Abstract
Intracranial electrical recordings and stimulation of neurosurgical patients have been central to the advancement of human neuroscience. The use of these methods has rapidly expanded over the last decade due to theoretical and technical advances, as well as the growing number of neurosurgical patients undergoing functional procedures for indications such as epilepsy, tumor resection, and movement disorders. These methods pose the potential for ethical conflict, as they involve basic neuroscientific research utilizing invasive procedures in human patients undergoing treatment for neurological illnesses. This review addresses technical aspects, clinical contexts, and issues of ethical concern, utilizing a framework that is informed by, but also departs from, existing bioethical literature on matters in clinical research. We conclude with proposals for improving informed consent processes to address potential problems specific to intracranial electrophysiology research, a general schema for scrutinizing research-related risk associated with different methods, and a call for the development of consensus to ensure continuing scientific progress alongside crucial patient protections in this promising area of human neuroscience.
Keywords: Bioethical issues, Electrocorticography, Deep brain stimulation, Human experimentation, Informed consent, Neurosurgery, Therapeutic misconception
ABBREVIATIONS
- BRAIN
Brain Research through Advancing Innovative Neurotechnologies
- ECoG
electrocorticography
- EEG
electroencephalography
Human cognitive neuroscience research has recently seen rapid growth in the use of invasive intracranial techniques. Unlike noninvasive techniques such as scalp electroencephalography (EEG) and functional MRI, invasive techniques such as electrophysiological recordings and electrical stimulation offer excellent resolution in both the spatial and temporal domains (Figure 1). The direct proximity of intracranial recording electrodes to neural sources also provides for a greater signal-to-noise ratio than noninvasive methods.1,2 While intracranial electrophysiological techniques have been used since the pioneering work of Jasper and Penfield in the 1950s, their application has expanded in recent decades (Figure 2) due to advances in systems neuroscience as well as technical advances in computer processing speed for signal processing and multivariate statistical analysis of multichannel data. Recent studies have made fundamental contributions to our current knowledge on vision, speech, decision making, memory, and sensorimotor processing in the human brain.3-13
FIGURE 1.

Spatial and temporal resolution. Comparison of spatial and temporal resolution of prevailing invasive methods in human neurophysiology (ECoG, electrocorticography; sEEG, stereotactic electroencephalography) to alternatives such as EEG, MEG, and functional MRI (fMRI). In dashed blue outline, “microECoG” represents ongoing development of higher-density recording techniques.
FIGURE 2.

Expansion of intracranial electrophysiology research, 1950-2014. Plotted in bars against the right axis are PubMed references for human research utilizing electrocorticography (ECoG) and stereotactic electroencephalography (sEEG). For trend comparison, plotted as lines against the left axis at 40:1 scale are references for 2 established noninvasive human neuroscience methods, EEG (subtracting out references for ECoG and sEEG) and functional MRI (fMRI).
In addition to advances in basic neuroscience, research using intracranial electrophysiology has practical translational applications for patients with neurological injury and disease. For example, one promising potential application is the use of implantable arrays as components of brain–computer interfaces for patients with severe motor impairment due to spinal cord injury, stroke, limb amputation, or neuromuscular disease.14,15 Another application is the determination of potential biomarkers of disease states (eg, Parkinson's, depression, anxiety) that may serve as feedback control signals for closed loop neuromodulation.16 As evidence of broad enthusiasm for the development of these and other advanced techniques in neuroscientific research, human intracranial research has been included for targeted funding in the President's BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative.17
With this enthusiasm, there is also need for ethical caution in this setting of human subject research. In particular, intracranial electrophysiological research can only be performed in a neurosurgical context. Thus, these studies are carried out in patients who already have an indication for functional neurosurgical treatment, such as medically refractory epilepsy, brain tumor, or Parkinson's disease. These medical circumstances, and the fact that clinical care and research often take place simultaneously, have the potential to affect patients’ ability to understand and consent to voluntary research protocols. Heterogeneity across sites in clinical protocols using these techniques has been documented;18,19 expansion in the number of investigators and centers performing electrophysiology research (Figure 2) may lead to similar heterogeneity in consent practices and subject selection. While local institutional review boards offer some degree of patient protection, numerous empirical studies have also documented inconsistencies in institutional review board determinations across different sites.20 The absence of consensus across centers raises concerns for transparency and patient trust, as well as the potential for abuse.
In this article, we present an ethical framework for this research that draws upon, but also departs in important ways from, a rich existing literature on the ethics of clinical research. We highlight domains of potential conflict that are particularly salient in human intracranial neuroscience, primarily informed by our own local experience. (This review addresses ethical concerns arising with adult patients, as research involvement of pediatric patients raises quite different issues.) We conclude by suggesting the need for developing consensus guidelines for self-regulation in this important and growing area of research.
