Abstract
Background
To investigate the relationship between the presence of fragmented QRS (fQRS) on electrocardiogram (ECG) and plasma NT-proBNP levels in patients with ST-elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI). The in-hospital prognostic value of the presence of fQRS was also assessed.
Methods
In this retrospective study, 216 patients with STEMI who were undergoing primary PCI were included. fQRS was identified in ECG following primary PCI. The fQRS included various morphologies of the QRS (< 120 ms), which included an additional R wave (R′) or notching in the nadir of the S wave, or > 1R′ (fragmentation) in 2 contiguous leads, corresponding to a major coronary artery territory. N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were measured immediately, 24 and 48 hours after admition and the peak level was adopted. Patients were divided into two groups based on the presence (n = 126) or absence (n = 90) of a fQRS on ECG.
Results
NT-proBNP level was found to be significantly higher in fQRS (+) group compared to the fQRS (-) group (p < 0.001). The in-hospital advanced heart failure were found to be significantly more frequent in the fQRS (+) group. In logistic regression analysis, the fQRS ≥ 3 leads, fQRS in anterior leads, and NT-proBNP were independently related with in-hospital major adverse cardiac events that includes cardiovascular mortality, reinfarction, advanced heart failure, repeat target vessel revascularization, ventricular arrhythmias, atrioventricular block or sroke in the hospital. The area under the curve using the NTpro-BNP level for the prediction of fQRS was 0.809.
Conclusions
The presence of fQRS is significantly associated with NT-proBNP and left ventricular ejection fraction, which can predict left ventricular function in patients with STEMI. fQRS is a prognostic marker of impaired regional ventricular systolic function.
Keywords: Fragmented QRS complex, Myocardial infarction, N-terminal pro-brain natriuretic peptide, Percutaneous coronary intervention
INTRODUCTION
Fragmented QRS (fQRS) complexes including narrow or wide QRS complexes are frequently recorded on surface electrocardiograms (ECGs). In previous studies, an fQRS complex on surface ECG has been found to be a predictor of adverse cardiovascular events such as cardiac death and heart failure.1
Primary percutaneous coronary intervention (PCI) is a type of coronary reperfusion therapy that can produce recanalization and improve myocardial perfusion in patients with ST elevation myocardial infarction (STEMI).2 N-terminal pro-brain natriuretic peptide (NT-proBNP) is produced and released by cardiac ventricles in response to ventricular wall stress overload, and its release is stimulated by myocardial tissue perfusion.3 NT-proBNP is used as a biomarker to predict both short- and long-term mortality in patients with acute coronary syndrome (ACS), and it is closely linked to levels of inflammation, neurohormonal responses, and heart function in patients with ACS.4
The relationship between fQRS and NT-proBNP has not previously been investigated in patients with STEMI undergoing primary PCI. The objective of this study was to investigate the association between fQRS and NT-proBNP levels and to assess the clinical significance of fQRS and NT-proBNP in patients with STEMI undergoing primary PCI.5
METHODS
Patients
In this retrospective study, patients with a diagnosis of STEMI who underwent primary PCI between June 2014 and February 2015 were enrolled. The inclusion criteria were: (1) presenting within 12 h from the onset of symptoms, defined as typical chest pain lasting for > 30 min; (2) ST-segment elevation ≥ 1 mm in two contiguous electrocardiographic leads or new onset of complete left bundle-branch block; and (3) having undergone primary PCI including balloon angioplasty, thrombus aspiration, and/or stent implantation. The exclusion criteria included an age > 85 years, a medical history of cardiopulmonary resuscitation, congenital heart disease, severe valvular heart disease, severe organ dysfunction such as liver or kidney failure, malignancy, presence of bundle branch blocks, Wolff-Parkinson-White syndrome, Brugada syndrome, and the implantation of a permanent pacemaker.
