 day follow-up, with 1 in 7 residents prescribed opioids long-term. We identified interesting patterns of nonopioid analgesics, adjuvants, and nonpharmacologic pain management use in opioid users and non-users that begin to fill knowledge gaps in nursing home resident pain management. Although we reported a lower prevalence of persistent pain than older studies,12,40 the extent to which this is due to the opioids, other medications, or methodologic differences cannot be disentangled.15 Given no studies demonstrate the long-term effectiveness of opioids and concerns that nursing home residents may be more vulnerable to adverse side effects of opioids,9,20–23 our findings inform discussions about improving opioid use with other pain management strategies in nursing homes.
The high prevalence of long-term opioid use in nursing homes is more than twofold the prescribing seen in community-dwelling older adults.10,11 This may be warranted due to residents’ pain/painful comorbidity burden and the historical undertreatment of pain in this care setting,12–16,36 which has distressing consequences including poor quality of life, decreased physical functioning, anxiety, and depression.16–18 Similar to community-dwelling populations, most residents received only short-acting opioids.41 This may be insufficient for managing chronic pain, which may require scheduled, long-acting opioids for adequate pain management.26 However, the risks of opioid use are not adequately understood, as few studies of opioid effectiveness and safety have examined nursing home residents.9,42 The high frequency of fentanyl initiation in opioid-naïve residents also raises concerns of suboptimal opioid prescribing.43,44 In community-dwelling populations, opioids have been linked to falls, fractures, overdoses, and all-cause mortality; further work is needed to characterize risks in nursing home residents.6,9
Our findings suggest that increased use of nonopioid analgesics and nonpharmacologic pain management may be potential areas for improvement in nursing homes, though these recommendations are not without limitations.6,26 Nonopioid medications used for pain are recommended as the first line treatment for chronic nonmalignant pain and can be concurrently used with opioids to provide potentially greater benefits to residents.6 We found that pain adjuvants/other medications for pain were only prescribed to approximately half of opioid users during follow-up. Whether this is appropriate remains unclear because these agents also have potential risks. For example, NSAIDS are known to be associated with hepatic, gastrointestinal, renal, and cardiovascular events in older adults and may not be appropriate opioid substitutes.26,35 AGS and CDC guidelines recommend nonpharmacologic pain management, which can be combined with opioid therapy to provide potentially greater pain relief to residents.6,45 We found that nonpharmacologic therapies were used in only one-quarter of opioid users. Although we could not ascertain specific nonpharmacologic interventions used with the MDS 3.0, common approaches in nursing homes include bio-feedback, applying heat/cold, massage, physical therapy, nerve block, stretching/strengthening exercises), and electrical stimulation.46 Their use – along with other nonopioid analgesics – are associated with short-term benefits and lower risks than opioids,6 but may have limited applicability to cognitively impaired residents and may be difficult to implement given nursing home staffing and reimbursement constraints.
We noted several potentially modifiable risk factors for opioid prescribing, particularly in long-term users. Long-term users had higher daily doses than short- and medium-term users. Although long-term users may need higher doses due to increased opioid tolerance, many adverse events linked to opioids are dose-dependent,9 and the CDC prescribing guidelines recommend reassessing individual risks and benefits at doses ≥50 OME/day and avoiding or carefully justifying doses ≥90 OME/day.6 Opioid users had a high prevalence of anxiolytic/hypnotic use. Direct measurement of benzodiazepines was not possible because they were not covered by Part D. Yet, before Part D, benzodiazepine use was more common than other anxiolytics/hypnotics in nursing homes.47 Benzodiazepines should never be co-prescribed with opioids,6 though further work is needed to evaluate this issue. Finally, 13% of opioid users received ≥2 medications known to increase the risks of falls/fractures during follow-up (antipsychotics, anxiolytics, and/or hypnotics).35 When possible, prescribers should optimize concurrent psychopharmacologic use to address concerns of drug-drug interactions and the co-occurrence of anxiety and depression with pain, which can interfere with pain management.6,35 Although reductions in antipsychotic use have occurred since 2012, antipsychotics, anxiolytics and hypnotics remain commonly used.48,49
Findings that long-term opioid use was higher in women, non-Hispanic whites, those with severe physical impairment, and those with no/mild cognitive impairment are consistent with prior studies examining the correlates of untreated or undertreated persistent pain in long-stay residents.14,15 Contrasting with prior studies,14,15 we did not observe a strong relationship between age and opioid use, perhaps due to the higher burden of certain painful comorbidities (e.g., arthritis) in those ≥85 years old, though caution is warranted when using opioids long-term in this population due to residents’ increased frailty. Identifying whether some residents are more susceptible to opioid-related adverse events is warranted.
