Abstract
Background and Aims
Chronic hepatitis C virus (HCV)-related complications have increased over the past decade.
Methods
We used join-point regression modeling to investigate trends in these complications from 2006–2015, and the impact of demographics on these trends. Using data from the Chronic Hepatitis Cohort Study (CHeCS), we identified points at which the trend significantly changed, and estimated the annual percent change (APC) in rates of cirrhosis, decompensated cirrhosis, and all-cause mortality, adjusted by race, sex, and age.
Results
Among 11,167 adults with chronic HCV infection, prevalence of cirrhosis increased from 20.8% to 27.6% from 2006 to 2015 with adjusted annual percentage change (aAPC) of 1.2 (p<0.01). Although incidence of all-cause mortality increased from 1.8% in 2006 to 2.9% in 2015, a join-point was identified at 2010, with aAPCs of 9.6 before (2006<2010; p<0.01) and −5.2 after (2010≤2015; p<0.01), indicating a decrease in mortality from 2010 and onward. Likewise, although overall prevalence of decompensated cirrhosis increased from 9.3% in 2006 to 10.4% in 2015, this increase was confined to patients 60 or older (aAPC=1.5; p=0.023). Asian American and Black/African American patients demonstrated significantly higher rates of cirrhosis than White patients, while older patients and men demonstrated higher rates of cirrhosis and mortality.
Conclusions
Although cirrhosis and mortality among HCV-infected patients in the US have increased in the past decade, the mortality has decreased in recent years.
Keywords: African Americans, Asian Americans, cirrhosis, decompensated cirrhosis
INTRODUCTION
As many as 65–75% of the estimated 3.5 million hepatitis C virus (HCV)-infected Americans are unaware of their infection status and have not received necessary care and treatment (1, 2), and remain at high risk for progression to cirrhosis (3), liver failure, and hepatocellular carcinoma (2, 4–6). Although rates of new HCV infections have declined since a peak in the 1990s (7), overall prevalence has increased as patients discover their infection status. Given that individuals born between 1945 and 1965 represent roughly 75% of all US HCV cases, epidemiologists have anticipated a rapid upsurge of HCV-related liver disease and cirrhosis as this birth cohort ages (8). Moreover, although HCV disproportionately affects men and African Americans(9), changes in rates of these complications in these demographics have not been well-studied.
This rising burden of HCV-related complications is now being realized. A recent study of predominantly male US veterans (N>47,000) showed that the prevalence of cirrhosis increased from 9% in 1996 to 18.5% in 2006; decompensated cirrhosis increased from 5% to 11% over this same period(10). Similarly, a recent general population study using NHANES data found that HCV patients demonstrated increasing prevalence of cirrhosis, from 6.6% (in 1988–1994) to 17.0% (2007–2012)(11). This latter study was limited to a small sample (n=725). We sought to determine how the actual trends of cirrhosis and other complications have changed over the past decade, and whether race, sex and age impact these trends.
The Chronic Hepatitis Cohort Study (CHeCS) includes over 11,000 HCV patients receiving care at one of four large health systems. Because CHeCS comprises a geographically- and racially-diverse sample of patients receiving routine clinical care, it is broadly generalizable to HCV patients throughout the US (12). We applied join-point modeling to comprehensive medical record data to investigate the dynamics of trends of cirrhosis, decompensated cirrhosis, and mortality among HCV patients from 2006 to 2015.
METHODS
CHeCS includes patients ≥18 years old who received services on or after January 1, 2006 at one of four health care systems—Henry Ford Health System; Geisinger Health System; Kaiser Permanente Northwest; and Kaiser Permanente Hawai’i. The study follows all guidelines of the US Department of Health and Human Services regarding the protection of human subjects; our protocol was approved and renewed annually by the Institutional Review Boards of Henry Ford Health System, Geisinger Health System; Kaiser Permanente Northwest; and Kaiser Permanente Hawai’i. Due to the de-identified nature of this observational study, requirements for written informed consent were waived.
CHeCS methods have been described (12, 13). Briefly, CHeCS patients are identified electronically using a combination of laboratory and ICD9-based criteria. Chronic HCV infection is confirmed through chart abstraction (12). For each data collection cycle, a random sample of new patients is added to the cohort, while existing patients continue to be followed. In the present analysis, we examined annual prevalence of cirrhosis and decompensated cirrhosis, and incidence of all-cause mortality, for the 2006–2015 period. Patients were included in the sample if they had been diagnosed prior to or during the given year, and their last encounter was during or after the given year. HBV/HCV co-infected patients were excluded; HIV/HCV co-infected patients were included in analyses.
Our classification of decompensated cirrhosis is based on a statistically rigorous classification and regression tree (CART) model that was validated against data from chart abstraction. This method classifies a cirrhotic patient to be decompensated cirrhosis if there was at least one of ICD9 codes for decompensation-related conditions, yielded validated AUROC of 92% and PPV of 85% (14).