A total of 241 patients were screened, of whom 216 were eligible for the study based on the inclusion and exclusion criteria. The patients were divided into two groups based on the presence or absence of fQRS after primary PCI. The fQRS included various morphologies of the QRS (< 120 ms) such as an additional R wave (R′) or notching in the nadir of the S wave, or > 1R′ (fragmentation) in two contiguous leads corresponding to a major coronary artery territory.6 The presence of fQRS (n = 126) was defined as fQRS (+), and the absence of fQRS (n = 90) was defined as fQRS (–). All primary PCIs were performed at our hospital (Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China), which is a single high-volume tertiary interventional treatment center (> 3000 PCI cases per year), by experienced interventional cardiologists who performed an average of > 300 PCI cases per year and were independent of the present study. The baseline demographics and angiographic characteristics, complications, laboratory and physical examination data on hospitalization were recorded by systematically reviewing the patients’ files. The hospital’s Ethics Committee approved the study protocol.
Coronary angiography, primary angioplasty and stenting
Primary PCI was usually performed using the percutaneous radial artery approach, and the femoral approach was used when an intra-aortic balloon pump was required. All angiographic data of the patients were assessed using a conventional technique from the catheterization laboratory records. The target artery was defined as being clinically significant when vessel stenosis was > 50%. Blood flow in the infarct-related artery (IRA) that received only primary PCI was graded based on the thrombolysis in myocardial infarction (TIMI) trial. A chewable loading dose of 300 mg aspirin and 180 mg ticagrelor with heparin (100 IU/kg) was provided before the PCI. Success of the procedure was defined as stenosis of the IRA < 20% with TIMI III flow after primary PCI. After primary PCI, all of the patients were transferred to our cardiac care unit and given standardized treatment for STEMI, which consisted of 100 mg aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, 20 mg atorvastatin once a day, and 90 mg ticagrelor, subcutaneous low-molecular weight heparin twice a day. Tirofiban was provided at the discretion of the interventional cardiologist.
Analysis of data
Twelve-lead ECG was recorded in each patient immediately on hospitalization, after primary PCI, and then at 24 h and 48 h. All ECGs (GE MAC1200, GE Healthcare, Fairfield, Connecticut, USA) were analyzed by two independent clinicians who were blinded to the study design, and clinical and angiographic data were analyzed during the first 48 h. QRS duration was measured using the longest QRS complex in any lead by both manual measurements and digital records from the ECG machine. All transthoracic echocardiographic examinations were performed using a machine (Philips CX50; Philips, Amsterdam, Netherlands) equipped with 5-1 MHz transducer.
Venous blood was collected from all patients within 72 h after hospitalization. NT-proBNP, creatine kinase-MB (CK-MB), and troponin I were measured daily. The blood samples were collected on admission and at 24 and 48 h in every patient. Plasma NT-proBNP levels were measured using an Elecsys 2010 analyzer, a commercially available electrochemiluminescent sandwich immunoassay (Elecsys proBNP; Roche Diagnostics, Mannheim, Germany). The 12-h fasting serum levels of blood glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol were measured using standard methods. Other biochemical measurements were performed using standard methods.
Definitions
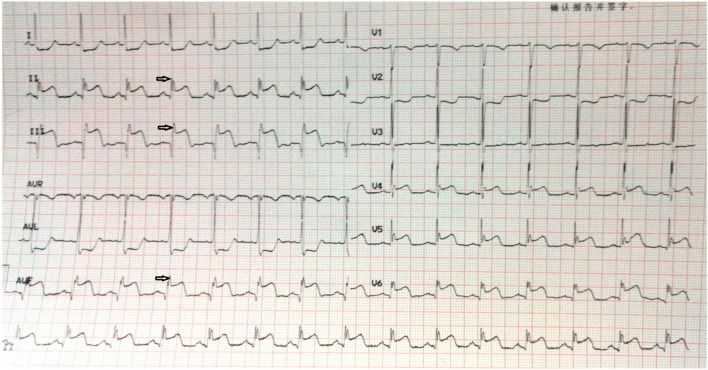
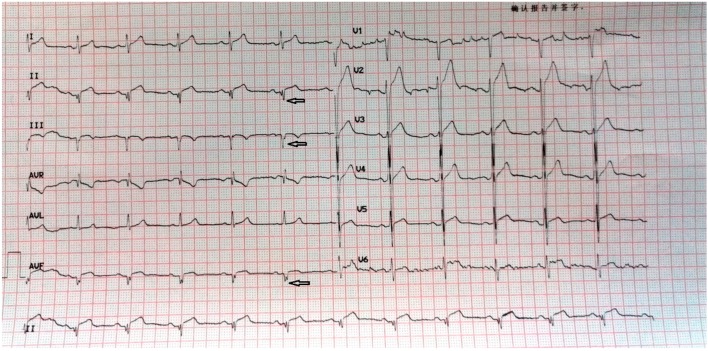
An fQRS complex was defined using the following criteria: (1) the presence of notched R or S, or the existence of an additional wave-like RSR’ pattern in the original QRS complex; (2) waves with or without a Q wave; (3) a duration of < 120 ms; and (4) no accompanying typical bundle branch block.6 Figures 1 and 2 represent examples of ECGs with fQRS complexes that were recorded on admission and after primary PCI.
