Abstract
Background
Multiple studies have been reported regarding preeclampsia as a possible risk factor of cerebrovascular disease (CVD). However, the correlation of preeclampsia and CVD, whether it is a cause-effect relationship or they are sharing common predisposing condition, is not well understood. Therefore, the aim of this study was to investigate the association between the preeclampsia during pregnancy and development of postpartum CVD.
Methods
A total of 1,384,550 Korean women who had a delivery between January 1, 2010 and December 31, 2012, were enrolled. Women with the risk of CVD within 1 year prior to pregnancy were excluded based on the Charlson comorbidity index. Primary endpoint was the event of CVD within a year from delivery. After exclusion, 1,075,061 women were analyzed.
Results
During the follow-up of 1 year postpartum, there were 25,577 preeclampsia out of 1,072,041 women without postpartum CVD (2.39%), and 121 of 3,020 women with postpartum CVD had preeclampsia before delivery (4.01%). In multivariate logistic regression analysis, women who had preeclampsia during pregnancy showed a higher risk for postpartum CVD (odds ratio, 1.64; 95% confidence interval, 1.37–1.98).
Conclusion
The incidence of CVD after delivery was higher in women who had preeclampsia during pregnancy.
Keywords: Cerebrovascular Disease, Pregnancy, Postpartum Period, Preeclampsia, Insurance Claim Reporting, Korea
Graphical Abstract
INTRODUCTION
Cerebrovascular disease (CVD) is a leading cause of mortality and morbidity in women, accounting for about 50% of total cardiovascular deaths in the United States. Besides, about two thirds of survivors developed residual disability. Although women younger than 30 years have a stroke incidence less than 5%, the effect of stroke on long-term disability is even greater in this age group. According to the American Stroke Association, the risk for stroke is higher in pregnant women than in non-pregnant young women, with the highest stroke risk occurring in the third trimester and postpartum.1,2 The physiologic changes of pregnancy, specifically venous stasis, edema, and hypercoagulability caused by activated protein C resistance, lower levels of protein S, and increase fibrinogen, when combined, increase risk for CVD during pregnancy and the postpartum period.3
Preeclampsia is a leading cause of severe maternal morbidity, mortality, and adverse perinatal outcomes, which complicates approximately 2%–8% of all pregnancies; it is characterized by dysfunctional remodeling of maternal vessels at the implantation site.4 Increased placental oxidative stress by shallow invasion of trophoblasts leads to endothelial dysfunction and a systemic inflammatory response.5 Many studies have reported that preeclampsia increases risks for cardiovascular disease.6,7,8,9 Preeclampsia is also one of the common risk factors for CVD1,10 and high systolic blood pressure (BP) is the top risk factor for stroke, regardless of age or ethnicity.10,11,12
Moreover, many studies have reported about preconception risk factors of preeclampsia such as pre-pregnancy obesity, uncontrolled BP; and maternal diseases including lupus, diabetes or thrombocytopenia, and maternal gene polymorphism.13,14,15,16 As previously mentioned, preeclampsia increases the risk for CVD. However, additional studies are required to determine causal relationships between preeclampsia and CVD. It is difficult to distinguish whether preeclampsia itself is a risk factor of CVD, as the risk factors of both preeclampsia and CVD are similar. Furthermore, although preeclamptic patients recover after delivery or termination of pregnancy,5 no evidence has been reported regarding how long the risk for CVD after delivery of a preeclamptic pregnancy will be sustained.
Preeclampsia has been reported as one of the risk factors for CVD,17 however, it is unclear if preeclampsia causes CVD itself or if a predisposing condition for preeclampsia in the pre-pregnancy period also attributes to CVD. It is vitally important to investigate whether preeclampsia could be a risk factor of CVD within 12 months after delivery in women without risk factors of CVD before pregnancy. Therefore, the aim of this study was to investigate the association between the development of postpartum cerebrovascular disease (PCVD) and preeclampsia during pregnancy. Additionally, we aimed to evaluate the temporal distribution pattern of the incidence of PCVD within 12 months in Korean population.
