Abstract
Background/Objectives
Approximately half of patients newly admitted to long-term care nursing homes experienced a prior hospitalization followed by discharge to a skilled nursing facility (SNF). We examined the characteristics associated with new institutionalizations among older adults on this care trajectory.
Design
Retrospective cohort study
Setting
SNFs and long-term care nursing homes
Participants
Medicare fee-for-service beneficiaries admitted to 7,442 SNFs in 2013 (N=597,986)
Measurements
Demographic and clinical characteristics from Medicare data and the Minimum Data Set. We defined “new institutionalization” as long-term care nursing home residence for >90 non-SNF days, starting within six months of hospital discharge.
Results
Among individuals who survived six months following hospital discharge, the overall rate of new long-term care institutionalizations was 10.0% (N=59,736). Older age, white race/ethnicity, being unmarried, Medicaid eligibility, higher income, more comorbidities, cognitive impairment, depression, functional limitations, hallucinations/delusions, aggressive behavior, incontinence, and pressure ulcers were associated with higher adjusted odds of new long-term care institutionalization. In analyses stratified by race/ethnicity, higher income was associated with decreased odds among whites (OR=0.92, 95% CI: 0.89-0.96) and increased odds among blacks (OR=1.40, 95% CI: 1.27-1.55) and Hispanics (OR=1.44, 95% CI: 1.25-1.66). Moderate/severe depression, functional limitations, hallucinations/delusions, aggressive behavior, and being unmarried were stronger risk factors for long-term care among cognitively intact individuals than among those with moderate/severe impairment. Being unmarried and having more comorbidities were stronger predictors among those 66-70 years than those 81-85 and ≥91 years.
Conclusion
Associations between risk factors and new long-term care institutionalizations varied across race/ethnicity, age group, and level of cognitive function. Programs that target older adults at increased risk may be an effective strategy for reducing new institutionalizations and fostering aging in place.
Keywords: Medicare, nursing home, race/ethnicity, cognitive status, post-acute care
Introduction
Aging in place is an important, patient-centered outcome.1 Considerable resources are dedicated to providing services that allow older individuals to remain in the community rather than in institutional settings.2-4 Older age,5-13 cognitive impairment,6,9,11-16 functional impairment,6-9,13,15,17 and lack of social support8-10,12,14,15,18 are known risk factors for institutionalization in nursing homes. Additionally, nursing home placement is more common among whites than among minority populations.10,14,19,20 These prior studies differed in the population studied (community cohort vs hospitalized patients) and outcome (long-term care nursing home vs any nursing facility).
Older adults who are hospitalized are frequently discharged to skilled nursing facilities (SNFs) for further recuperative care.21 Even with these additional services, not all patients are able to return to the community. Because institutionalization in long-term care nursing homes often follows hospitalization and then a SNF stay,11,14,22 a better understanding of the risk factors for institutionalization along this care trajectory can help inform prevention efforts.
We used a national cohort of Medicare beneficiaries to examine the characteristics associated with new institutionalizations in long-term care nursing homes among older adults who experienced a hospitalization followed by a SNF stay. We were particularly interested in whether risk factors varied across race/ethnicity, age group, and level of cognitive function. Based on previous research12,19,20 and clinical experience, we hypothesized that the associations between known risk factors and institutionalization in long-term care nursing homes would be weaker as age increased and as cognitive function decreased. We also hypothesized that risk factors would differ across race/ethnicities.
Methods
Data Sources
We used the following 100% Medicare files from 2012-2014: Medicare Provider Analysis and Review (MedPAR), Beneficiary Summary, and Resident Assessment Instrument Minimum Data Set 3.0 (MDS). The MedPAR, Beneficiary Summary, and MDS files were linked using unique encrypted beneficiary identification numbers. We used Medicare Provider of Services files to get information on the location (zip code) of SNFs.23 Median household income in the beneficiary's zip code of residence was obtained from the 2013 American Community Survey estimates of the U.S. Census Bureau.24 A data use agreement (DUA) was obtained from CMS. The research was approved by the UTMB Institutional Review Board.
Study Population
We identified individuals who received SNF services within three days of hospital discharge in 2013 and selected the first occurrence if a patient had more than one over the year (N=1,652,492). To ensure we had complete data on the predictors of interest, some of which required a one-year look back, and the outcome, which required a six-month follow-up, we excluded individuals under 66 years of age on 1/1/2013 and those without continuous Part A (no Medicare Advantage) enrollment over the one year prior to and the six months following the index hospitalization (N=343,883). We also excluded SNF patients without complete MDS assessment data (N=244,897). Our outcome was “new” institutionalizations, so we excluded individuals who resided in long-term care nursing homes at any time over the six months prior to their index hospitalization (N=145,163). We also excluded patients living outside the United States and those with outlier hospital lengths of stay, which we defined as greater than two standard deviations from the geometric mean for their Diagnosis-Related Group Major Diagnostic Category (DRG MDC) (N=41,390). Our goal was to ensure the cohort was representative of typical patients, not those experiencing excessively long hospitalizations due to factors not captured in administrative data. We excluded individuals in SNF facilities with fewer than 25 discharges (N=66,506) and those who did not survive for six months following hospital discharge (N=212,667). The final cohort included 597,986 individuals (Supplementary Figure S1).