Figure 1.
A 57-year-old male patient was admitted to our hospital with squeezing chest pain for 4 hours. On admission, electrocardiography (ECG) showed ST-segment elevation at inferior derivations (II, III, aVF) with fQRS complexes. He was transferred to our cath laboratory for primary PCI, and a totally occluded right coronary artery (RCA) was revascularized with balloon dilatation and stenting (TIMI III flow). PCI, percutaneous coronary intervention.
Figure 2.
A 65-year-old male patient was admitted to our hospital with squeezing chest pain for 8 hours. Primary PCI was performed for a totally occluded right artery (RCA) (TIMI III flow = 3). Post-PCI ECG revealed isoelectric ST-segment at all precordial leads with fQRS complexes at II, III and aVF derivations. PCI, percutaneous coronary intervention.
Reperfusion time was defined as symptom-to-balloon time, and door-to-balloon time was defined as the time between hospitalization and balloon dilation. Advanced heart failure was defined as a New York Heart Association (NYHA) functional classification of ≥ 3.
Diabetes mellitus was diagnosed when a patient was treated with insulin or oral hypoglycemic drugs, when the casual plasma glucose level was > 11.1 mmol/L, fasting plasma glucose level was > 7 mmol/L, or the glycosylated hemoglobin level was > 6.5% in patients who were not receiving retreatment with insulin or oral hypoglycemic drugs. Hypertension was diagnosed when the systolic arterial pressure was ≥ 140 mmHg and/or diastolic arterial pressure was ≥ 90 mmHg, and when the patient had used antihypertensive drugs for a long time. Hyperlipidemia was defined as a fasting total serum cholesterol level > 5.17 mmol/L, LDL-C level > 3.15 mmol/L, or serum triglyceride level > 1.70 mmol/L, or if the patient used lipid-lowering drugs due to a medical history of hypercholesterolemia. Smoking was defined as the current regular use of cigarettes or if the patient had quit smoking within the last year.
Study endpoints
Major adverse cardiac events (MACEs) included cardiovascular mortality, reinfarction, advanced heart failure, repeat target vessel revascularization (TVR), ventricular arrhythmias, atrioventricular block or stroke in the hospital. Cardiovascular mortality was defined as unexplained sudden cardiac death, death due to acute STEMI, decompensated heart failure, or hemodynamically significant arrhythmia. Repeat TVR was defined as the need for PCI due to restenosis or reocclusion of the IRA. Reinfarction was defined as the sudden occurrence of cardiac symptoms (especially chest pain) accompanied with increased levels of cardiac markers and/or electrocardiographic evidence of myocardial infarction (MI) after primary PCI in the first 24 h.
Statistical analysis
Quantitative variables were expressed as mean value ± standard deviation, and qualitative variables were expressed as a percentage (%). A comparison of parametric values between the two groups was performed using means of two independent sample Student’s t or Mann-Whitney U test. Categorical variables were also compared using the chi-square or Fisher’s exact test. ROC curves analysis was performed to determine the cut-off values of NT-proBNP for fQRS. Univariate and multivariate logistic regression analysis were performed in order to identify independent predictors of in-hospital MACE. MACE was the dependent variable and variables such as age, men, NT-proBNP, peak CK-MB and all 3 fQRS were controlled in the multivariate logistic regression analyses. Statistical significance was indicated when a two-sided p-value was < 0.05. All statistical analyses were performed using the IBM SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL).