Due to the observational nature of this study, availability of cirrhosis data varied. Roughly 20% of our sample had liver biopsy data; 60% had laboratory data for calculation of FIB4. To overcome this variation, we implemented a hierarchical classification algorithm to identify cirrhosis: 1) decompensated cirrhosis identified using the CART model; 2) “F4” liver biopsy; 3) FIB4>5.88; 4) presence of ICD9/10 diagnosis codes for cirrhosis in the medical record. CHeCS has previously validated the use of the Fibrosis-4 (FIB4) serum-based biomarker in HCV patients; we found that a FIB4 score >5.88, calculated from routine laboratory results, accurately classifies cirrhosis (Metavir fibrosis stage F4; area under the receiver operator characteristic curve [AUROC]=85% and positive predictive value [PPV]=82%)(15). Decompensated cirrhosis was determined using a similar approach. Cirrhosis and decompensated cirrhosis were assumed to persist unless records indicated receipt of a liver transplant.
Statistical Analysis
Outcomes of interest included prevalence of cirrhosis and decompensated cirrhosis, as well as incidence of all-cause mortality (based on our work showing that liver-related mortality is under-reported in routine care settings(16)). Covariates of interest included: age during the given year (categorized as <40, 40<50, 50<60, and ≥60); sex; and race (categorized as Black/African American, Asian American/Pacific Islander [AAPI], White, and Other/Unknown). Age was used instead of birth cohort in order to assess the effect of aging within the cohort.
Summary statistics are presented as percentages (for categorical variables) and mean/standard deviations (for continuous variables) for each year. Chi-square tests were used to study time (year) effects on differences in patient characteristics across time.
To study the dynamics of longitudinal trends in the outcomes of interest, we adapted and extended a two-step join-point Poisson regression modeling approach(17) by fitting a series of straight lines on a log scale to the trend; each join-point represents a statistically significant (p<0.05) change in trend (i.e., slope of the line segment). For example, a single join-point splits the trend line into two line segments, whereas zero join-points indicates that the best fit to the trend consists of only a single line segment. In the first step, we identified the optimal join-point(s) using a nonlinear modeling approach. Next, multivariable analyses were performed based on the selected join-point(s) as well as potential stratification variables. Interactions were tested only for individual variables that were significant. Variables were retained in the final model if estimated annual percentage changes (APCs) before and after the join-point were significant (p<0.05). We also evaluated whether the APC of each line segment differed from no change (APC=0). For each outcome of interest, we estimated adjusted APCs (aAPC) and 95% confidence intervals (CI), as well as adjusted prevalence rates by year or by age, race, or sex. Study sites were included in all analysis as the stratification variable.
We did not use age-standardized rates in the model to avoid masking the known birth-cohort effect in HCV patients born between 1945 and 1965(8); this is consistent with the approach used in a recent study of cirrhosis prevalence among US veterans (18). However, age was included as a stratification variable. SAS Version 9.4 (SAS Institute Inc, Cary NC) was used for all analyses.
RESULTS
We identified 11,167 confirmed HCV patients with 80,988 person-years of observation across the 2006–2015 study period, with 3856 cases of prevalent cirrhosis (34.5% cumulative, including decompensated), 2027 cases of prevalent decompensated cirrhosis (18.2% cumulative), and 2246 incident deaths (20.1%). Among the 11,167 patients, 8185 had their initial HCV diagnosis prior to or during 2006, while the remaining 2982 were newly diagnosed during the study period. The cohort size varied across time—from 8185 in 2006, to 6100 in 2015—due to the addition of incident HCV cases and the loss of patients who died or left the health systems. Among 2027 patients with decompensated cirrhosis, 30% had diagnosis or procedure codes related to liver transplant in the EHR; 39% had codes related to hepatocellular carcinoma (HCC); 23% had codes related to encephalopathy; 65% had codes related to ascites; and those symptoms were not mutually exclusive. Among 2246 HVC patients who died from 2006–2015, reports of liver-related death varied from year to year, ranging from 39% to 46%, with data missing for an additional 7–10% annually.
Select patient demographics are presented in Table 1. Notably, our cohort aged significantly over time. In 2006, only 14% of patients were >60 years old; this proportion increased to 49% by 2014. Likewise, the proportion of female patients increased from 40% to 43% over the same time period. The overall unadjusted prevalence of cirrhosis, decompensated cirrhosis, and incidence of all-cause mortality is illustrated in Figure 1. There were three treatment eras during this ten year period, which are indicated in Figure 1: the ribavirin + interferon combination therapy era (2006–2010); the triple therapy era (2011–2013), and the DAA era (2014–2015). Annual rates of HCV treatment were low, but increased from less than 5% in 2006 to roughly 15% in 2015. We did note increased rates of treatment among cirrhotic patients (from 23% in 2006 to 53% in 2015).