Outcome
The outcome was “new institutionalization”, defined as residence in a long-term care nursing home for at least 90 days starting within six months of discharge from a hospital to a SNF. Days in SNF care did not count towards the 90 days. We differentiated long-term care days from SNF days using methods similar to those of Intrator, et al.25,26 We identified claims for SNF stays in the MedPAR files; MDS episodes outside of SNF dates were considered long-term care stays. We have validated this approach against Medicaid data, with 91% sensitivity and 87% positive predictive value.26
Characteristics
We extracted information on individuals' age, sex, race/ethnicity, and Medicaid eligibility (i.e., simultaneous enrollment in Medicare and Medicaid)27 from the Beneficiary Summary files. We obtained information on the length of stay and primary diagnosis (DRG MDC) for the index hospitalization from the MedPAR files. We also extracted all diagnoses from the index and other hospitalizations over the prior year to identify Elixhauser comorbidities.28
The MDS is a comprehensive assessment administered to prospective payment system SNF patients at multiple time points during their stay, including within eight days of admission.29 The instrument includes standardized items related to patients' hearing, speech, vision, cognition, mood, behavior, functional status, bowel and bladder management, health conditions, swallowing/nutritional status, oral/dental status, skin conditions, medications, and receipt of special treatments or procedures.29 The MDS 3.0 items demonstrate good reliability and validity.30 We used the first MDS assessment from the patient's SNF stay to get information on their marital status (married/unmarried), cognitive status (cognitively intact, mildly impaired, moderately/severely impaired),31 mood (no depression, minimal/mild depression, moderate/severe depression),32,33 functional status (six activities of daily living [ADL] items), and prognosis (life expectancy <6 months, yes/no). Additionally, we used these assessment files to determine whether individuals experienced hallucinations or delusions (yes/no); displayed aggressive behavior (none, moderate, severe);34 were continent (continent of bladder and bowel, yes/no); had a urinary catheter (yes/no); had an ostomy (yes/no); had a pressure ulcer (any/none); were on a ventilator or respirator (any/none); or received an insulin injection, oxygen therapy, cancer treatment, tracheostomy care, intravenous medication, blood transfusion, dialysis, or hospice care. The look-back period for the included MDS items is seven days, with the exception of mood which has a two-week look-back.29 We used questions from the MDS 3.0 to create our modified Aggressive Behavior Scale categorical variable. The original scale was created using items in the MDS 2.0 and we used the equivalent items from the MDS 3.0.34 Tetrachoric or polychoric correlations were performed to measure the correlations between the functional status items (six ADL items) and between the bladder/bowel items.35 The largest tetrachoric correlation between the functional items was 0.90. Because of this intercorrelation, we created a composite score (range 0-24) for functional status (higher scores indicating greater assistance with ADLs). The polychoric correlations between the bladder/bowel items were ≥0.76. Therefore, we created a single two-category predictor: continent of bladder and bowel (yes/no).
Data Analysis
We calculated the rates of new institutionalizations for the overall cohort and by patient characteristics. We then examined if rates varied at the state-level. Medicaid, rather than Medicare, is the primary payer for long-term care services and supports.2 The proportion of Medicaid spending for home- and community-based long-term care services varies by state.2 Rates varied across states, so all subsequent analyses included patient's state of residence as a fixed effect. We used logistic regression to determine the association between patient characteristics and new institutionalizations. We performed unadjusted and adjusted analyses. Adjusted analyses included the patient characteristics listed in Table 1, DRG MDCs (Supplementary Table S1), and state of residence. We estimated a fully interacted (i.e., all possible two-way interaction terms) logistic regression model. We then performed stratified analyses for all significant interaction terms. Analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC).
Table 1. Observed rates and unadjusted and adjusted odds of new institutionalizations by patient characteristics.
| Patient characteristic | N | Observed Rates | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| Overall | 597,986 | 10.0% | ||
| Race/ethnicity | ||||