This study has several strengths and limitations. The national MDS 3.0 data linked to Part D claims provided national, comprehensive information on long-stay residents who were Medicare beneficiaries. We provided detailed information on opioid use not previously examined including specific opioids used, dosage strength, and length of opioid use over 120 days of follow-up. We characterized nonopioid pharmacologic alternatives for pain, nonpharmacologic pain management, and concurrent psychopharmacologic medication use. While the data are from 2012, they provide an important, more recent snapshot on opioid prescribing during the height of the opioid epidemic.4,14,15 Although we had loss to follow-up by requiring residents to be in the nursing home for 120 days, the sensitivity analysis suggests our estimates may be conservative because those lost to follow-up had higher opioid use. We recognize that classifying opioid use by cumulative number of days discarded important information on patterns of opioid use. We believe this affected our results focusing on long-term opioid use minimally. Operationalizing medication use through Part D claims may overestimate opioid use if medications were not used by residents; multiple opioid claims among those prescribed opioids suggest that this issue may be minimal. We cannot know from Part D claims how medications were administered, though data from MDS assessments show that most long-term opioid users received scheduled analgesics. We have limited information on indications for medication use, resulting in potential misclassification (e.g., medications classified as pain adjuvants when they are prescribed for other indications). We could not evaluate over-the-counter medications from Part D (e.g., over-the-counter NSAIDS). No information on resident or staff pain management preferences was available.
In conclusion, long-term opioid use in older nursing home residents is twice as prevalent than in community settings.11 Cautious and consistent monitoring of opioid doses, optimizing concurrent psychopharmacologic, and increasing use of nonopioid analgesics and adjuvants and nonpharmacologic interventions hen appropriate may be warranted to improve the quality opioid use in nursing homes. Interventions to improve opioid prescribing should incorporate complex systems approaches that engage all providers including physicians, nurses, pharmacists, and other staff to improve opioid prescribing (e.g., through education, increased use of alternatives, and adverse event monitoring).50 Comparative effectiveness studies that focus on physical functioning, pain control, and quality of life endpoints, and comparative safety studies of opioids in nursing homes could help healthcare providers, residents and their families make informed decisions on opioid use.
Supplementary Material
Supplementary Figure S1. Selection of participants into study
Supplementary Table S1. Specific opioid medications prescribed to study participants during the 120 day follow-up window.
Supplementary Table S2. Specific NSAIDS, pain adjuvants, and other medications used for pain and prescribed to study participants (overall and stratified by any opioid use and length of opioid use) during the 120 day follow-up window.
Supplementary Table S3. Association between resident characteristics and long-term opioid use, restricted to residents in persistent pain.
Supplementary Text S1. Examining opioids prescribed to censored residents
Acknowledgments
Funding Sources: This work was funded by the following NIH grants: 1TL1TR001454, 1R56NR015498-01
Conflict of interest:
| Elements of Financial/Personal Conflicts | JNH | SAC | CMU | AH | JT | KLL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | x | X | X | ||||||
| Grants/Funds | X | X | X | x | X | X | ||||||
| Honoraria | X | X | X | x | X | X | ||||||
| Speaker Forum | X | X | X | x | X | X | ||||||
| Consultant | X | X | X | x | X | X | ||||||
| Stocks | X | X | X | x | X | X | ||||||
| Royalties | X | X | X | x | X | X | ||||||
| Expert Testimony | X | X | X | x | X | X | ||||||
| Board Member | X | X | X | x | X | X | ||||||
| Patents | X | X | X | x | X | X | ||||||
| Personal Relationship | X | X | X | x | X | X | ||||||
Financial Conflicts: This work was funded by the following NIH grants: 1TL1TR001454 (Hunnicutt, Lapane), 1R56NR015498-01 (Lapane, Tjia, Ulbricht). Dr. Tjia was also supported by a Sojourns Scholar Leadership Award from the Cambia Health Foundation and is a consultant for the CVS Caremark Pharmacy and Therapeutics committee. Drs. Hume and Chrysanthopoulou report no financial conflicts.