METHODS
Health care delivery system in Korea
Study data for 2009–2013 were collected from the Korea National Health Insurance (KNHI) claims database of the Health Insurance Review and Assessment Service (HIRA). In Korea, 97% of the population is obligated to enroll in the KNHI program. Healthcare providers are required by health insurance policies to allow HIRA to review the medical costs incurred. The remaining 3% of the population is covered under the Medical Aid Program. Thus, the HIRA database contains information on all claims for approximately 50 million Koreans, and nearly all information about the volume of disease can be obtained from this centralized database with the exception of procedures not covered by insurance, such as cosmetic operations. Many epidemiological analyses have been published from this database. According to the Act on the Protection of Personal Information Maintained by Public Agencies, HIRA prepares the claims data by concealing individual identities.18 The database we received included an unidentifiable code representing each individual together with his/her age, diagnosis, and a list of prescribed procedures.
Study population
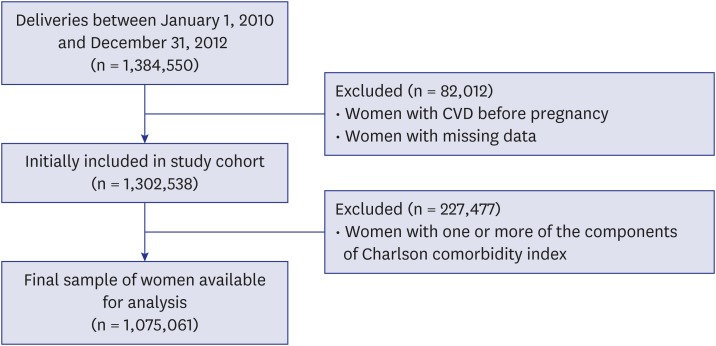
Fig. 1 shows the flow chart illustrating the inclusion and exclusion of study participants. The International Classification of Diseases, Tenth Revision (ICD-10) diagnostic code was used to identify all women who gave birth and women who were diagnosed as having CVD during the study period. The current study included only women who had given birth between January 1, 2010 and December 31, 2012.
Fig. 1.
Cohort flowchart illustrating the inclusion and exclusion of participants. Women with CVD before pregnancy and with missing data were excluded and women with one or more predisposing factors for the CVD were excluded.
CVD = cerebrovascular disease.
Women were classified as having CVD if they were newly diagnosed having CVD (ICD-10 code, I60–I69) from delivery to 1 year postpartum. The time of each patient's initial diagnosis was confirmed by the lack of a medical claim for CVD as a primary or secondary disease before delivery from January 1, 2009. To evaluate the effect of pregnancy itself on the development of CVD, women who had one or more of the components of the Charlson comorbidity index before pregnancy were excluded.19
Data of the women's characteristics such as age, parity, multiple pregnancies (defined as twins or higher-order gestation), delivery mode (vaginal delivery or cesarean section), and complications of pregnancy (e.g., gestational diabetes mellitus, and preeclampsia), were obtained.
Statistical analysis
The Student's t-test was used to compare continuous variables between groups, whereas the χ2 test was used to compare categorical variables. A model of multivariate logistic regression analysis was performed to evaluate the risk of the development of CVD after delivery. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 12.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Yonsei University Medical Center (approval No. 4-2016-1027). No individual informed consent was required in this study.
RESULTS
Among 1,075,061 women, the prevalence of CVD within 12 months of the postpartum period was 0.28%. About 2.4% of women without PCVD had preeclampsia during the prenatal period while 4.0% of women with postpartum CVD had preeclampsia during the prenatal period (P < 0.01).
Basic characteristics of the study population according to the development of CVD within 12 months of the postpartum period are presented in Table 1. The development of PCVD has statistically significant association with old age (more than 35 years, P < 0.01), parity (P < 0.01), mode of delivery (P < 0.01), occurrence of preeclampsia (P < 0.01), placental abruption (P < 0.04), and thromboembolism (P < 0.01) during pregnancy.