RESULTS
Two hundred and sixteen patients (mean age 59.91 ± 10.97 years, 161 men and 55 women) were enrolled. The patients were categorized into an fQRS (+) group (n = 126) and fQRS (–) group (n = 90) based on the presence of fQRS on ECG at hospitalization. The baseline demographic and angiographic characteristics of the two groups are presented in Table 1. A comparison of the two groups showed some differences with regards to demographic and angiographic characteristics including hyperlipidemia, a medical history of MI, anterior MI, NYHA class, and culprit lesion: the patients in the fQRS (+) group had a higher rate of hyperlipidemia, lower levels of heart function, and a higher prevalence of anterior MI and a medical history of MI at hospitalization compared to the fQRS (–) group.
Table 1. Baseline demographic and angiographic characteristics of fQRS(+) and fQRS(−) groups.
| fQRS(+) group (n = 126) | fQRS(–) group (n = 90) | p value | |
| Age, years | 60.63 ± 10.69 | 58.84 ± 11.41 | 0.242 |
| Men, n (%) | 95 (75.40) | 66 (73.33) | 0.753 |
| Current smoker, n (%) | 71 (56.35) | 58 (64.44) | 0.262 |
| Hypertension, n (%) | 61 (48.41) | 47 (52.22) | 0.679 |
| Hyperlipidemia, n (%) | 87 (68.66) | 42 (46.67) | 0.001 |
| Diabetes, n (%) | 31 (24.60) | 15 (16.67) | 0.180 |
| Medical history of MI, n (%) | 16 (12.70) | 5 (5.56) | 0.047 |
| Previous PCI, n (%) | 17 (13.49) | 11 (12.22) | 0.840 |
| SBP, mmHg | 113.89 ± 27.32 | 117.12 ± 29.91 | 0.096 |
| DBP, mmHg | 70.50 ± 17.33 | 69.30 ± 15.90 | 0.295 |
| Reperfusion time, min | 275.00 ± 189.82 | 275.82 ± 192.44 | 0.977 |
| Door-to-balloon time, min | 71.16 ± 25.64 | 70.30 ± 22.99 | 0.799 |
| Pre-TIMI flow, n (%) | |||
| 0 | 82 (65.08) | 55 (61.11) | 0.569 |
| I | 25 (19.84) | 21 (23.33) | 0.614 |
| II | 7 (5.56) | 7 (7.78) | 0.581 |
| III | 10 (7.94) | 9 (10.00) | 0.632 |
| Post-TIMI flow, n (%) | |||
| 0 | 2 (1.59) | 0 (0.00) | - |
| I | 0 (0.00) | 0 (0.00) | - |
| II | 26 (20.63) | 13 (14.44) | 0.284 |
| III | 98 (77.78) | 77 (85.56) | 0.163 |
| NYHA class, n (%) | |||
| I | 80 (63.50) | 83 (92.22) | < 0.001 |
| II | 9 (7.14) | 3 (3.33) | 0.367 |
| III | 16 (12.70) | 3 (3.33) | 0.026 |
| IV | 11 (8.73) | 1 (1.11) | 0.016 |
| Culprit lesion, n (%) | |||
| LAD | 74 (58.73) | 37 (41.11) | 0.013 |
| LCX | 12 (9.52) | 12 (13.33) | 0.389 |
| RCA | 40 (31.75) | 41 (45.56) | 0.046 |
| Stent length, mean, mm | 25.70 ± 7.14 | 25.26 ± 5.70 | 0.643 |
| Stent diameter, mean, mm | 3.07 ± 0.38 | 3.02 ± 0.42 | 0.314 |
| Tirofiban use, n (%) | 39 (31.00) | 32 (35.56) | 0.229 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
CABG, coronary artery bypass grafting; DBP, diastolic blood pressure; fQRS, fragmented QRS; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; TIMI, thrombolysis in myocardial infarction.