| White | 524,253 | 9.7% | Reference | Reference |
| Black | 40,112 | 12.3% | 1.33 (1.27, 1.40) | 0.87 (0.79, 0.96) |
| Hispanic | 19,406 | 11.7% | 1.31 (1.27, 1.35) | 0.72 (0.61, 0.83) |
| Others | 14,215 | 12.5% | 1.23 (1.18, 1.29) | 0.83 (0.70, 0.99) |
| Sex | ||||
| Male | 190,505 | 9.3% | Reference | Reference |
| Female | 407,481 | 10.3% | 1.13 (1.11, 1.15) | 0.90 (0.86, 0.94) |
| Age, years | ||||
| 66-70 | 51,596 | 5.6% | Reference | Reference |
| 71-75 | 84,573 | 6.0% | 1.07 (1.02, 1.12) | 1.18 (1.01, 1.39) |
| 76-80 | 107,537 | 7.4% | 1.35 (1.29, 1.41) | 1.45 (1.24, 1.69) |
| 81-85 | 131,615 | 9.4% | 1.74 (1.67, 1.81) | 1.75 (1.51, 2.03) |
| 86-90 | 129,102 | 12.3% | 2.36 (2.26, 2.45) | 2.12 (1.83, 2.46) |
| ≥91 | 93,563 | 16.7% | 3.35 (3.22, 3.49) | 2.98 (2.55, 3.47) |
| Marital statusb | ||||
| Married | 211,357 | 6.8% | Reference | Reference |
| Unmarried | 373,767 | 11.8% | 1.85 (1.82, 1.89) | 1.30 (1.27, 1.34) |
| Medicaid eligibility | ||||
| No | 494,967 | 7.1% | Reference | Reference |
| Yes | 103,019 | 24.13% | 4.20 (4.12, 4.27) | 4.04 (3.79, 4.30) |
| Number of comorbidities | ||||
| 0,1 | 45,155 | 6.0% | Reference | Reference |
| 2 | 69,250 | 8.0% | 1.36 (1.29, 1.42) | 1.08 (1.01, 1.16) |
| 3 | 86,515 | 9.6% | 1.66 (1.59, 1.74) | 1.15 (1.07, 1.23) |
| 4 | 88,550 | 10.6% | 1.84 (1.76, 1.92) | 1.14 (1.06, 1.22) |
| ≥5 | 308,516 | 11.0% | 1.92 (1.84, 2.00) | 1.10 (1.04, 1.18) |
| Income quartilec | ||||
| ≤41,989 | 145,063 | 11.5% | Reference | Reference |
| 41,990-52,611 | 147,555 | 10.4% | 0.89 (0.87, 0.91) | 1.08 (1.01, 1.14) |
| 52,612-69,475 | 151,034 | 9.3% | 0.79 (0.77, 0.81) | 1.12 (1.05, 1.16) |
| ≥69,475 | 154,334 | 8.8% | 0.74 (0.72, 0.76) | 1.17 (1.10, 1.25) |
| Distance from SNF (km)d | ||||
| 0 | 162,578 | 11.8% | Reference | Reference |
| 0.1-7.1 | 135,250 | 8.5% | 0.69 (0.68, 0.71) | 0.69 (0.64, 0.75) |
| 7.2-15.7 | 150,252 | 9.0% | 0.74 (0.72, 0.76) | 0.76 (0.70, 0.83) |
| ≥15.8 | 149,906 | 10.4% | 0.87 (0.85, 0.89) | 0.85 (0.78, 0.92) |
| Length of hospital staye | ||||
| Quartile 1 | 227,124 | 8.8% | Reference | Reference |
| Quartile 2 | 150,731 | 10.6% | 1.23 (1.20, 1.26) | 0.94 (0.92, 0.97) |
| Quartile 3 | 103,251 | 10.5% | 1.21 (1.18, 1.24) | 0.95 (0.92, 0.98) |
| Quartile 4 | 116,880 | 11.1% | 1.29 (1.26, 1.32) | 1.04 (1.00, 1.07) |
| Type of DRG | ||||
| Surgical | 249,769 | 4.5% | Reference | Reference |
| Medical | 348,217 | 14.0% | 3.47 (3.40, 3.55) | 2.00 (1.94, 2.07) |
| Cognitive function | ||||
| Cognitively intact | 398,688 | 5.3% | Reference | Reference |
| Mildly impaired | 125,831 | 14.1% | 2.95 (2.88, 3.01) | 1.16 (0.99, 1.36) |
| Moderately or severely impaired | 73,467 | 28.6% | 7.21 (7.06, 7.36) | 1.59 (1.35, 1.89) |
| Mood | ||||
| No depression | 243,063 | 8.6% | Reference | Reference |
| Minimal or mild depression | 327,308 | 10.5% | 1.25 (1.23, 1.28) | 1.09 (1.04, 1.13) |
| Moderate or severe depression | 27,615 | 15.7% | 1.98 (1.91, 2.05) | 1.25 (1.14, 1.38) |
| ADL (score range 0-24) | ||||
| 0-6 | 25,595 | 3.4% | Reference | Reference |
| 7-12 | 138,442 | 4.5% | 1.32 (1.22, 1.41) | 0.99 (0.81, 1.20) |
| 13-18 | 388,478 | 10.4% | 3.25 (3.03, 3.47) | 1.38 (1.14, 1.67) |
| 19-24 | 45,471 | 27.2% | 10.48 (9.77, 11.24) | 2.31 (1.90, 2.80) |
| Hallucinations/Delusions | ||||
| No | 589,129 | 9.8% | Reference | Reference |
| Yes | 8,857 | 25.6% | 3.18 (3.03, 3.34) | 1.36 (1.28, 1.45) |
| Aggressive Behavior | ||||
| None | 561,798 | 9.2% | Reference | Reference |
| Moderate | 28,567 | 20.2% | 2.51 (2.43, 2.58) | 1.33 (1.29, 1.38) |
| Severe | 7,621 | 31.3% | 4.51 (4.29, 4.73) | 1.66 (1.55, 1.78) |
| Continent of bladder and bowel | ||||
| Yes | 306,092 | 4.6% | Reference | Reference |
| No | 291,894 | 15.6% | 3.82 (3.74, 3.89) | 1.85 (1.81, 1.89) |
| Ostomy | ||||
| No | 587,701 | 10.03% | Reference | Reference |
| Yes | 10,285 | 7.90% | 0.77 (0.72, 0.83) | 0.99 (0.92, 1.07) |
| Urinary catheter | ||||
| No | 546,538 | 9.60% | Reference | Reference |
| Yes | 51,448 | 14.18% | 1.56 (1.52, 1.60) | 1.26 (1.23, 1.30) |
| Insulin injection | ||||
| No | 486,857 | 9.9% | Reference | Reference |
| Yes | 111,129 | 10.6% | 1.08 (1.06, 1.10) | 1.01 (0.99, 1.04) |
| Pressure ulcer | ||||
| None | 528,169 | 9.4% | Reference | Reference |
| Any | 69,817 | 14.1% | 1.58 (1.54, 1.62) | 1.28 (1.21, 1.35) |
| Oxygen therapy | ||||
| No | 473,922 | 9.8% | Reference | Reference |
| Any | 124,064 | 10.6% | 1.08 (1.06, 1.11) | 0.89 (0.87, 0.92) |
| IV medication | ||||
| No | 558,084 | 10.1% | Reference | Reference |
| Yes | 39,902 | 7.9% | 0.76 (0.73, 0.79) | 0.74 (0.71, 0.77) |
Abbreviations: OR, odds ratio; CI, confidence interval; SNF, skilled nursing facility; DRG, Diagnosis-Related Group; ADL, activities of daily living; IV, intravenous.