TECHNICAL ASPECTS
Intracranial methods were pioneered and made popular in the 1940s and 1950s by Penfield and colleagues,21 who extended the stimulation protocols in their Montreal Procedure to include direct neural recordings as a measure of seizure activity. The earliest instances of this procedure represent examples of basic research being carried out in the clinical realm, as Penfield and colleagues used electrical stimulation to define fundamental characteristics of functional brain organization, including sensory, motor, and speech maps.
In the intervening decades, several different technical approaches have been developed to allow recording of population neural activity and electrical stimulation in neurosurgical patients (Table 1, Figure 3). Each technique has its own set of risks and research advantages and disadvantages, providing a range of possible approaches to human neurophysiology.
TABLE 1.
Techniques for Intracranial Electrode Placement
| Surface electrode grids (eg, 8 × 8, 16 × 16) | Surface electrode strips (eg, 1 × 6, 2 × 14) | Penetrating electrodes (eg DBS electrode, depth electrode for sEEG) | |
|---|---|---|---|
| Placement | Placed directly over cortical surface after craniotomy | Narrow profile allows for manipulation beyond craniotomy margin or through burr holes | Stereotactic placement under imaging guidance through burr holes or craniotomy |
| Risks | Cortical surface exposure; hemorrhage; infection | Cortical surface exposure (if craniotomy is used); hemorrhage; infection; risk of injury to bridging veins when manipulated beyond craniotomy margins | Infection; hemorrhage; local tissue injury from penetrating probes |
| Advantages | Extensive exposure of multiple cortical regions simultaneously; usually gyral surface only | Coverage of targeted cortical surface regions not covered by grids or beyond the margins of a craniotomy; reduced cortical surface exposure | 3-D selective sampling of deep and superficial cortex; access to sulci |
| Disadvantages | Limited coverage of sulci and areas not directly exposed by craniotomy | Limited coverage of targeted regions | Limited coverage of targeted regions (eg, typically < 5 electrodes in regions of interest) |
FIGURE 3.
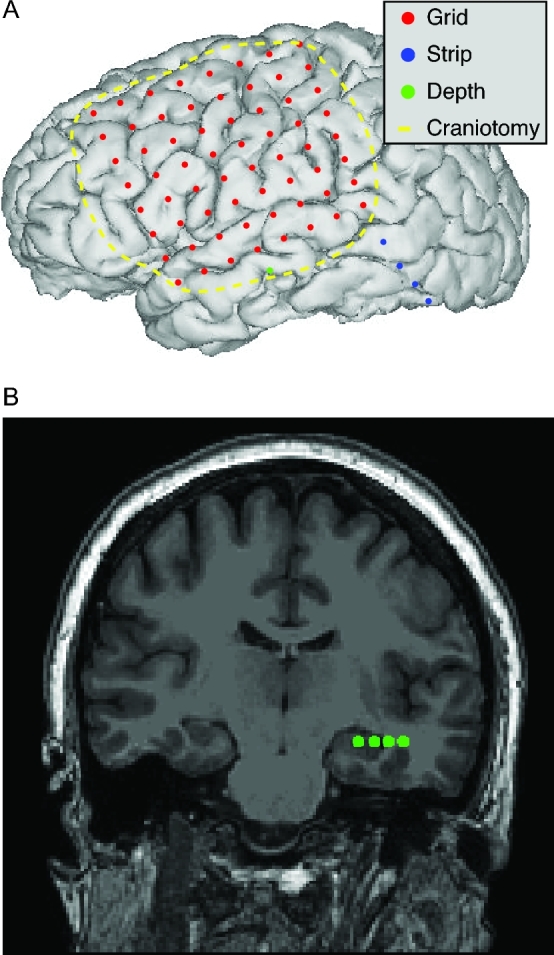
Techniques for intracranial electrode placement. A, Reconstruction of postoperative image from epilepsy patient undergoing electrode placement, illustrating placement of electrocorticography grid (depicted in red), electrocorticography strip (depicted in blue, extending outside craniotomy margin), and penetrating depth electrode (depicted in green). B, Volumetric MRI slice from the same patient demonstrating the location of the depth electrode probe (contacts depicted in green). Other techniques not depicted include stereotactic electroencephalography and microelectrode recording for deep brain stimulation using burr holes rather than craniotomy.
CLINICAL CONTEXT AND DECISION MAKING
Because intracranial techniques involve the placement of electrodes either below the dura (as in electrocorticography [ECoG] and electrical cortical stimulation) or within the brain parenchyma (as in stereotactic EEG and deep brain stimulation [DBS]), human research can only be performed with patients who already have a clinical indication for neurosurgical intervention with electrophysiological techniques. Common clinical indications that may also provide opportunities for research include DBS implantation for movement disorders and resection planning for epilepsy and tumors. Across different clinical indications, electrodes may be used to record activity or deliver stimulation in both intraoperative and extraoperative settings.