Laboratory characteristics of the fQRS (+) and fQRS (–) groups are summarized in Table 2. Patients in the fQRS (+) group had significantly higher levels of peak CK-MB, troponin I, leukocyte count, and NT-proBNP compared to the fQRS (–) group. The optimal cutoff value for NT-proBNP level was 801.5 pg/ml, with a specificity of 70% and sensitivity of 80.8%. The area under the curve (AUC) using this NT-proBNP level to predict fQRS was 0.809 (p < 0.001) (Figure 3).
Table 2. Baseline laboratory characteristics of fQRS (+) and fQRS (−) groups.
| fQRS (+) group (n = 126) | fQRS (–) group (n = 90) | p value | |
| Platelet counts, ×109/L | 220.54 ± 78.40 | 218.89 ± 53.75 | 0.729 |
| WBC, ×109/L | 11.80 ± 4.62 | 10.71 ± 3.70 | < 0.001 |
| Hemoglobin, g/L | 134.48 ± 18.92 | 137.12 ± 18.74 | 0.057 |
| Glucose, mmol/L | 8.16 ± 3.63 | 7.56 ± 3.59 | 0.341 |
| Creatinine, μmol/L | 85.76 ± 20.36 | 86.40 ± 22.16 | 0.753 |
| Troponin I, μg/L | 241.33 ± 21.67 | 151.40 ± 16.14 | 0.002 |
| Peak CK-MB, μg/L | 238.64 ± 21.97 | 213.15 ± 22.59 | 0.001 |
| Total cholesterol, mol/L | 4.71 ± 1.14 | 4.47 ± 1.06 | 0.220 |
| LDL-cholesterol, mol/L | 2.99 ± 0.90 | 2.86 ± 0.81 | 0.395 |
| HDL-cholesterol, mol/L | 1.28 ± 0.43 | 1.16 ± 0.28 | 0.074 |
| Triglyceride, mol/L | 1.91 ± 1.52 | 1.98 ± 1.17 | 0.770 |
| hs-CRP | 10.15 ± 4.28 | 10.00 ± 4.59 | 0.832 |
| NT-proBNP, pg/mL | 3002.15 ± 298.71 | 902.26 ± 110.56 | < 0.001 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
CK-MB, creatine kinase-MB; fQRS, fragmented QRS; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; WBC, white blood cell.
Figure 3.
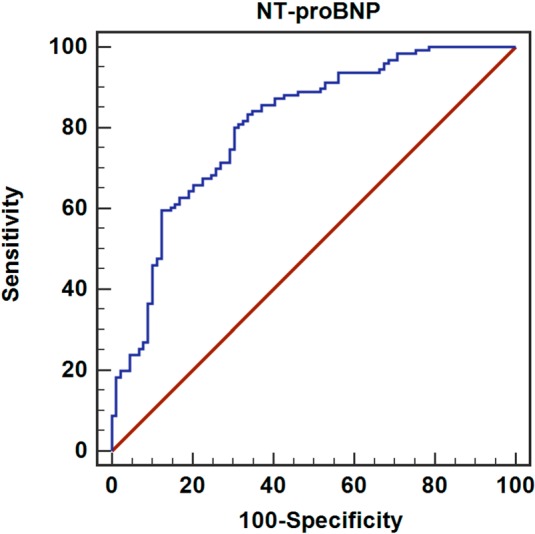
ROC curve analysis. Diagnostic value of NT-proBNP for fQRS. The optimal cutoff value of NT-proBNP level was 801.5 pg/ml, with a specificity of 70% and sensitivity of 80.8%. The AUC using this NT-proBNP level to predict fQRS was 0.809 (p < 0.001). AUC, area under the curve; fQRS, Fragmented QRS; NT-proBNP, N-terminal pro-brain natriuretic peptide; ROC, receiver operating characteristic curve.
The detailed ECG data are presented in groups determined by the presence or absence of fQRS after primary PCI in Table 3. Delta QRS duration and percentage of total ST resolution were significantly lower in the fQRS (+) group compared to the fQRS (–) group. However, the presence of the Q wave was significantly higher in the fQRS (+) group than in the fQRS (–) group. The patients with ≥ 3 leads with fQRS (n = 43) had a higher prevalence of anterior wall MI.