Table 1.
Study Population Demographics and Outcomes by Year, 2006–2015
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Full sample | 8185 | 8785 | 9022 | 9027 | 8886 | 8838 | 8239 | 7516 | 6946 | 6100 | |||||||||||
| Race | Asian/other | 567 | 6.9 | 607 | 6.9 | 624 | 6.9 | 616 | 6.8 | 599 | 6.7 | 591 | 6.7 | 547 | 6.6 | 510 | 6.8 | 478 | 6.9 | 418 | 6.9 |
| Black | 1967 | 24.0 | 2093 | 23.8 | 2134 | 23.7 | 2142 | 23.7 | 2095 | 23.6 | 2044 | 23.1 | 1918 | 23.3 | 1753 | 23.3 | 1598 | 23.0 | 1453 | 23.8 | |
| White | 5247 | 64.1 | 5664 | 64.5 | 5862 | 65.0 | 5897 | 65.3 | 5860 | 65.9 | 5912 | 66.9 | 5527 | 67.1 | 5032 | 67.0 | 4663 | 67.1 | 4070 | 66.7 | |
| Unknown | 404 | 4.9 | 421 | 4.8 | 402 | 4.5 | 372 | 4.1 | 332 | 3.7 | 291 | 3.3 | 247 | 3.0 | 221 | 2.9 | 207 | 3.0 | 159 | 2.6 | |
| Sex | Female | 3242 | 39.6 | 3476 | 39.6 | 3605 | 40.0 | 3662 | 40.6 | 3652 | 41.1 | 3667 | 41.5 | 3476 | 42.2 | 3215 | 42.8 | 3028 | 43.6 | 2674 | 43.8 |
| Male | 4943 | 60.4 | 5309 | 60.4 | 5417 | 60.0 | 5365 | 59.4 | 5234 | 58.9 | 5171 | 58.5 | 4763 | 57.8 | 4301 | 57.2 | 3918 | 56.4 | 3426 | 56.2 | |
| Age | <40 | 800 | 9.8 | 842 | 9.6 | 823 | 9.1 | 785 | 8.7 | 792 | 8.9 | 817 | 9.2 | 800 | 9.7 | 696 | 9.3 | 626 | 9.0 | 522 | 8.6 |
| 40<50 | 1993 | 24.3 | 1870 | 21.3 | 1735 | 19.2 | 1562 | 17.3 | 1357 | 15.3 | 1178 | 13.3 | 947 | 11.5 | 741 | 9.9 | 604 | 8.7 | 452 | 7.4 | |
| 50<60 | 4161 | 50.8 | 4580 | 52.1 | 4686 | 51.9 | 4532 | 50.2 | 4248 | 47.8 | 3999 | 45.2 | 3429 | 41.6 | 2875 | 38.3 | 2375 | 34.2 | 1822 | 29.9 | |
| ≥60 | 1231 | 15.0 | 1493 | 17.0 | 1778 | 19.7 | 2148 | 23.8 | 2489 | 28.0 | 2844 | 32.2 | 3063 | 37.2 | 3204 | 42.6 | 3341 | 48.1 | 3304 | 54.2 | |
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Outcome | Cirrhosis | 1700 | 20.8 | 1935 | 22.0 | 2023 | 22.4 | 2088 | 23.1 | 2048 | 23.0 | 2090 | 23.6 | 1977 | 24.0 | 1897 | 25.2 | 1791 | 25.8 | 1684 | 27.6 |
| DC | 762 | 9.3 | 811 | 9.2 | 652 | 7.2 | 839 | 9.3 | 807 | 9.1 | 805 | 9.1 | 785 | 9.5 | 703 | 9.4 | 664 | 9.6 | 636 | 10.4 | |
| Mortality | 150 | 1.8 | 196 | 2.2 | 228 | 2.5 | 245 | 2.7 | 280 | 3.2 | 270 | 3.1 | 263 | 3.2 | 236 | 3.1 | 204 | 2.9 | 174 | 2.9 | |
Figure 1.