Intraoperative Applications
Intracranial electrophysiological techniques are often employed for clinical purposes in a wide range of neurosurgical procedures, which may also present opportunities for their use in research. During resection planning for epilepsy and tumors, ECoG may be used to provide information about the function and physiology of the targeted region, and electrical stimulation may be used to map nearby “eloquent” cortical regions to be avoided in the resection. Similarly, during surgical implantation of DBS electrodes, neural recordings and electrical stimulation from these electrodes are used to confirm proper placement. Neuroscientific studies can be performed in this setting with a cooperative and awake subject intraoperatively, but are limited by time available for testing and the limited range of research tasks that can be feasibly conducted in the operating room.
Extraoperative Applications With Temporary Electrodes
In some patients referred for epilepsy surgery, a resectable epileptogenic focus is suspected, but noninvasive tests to localize this target are inconclusive or conflicting. These patients may then undergo extended extraoperative ECoG monitoring, usually in a 2-stage process. First, electrodes are implanted surrounding the suspected seizure focus under general anesthesia. After this initial surgery, antiepileptic medications are withdrawn and patients are observed in a hospital monitoring unit over 1 to 2 wk for seizures to identify unambiguously the seizure onset zone, and to map the surgical margin. This extended period of monitoring for seizure activity is a window during which cognitive and behavioral tasks can be presented while electrophysiological data are recorded. In addition, patients may undergo electrical stimulation for more detailed mapping of eloquent regions, during which time neuroscientific data regarding the function of these regions may be collected. After monitoring, the patient is returned to the operating room for a second surgery to remove the electrodes; if an epileptogenic focus has been identified during monitoring, surgical resection is usually performed at the same time.
Extraoperative Applications With Chronically Implanted Electrodes
Newer implanted neurostimulators, such as the investigational Medtronic Activa PC+S DBS device (Medtronic, Minneapolis, Minnesota) and the NeuroPace RNS System for epilepsy (NeuroPace, Mountain View, California), also present opportunities for performing intracranial recordings in the outpatient or even the home setting. While these devices are implanted in patients for clinical indications (such as Parkinson's disease and partial-onset seizures without a resectable focus), they include added functions with research applications, such as the ability to store neural recordings from implanted electrodes and to wirelessly stream data to external computers. Cognitive task paradigms can then be presented to patients during outpatient follow-up visits or in dedicated behavioral testing facilities, avoiding the limitations of testing in the inpatient hospital setting.22 Still, these secondary research uses of these devices should not compromise the primary therapeutic purposes for which they were originally indicated and implanted.
Associated Risks
Surgical approaches and techniques for electrode placement are governed by patients’ clinical indications for intervention. Published data on risks associated with electrode placement are subject to several limitations; while single-center case series may have a limited sample size to estimate uncommon risks (and sites with fewer complications may be likelier than sites with more complications to publish their outcomes), meta-analyses may generalize across heterogeneous studies with different reported measures and encompassing a variety of surgical approaches. In a meta-analysis of 2542 patients implanted with subdural grids for extraoperative monitoring prior to epilepsy resection, both with and without depth electrodes, the estimated pooled prevalence of pyogenic central nervous system infection was 2.3%, of intracranial hemorrhage was 4.0%, and of transient new neurological deficits was 4.6%, with 3.5% of patients requiring additional surgical procedures to manage adverse events.23 Similar complication rates were reported from a national hospital database in addition to an estimated 11.7% rate of cerebrospinal fluid leakage, a complication that often is unreported in published case series.24
Comparing different types of procedures, several case series document a higher rate of complications with surface electrode grids than with surface electrode strips.25-28 Comparing surface electrodes with depth electrodes given similar clinical indications (such as epilepsy resection planning), many studies are limited by small samples of patients with depth electrodes. In 2 studies, depth electrodes were associated with fewer complications than surface electrodes25 without a difference in clinically significant findings;28 while surface electrodes are associated with extra-axial fluid collections and subdural hemorrhage, depth electrodes raise concerns for intraparenchymal and intraventricular hemorrhage. A systematic review of functional procedures in deep structures (including DBS and lesioning) reported an estimated 5.0% rate of hemorrhagic complications, including a 2.1% rate of symptomatic hemorrhages and 1.1% of permanent neurological deficits or death.29 These risks may be reduced by imaging-based targeting techniques to reduce the risk of traversing sulci, ventricles, or blood vessels.