Odds ratios are from a logistic regression model adjusted for all characteristics presented in the table, plus the Diagnosis-Related Group Major Diagnostic Categories (Supplementary Table S1), and state of residence. Also included in the model were a number of characteristics from the MDS with very low prevalence, including poor prognosis, cancer treatment, tracheostomy, ventilator, recent transfusion, dialysis and hospice care. The C statistic for the model was 0.83.
Marital status missing for 12,862 individuals.
Household income level in the beneficiary's zip code of residence, obtained from the 2013 American Community Survey (ACS) estimates of the U.S. Census Bureau.
Distance between individuals' zip code of residence and the skilled nursing facility.
Length of stay quartiles based on expected length of stay for admitting diagnosis-related group major diagnostic category (DRG-MDC).
Results
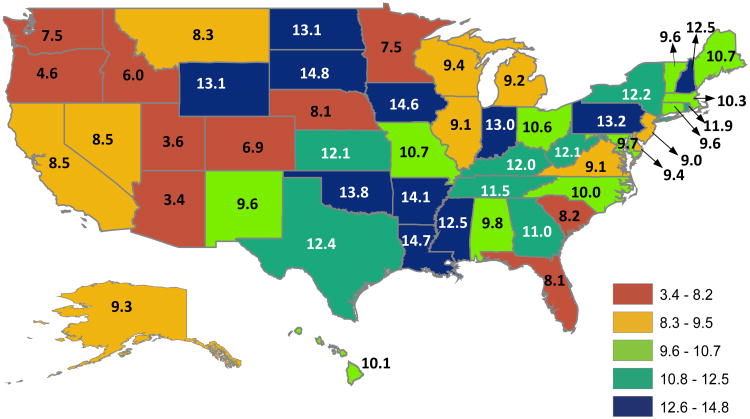
Cohort characteristics are presented in Table 1. The average age was 81.7 (SD 7.85) years; 87.7% were white and 68.1% were female. The overall rate of new long-term care institutionalizations was 10.0% (N=59,736). The median length of stay over the year following admission to long-term care was 252 (IQR: 181, 295) days. Only 7.1% (N=4,228) were discharged to the community within one year of admission and 18.5% (N=11,040) died. Rates of new institutionalizations varied across states (Figure 1). In general, rates were lowest in the West and Southwest (Arizona, 3.4%; Utah, 3.6%; Oregon, 4.6%) and highest in the central U.S (South Dakota, 14.8%; Louisiana, 14.7%; Iowa, 14.6%).
Figure 1.
State-level variation in the observed rates of new institutionalizations among a national cohort of older adults discharged to skilled nursing facilities following hospitalization (N=597,986). The overall rate was 10.0%.
Unadjusted and adjusted odds ratios (ORs) from the models estimating new institutionalization are presented in Table 1. The C-statistic for the multivariate logistic regression model was 0.83. As expected, older age (OR=2.98, 95% confidence interval [CI]: 2.55-3.47, ≥91 vs 66-70 years), being unmarried (OR=1.30, 95% CI: 1.27-1.34), and being eligible for Medicaid (OR=4.04, 95% CI: 3.79-4.30) were associated with higher odds of new long-term care institutionalizations. Residing in zip codes further from the SNF was associated with lower odds (OR=0.85, 95% CI: 0.78-0.92, ≥15.8 km vs zip code with SNF). In unadjusted analyses, blacks and Hispanics had higher odds of new institutionalizations than whites; however, this association reversed after adjustment for clinical and sociodemographic characteristics (OR=0.87, 95% CI: 0.79-0.96 for blacks and OR=0.72, 95% CI: 0.61-0.83 for Hispanics). Similarly, females had higher odds than males in unadjusted analyses, but lower odds in adjusted analyses (OR=0.90, 95%CI: 0.86-0.94). In unadjusted analyses, individuals residing in zip codes with higher median incomes had lower odds of new institutionalizations, but higher odds after adjustment (OR=1.17, 95% CI: 1.10-1.25, top vs bottom quartiles).
Individuals with more comorbidities had higher odds of new long-term care institutionalizations. There were also a number of clinical characteristics from the MDS that predicted subsequent institutionalization. These included cognitive impairment (OR=1.59, 95% CI: 1.35-1.89, moderately/severely impaired vs intact), depression (OR=1.25, 95% CI: 1.14-1.38, moderate/severe vs none), and functional limitations (OR=2.31, 95% CI: 1.90-2.80, ADL scores 19-24 vs 0-6). Patients experiencing hallucinations/delusions (OR=1.36, 95% CI: 1.28-1.45) or displaying aggressive behavior (OR=1.66, 95% CI: 1.55-1.78, severe vs none) also had higher odds, as did individuals with incontinence (OR=1.859, 95% CI: 1.81-1.898, incontinent vs continent of bladder and bowel) or pressure ulcers (OR=1.28, 95% CI: 1.21-1.35).