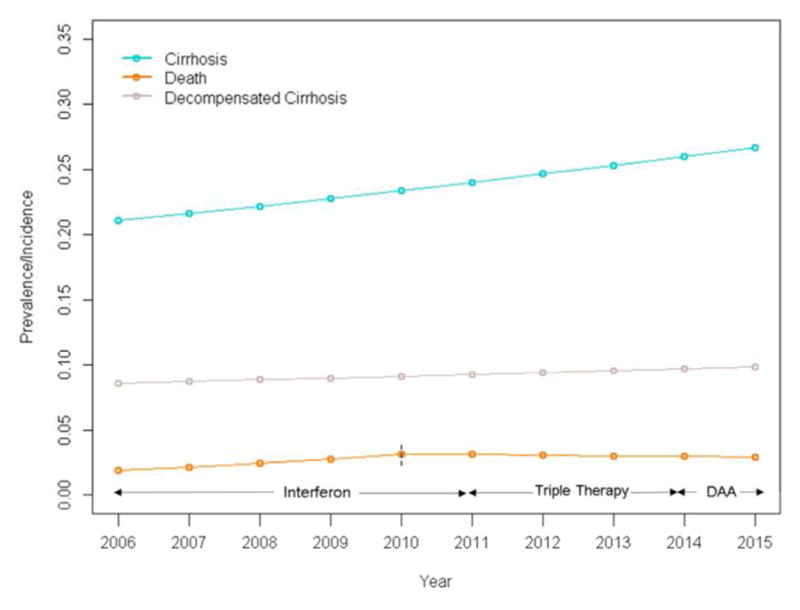
Prevalence of cirrhosis and decompensated cirrhosis, and incidence of all-cause mortality in CHeCS HCV patients from 2006–2015 (join-points for all-cause mortality in 2010).
Prevalence of cirrhosis
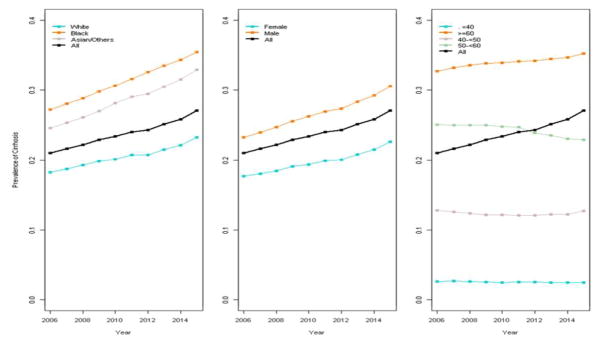
Prevalence of cirrhosis (including decompensated cirrhosis) increased from 20.8% (in 2006) to 27.6% (in 2015), with no join-point identified. The overall adjusted APCs (aAPC, adjusted for race, sex, and age category, Figure 2) was 1.2 (p<0.01). We did not find any interactions between time and covariates (age, sex or age), indicating that—although each factor independently impacted prevalence rates—their effect was consistent over time. Notably, both Black/African American and AAPI patients demonstrated significantly higher prevalence of cirrhosis than White patients (p<0.01). Older patients (especially those ≥60) and men also demonstrated significantly higher prevalence than younger patients or women, respectively. Consistent with the overall results, increases in cirrhosis rates among these groups were consistent over time.
Figure 2.
Prevalence of cirrhosis by race, sex, and age in CHeCS HCV patients from 2006–2015. No join-points and only one segment were identified. Line segments illustrate the adjusted annual percent change (aAPC).
Prevalence of decompensated cirrhosis
The model for prevalence of decompensated cirrhosis also fitted to a straight line (no join-points) with APC of 1.5 (p<0.01), indicating that the increase from 9.3% (in 2006) to 10.4% (in 2015) was consistent across the study period. However, the multivariate analysis showed a significant interaction between age and time; this indicates that the trend (APC) varied across age groups. We found that higher prevalence and increasing trends were both confined to patients ≥60 (aAPC=1.5, p=0.023). Lower prevalence and declining trends were observed in patients 40–<50 and 50–<60 (aAPCs=−3.2 and −7.9, respectively; p<0.01 for both); no change was observed among patients <40 (Figure 3). Men demonstrated higher prevalence of decompensated cirrhosis than women, while higher rates of decompensated cirrhosis were found in Black/African American and AAPI patients than White patients (p<0.01).
Figure 3.
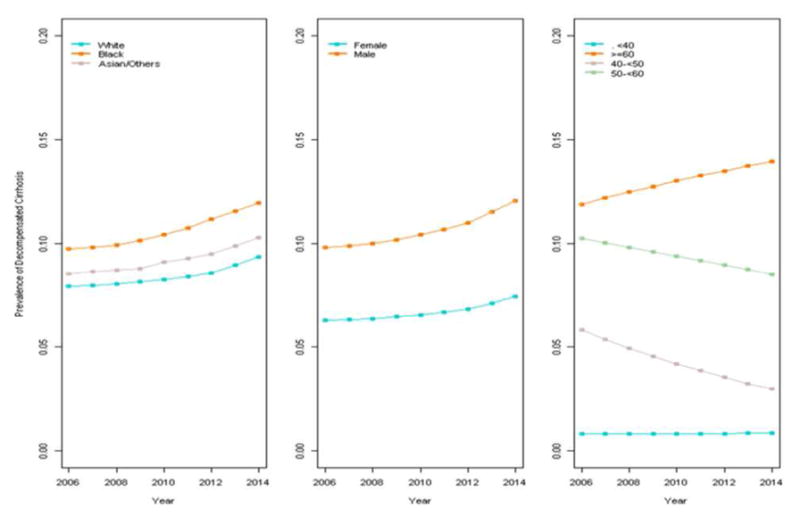
Prevalence of decompensated cirrhosis by race, sex, and age in CHeCS HCV patients from 2006–2015. No join-points and only one segment were identified. Line segments illustrate the adjusted annual percent change (aAPC).