Table 3. Detailed information from ECG after primary PCI.
| fQRS (+) group (n = 126) | fQRS (–) group (n = 90) | p value | |
| Delta PR interval, ms | -1.2 ± 4.4 | -1.3 ± 4.1 | 0.958 |
| Delta QRS duration, ms | -4.7 ± 10.8 | -6.4 ± 13.5 | 0.024 |
| Delta QTc interval, ms | -2.7 ± 8.4 | -3.3 ± 9.1 | 0.174 |
| Percentage of total ST resolution (%) | 48 ± 44 | 63 ± 28 | 0.008 |
| Presence of Q Wave, n (%) | 119 (94.4) | 67 (74.4) | < 0.001 |
| fQRS on anterior leads, n (%) | 74 (58.73) | - | - |
| fQRS on inferior leads, n (%) | 52 (41.27) | - | - |
| fQRS ≥ 3 leads, n (%) | 52 (41.27) | - | - |
| fQRS ≥ 3 on anterior leads, n (%) | 43 (58.11) | - | - |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
fQRS, fragmented QRS; PCI, percutaneous coronary intervention.
The echocardiographic characteristics of the fQRS (+) and fQRS (–) groups are presented in Table 4. Left ventricle end-systolic diameter, left ventricle end-diastolic diameter, and left atrial diameter were significantly higher in the fQRS (+) group compared to the fQRS (–) group. However, left ventricular ejection fraction (LVEF) was significantly lower in the fQRS (+) group than in the fQRS (–) group. There was no significant difference between the two groups with regards to the degree of mitral regurgitation.
Table 4. Echocardiographic characteristics of fQRS (+) and fQRS (−) groups.
| fQRS (+) group (n = 126) | fQRS (–) group (n = 90) | p value | |
| LVEF, % | 53.31 ± 9.35 | 58.51 ± 6.67 | < 0.001 |
| LVESD, mm | 31.33 ± 7.27 | 27.69 ± 5.59 | < 0.001 |
| LVEDD, mm | 47.17 ± 6.86 | 45.07 ± 5.56 | 0.021 |
| Left atrial diameter, mm | 34.09 ± 5.99 | 32.00 ± 5.37 | 0.011 |
| Degree of mitral regurgitation | |||
| 0 | 53 | 46 | 0.214 |
| 1 | 9 | 7 | 0.861 |
| 2 | 51 | 30 | 0.320 |
| 3 | 10 | 4 | 0.405 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
fQRS, fragmented QRS; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricle end-systolic diameter.
A comparison of the two groups with regards to in-hospital MACEs is shown in Table 5. The in-hospital rates of advanced heart failure and MACEs were significantly higher in the fQRS (+) group. However, there were no significant differences in cardiovascular mortality, reinfarction, and other adverse cardiovascular events between the two groups.
Table 5. Comparison of fQRS (+) and fQRS (−) groups with regard to in-hospital MACE.
| fQRS (+) group (n = 126) | fQRS (–) group (n = 90) | p value | |
| Cardiovascular mortality, n (%) | 6 (4.76) | 1 (1.11) | 0.243 |
| Reinfarction, n (%) | 1 (0.79) | 1 (1.11) | 0.810 |
| TVR, n (%) | 1 (0.79) | 1 (1.11) | 0.810 |
| Advanced heart failure, n (%) | 27 (21.43) | 4 (4.44) | < 0.001 |
| Stroke, n (%) | 11 (8.73) | 7 (7.78) | 0.803 |
| VT/VF, n (%) | 8 (6.35) | 6 (6.67) | 0.926 |
| AVB, n (%) | 10 (7.94) | 7 (7.78) | 0.966 |
| MACE | 64 (50.79) | 27 (30.00) | 0.003 |
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively.
AVB, atrioventricular block; fQRS, fragmented QRS; MACE, major adverse cardiac event; TVR, target vessel revascularization; VF, ventricular fibrillation; VT, ventricular tachycardia.