Subgroup analyses
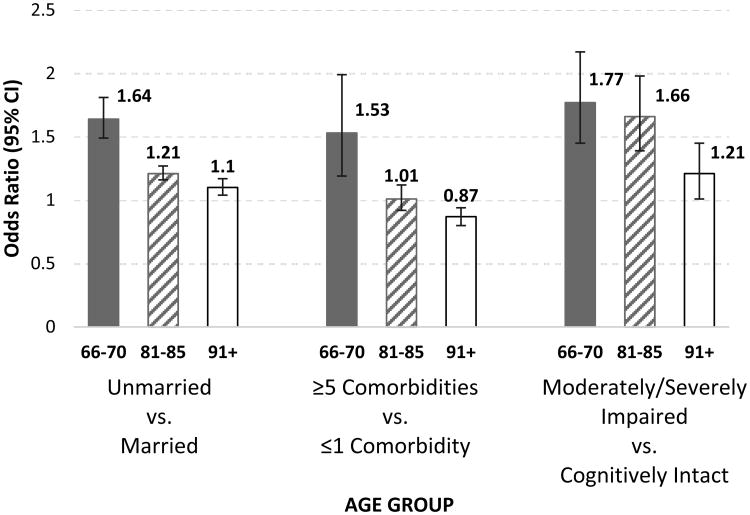
The odds ratios from all stratified analyses for significant interaction terms are presented in Supplementary Table S2. There were a number of significant interactions by race/ethnicity, age group, and level of cognitive function. In Figure 2, we show selected adjusted odds ratios stratified by age. Being unmarried and having more comorbidities were stronger predictors in the 66-70 years age group than the 81-85 and ≥91 years age groups. For example, the odds of institutionalization for individuals 66-70 years who were unmarried versus married was 1.64 (95% CI: 1.49-1.81), while the odds for those ≥91 years was 1.10 (95% CI: 1.04-1.17).
Figure 2.
Odds of new institutionalization from age group stratified analyses adjusted for all patient characteristics listed in Table 1, plus the DRG MDCs in Supplementary Table S1, and state of residence. Abbreviation: CI, confidence interval.
We present adjusted odds ratios from the stratified analyses for race/ethnicity in Table 2. Higher income was associated with decreased odds of new institutionalization among whites (OR=0.92, 95% CI: 0.89-0.96) and increased odds among blacks (OR=1.40, 95% CI: 1.27-1.55) and Hispanics (OR=1.44, 95% CI: 1.25-1.66). Moderate to severe cognitive impairment was a stronger predictor of new institutionalization among whites (OR=1.76, 95% CI: 1.49-2.08) than among blacks (OR=1.38, 95% CI: 1.16-1.65). Selected adjusted odds ratios from stratified analyses for cognitive function are also presented in Table 2. Moderate/severe depression, functional limitations, hallucinations/delusions, aggressive behavior, and being unmarried were stronger risk factors among individuals who were cognitively intact than those with moderate/severe cognitive impairment.
Table 2. Selected adjusted odds ratios from stratified analyses for race/ethnicity and level of cognitive function.
| Race/Ethnicity | |||||
|---|---|---|---|---|---|
| Patient characteristic | White | Black | Hispanic | Others | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Incomequartilea | |||||
| ≤41,989 | Reference | Reference | Reference | Reference | |
| ≥69,475 | 0.92 (0.89, 0.96) | 1.40 (1.27, 1.55) | 1.44 (1.25, 1.66) | 1.02 (0.87, 1.19) | |
| Cognitive function | |||||
| Cognitively intact | Reference | Reference | Reference | Reference | |
| Moderately or severely impaired | 1.76 (1.49, 2.08) | 1.38 (1.16, 1.65) | 1.63 (1.33, 1.99) | 1.63 (1.32, 2.02) | |
| Cognitive Function | |||||
| Patient characteristic | Cognitively intact | Moderately/Severely impaired | |||
| OR (95% CI) | OR (95% CI) | ||||
| Mood | |||||
| No depression | Reference | Reference | |||
| Moderate or severe depression | 1.49 (1.33, 1.66) | 1.04 (0.93, 1.16) | |||
| ADL (score range 0-24) | |||||
| 0-6 | Reference | Reference | |||
| 19-24 | 4.43 (3.58, 5.48) | 1.20 (0.94, 1.55) | |||
| Hallucinations/Delusions | |||||
| No | Reference | Reference | |||
| Yes | 1.45 (1.27, 1.66) | 1.20 (1.11, 1.30) | |||
| Aggressive Behavior Scale | |||||
| None | Reference | Reference | |||
| Severe | 2.12 (1.81, 2.48) | 1.46 (1.36, 1.57) | |||
| Marital status | |||||
| Married | Reference | Reference | |||
| Unmarried | 1.43 (1.37, 1.48) | 1.18 (1.13, 1.23) | |||
Abbreviations: OR, odds ratio; CI, confidence interval; ADL, activities of daily living
Note: Odds ratios are from logistic regression models adjusted for all patient characteristics listed in Table 1, Diagnosis-Related Group Major Diagnostic Categories (Supplementary Table S1), and state of residence.
Household income level in the beneficiary's zip code of residence, obtained from the 2013 American Community Survey (ACS) estimates of the U.S. Census Bureau.