Incidence of all-cause mortality
Overall incidence of all-cause mortality increased across the study period, from 1.8% (in 2006) to 2.9% (in 2015). However, this increase was confined almost entirely to the period prior to the 2010 join-point (Figure 1, Table 2). APCs were 13.7 and −1.6 (p<0.01 and 0.28) before and after 2010, respectively; aAPCs were 9.6 and −5.2 (p<0.01 for both), indicating that there was a significant decrease in incidence of all-cause mortality after 2010. We observed that age, sex contributed independently to incidence of mortality (Figure 4), but trends remained consistent across time among groups. Older patients (especially those ≥60) and men again demonstrated the highest incidence of mortality (Figure 4). Despite higher rates of cirrhosis among Black/African American and AAPI patients compared to White, there was no significant difference in mortality by race (p=0.13).
Table 2.
Unadjusted and adjusted annual percentage change and join-points for hepatitis C-related outcomes.
| Annual percentage change (APC) | First segment | Second segment | ||
|---|---|---|---|---|
| Interval | Interval | |||
| APC (95%CI) | p-value | APC (95%CI) | p-value | |
| Cirrhosis | 2006≤2015 | |||
| Unadjusted | 2.7 (2.1, 3.2) | <0.01 | ||
| Adjusted | 1.2 (0.7, 1.8) | <0.01 | ||
| Decompensated Cirrhosis | 2006≤2015 | |||
| Unadjusted | 1.5 (0.7, 2.4) | <0.01 | ||
| Adjusted (<40) | −3.7 (−11.9, 5.3) | 0.40 | ||
| Adjusted (40<50) | −7.9 (−10.9, −4.8) | <.0001 | ||
| Adjusted (50<60) | −3.2 (−4.4, −1.9) | <.0001 | ||
| Adjusted (≥60) | 1.5 (0.2, 2.8) | 0.02 | ||
| All-cause Mortality | 2006<2010 | 2010≤2015 | ||
| Unadjusted | 13.7 (9.4, 18.1) | <0.01 | −1.6 (−4.4, 1.3) | 0.28 |
| Adjusted | 9.6 (5.5, 13.8) | <0.01 | −5.2 (−7.9, −2.4) | <0.01 |
Figure 4.
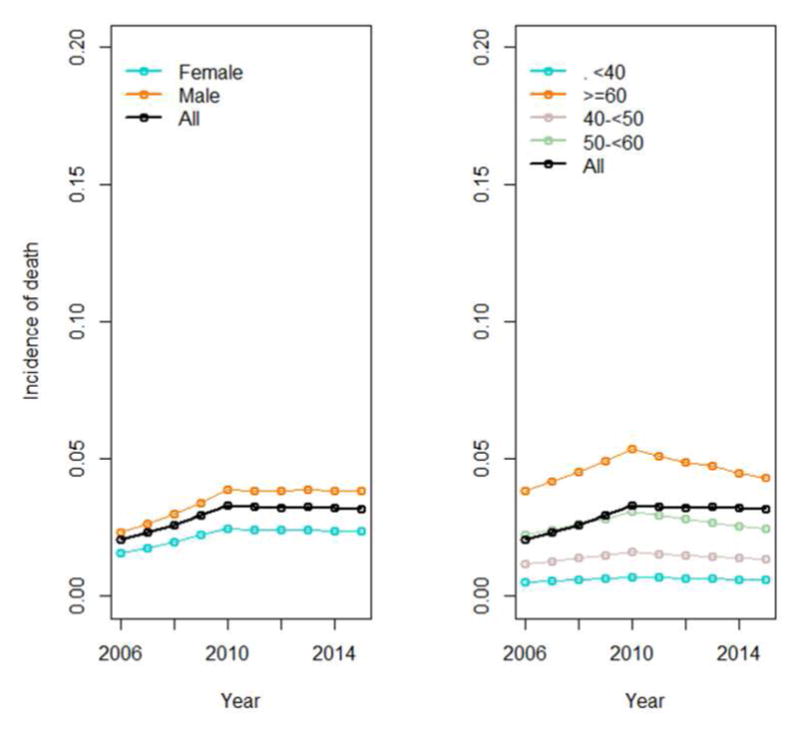
Incidence of all-cause mortality by sex and age in CHeCS HCV patients from 2006–2015. One join-point (2010) and two segments were identified. Line segments illustrate the adjusted annual percent change (aAPC).