Personal Conflicts: Mr. Hunnicutt and Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, and Tjia report no personal conflicts.
Full or adequate disclosure: Mr. Hunnicutt and Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, and Tjia addressed each of the specific categories of financial and personal conflicts.
Potential conflicts: Mr. Hunnicutt and Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, and Tjia report no potential conflicts.
Author Contributions: Mr. Hunnicutt had full access to all of the data and takes responsibility for the integrity and accuracy of the data analysis. Study concept and design: Mr. Hunnicutt, Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, Tjia. Acquisition of data: Dr. Lapane. Preparation of manuscript: Mr. Hunnicutt, Dr. Lapane. Critical revision of manuscript: Mr. Hunnicutt, Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, Tjia. Statistical analysis and interpretation: Mr. Hunnicutt, Drs. Lapane and Chrysanthopoulou. Obtained funding: Mr. Hunnicutt; Dr. Lapane. Final approval of the manuscript: Mr. Hunnicutt, Drs. Lapane, Chrysanthopoulou, Ulbricht, Hume, Tjia.
Sponsor’s Role: None of the funders had a role in design, methods, data collection, analysis, or preparation of this paper.
References
- 1.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers--United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 2.Rudd R, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 3.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 4.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 5.Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376:663–673. doi: 10.1056/NEJMsa1610524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353–1369. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician. 2012;15:S67–116. [PubMed] [Google Scholar]
- 9.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 10.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo YF, Raji MA, Chen NW, et al. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129:221.e21–30. doi: 10.1016/j.amjmed.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Won AB, Lapane KL, Vallow S, et al. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52:867–874. doi: 10.1111/j.1532-5415.2004.52251.x. [DOI] [PubMed] [Google Scholar]
- 13.Pimentel CB, Briesacher BA, Gurwitz JH, et al. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63:633–641. doi: 10.1111/jgs.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fain K, Alexander GC, Dore DD, et al. Frequency and predictors of analgesic prescribing in US nursing home residents with persistent pain. J Am Geriatr Soc. 2017;65:286–293. doi: 10.1111/jgs.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunnicutt JN, Ulbricht CM, Tjia J, et al. Pain and pharmacologic pain management in long-stay nursing home residents. Pain. 2017;158:1091–1099. doi: 10.1097/j.pain.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won A, Lapane K, Gambassi G, et al. Correlates and management of nonmalignant pain in the nursing home. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. J Am Geriatr Soc. 1999;47:936–942. doi: 10.1111/j.1532-5415.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 17.Lapane KL, Quilliam BJ, Chow W, et al. The association between pain and measures of well-being among nursing home residents. J Am Med Dir Assoc. 2013;13:344–349. doi: 10.1016/j.jamda.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Cadogan MP, Edelen MO, Lorenz KA, et al. The relationship of reported pain severity to perceived effect on function of nursing home residents. J Gerontol A Biol Sci Med Sci. 2008;63:969–973. doi: 10.1093/gerona/63.9.969. [DOI] [PubMed] [Google Scholar]
- 19.Lapane KL, Quilliam BJ, Chow W, et al. Pharmacologic management of non cancer pain among nursing home residents. J Pain Symptom Manage. 2013;45:33–42. doi: 10.1016/j.jpainsymman.2011.12.285. [DOI] [PubMed] [Google Scholar]
- 20.Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629–1634. doi: 10.1001/archinte.161.13.1629. [DOI] [PubMed] [Google Scholar]
- 21.Freedman VA, Spillman BC. The residential continuum from home to nursing home: size, characteristics and unmet needs of older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69:S42–50. doi: 10.1093/geronb/gbu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jokanovic N, Tan EC, Dooley MJ, et al. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16:535.e1–12. doi: 10.1016/j.jamda.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Makris UE, Abrams RC, Gurland B, et al. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312:825–836. doi: 10.1001/jama.2014.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. N Engl J Med. 2016;374:1480–1485. doi: 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- 26.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 27.Saliba D, Buchanan J. Development and validation of a revised nursing home assessment tool: MDS 3.0. Santa Monica, California: Rand Corp; 2008. [Google Scholar]
- 28.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13:602–610. doi: 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Saliba D, Jones M, Streim J, et al. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;14:595–601. doi: 10.1016/j.jamda.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Wei YJ, Simoni-Wastila L, Zuckerman IH, et al. Algorithm for identifying nursing home days using Medicare claims and Minimum Data Set Assessment data. Med Care. 2016;54:e73–e77. doi: 10.1097/MLR.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 31.Leslie RS. Using arrays to calculate medication utilization. [Accessed April 21, 2017];SAS Global forum (online) Available at: http://www2.sas.com/proceedings/forum2007/043-2007.pdf.