Detail data of the predictors of in-hospital MACEs using logistic regression analysis are listed in Table 6. fQRS ≥ 3 leads (OR, 5.02; p = 0.007), fQRS on anterior leads (OR, 3.70; p = 0.007), and NT-proBNP (OR 1.052; p = 0.023) were independently associated with in-hospital MACEs.
Table 6. Univariate and multivariate logistic regression analysis for predictors of in-hospital MACE.
| Variables | Univariate | Multivariate | ||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age, years | 1.00 (0.98-1.03) | 0.99 | 0.98 (0.94-1.02) | 0.331 |
| Men | 2.02 (1.11-3.67) | 0.021 | 1.90 (0.71-5.06) | 0.200 |
| fQRS in inferior leads | 1.83 (1.09-3.92) | 0.04 | 1.063 (1.032-1.094) | 0.33 |
| fQRS in anterior leads | 10.78 (2.64-42.94) | < 0.001 | 3.70 (1.68-10.02) | 0.001 |
| fQRS ≥ 3 leads | 19.87 (6.29-60.77) | < 0.001 | 5.02 (1.51-49.83) | 0.007 |
| NT-proBNP, pg/mL | 1.10 (1.01-1.21) | < 0.001 | 1.052 (1.029-1.076) | 0.023 |
| Peak CK-MB, μg/L | 1.002 (1.00-1.003) | 0.012 | 1.001 (0.99-1.003) | 0.408 |
CI, confidence interval; CK-MB, creatine kinase-MB; fQRS, fragmented QRS complex; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; TIMI, thrombolysis in myocardial infarction.
Variables such as age, men, NT-proBNP, peak CK-MB and all 3 fQRS were controlled in the multivariate logistic regression analyses.
DISCUSSION
The objective of the present study was to assess the relationship between the presence of fQRS and NT-proBNP for STEMI in patients who underwent primary PCI. The main findings of this study were as follows: (1) the NT-proBNP level was significantly higher in the fQRS (+) group compared to the fQRS (–) group; (2) fQRS in ≥ 3 leads, fQRS in anterior leads, and plasma NT-proBNP level were independent predictors of in-hospital MACEs; (3) when NT-proBNP levels was ≥ 801.5 pg/ml, the sensitivity and specificity in fQRS were 80.8% and 70%, respectively; and (4) the in-hospital advanced heart failure rate was significantly higher in the fQRS (+) group. To the best of our knowledge, this is the first study to show a relationship between the fQRS complex and NT-proBNP, which is a marker of myocardial tissue perfusion and ventricular dysfunction induced by myocardial ischemia, based on data from patients with STEMI undergoing primary PCI.7
A routine 12-lead ECG is the gold standard tool for the rapid diagnosis of STEMI.8 The diagnosis of STEMI and assessments of reperfusion can be performed through dynamic changes in standard ECG, which include pathological Q-wave, T-wave, and ST-segment. The frequency of fQRS on standard ECG has been previously reported to range from 34.9% to 60.1% in ACS.9 Das et al. showed that the location of the leads with fQRS complex on 12-lead ECG reflected the infarct size and location of ischemia, myocardial fibrosis, or scar tissue in the ventricles.10 Previous studies have also demonstrated the relationship between perfusion defect, ischemic scar tissue, and fQRS.11 fQRS manifests as cardiac arrhythmias and conduction disturbances, which seem to be related to myocardial inflammation, focal fibrosis, and ischemia within the conduction system, possibly due to nonhomogeneous activation of ischemic or infarcted myocardial tissue.12,13 Perfusion defects caused by infarct or ischemia are associated with a poor prognosis owing to the risk of malignant arrhythmias and heart failure.14 Stavileci et al. also demonstrated an association between persistent fQRS on ECG at hospitalization with a poor prognosis in acute STEMI.12 In addition, Kalkan et al. reported that fQRS was a marker of elevated pressure of myocardial perfusion abnormalities and functional deterioration of the left ventricle,15 and Erdem et al. demonstrated a significant negative correlation between fQRS and left ventricular ejection fraction (LVEF) in patients with acute STEMI.16