DISCUSSION
Consistent with recent studies, we found a marked increase in cirrhosis prevalence from 2006 to 2015 in a diverse, non-veteran-based cohort of over 11,000 HCV patients. Likewise, even though the overall rate of all-cause mortality roughly doubled from 2006 to 2010, incidence declined after 2010. Trends in prevalence of decompensated cirrhosis varied by age; although prevalence increased significantly in patients ≥ 60 years old, rates plateaued or declined among younger age groups.
Our observation of increasing prevalence of cirrhosis but decreasing rates of decompensated cirrhosis and all-cause mortality suggests that cirrhotic patients are living longer. This may be a result of more frequent antiviral treatment among cirrhotic patients (we observed an increase from 22% in 2006 to 53% in 2015), reducing the number of patients who progress to decompensated cirrhosis and death. Although there is some evidence that liver fibrosis may regress after SVR, we cannot adequately capture this regression. Being conservative, we considered cirrhosis to persist in the absence of a liver transplant,
We found significant disparities in the prevalence of HCV-related complications across age, sex, and racial categories. HCV patients ≥60 years old had the highest prevalence of liver-related complications and all-cause mortality; similar findings have been reported among US veterans (19). Sex also independently influenced all outcomes (Figures 2–4), although to a lesser extent than age. Men had the highest prevalence of cirrhosis and decompensated cirrhosis, as well as incidence of mortality.
We observed that decompensated cirrhosis increased only 1 percentage point in 10 years, and only among patients in the oldest age group (≥60), despite larger increases in the prevalence of cirrhosis across all age groups. We speculate that this may be due to improvements in HCV treatment, particularly among patients with advanced fibrosis (6, 20). Although we observed an overall increase in the incidence of all cause-mortality (from 1.8% in 2006 to 2.9% in 2015), our analyses indicated that the rate of increase reversed after 2010; this may also be related to better HCV treatment options as well as improved survival in patients with late-stage liver disease(21, 22).
Notably, Black/African American patients in our cohort demonstrated the highest prevalence of cirrhosis, even after controlling for age. This finding is in contrast to studies that have found lower risk of cirrhosis among Black patients with HCV (23–25). A number of previous studies have shown that Black/African Americans were historically less likely to receive interferon-based HCV treatment (26–29); our own recent study of the factors associated with DAA uptake has demonstrated the same pattern (30). The higher prevalence of cirrhosis observed in this racial group may be related to differences in receipt of therapy or access to care. Future studies investigating the causes of these disparities are warranted.
Our study has several limitations. We used all-cause mortality instead of HCV-related mortality because a previous CHeCS analysis demonstrated that liver-related mortality is under-reported in routine-care settings(16). Although our analysis is based on ongoing “dynamic” cohort accrual from health system populations, the cohort has aged significantly over time; patients ≥60 years made up only 15% of our 2006 cohort, but more than a half (53%) of the cohort in 2015. However, we tested for interactions between age and time trends. Likewise, we did not adjust for the effect of treatment or SVR on these trends because our analysis was designed to describe overall temporal changes HCV-related complications, rather than individual treatment effect on a patient’s trajectory, which has been studied thoroughly for interferon-based treatment (6, 20). It would not be appropriate to assess treatment effect in the present trend analysis, because HCV treatment-related factors, including eligibility for treatment/treatment uptake, treatment failure (especially for interferon-based treatment), treatment response (SVR) and the rate of SVR responses, are a function of the era of HCV treatment. Nevertheless treatment impact may be minimal in the present trend analysis given low HCV treatment uptake and SVR responses in this population.
Because our cohort consists of individuals with at least some contact with the health system, we are unable to estimate the prevalence of outcomes in HCV-positive individuals who remain undiagnosed or those without ongoing contact with a health care system. Such individuals are perhaps most at risk for poor outcomes. In addition, our study is based primarily upon health records data and may fail to capture factors that potentially affect clinical outcomes, such as disease duration or undiagnosed substance or alcohol abuse. Our study is also limited generally to the era of interferon-based therapy through the introduction of triple therapy that included the initial protease inhibitors boceprevir and telaprevir.
In conclusion, although overall prevalence of cirrhosis increased by roughly 40% among HCV-infected patients in the US, all-cause mortality declined significantly in recent years. Likewise, increasing prevalence of decompensated cirrhosis was confined to patients over 60. Accelerating uptake of highly effective direct-acting all-oral regimens may ultimately reverse the increase of liver-related complications in this population.
Key Points.