We performed sensitivity analyses in a cohort that included those who died over the six months following hospital discharge (N=810,653), with “new institutionalization” defined as residence in a long-term care nursing home for at least 90 days or until death, starting within six months of discharge from a hospital to a SNF. Adjusted odds ratios for the sensitivity analysis are presented in Supplementary Table S3. The overall rate of new institutionalizations was 12.8%. The direction of associations remained the same as the original analysis except for income in the highest quartile, which was associated with slightly lower odds (OR=0.96, 95%CI: 0.94-0.98) in the sensitivity analysis and higher odds in the original analysis. Insulin injection was associated with the outcome in the sensitivity analysis (OR=1.03, 95%CI: 1.01-1.05), but not in the original analysis; whereas pressure ulcers were not associated with the outcome in the sensitivity analysis, but were in the original analysis. Cognitive impairment was more strongly associated with the outcome in the sensitivity analysis than the original analysis.
Discussion
In this national cohort of community-dwelling older adults discharged to SNFs following hospitalization, among those who survived for six months 10.0% had a subsequent long-term care admission. This rate is higher than the new long-term care institutionalization rate of 3.6% for Medicare beneficiaries discharged from the hospital36 because individuals discharged to a SNF are at an increased risk of institutionalization.11,14,22
Previous research indicates that whites are more likely to be institutionalized in nursing homes than other race/ethnicities,10,13,20,37 while the relationship between socioeconomic status and risk for institutionalization is less consistent.10,13 Our results also found a higher risk for whites, after adjusting for patient characteristics such as demographics, socioeconomic status and comorbidities. Furthermore, there were a number of significant interactions between race/ethnicity and other patient characteristics. In our cohort, higher income had a protective effect among whites; however, among both blacks and Hispanics, higher income was associated with greater odds of institutionalization. These findings differ from previous studies reporting lower risk of institutionalization among blacks with better socioeconomic status.20,37 The inconsistency may be related to the different socioeconomic status indicator variables used and/or to cohort differences. We used the median income in an individuals' zip code of residence as a proxy for socioeconomic status. Prior studies used self-reported non-housing wealth37 or dichotomized income to above/below $10,000.20 We studied individuals discharged from hospitals to SNFs. This represents a specific and common trajectory leading to institutionalization. The interplay of race/ethnicity and economic resources may be different along this pathway compared to other pathways (e.g., direct admission to long-term care from home). Finally, we studied risk of long-term care nursing homes whereas other studies conflate SNF and long-term care stays under the rubric of “nursing home”.
While the association between older age and risk for institutionalization is well-established,5-13 less is known on whether risk factors differ between age groups within the older adult population. In general, the strength of associations between risk factors and new institutionalizations diminished as age increased. In some cases, the association disappeared, such as with comorbidities.
A strength of our study is that we used risk factors assessed at the time of a precipitating event (i.e., hospitalization followed by SNF admission), not at the time of institutionalization. This allows findings to inform proactive efforts focused on preventing institutional care. Avoiding unnecessary institutionalizations is a reasonable objective, as providing home-based services may delay nursing home entry.38 The variation in rates across states supports the concept that some future institutionalizations among individuals discharged from acute care hospitals to SNFs are potentially preventable. Comprehensive prevention efforts may need to target persons at increased risk. In addition to modifying risk factors that are mutable, efforts may need to focus on addressing gaps in home and community-based services and supports. Not all new institutionalizations will be avoidable with the current services and supports available. However, a clear picture of the individuals at higher risk for institutionalization will guide resource development and foster aging in place initiatives. Home- and community-based long-term care services and supports can be tailored to the needs of those who currently continue to require institutional-level care. Our findings extend previous research and help inform these efforts.
Successful discharge to the community, defined as discharging to a community setting and remaining there without an unplanned rehospitalization or death for 31 days, is a new quality measure for SNFs.39 We present the patient characteristics associated with increased risk of institutionalization following SNF discharge. These findings may help inform risk-adjustment for the new community discharge quality metric.
Limitations
We used Medicare claims and assessment data to address our study objectives. Although this provided a national sample, these data were not collected for research purposes. Information regarding the accuracy of data entry is not available. Use of this data limits the generalizability of our findings to the Medicare fee-for-service population.
Our definition of “long-term care” differs from the definition used by the Centers for Medicare & Medicaid Services (CMS) for quality reporting. CMS dichotomizes patients in nursing homes as short-stay or long-stay based on how long they have been in the facility. Individuals residing in a nursing home for more than 100 days are considered long-stay residents.40 We defined “long-term care” as residing in a nursing home for 90 days, excluding any days in which the individual received SNF services. Our approach allowed us to differentiate between the two disparate populations within nursing homes, those receiving post-acute recuperative SNF services and those residing in the facility in a more permanent capacity. The importance of distinguishing between these populations is highlighted in other work.25,26,41
We were interested in new institutionalizations among individuals discharged from acute care hospitals to SNFs. This represents a vulnerable population at high risk for future institutionalization, but limits the generalizability of our findings to others on this care trajectory. An important area for future research will be examining risk factors along other pathways (e.g., other post-acute care settings). Future research is also needed to better understand the role SNF quality plays in risk of institutionalization. SNF quality may have a mediating effect on risk for future institutionalization, as there may be certain characteristics (e.g. socioeconomic status) that increase the likelihood of patients going to high versus low quality facilities.