Table 1. Basic characteristics of study population.
| Parameters | Postpartum | P value | |
|---|---|---|---|
| Normal (n = 1,072,041) | CVD (n = 3,020) | ||
| Age, yr | 30.76 ± 4.10 | 31.08 ± 4.50 | < 0.01 |
| Old age (≥ 35 yr) | 179,468 | 620 | < 0.01 |
| Primiparity | 563,798 | 1,406 | < 0.01 |
| Multiple pregnancy | 15,063 | 43 | 0.93 |
| Cesarean delivery | 376,427 (35) | 1,206 (40) | < 0.01 |
| Induction | 253,377 | 669 | 0.06 |
| Vacuum delivery | 66,669 | 184 | 0.77 |
| Preeclampsia | 25,577 (2.39) | 121 (4.01) | < 0.01 |
| Gestational diabetes mellitus | 20,876 | 66 | 0.34 |
| Placenta previa | 8,993 | 23 | 0.64 |
| Placental abruption | 3,973 | 18 | 0.04 |
| Peripartum hysterectomy | 995 | 5 | 0.19 |
| Uterine arterial embolization | 890 | 4 | 0.35 |
| Postpartum hemorrhage | 77,920 | 208 | 0.42 |
| Thromboembolism | 1,040 | 17 | < 0.01 |
Values are presented as number (%) or mean ± standard deviation.
CVD = cerebrovascular disease.
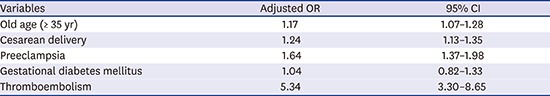
Table 2 shows the adjusted odds ratio (OR) for CVD within 12 months of the postpartum period according to multivariate logistic regression analysis. The incidence of CVD within 12 months of the postpartum period was higher in women with an older age (OR, 1.17; 95% confidence interval [CI], 1.07–1.28), cesarean delivery (OR, 1.24; 95% CI, 1.13–1.35), preeclampsia (OR, 1.64; 95% CI, 1.37–1.98), and thromboembolism (OR, 5.34; 95% CI, 3.30–8.65). Primiparous women had a lower incidence of PCVD (OR, 0.79; 95% CI, 0.73–0.85).
Table 2. Multivariate logistic regression analysis for postpartum CVD.
| Variables | Adjusted OR | 95% CI |
|---|---|---|
| Old age (≥ 35 yr) | 1.17 | 1.07–1.28 |
| Primiparity | 0.79 | 0.73–0.85 |
| Multiple pregnancy | 0.91 | 0.67–1.24 |
| Cesarean delivery | 1.24 | 1.13–1.35 |
| Induction | 1.05 | 0.95–1.16 |
| Vacuum delivery | 1.17 | 0.99–1.37 |
| Preeclampsia | 1.64 | 1.37–1.98 |
| Gestational diabetes mellitus | 1.04 | 0.82–1.33 |
| Placenta previa | 0.76 | 0.50–1.16 |
| Placental abruption | 1.40 | 0.88–2.23 |
| Peripartum hysterectomy | 1.45 | 0.59–3.58 |
| Uterine arterial embolization | 1.41 | 0.52–3.85 |
| Postpartum hemorrhage | 0.93 | 0.80–1.07 |
| Thromboembolism | 5.34 | 3.30–8.65 |
CVD = cerebrovascular disease, OR = odds ratio, CI = confidence interval.