The primary risk of electrical stimulation mapping is provoking a seizure. This risk may depend significantly on technique; in a review of risks in intraoperative mapping for epilepsy surgery, the risk of seizure was 1.2% with the train-of-5 technique and 9.5% with the 60-Hz technique.30
ISSUES OF ETHICAL CONCERN
Informed Consent, Undue Influence, and Capacity
A basic ethical prerequisite of human subject research is the freedom of each potential subject to either willingly participate or refuse participation, based upon their informed understanding of the risks, benefits, and nature of the research. Intracranial electrophysiological research is subject to the same requirements as other human subject research, but the neurosurgical treatment context of this research raises 3 special concerns. First, participants are also patients undergoing surgery for serious neurological conditions, which may result (even if unintended) in undue influence to participate when the physician is an investigator. It is crucial that patients be aware that their care is not conditional on their participation in research. In other research performed in clinical contexts (such as clinical trials), some protocols preclude treating physicians from obtaining consent for research to avoid undue influence. However, there is no consensus on this issue, and some have argued that a patient's treating physician is often best positioned to explain the research protocol and to clarify the distinction between clinical and scientific components.31 For the complex interventions involved in intracranial electrophysiology, a research coordinator or fellow without medical or surgical training and who is not clinically involved in invasive procedures may be poorly equipped to answer patients’ questions about the medical risks of the research.
Second, it may be difficult for patients to distinguish among interventions that are part of the research protocol, interventions that are part of their clinical care, and interventions that serve combined research and clinical purposes. In the course of hospitalization, patients may interact with dozens of people in different research and clinical roles, and many of the clinical/behavioral tests that they perform are qualitatively similar to research tasks. For example, when a patient undergoing extended ECoG is approached in her hospital bed to perform a series of language tasks, it may be difficult for the patient to tell whether this is a research protocol that she may reasonably decline (if tired or bored), or part of her clinical management that is necessary to minimize the risk of postoperative language deficits.
Finally, while the complex interplay of clinical and research components in intracranial electrophysiology would pose challenges for cognitively normal people to make truly informed decisions about participation, potential research participants are also patients with serious conditions such as epilepsy and Parkinson's disease that can impair cognition and may be associated with psychiatric comorbidity. Of course, a neuropsychiatric diagnosis does not in itself mean the patient cannot consent to research; assessments of capacity are specific to a potential subject's understanding, appreciation, reasoning, and choice about the benefits and risks of a proposed intervention.32 Consent capacity is also specific to a given decision; thus, the decision to undergo treatment (for one's own benefit) is a separate decision from the decision to enroll in research (to contribute to generalizable neuroscientific knowledge), and should be evaluated separately. At the same time, a finding of incapacity to consent to research may prompt a more thorough assessment of the patient's understanding of the clinical intervention, or even of the appropriateness of intervention if the patient is cognitively impaired. If patients are found to lack capacity to consent to research but are still candidates for clinical intervention, then proxy consent for inclusion in research may be sought from legally authorized representatives if these are defined by state law,33 with the guidance of the institutional review board.
Consent for Continuing Participation
While intraoperative research activities during surgery are time limited, extraoperative ECoG and electrical stimulation may take place over the course of days or weeks of inpatient care. Our practice in these cases is to obtain written informed consent before the onset of research, and for this consent to cover future sessions that fall under the described research project. For subsequent cognitive and behavioral testing sessions, our research teams will typically obtain oral consent prior to each session of data collection (which typically last 30-60 min, depending on subject tolerance and participation).
Patients’ physical and emotional states can fluctuate dramatically during an extended hospitalization, and after surgery many patients require pain medication, including opiates, that can affect their judgment and awareness. When the researchers arrive at the patient's room to obtain oral consent for a research session and the patient declines, how are the researchers to interpret this?
In our view, it is appropriate in such cases to thank subjects for their participation, briefly explain upcoming procedures (including the distinction between research and clinical procedures), and remind them of their right to forgo optional elements of the research protocol or to withdraw entirely.34 In many cases, patients may simply wish to postpone a research session to a later time, or may object to specific research paradigms as repetitive or boring. Repeated postponements or refusals might prompt a broader discussion about participants’ willingness to continue in research, including a reminder that the rest of their care is not conditional on research participation.
Subject Selection and Electrode Placement
Another ethical prerequisite of research is that risks should be proportionate to the benefits of research, and that these risks should be minimized.35 Human intracranial electrophysiology research presents an atypical case. In general, it would be unethical to expose healthy subjects to the risks of invasive recording and stimulation, such as bleeding, infection, direct brain injury, and seizures, only for the sake of advancing scientific understanding. What makes this research ethical in a neurosurgical context is that these patients already have a medical indication for invasive recording and stimulation, and therefore participation in research does not expose them to disproportionate additional risks.
It is therefore crucial that such research be performed only in patients who have a clinical indication for invasive electrophysiology. This is complicated by the fact that clinical decision making in borderline cases is not always clear cut; for example, in the case of epilepsy surgery, 2 clinicians might sometimes disagree about whether noninvasive tests provide sufficient data to localize a resection target without extended ECoG monitoring. It may then be challenging to ensure that decisions about subject selection for electrophysiological techniques are made on purely clinical grounds and not influenced by scientific considerations. In our center, decisions about the necessity of intracranial electrophysiology for a given patient are made at an interdisciplinary consensus conference that includes clinicians who share clinical responsibility for the patient but are not involved in research.