Conclusions
Tailoring programs to address the needs of patients with risk factors for institutionalization in long-term care nursing homes may improve prevention efforts. We examined characteristics associated with new institutionalizations among individuals receiving SNF services following hospitalization. In this vulnerable population, risk factors varied across race/ethnicity, age group, and level of cognitive function. We observed a variation in rates across states, suggesting that some new institutionalizations may be preventable. A better understanding of the complex interplay between risk factors and Medicaid policies may further inform targeted prevention efforts.
Supplementary Material
Supplementary Figure S1. Flow chart presenting number of eligible cases remaining at each step as exclusion criteria applied.
Supplementary Table S1. New institutionalizations by patients' hospital diagnostic-related group major diagnostic category (DRG-MDC).
Supplementary Table S2. Stratified analyses for all significant interaction terms in the fully interacted (i.e. all possible two-way interaction terms) logistic regression model predicting new institutionalizations.
Supplementary Table S3. Observed rates and adjusted odds of residence in a long-term care nursing home for at least 90 days or until death, starting within six months of discharge from a hospital to a SNF. In this analysis we did not exclude those who died within six months of hospital discharge, as we did in the main analyses.
Acknowledgments
Funding Sources: The study was supported by the National Institutes of Health (R01AG33134; K05CA134923; R01HD069443; K12HD055929; U54GM104941; P2CHD065702, and P30AG024832); the Agency for Healthcare Research and Quality (R24HS22134); and the Foundation for Physical Therapy's Center of Excellence in Physical Therapy Health Services and Health Policy Research and Training Grant.
Sponsor's Role: The study was supported by the National Institutes of Health (R01AG33134; K05CA134923; R01HD069443; K12HD055929; U54GM104941; P2CHD065702, and P30AG024832); the Agency for Healthcare Research and Quality (R24HS22134); and the Foundation for Physical Therapy's Center of Excellence in Physical Therapy Health Services and Health Policy Research and Training Grant. The funding organizations had no role in the design of the study; the collection, management, analysis, and interpretation of the data; or the preparation of the manuscript.
Footnotes
Conflict of Interest: The authors have no financial or personal conflicts of interest to disclose.
Author Contributions: Study concept and design: All authors. Acquisition of data: Goodwin. Analysis and interpretation of data: All authors. Preparation of the manuscript: Middleton. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Li and Kuo. Obtained funding: Goodwin. Study supervision: Goodwin.
References
- 1.AARP. Home and Community Preferences of the 45+ Population. [Accessed March 16, 2017];2014 Available at: http://www.aarp.org/content/dam/aarp/research/surveys_statistics/il/2015/2014-Home-Community-45plus-res-il.pdf.
- 2.Eiken S, Sredl K, Burwell B, et al. Medicaid Expenditure for Long-Term Services and Supports (LTSS) in FY 2014: Managed LTSS Reached15 Percent of LTSS Spending. [Accessed June 2, 2016]; Available at: https://www.medicaid.gov/medicaid-chip-program-information/by-topics/long-term-services-and-supports/downloads/ltss-expenditures-2014.pdf.
- 3.Blackburn J, Locher JL, Morrisey MA, et al. The effects of state-level expenditures for home- and community-based services on the risk of becoming a long-stay nursing home resident after hip fracture. Osteoporos Int. 2016;27(3):953–961. doi: 10.1007/s00198-015-3327-3. [DOI] [PubMed] [Google Scholar]
- 4.Patient Protection and Affordable Care Act, PL 111-148. [Accessed June 16, 2016]; Available at: http://www.hhs.gov/healthcare/about-the-law/read-the-law/index.html.
- 5.Ahmed A, Allman RM, DeLong JF. Predictors of nursing home admission for older adults hospitalized with heart failure. Arch Gerontol Geriatr. 2003;36(2):117–126. doi: 10.1016/s0167-4943(02)00063-8. [DOI] [PubMed] [Google Scholar]
- 6.Foley DJ, Ostfeld AM, Branch LG, et al. The risk of nursing home admission in three communities. J Aging Health. 1992;4(2):155–173. doi: 10.1177/089826439200400201. [DOI] [PubMed] [Google Scholar]
- 7.Jette AM, Branch LG, Sleeper LA, et al. High-risk profiles for nursing home admission. Gerontologist. 1992;32(5):634–640. doi: 10.1093/geront/32.5.634. [DOI] [PubMed] [Google Scholar]
- 8.Smith ER, Stevens AB. Predictors of discharges to a nursing home in a hospital-based cohort. J Am Med Dir Assoc. 2009;10(9):623–629. doi: 10.1016/j.jamda.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha AJ, Pandav R, Shen C, et al. Predictors of nursing facility admission: a 12-year epidemiological study in the United States. J Am Geriatr Soc. 2004;52(3):434–439. doi: 10.1111/j.1532-5415.2004.52118.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin JS, Howrey B, Zhang DD, et al. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1321–1327. doi: 10.1093/gerona/glr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luppa M, Riedel-Heller SG, Luck T, et al. Age-related predictors of institutionalization: results of the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Soc Psychiatry Psychiatr Epidemiol. 2012;47(2):263–270. doi: 10.1007/s00127-010-0333-9. [DOI] [PubMed] [Google Scholar]
- 13.Luppa M, Luck T, Weyerer S, et al. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39(1):31–38. doi: 10.1093/ageing/afp202. [DOI] [PubMed] [Google Scholar]
- 14.Greiner MA, Qualls LG, Iwata I, et al. Predicting nursing home placement among home- and community-based services program participants. Am J Manag Care. 2014;20(12):e535–536. [PubMed] [Google Scholar]