We applied join-point modeling to data from the Chronic Hepatitis Cohort Study to study changing trends in rates of cirrhosis, decompensated cirrhosis, and all-cause mortality among a geographically- and racially-diverse sample of over 11,000 hepatitis C patients
Increasing prevalence of cirrhosis and mortality in HCV patients has slowed down or leveled off in recent years
Increasing prevalence of decompensated cirrhosis is confined to patients over 60
African Americans and Asian Americans demonstrate higher rates of cirrhosis than White patients
Acknowledgments
Financial support
Henry Ford Health System receives funding for CHeCS from the Centers for Disease Control and Prevention and from Gilead Sciences. CHeCS was previously funded through May 2016 by the CDC Foundation, which received grants from AbbVie; Genentech, A Member of the Roche Group; Gilead Sciences; Janssen Pharmaceuticals, Inc. and Vertex Pharmaceuticals; past partial funders include Bristol-Myers Squibb.
Granting corporations do not have access to CHeCS data and do not play a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript.
Dr. Lu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The CHeCS Investigators include the following investigators and sites: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, Anne C. Moorman, Jim Xing, and Yuna Zhong, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Jia Li, Loralee B. Rupp, Nonna Akkerman, Wu, Kuan-Han, Talan Zhang, Sheri Trudeau, and Yueren Zhou, Henry Ford Health System, Detroit, Michigan; Joseph A. Boscarino, Zahra S. Daar, and Robert E. Smith, Center for Health Research, Geisinger Health System, Danville, Pennsylvania; Yihe G. Daida, Connie M. Trinacty, and Carmen P. Wong, The Center for Health Research, Kaiser Permanente-Hawaii, Honolulu, Hawaii; Mark A. Schmidt, Judy L. Donald, and Erin M. Keast, The Center for Health Research, Kaiser Permanente-Northwest, Portland, OR.
Abbreviations
- HCV
hepatitis C virus
- CHeCS
The Chronic Hepatitis Cohort Study
- APC
annual percentage change
- aAPC
adjusted annual percentage change
- NHANES
National Health and Nutrition Examination Survey
- ICD9
International Classification of Diseases, 9th edition
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- FIB4
Fibrosis-4 Index
- AUROC
area under the receiver operating characteristic curve
- PPV
positive predictive value
- CART
Classification and Regression Tree
- AAPI
Asian American or Pacific Islander
- CI
confidence interval
Footnotes
Disclaimer:
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the US Department of Health and Human Services, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.
Conflict of interest
Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Bristol-Myers Squibb, Gilead Pharmaceuticals, GlaxoSmithKline, Intercept Pharmaceuticals, Merck, and Vertex Pharmaceuticals. He is also ad hoc advisor for AbbVie, CVS Caremark, Gilead Sciences, Intercept and Merck; Mei Lu, Jia Li, Lora Rupp, Yueren Zhou, Sheri Trudeau, Yihe G. Daida, Mark A. Schmidt, Joseph A. Boscarino receive grant/research support from Gilead and Intercept Pharmaceuticals.
The other authors have no potential conflicts of interest.
References
- 1.EDLIN BR, ECKHARDT BJ, SHU MA, HOLMBERG SD, SWAN T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology (Baltimore, Md) 2015;62(5):1353–63. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HOLMBERG SD, LU M, RUPP LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(2):240–6. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PREVENTION CFDCA. Viral hepatitis. [cited 1/7/14]; Available from: http://www.cdc.gov/hepatitis.