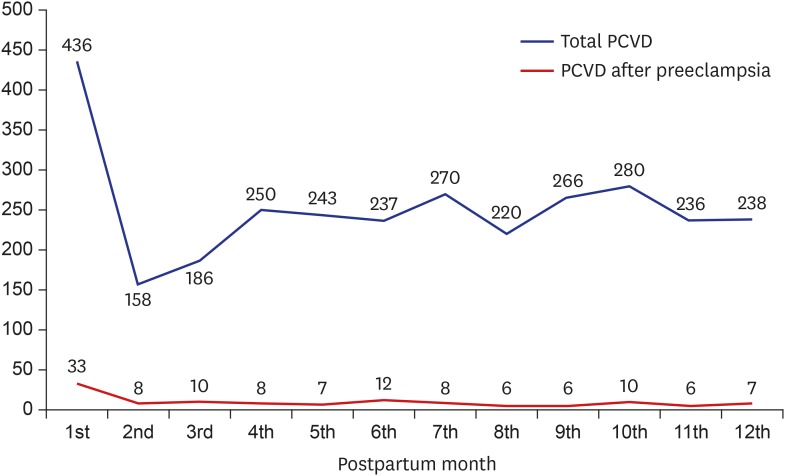
The total incidence of CVD for each month of the postpartum period and the incidence of CVD in women with preeclampsia during pregnancy are presented in Fig. 2. The incidence of CVD within 1 month after delivery was double the rate of that for the other months. Similarly, women who had preeclampsia during pregnancy had the highest incidence of CVD within 1 month after delivery, and the incidence within 1 month after delivery was approximately three times that of the other months.
Fig. 2.
Incidence of PCVD for each month of the postpartum period. The incidence of PCVD within 1 month after delivery was double the rate of that for later months. Similarly, women who had preeclampsia during pregnancy had the highest incidence of PCVD within 1 month after delivery, and the incidence within 1 month after delivery was approximately three times that of later months.
PCVD = postpartum cerebrovascular disease.
DISCUSSION
In this study, we found that the incidence of PCVD was increased when the pregnant women had preeclampsia during pregnancy. Preeclampsia increased the odds of CVD by 1.7-fold in this study, which was relatively lower than the results from other studies reporting an increased risk of up to 3.7-fold.7,8,10,11,12,13,14,15 The reason for relatively lower odds of PCVD in this study was attributed to the exclusion of women with a high risk of CVD during the preconception period. Nevertheless, it is obvious that preeclampsia independently increased the incidence of CVD. Our results support that preeclampsia itself may be an independent risk factor for CVD in the postpartum period.
We also found that the patients who had preeclampsia during pregnancy had a higher risk of CVD until 12 months after delivery than normotensive women. This means that the risk for PCVD might be continuously increased in the postpartum period. Although many studies have reported increased risks for PCVD in pregnant women who experienced preeclampsia, they were heterogenous between studies in the follow-up period and mean age of patients.8,14,16,17,20,21 Most follow-up studies on PCVD after preeclampsia assessed the increased risk for PCVD after the follow-up period without continuous assessment during the period. However, Tang et al.20 reported that preeclampsia/eclampsia increased risk for postpartum CVD until 12 months after delivery and they set the interval to examine the risk of PCVD as 3 months until 12 months after delivery. In our study, we monitored patients for an increased risk of PCVD monthly for 12 months after delivery.
Pregnancy-associated hypertension including preeclampsia is defined as BP that is generally normalized 12 weeks after delivery, and patients whose BP becomes normalized do not need to be followed up at a hospital.5,22 The task force by the American Congress of Obstetrics and Gynecology recommended that health providers should continue BP monitoring for postpartum surveillance for 7–10 days after delivery, regardless of hospitalization or outpatient follow-up, and it suggested that patients should promptly report signs and symptoms of preeclampsia.22 Nevertheless, based on our results, we think that patients who had preeclampsia during pregnancy should be instructed to self-monitor their BP at least 12 months after delivery. Furthermore, continuous monitoring for PCVD should be recommended for at least 12 months after delivery.