Once a decision has been made on clinical grounds to expose a patient to the risks associated with recording and/or stimulation, a series of other important decisions must be made about the extent of coverage; for instance, the type and size of electrode arrays, how far away from a suspected epileptogenic focus they should extend, whether penetrating electrodes should be used, and which regions should be mapped with electrical stimulation. In these cases, in which the main risks associated with surgery and electrophysiological interventions are already assumed on clinical grounds, ethical questions remain about the potential influence of research considerations on the number or location of implanted electrodes. In the final section, we consider proposals for achieving consensus on this issue.
Dual Roles of Clinician and Investigator
Underlying many of these concerns, such as the possibility of undue influence or the appropriate role of research considerations in electrode placement, is the potential for role conflict. As clinicians, neurosurgeons and other members of the clinical team are obligated to pursue the well-being of their individual patients. At the same time, as scientific investigators they are also obligated to conduct rigorous research that contributes to generalizable knowledge that may improve and inform the care of other patients in the future. These dual roles can also create confusion in patients. For instance, a neurosurgeon might encourage a patient with Parkinson's disease to undergo DBS instead of continued medical management, as medical advice aimed at the patient's own well-being. But the same neurosurgeon might also encourage this patient to participate in an intraoperative research protocol on the electrophysiology of the basal ganglia—this is not advice directed at the patient's own well-being, but is instead an appeal to the patient's altruism to contribute to broader scientific understanding. Of note, this situation in itself is not unique to electrophysiological studies. For example, typical randomized clinical trials involving patients with a medical condition of interest are usually conducted in clinical settings, where investigators often have this dual role.36
While earlier work sought to draw a clear distinction between clinical and research activities,36-38 such activities may not be neatly separable in clinical trials or in human electrophysiology.39 For example, if a patient has a suspected epileptogenic focus adjacent to functionally important language regions, electrical stimulation mapping of the language network prior to resection is considered the best way to preserve function in that individual patient.40 However, the data gathered from one patient's language map can be combined with data from many other patients to contribute to generalizable knowledge about language systems in the human brain.41,42 In this case, the same activity of electrical stimulation mapping might serve the clinical aim of promoting the medical interests of the patient while also serving the research aim of advancing our understanding of the neural bases of language (potentially contributing to the future medical benefit of patients with language disorders).
While bioethicists continue to debate the interplay of these dual roles, the extensive literature on ethical conduct in clinical trials does provide a template for how clinical responsibilities of care for the individual patient can be balanced with scientific responsibilities to contribute to generalizable knowledge. In some medical subspecialties, such as pediatric oncology, a sizable majority of patients are enrolled in clinical trials, and research participation is considered a marker of high-quality clinical care. Participation in intracranial electrophysiology research may offer similar benefits to neurosurgical patients, in the form of more time with treating physicians, greater scrutiny over details of their care, and the possibility that investigational imaging and electrophysiological studies may inform the management of their individual case.
Systems of Care and Research
Even purely clinical applications of electrophysiological techniques will typically require the involvement of interdisciplinary teams in patients’ care; inclusion of research protocols in clinical settings will add to this complexity. Appropriate coordination across clinical and research teams requires clear delineation of responsibility and open communication among clinicians, investigators, patients, and patients’ families.
A further consideration is of cost and resource allocation. In the United States, the costs associated with clinical care are often borne by private third-party health insurance companies, whose fees are ultimately paid by patients’ employers or patients themselves; in countries with national health systems (and for US Medicare and Medicaid enrollees), these costs are covered by public sources. As noted above, it can be difficult to distinguish between practice and research activities, but careful accounting is necessary to ensure that research-related costs are not inappropriately shifted to health insurance systems.
PROPOSALS
Informed Consent Procedures
As noted above, there is a risk of undue influence when a treating neurosurgeon (or other member of the clinical team) approaches patients for consent to participate in intracranial electrophysiology research. However, nonclinician members of the research team may be poorly equipped to answer questions about the nature and risks of research participation and may experience their own conflicts of interest in seeking to enroll patients for studies. The question of who should obtain consent may need to be made on a case-by-case basis by institutional review boards, based on the particular features of a given study.31 In either case, other protections against undue influence are also needed, including appropriate training of clinician investigators around potential conflicts between therapeutic and scientific aims, and meaningful assurances to patients that research participation is voluntary and not a condition of their clinical care. Finally, nonclinician personnel often do not receive formal training in interaction with clinical populations, and thus may not recognize when patients are confused about important matters such as the distinction between research and clinical care. If nonclinicians are to obtain consent, they should receive instruction in such interactions and on surgical methods and risks. Even when nonclinicians are not involved in the consent process, formal bedside training may help them to recognize and resolve misconceptions when they arise.