- 4.GORDON SC, POCKROS PJ, TERRAULT NA, et al. Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology (Baltimore, Md) 2012;56(5):1651–60. doi: 10.1002/hep.25842. [DOI] [PubMed] [Google Scholar]
- 5.GORDON SC, LAMERATO LE, RUPP LB, et al. Prevalence of cirrhosis in hepatitis C patients in the Chronic Hepatitis Cohort Study (CHeCS): a retrospective and prospective observational study. The American journal of gastroenterology. 2015;110(8):1169–77. doi: 10.1038/ajg.2015.203. quiz 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LU M, LI J, RUPP LB, et al. Hepatitis C treatment failure is associated with increased risk of hepatocellular carcinoma. Journal of viral hepatitis. 2016;23(9):718–29. doi: 10.1111/jvh.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WASLEY A, GRYTDAL S, GALLAGHER K CENTERS FOR DISEASE C, PREVENTION. Surveillance for acute viral hepatitis--United States, 2006. MMWR Surveill Summ. 2008;57(2):1–24. [PubMed] [Google Scholar]
- 8.SAAB S, RHEEM J, SUNDARAM V. Hepatitis C Infection in the Elderly. Digestive diseases and sciences. 2015;60(11):3170–80. doi: 10.1007/s10620-015-3717-6. [DOI] [PubMed] [Google Scholar]
- 9.PREVENTION CFDCA. Hepatitis C: testing baby boomers saves lives. [cited 2014 1/7/14]; Available from: http://www.cdc.gov/features/vitalsigns/hepatitisc/
- 10.KANWAL F, HOANG T, KRAMER JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–88. e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UDOMPAP P, MANNALITHARA A, HEO NY, KIM D, KIM WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. Journal of hepatology. 2016;64(5):1027–32. doi: 10.1016/j.jhep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MOORMAN AC, GORDON SC, RUPP LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56(1):40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 13.LU M, RUPP LB, MOORMAN AC, et al. Comparative effectiveness research of chronic hepatitis B and C cohort study (CHeCS): improving data collection and cohort identification. Digestive diseases and sciences. 2014;59(12):3053–61. doi: 10.1007/s10620-014-3272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LU MCW, RABIN D, RUPP L, TRUDEAU S, LI J, GORDON S. Validity of an automated algorithm using diagnosis and procedure codes to identify decompensated cirrhosis using electronic health records (EHR) Clinical Epidemiology. 2017 doi: 10.2147/CLEP.S136134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LI J, GORDON SC, RUPP LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. Journal of viral hepatitis. 2014;21(12):930–7. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 16.MAHAJAN R, XING J, LIU SJ, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(8):1055–61. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 17.KIM HJ, FAY MP, FEUER EJ, MIDTHUNE DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.BESTE LA, LEIPERTZ SL, GREEN PK, DOMINITZ JA, ROSS D, IOANNOU GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–82. e5. doi: 10.1053/j.gastro.2015.07.056. quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 19.EL-SERAG HB, KRAMER J, DUAN Z, KANWAL F. Epidemiology and outcomes of hepatitis C infection in elderly US Veterans. Journal of viral hepatitis. 2016 doi: 10.1111/jvh.12533. [DOI] [PubMed] [Google Scholar]
- 20.LU M, LI J, ZHANG T, et al. Serum Biomarkers Indicate Long-term Reduction in Liver Fibrosis in Patients With Sustained Virological Response to Treatment for HCV Infection. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2016;14(7):1044–55. e3. doi: 10.1016/j.cgh.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SCHMIDT ML, BARRITT AS, ORMAN ES, HAYASHI PH. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology. 2015;148(5):967–77. e2. doi: 10.1053/j.gastro.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KANWAL F. Decreasing mortality in patients hospitalized with cirrhosis. Gastroenterology. 2015;148(5):897–900. doi: 10.1053/j.gastro.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 23.EL-SERAG HB, KRAMER J, DUAN Z, KANWAL F. Racial Differences in the Progression to Cirrhosis and Hepatocellular Carcinoma in HCV-Infected Veterans. The American journal of gastroenterology. 2014;109(9):1427–35. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 24.KOHLA M, IWATA S, EA R, et al. Histological versus clinical cirrhosis in chronic hepatitis C: does race/ethnicity really matter? Digestive diseases and sciences. 2012;57(3):771–6. doi: 10.1007/s10620-011-1908-3. [DOI] [PubMed] [Google Scholar]
- 25.TERRAULT NA, IM K, BOYLAN R, et al. Fibrosis progression in African Americans and Caucasian Americans with chronic hepatitis C. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008;6(12):1403–11. doi: 10.1016/j.cgh.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KHOKHAR OS, LEWIS JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Digestive diseases and sciences. 2007;52(5):1168–76. doi: 10.1007/s10620-006-9579-1. [DOI] [PubMed] [Google Scholar]
- 27.BORUM ML, IGIEHON E, SHAFA S. African Americans may differ in their reasons for declining hepatitis C therapy compared to non-African Americans. Digestive diseases and sciences. 2009;54(7):1604. doi: 10.1007/s10620-009-0806-4. author reply 04–5. [DOI] [PubMed] [Google Scholar]
- 28.HARE CB, MORRIS JA, CHU A, et al. Comparison of characteristics of treated and non-treated patients with Hepatitis C infection. Pharmacoepidemiology and drug safety. 2006;15(2):71–6. doi: 10.1002/pds.1146. [DOI] [PubMed] [Google Scholar]
- 29.MELIA MT, MUIR AJ, MCCONE J, et al. Racial differences in hepatitis C treatment eligibility. Hepatology (Baltimore, Md) 2011;54(1):70–8. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SPRADLING PR, XJ R, UPP L, GORDON S, LU M, TESHALE T, BOSCARINO J, SCHMIDT M, DAIDA Y, HOLMBERG S. Uptake of and factors associated with direct-acting antiviral therapy among patients in the Chronic Hepatitis Cohort Study, 2014–2015. Journal of clinical gastroenterology. 2017 doi: 10.1097/MCG.0000000000000857. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]