In this study, the incidence of CVD was 2.8 per 100,000 deliveries, which is comparable to the results from other studies.7,8,11,14,17,23 Interestingly, the incidence of PCVD tended to be similar each month, except for the first month after delivery, the rate approximately doubled in the first month compared to the average of the other months in both preeclamptic patients and non-preeclamptic women. Our results indicate a considerably increasing risk for PCVD in pregnant women with and without preeclampsia within 12 months after delivery. As previously mentioned, physicians have to follow-up and manage these patients in terms of aspects of morbidity because women with CVD in the childbearing period develop long-term disability for the rest of their lives.11,24
This pattern of incidence of PCVD can be explained in terms of hemodynamic changes during pregnancy and the postpartum period. As previously stated, the exact mechanism for the preponderance of stroke during the postpartum period is unknown; hemostatic change leads to an increased level of clotting factors and fibrinogen, along with decreased anticoagulation and fibrinolytic activity, and a peak level of hypercoagulability is reached around delivery and immediately postpartum. Factor VII, fibrinogen, and protein C resistance as well as levels of endothelial-derived plasminogen activator inhibitor 1, placenta-derived plasminogen activator inhibitor 2, and thrombin activatable fibrinolysis inhibitor increase during pregnancy, while anticoagulants, including protein S and the tissue plasminogen activator decrease during pregnancy.3,25 These changes gradually return to the nonpregnant state around 4 weeks postpartum. Hemostatic changes are probably related to hormonal changes during pregnancy to protect against fatal hemorrhage at the time of placental separation.3,25 Some parameters that reflect endothelial cell damage, including the circulating endothelial cell number, soluble vascular cell adhesion molecule-1, and soluble form of vascular endothelial growth factor receptor-1, have been reported, and these parameters remain at higher levels in pregnant women with preeclampsia than in those without preeclampsia (i.e., a normal pregnancy) after delivery.26,27,28 Additionally, remaining endothelial dysfunction could result in microangiopathy in the cerebrovascular system. These previous studies supported that preeclampsia increased the risk of PCVD in pregnant women.
In the present study, old age, cesarean delivery, and thromboembolism were associated with an increased risk of CVD in the postpartum period. These findings are consistent with those of other studies.3,11,29,30,31
This study stands out from previous studies with the following reasons. First, the number of subjects enrolled was very large. It is difficult to find studies with over 1 million subjects. Thanks to national healthcare database with almost every single Korean women's delivery records, this cohort minimizes the selection bias. Secondary, this study looked at CVD over 12 months of postpartum period, in contrary to previous studies only focused on OR of from the single moment, even with more 1 million subjects.12,13,32,33 Third, this study offered frequent and intense monthly follow-up for the 12 months postpartum. In contrast, other previous studies designed for a long-term follow-up only focused on data at 15–40 years after delivery, when most of the subjects become postmenopausal women.12,13,21,34 With our study design, we found that the risk for the postpartum CVD gets consistently increased until 12 months after delivery even with BP being normalized within 12 weeks after birth in preeclampsia group. Fourth, the risk of peripartum stroke in preeclamptic/eclamptic pregnancies was previously analyzed by Tang et al.20 in Taiwan. The number of subject was very large (n = 1,132,019), however, this study was limited as the cohort did not exclude the patients who already had predisposing risks factors of CVD such as obesity, diabetes mellitus, high cholesterol and smoking prior to pregnancy. Moreover, Tang et al.'s study20 compared the risks between hemorrhagic and ischemic stroke in preeclampsia, while our study compared the risks of postpartum CVD between in the preeclampsia and in the normal pregnancies.
Several limitations of our study should be considered when interpreting our findings. First, the diagnosis of preeclampsia was based on insurance claims data from the KNHI claims database, which was designed for reimbursement and not for research purposes. Thus, the main limitation is the validity of the diagnosis of preeclampsia in this database. However, the KNHI claims data, which include the entire Korean population, have greater reliability than data from other sources for estimating healthcare utilization in Korea.35 One previous validation study comparing the diagnoses derived from the database with the actual diagnosis recorded in patients' medical records found that the overall positive predictive value of the diagnoses was 83.4% in cases of hospitalized patients.36 Lastly, we are limited in our ability to evaluate the association between preeclampsia and PCVD according to the time of onset.
Nevertheless, the strength of the present study was that it used data from a population-based registry with large numbers. Moreover, we suggested that preeclampsia could be an independent risk factor of PCVD after delivery.
In conclusion, higher incidence of CVD within 12 months after delivery is associated with the development of preeclampsia during pregnancy.