Patients may be confused about the complex relationship between the clinical and scientific aims of invasive electrophysiological studies. If patients do not understand that some research interventions are directed at the scientific aim of contributing to generalizable knowledge rather than their own clinical benefit, their informed consent is undermined. Standard informed consent procedures may encourage subjects to merely memorize and repeat study risks and benefits without true appreciation. Innovative procedures incorporating teach-back/teach-to-goal methods and targeted assessment of patient comprehension may provide greater assurance that consent is valid.43
Two Dimensions of Ethical Concern/Scrutiny
Decisions about subject selection should always be made on purely clinical grounds; for assurance in borderline cases, we encourage that such decisions include the input of clinicians (such as neurologists and psychologists) who share clinical responsibility for the patient and are not involved in research. After subject selection, other decisions must be made about the sites of electrical recording and stimulation. Some proposed interventions may provide potentially important neuroscientific data, but are not directed at the patient's clinical benefit. For example, in patients with Parkinson's disease undergoing DBS, some investigators have integrated the temporary use of subdural electrode strips that are introduced through the same burr holes used to place the penetrating electrodes, which allows recording from cortical regions concurrently with basal ganglia targets.16 Similarly, in epilepsy surgery, depth electrodes with microwires in medial temporal structures now allow for recording from single neurons; these microwires have a potential to damage tissue and thus pose risk to patients, although they are typically only implanted in tissue that clinicians are relatively certain will be resected.44
In decisions about electrode placement and research design, we propose 2 dimensions according to which potential research applications should be scrutinized (Figure 4). The first is the extent to which the patient's intervention is modified by scientific rather than clinical considerations. (This is analogous to the ethics of clinical trials, where some study procedures such as blood draws or lumbar punctures for monitoring treatment effects are undertaken as part of the study protocol, rather than from clinical need.) At one end, an individual subject's data collected for a clinical purpose (as in the example of electrical cortical stimulation mapping of language centers) are combined with data from other patients as a contribution to generalizable knowledge, without exposing the patient to additional procedures or risks. In the middle of this spectrum, patients may undergo additional noninvasive procedures (such as performing behavioral tasks) while clinically indicated recording electrodes are in place. This requires patient participation and could expose the patient to some additional risks and/or unnecessary pain, for example, if testing is done intraoperatively and extends the period of time spent in the operating room. Additional scrutiny should be applied to protocols in which invasive procedures could be modified or augmented for the sake of the scientific aim; for example, if additional electrodes are placed in an epilepsy patient to include a cortical region of research interest that is not also part of the suspected epileptogenic zone.
FIGURE 4.

Two dimensions of ethical scrutiny.
The second dimension of ethical scrutiny concerns the magnitude of risks involved. These risks vary principally by the invasiveness of the electrophysiological techniques employed. In general, modification of protocols involving penetrating electrodes should generally invite the highest degree of scrutiny, given the risks of local tissue injury and potential intracerebral hemorrhage associated with these techniques.
Developing Dialogue and Consensus
These proposals are presented not as definitive, but instead as a contribution to what we hope will be a broad conversation among clinicians, investigators, patient-participants, institutional review boards, and the public about ethical issues in advancing neurotechnologies (Table 2). This important conversation can be enhanced by the inclusion of bioethics specialists on research teams; as a model of fostering interdisciplinary collaboration in research, the US BRAIN Initiative has recently issued funding opportunities for research on the ethical implications of neurotechnology. Given the recent and continuing expansion in the number of centers performing invasive electrophysiology research worldwide and rapid innovations in electrophysiological techniques, we see an urgent need for establishing common standards and consensus around their ethical development and application. Human intracranial electrophysiological research holds tremendous promise for advancing our understanding of fundamental neural processes and for the relief of suffering from neurological injury and disease. This opportunity is made possible by the altruism and trust of patients who are themselves undergoing neurosurgical treatment for serious illnesses, yet still assume research-related risks to benefit others. The future of this research field depends on minimizing these risks and, when risks cannot be eliminated, ensuring that any remaining risks are fully justified and openly communicated to participants.
TABLE 2.
Proposed Ethical Standards for Consensus Deliberation
| 1. Patients should be assured that care is not conditional on their participation in research. |
| 2. Consent capacity for research is distinct from consent capacity for clinical interventions. |
| 3. Patients always retain the right to refuse participation in research interventions, including in extraoperative research that may take place over several days in a hospitalization. |
| 4. Repeated postponements or refusals of research protocols may indicate that a broader conversation regarding willingness to participate in research is appropriate, with reminders that patients can always withdraw from research without forgoing continued clinical care. |
| 5. Human intracranial research should only be performed in patients with a clinical indication for invasive electrophysiology. |
| 6. Involvement of clinicians with responsibility for the patient, who are not investigators, may provide further assurance that decisions about clinical applications of invasive electrophysiology are not influenced by scientific considerations. |
| 7. Clinician-investigators have dual roles that must be acknowledged and communicated openly with patients. |
| 8. It may be impractical to exclude treating physicians from obtaining patient consent for research participation; in either case, other safeguards against undue influence are needed, including meaningful assurance that patient care will not be compromised by patient refusals to participate in research. |
| 9. Nonclinician members of the research team who obtain consent require instruction on bedside interactions with patients and on surgical methods and risks, and even when not involved in the consent process may benefit from such training. |
| 10. Standard informed consent procedures may be insufficient to ensure that consent is valid, and innovative methods may be needed. |
| 11. We have proposed a dimensional framework for evaluating potential research applications of intracranial electrophysiology (Figure 4). |
Disclosures
This work was supported by the National Institutes of Health (U01NS098971, K23AG043553). The authors report no commercial conflicts of interest. Of note, the authors receive funding to perform neuroscientific research using the techniques under discussion in this work. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors thank Dr Philip Starr for helpful input, and thank Ali Zahir for assistance in preparing the manuscript.