- 32.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braden JB, Russo J, Fan MY, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25:733–737. doi: 10.1002/pds.3945. [DOI] [PubMed] [Google Scholar]
- 35.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 36.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 37.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare and Medicaid Services. Nursing Home Data Compendium 2015 edition. [Accessed April 21, 2017];CMS (online) Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf.
- 39.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 40.Teno JM, Weitzen S, Wetle T, et al. Persistent pain in nursing homes. JAMA. 2001;285:2081. doi: 10.1001/jama.285.16.2081-a. [DOI] [PubMed] [Google Scholar]
- 41.Von Korff M, Saunders K, Thomas Ray G, et al. Defacto Long-term Opioid Therapy for Non-Cancer Pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Won A, Lapane KL, Vallow S, et al. Long-term effects of analgesics in a population of elderly nursing home residents with persistent nonmalignant pain. J Gerontol Ser A Biol Sci Med Sci. 2006;61:165–169. doi: 10.1093/gerona/61.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pimentel CB, Gurwitz JH, Tjia J, et al. New initiation of long-acting opioids in long-stay nursing home residents. J Am Geriatr Soc. 2016;64:1772–1778. doi: 10.1111/jgs.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fain KM, Castillo-Salgado C, Dore DD, et al. Inappropriate fentanyl prescribing among nursing home residents in the United States. J Am Med Dir Assoc. 2017;18:138–144. doi: 10.1016/j.jamda.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Medicare & Medicaid Services. Chapter 3: Overview to the item-by-item guide to the MDS 3.0. [Accesssed July 5, 2017];Long-term Care Facility Resident Assesment Instrument 3.0 User’s Manual, version 1.14. 2016 :355–388. Available at: https://downloads.cms.gov/files/MDS-30-RAI-Manual-V114-October-2016.pdf.
- 47.Briesacher BA, Soumerai SB, Field TS, et al. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med. 2010;170:693–698. doi: 10.1001/archinternmed.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Medicare and Medicaid Services. Update report on the National Partnership to improve dementia care in nursing homes. [Accessed April 21, 2017];CMS (online) Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-16-28.pdf.
- 49.Tjia J, Hunnicutt JN, Hernon L, et al. Association of a communication training program with use of antipsychotics in nursing homes. [Accessed April 21, 2017];JAMA Intern Med. 2017 doi: 10.1001/jamainternmed.2017.0746. (epublished ahead of print) Available at: http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2618819. [DOI] [PMC free article] [PubMed]
- 50.El-Sayed AM, Galea S. Systems science and population health. 1. New York: Oxford University Press; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Selection of participants into study
Supplementary Table S1. Specific opioid medications prescribed to study participants during the 120 day follow-up window.
Supplementary Table S2. Specific NSAIDS, pain adjuvants, and other medications used for pain and prescribed to study participants (overall and stratified by any opioid use and length of opioid use) during the 120 day follow-up window.
Supplementary Table S3. Association between resident characteristics and long-term opioid use, restricted to residents in persistent pain.
Supplementary Text S1. Examining opioids prescribed to censored residents