Footnotes
Funding: This research was supported by a grant from the Korea Health Technology R & D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI15C0810).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Park Y, Cho GJ. Methodology: Cho GJ. Data curation: Kim LY, Lee TS. Formal analysis: Oh MJ, Kim YH.
References
- 1.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 2.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Elkind MS, et al. Risk of Pregnancy-associated stroke across age groups in New York State. JAMA Neurol. 2016;73(12):1461–1467. doi: 10.1001/jamaneurol.2016.3774. [DOI] [PubMed] [Google Scholar]
- 3.Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(6):978–984. doi: 10.1016/s0029-7844(99)00430-5. [DOI] [PubMed] [Google Scholar]
- 4.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald SE, Walker M, Ramshaw H, Godwin M, Chen XK, Smith G. Hypertensive disorders of pregnancy and long-term risk of hypertension: what do Ontario prenatal care providers know, and what do they communicate? J Obstet Gynaecol Can. 2007;29(9):705–710. doi: 10.1016/s1701-2163(16)32601-9. [DOI] [PubMed] [Google Scholar]
- 7.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 10.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16(2):206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 14.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 15.Berks D, Hoedjes M, Raat H, Duvekot JJ, Steegers EA, Habbema JD. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: a literature-based study. BJOG. 2013;120(8):924–931. doi: 10.1111/1471-0528.12191. [DOI] [PubMed] [Google Scholar]
- 16.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell C, Chireau M. Preeclampsia and stroke: risks during and after pregnancy. Stroke Res Treat. 2011;2011:858134. doi: 10.4061/2011/858134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, et al. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke. 2009;40(4):1162–1168. doi: 10.1161/STROKEAHA.108.540880. [DOI] [PubMed] [Google Scholar]
- 21.Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, et al. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke. 2006;37(4):1055–1059. doi: 10.1161/01.STR.0000206284.96739.ee. [DOI] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 23.Helms AK, Kittner SJ. Pregnancy and stroke. CNS Spectr. 2005;10(7):580–587. doi: 10.1017/s1092852900010221. [DOI] [PubMed] [Google Scholar]
- 24.Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke. 2014;45(7):1947–1950. doi: 10.1161/STROKEAHA.114.005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franchini M. Haemostasis and pregnancy. Thromb Haemost. 2006;95(3):401–413. doi: 10.1160/TH05-11-0753. [DOI] [PubMed] [Google Scholar]
- 26.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286(4):H1389–H1393. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 27.Blaauw J, Graaff R, van Pampus MG, van Doormaal JJ, Smit AJ, Rakhorst G, et al. Abnormal endothelium-dependent microvascular reactivity in recently preeclamptic women. Obstet Gynecol. 2005;105(3):626–632. doi: 10.1097/01.AOG.0000153490.41973.e0. [DOI] [PubMed] [Google Scholar]
- 28.Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B, Arikan H, Koc M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. Am J Obstet Gynecol. 2015;213(4):533.e1–533.e7. doi: 10.1016/j.ajog.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Lamminpää R, Vehviläinen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth. 2012;12(1):47. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274–1282. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 31.Gaillard R, Bakker R, Steegers EA, Hofman A, Jaddoe VW. Maternal age during pregnancy is associated with third trimester blood pressure level: the generation R study. Am J Hypertens. 2011;24(9):1046–1053. doi: 10.1038/ajh.2011.95. [DOI] [PubMed] [Google Scholar]
- 32.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124(25):2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 33.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 34.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77(2):154–158. doi: 10.1136/hrt.77.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang HY, Lim SJ, Suh HS, Liew D. Estimating the lifetime economic burden of stroke according to the age of onset in South Korea: a cost of illness study. BMC Public Health. 2011;11(1):646. doi: 10.1186/1471-2458-11-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park TH, Choi JC. Validation of stroke and thrombolytic therapy in Korean National Health Insurance claim data. J Clin Neurol. 2016;12(1):42–48. doi: 10.3988/jcn.2016.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]