Notes
This research was presented at the American Society for Bioethics and Humanities, October 8, 2016, Washington, DC, as a panel discussion and at the BRAIN Initiative Investigators Meeting, December 13, 2016, Bethesda, Maryland, as a panel discussion.
COMMENTS
This is a thoughtful paper that addresses important ethical considerations for both neurosurgeons and potential patients who might be involved in clinical trials. I agree that patients undergoing invasive techniques and behavioral investigations should be informed about the procedures and processes.
Richard Rapport
Seattle, Washington
The general rule in medical ethics is to separate as much as possible the role of treating physician and the role of the clinical or translational investigator. That distinction is often difficult to maintain in neurosurgery, and, ethical considerations aside, not always in the patients’ best interests. With that said, when, whether, and how to protect patients who are subjects of human experimentation under such circumstances is a critically important question. The authors have provided an excellent analysis of the problem and guidelines for good investigational practice.
One key observation is that the differences between research, clinical care, and combinations of the 2 are not always clear cut and evident. It is not surprising that patients might have difficulty telling these activities apart. It is important to note that sometimes the neurosurgeon may have the same difficulty simply because of liminal ambiguities. How does one break down what happens during the process of electrophysiological monitoring? Take the deceptively simple task of electrophysiological signal extraction, for example, once an electrode is in place: when is it acceptable to prolong a procedure to obtain a better signal, and how does one go about obtaining voluntary informed consent for that detail? Is there anything that qualifies for a “judgement call” by the surgeon, an extension of the surgeon's prerogative, or is that a flawed question to begin with? What level of granularity should the consent process involve?
It seems clear that the surgeon must disclose to the patient her or his role as an investigator as well as a surgeon while providing assurance and committing to the principle that the patient's welfare and benefit always come first. The principles outlined in this paper offer a very sound basis for practice.
T. Forcht Dagi
Newton Centre, Massachusetts
REFERENCES
- 1. Engel AK, Moll CKE, Fried I, Ojemann GA. Invasive recordings from the human brain: Clinical insights and beyond. Nat Rev Neurosci. 2005;6(1):35-47. [DOI] [PubMed] [Google Scholar]
- 2. Chang EF. Towards large-scale, human-based, mesoscopic neurotechnologies. Neuron. 2015;86(1):68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaghloul KA, Blanco JA, Weidemann CT et al. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323(5920):1496-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphey DK, Maunsell JHR, Beauchamp MS, Yoshor D. Perceiving electrical stimulation of identified human visual areas. Proc Nat Acad Sci USA. 2009;106(13):5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495(7441):327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan AM, Dykstra AR, Jayaram V et al. Speech-specific tuning of neurons in human superior temporal gyrus. Cereb Cortex. 2013;24(10):2679-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flinker A, Korzeniewska A, Shestyuk AY et al. Redefining the role of Broca's area in speech. Proc Nal Acad Sci USA. 2015;112(9):2871-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci. 2010;14(4):162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98(3):279-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leonard MK, Baud MO, Sjerps MJ, Chang EF. Perceptual restoration of masked speech in human cortex. Nat Commun. 2016;7:13619 doi:10.1038/ncomms13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343(6174):1006-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus dependence of gamma oscillations in human visual cortex. Cereb Cortex. 2014;25(9):2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nourski KV, Howard MA III. Invasive recordings in the human auditory cortex. Handb Clin Neurol. 2015; 129(1): 225-, 244. [DOI] [PubMed] [Google Scholar]
- 14. Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain–computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1(2):63-71. [DOI] [PubMed] [Google Scholar]
- 15. Schalk G, Leuthardt EC. Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng. 2011;4(1):140-154. [DOI] [PubMed] [Google Scholar]