- 15.Hajek A, Brettschneider C, Lange C, et al. Longitudinal Predictors of Institutionalization in Old Age. PLoS One. 2015;10(12):e0144203. doi: 10.1371/journal.pone.0144203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hongisto MT, Nuotio M, Luukkaala T, et al. Does cognitive/physical screening in an outpatient setting predict institutionalization after hip fracture? BMC Musculoskelet Disord. 2016;17(1):444. doi: 10.1186/s12891-016-1272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Bonsdorff M, Rantanen T, Laukkanen P, et al. Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology. 2006;52(6):359–365. doi: 10.1159/000094985. [DOI] [PubMed] [Google Scholar]
- 18.Pimouguet C, Rizzuto D, Schon P, et al. Impact of living alone on institutionalization and mortality: a population-based longitudinal study. Eur J Public Health. 2016;26(1):182–187. doi: 10.1093/eurpub/ckv052. [DOI] [PubMed] [Google Scholar]
- 19.Cai X, Temkin-Greener H. Nursing home admissions among Medicaid HCBS enrollees: Evidence of racial/ethnic disparities or differences? Med Care. 2015;53(7):566–573. doi: 10.1097/MLR.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 20.Akamigbo AB, Wolinsky FD. New evidence of racial differences in access and their effects on the use of nursing homes among older adults. Med Care. 2007;45(7):672–679. doi: 10.1097/MLR.0b013e3180455677. [DOI] [PubMed] [Google Scholar]
- 21.Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. [Accessed July 20, 2016];2016 Jun; Available at: http://medpac.gov/documents/data-book/june-2016-data-book-health-care-spending-and-the-medicare-program.pdf?sfvrsn=0.
- 22.Porell FW, Carter MW. Risk of mortality and nursing home institutionalization after injury. J Am Geriatr Soc. 2012;60(8):1498–1503. doi: 10.1111/j.1532-5415.2012.04053.x. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare & Medicaid Services. Provider of Services Current Files (POS) [Accessed September 8, 2016]; Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-Services/
- 24.United States Census Bureau. American FactFinder. [Accessed May 23, 2016]; Available at: http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- 25.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents' long-term care histories. Health Serv Res. 2011;46(1):120–137. doi: 10.1111/j.1475-6773.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin JS, Li S, Zhou J, et al. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. doi: 10.1186/s12913-017-2318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare & Medicaid Services. Medicare-Medicaid Coordination Office Fact Sheet-March 2017. [Accessed July 10, 2017]; Available at: https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/Downloads/MMCO_Factsheet.pdf.
- 28.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services. Long-Term Care Facility Resident Assessment Instrument 3.0 User's Manual. Version 1.13. 2015 Oct; [Google Scholar]
- 30.Saliba D, Buchanan J. Development & Validation of a Revised Nursing Home Assessment Tool: MDS 3.0. [Accessed May 9, 2017];2008 Available at: http://www.cms.hhs.gov/NursingHomeQualityInits/Downloads/MDS30FinalReport.pdf.
- 31.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2015 doi: 10.1097/MLR.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saliba D, DiFilippo S, Edelen MO, et al. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618–625. doi: 10.1016/j.jamda.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298–2303. doi: 10.1111/j.1532-5415.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 35.Drasgow F. Polychoric and polyserial correlations. In: Kotz L, Johnson N, editors. Encyclopedia of Statistical Sciences. New York: Wiley-Interscience; 1988. pp. 69–74. [Google Scholar]
- 36.Middleton A, Zhou J, Ottenbacher KJ, et al. Hospital Variation in Rates of New Institutionalizations Within 6 Months of Discharge. J Am Geriatr Soc. 2017;65(6):1206–1213. doi: 10.1111/jgs.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomeer MB, Mudrazija S, Angel JL. How do race and Hispanic ethnicity affect nursing home admission? Evidence from the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):628–638. doi: 10.1093/geronb/gbu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young Y, Kalamaras J, Kelly L, et al. Is aging in place delaying nursing home admission? J Am Med Dir Assoc. 2015;16(10):900 e901–906. doi: 10.1016/j.jamda.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 39.RTI International. Measure Specifications for Measures Adopted in the FY 2017 SNF QRP Final Rule. [Accessed 8/23/2016]; Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Downloads/Measure-Specifications-for-FY17-SNF-QRP-Final-Rule.pdf.
- 40.RTI International. MDS 3.0 Quality Measures User's Manual. [Accessed July 10, 2017]; Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Downloads/MDS-30-QM-Users-Manual-V11-Final.pdf.
- 41.Givens JL, Mitchell SL, Kuo S, et al. Skilled nursing facility admissions of nursing home residents with advanced dementia. J Am Geriatr Soc. 2013;61(10):1645–1650. doi: 10.1111/jgs.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Flow chart presenting number of eligible cases remaining at each step as exclusion criteria applied.
Supplementary Table S1. New institutionalizations by patients' hospital diagnostic-related group major diagnostic category (DRG-MDC).
Supplementary Table S2. Stratified analyses for all significant interaction terms in the fully interacted (i.e. all possible two-way interaction terms) logistic regression model predicting new institutionalizations.
Supplementary Table S3. Observed rates and adjusted odds of residence in a long-term care nursing home for at least 90 days or until death, starting within six months of discharge from a hospital to a SNF. In this analysis we did not exclude those who died within six months of hospital discharge, as we did in the main analyses.