- 16. de Hemptinne C, Swann NC, Ostrem JL et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nature Neurosci. 2015;18(5):779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jorgenson LA, Newsome WT, Anderson DJ et al. The BRAIN Initiative: developing technology to catalyse neuroscience discovery. Philos Trans R Soc B Biol Sci. 2015;370(1668):20140164 doi:10.1098/rstb.2014.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamberger MJ, Williams AC, Schevon CA. Extraoperative neurostimulation mapping: results from an international survey of epilepsy surgery programs. Epilepsia. 2014;55(6):933-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolston JD, Englot DJ, Knowlton RC, Chang EF. Rate and complications of adult epilepsy surgery in North America: analysis of multiple databases. Epilepsy Res. 2016;124:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abbott L, Grady C. A systematic review of the empirical literature evaluating IRBs: what we know and what we still need to learn. J Empir Res Hum Res Ethics. 2011;6(1):3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Brain. Boston, MA: Little Brown and Company; 1954: 122-126. [Google Scholar]
- 22. Rao VR, Leonard MK, Kleen JK, Lucas BA, Mirro EA, Chang EF. Chronic ambulatory electrocorticography from human speech cortex. NeuroImage. 2017;153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systematic review and meta‐analysis. Epilepsia. 2013;54(5):828-839. [DOI] [PubMed] [Google Scholar]
- 24. Rolston JD, Ouyang D, Englot DJ, Wang DD, Chang EF. National trends and complication rates for invasive extraoperative electrocorticography in the USA. J Clin Neurosci. 2015;22(5):823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wellmer J, von der Groeben F, Klarmann U et al. Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia. 2012;53(8):1322-1332. [DOI] [PubMed] [Google Scholar]
- 26. Vale FL, Pollock G, Dionisio J, Benbadis SR, Tatum WO. Outcome and complications of chronically implanted subdural electrodes for the treatment of medically resistant epilepsy. Clin Neurol Neurosurg. 2013;115(7):985-990. [DOI] [PubMed] [Google Scholar]
- 27. Hedegärd E, Bjellvi J, Edelvik A, Rydenhag B, Flink R, Malmgren K. Complications to invasive epilepsy surgery workup with subdural and depth electrodes: a prospective population-based observational study. J Neurol Neurosurg Psychiatry. 2014;85(7):716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt RF, Wu C, Lang MJ et al. Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia. 2016;57(10):1697-1708. [DOI] [PubMed] [Google Scholar]
- 29. Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg. 2012;116(1):84-94. [DOI] [PubMed] [Google Scholar]
- 30. Szelényi A, Joksimovic B, Seifert V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol. 2007;24(1):39-43. [DOI] [PubMed] [Google Scholar]
- 31. Shah A, Porter K, Juul S, Wilfond BS. Precluding consent by clinicians who are both the attending and the investigator: An outdated shibboleth? Am J Bioeth. 2015;15(4):80-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hooper SM, Chiong W. Decision-making capacity and frontal lobe dysfunction. In: Miller BL, Cummings J, eds. The Human Frontal Lobes. 3rd ed New York, NY: Guilford Press; In press. [Google Scholar]
- 33. Saks ER, Litt M, Dunn LB, Wimer J. Proxy consent to research: the legal landscape. Yale J Health Policy Law Ethics. 2008;8(1):37-92. [PubMed] [Google Scholar]
- 34. Wendler D, Rackoff J. Consent for continuing research participation: What is it and when should it be obtained? IRB. 2002;24(3):1-6. [PubMed] [Google Scholar]
- 35. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research The Belmont Report: Ethical principles and guidelines for the protection of human subject of research. Fed Regist. 1979;44(76):23192-23197. [Google Scholar]
- 36. Chiong W. The real problem with equipoise. Am J Bioeth. 2006;6(4):37-47. [DOI] [PubMed] [Google Scholar]
- 37. Miller FG, Brody H. A critique of clinical equipoise. Therapeutic misconception in the ethics of clinical trials. Hastings Cent Rep. 2003;33(3):19-28. [PubMed] [Google Scholar]
- 38. Weijer C. The ethical analysis of risk. J Law Med Ethics. 2000;28(4):344-361. [DOI] [PubMed] [Google Scholar]
- 39. Kass NE, Faden RR, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. The research-treatment distinction: A problematic approach for determining which activities should have ethical oversight. Hastings Cent Rep. 2013;43(1):4-15. [DOI] [PubMed] [Google Scholar]
- 40. Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17(4):477-489. [DOI] [PubMed] [Google Scholar]
- 41. Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain. 2014;137(10):2773-2782. [DOI] [PubMed] [Google Scholar]
- 42. Chang EF, Breshears JD, Raygor KP, Lau D, Molinaro AM, Berger MS. Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. J Neurosurg. 2017;126(1):14-121. [DOI] [PubMed] [Google Scholar]
- 43. Sudore RL, Landefeld CS, Williams BA, Barnes DE, Lindquist K, Schillinger D.. Use of a modified informed consent process among vulnerable patients: A descriptive study. J Gen Int Med. 2006;21(9):867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bitterman Y, Mukamel R, Malach R, Fried I, Nelken I. Ultra-fine frequency tuning revealed in single neurons of human auditory cortex. Nature. 2008;451(7175):197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]