fQRS has been reported to be significantly associated with in-hospital adverse cardiovascular events in patients with STEMI who underwent primary PCI.9,17 Recent studies have also shown that fQRS can occur within 24-48 h after the onset of symptoms and persist thereafter, and that this was considered to be a marker predicting MACEs in patients with coronary artery disease.18 fQRS may also be a surrogate marker of myocardial scarring and/or ischemia, which are significantly associated with systolic dysfunction.19 In our study, we showed by analyzing the adverse outcomes after acute myocardial infarction (AMI) that MACEs (cardiovascular mortality, reinfarction, advanced heart failure, or repeat target vessel revascularization) were significantly more frequent in the fQRS (+) group. The prognostic value of fragmented QRS on 12-lead ECG and BNP in patients with AMI was evaluated by Lorgis et al, who reported that persistent fQRS and BNP were associated with decreased survival in univariate analysis. However, in multivariate Cox regression analysis, they were not significant independent predictors of MACE. In addition, they found that fQRS was not a predictor of heart failure or ventricular arrhythmia in univariate analysis.20 Interestingly, even though fQRS was not an independent predictor of adverse outcomes after AMI in our study, fQRS ≥ 3 leads was an independent predictor of in-hospital MACEs. It has been reported that a larger number of fQRS complexes may be associated with a larger size of myocardial scars,21,22 a more depressed left ventricular (LV) systolic function, and intraventricular systolic dyssynchrony.23 These findings suggest that the number of leads with fQRS provides useful prognostic information for patients with MI.
In the present study, the level of NT-proBNP was independently associated with in-hospital MACEs and when NT-proBNP levels was > 801.5 pg/ml, the sensitivity and specificity in fQRS were 80.8% and 70%, respectively. Tensile cardiac ventricles synthesize and release proBNP which is then converted into NT-proBNP and bioactive BNP by protease when the ventricular wall pressure increases or ventricles dilate.24,25 Both NT-proBNP and BNP are released into the circulation at a ratio of 1:1, and myocardial tissue perfusion may also stimulate their release in the area of myocardial ischemia and infarction.3,26 After STEMI, increased levels of BNP have been found to be associated with the development of left ventricle remodeling and decreased LVEF. The first study to demonstrate an association between NT-proBNP and STEMI was published in 1998, and since then many studies have suggested that NT-proBNP levels observed in ACS are significantly associated with both short- and long-term cardiovascular mortality.3,27,28 Recent studies have shown that secretion of BNP and NT-proBNP, even in the absence of MI, is increased by transient or permanent ventricular dysfunction induced by myocardial ischemia. An increase in the plasma concentration of NT-proBNP has also been reported to be a reliable marker of ventricular dysfunction.29 Moreover, the degree of increase in NT-proBNP level is directly related to the extent of the ischemic or necrotic territory. The prognostic value of BNP and NT-proBNP has been shown in many studies. Evidence also shows that NT-proBNP is an indicator that can be used to assess the infarct size and LEVF in STEMI.30,31 Circulating levels of NT-proBNP and CKMB, which reflect pressure overload and myocardial damage, were significantly higher in the patients with fQRS in the present study. These results indicate that the presence of fQRS in patients with diastolic dysfunction could be associated with disease severity.
Limitations of the study
The present study has some limitations. The main limitation is the presence of significant referral bias due to the retrospective and single-center design. In addition, only in-hospital adverse cardiovascular events were analyzed. Data on long-term cardiovascular events and follow-up were lacking, and are planned to be included in a future study.
CONCLUSIONS
In conclusion, the presence of fQRS is significantly associated with NT-proBNP and LVEF, which can predict LV function in patients with STEMI. A significant direct relationship between fQRS and NT-proBNP was found in this study, and the number of fQRS leads on ECG was related to a higher rate of in-hospital adverse events in patients with STEMI undergoing primary PCI. fQRS is a prognostic marker of impaired regional ventricular systolic function.
Acknowledgments
None.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Ozcan F, Turak O, Canpolat U, et al. Myocardial tissue perfusion predicts the evolution of fragmented QRS in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Ann Noninvasive Electrocardiol. 2014;19:454–461. doi: 10.1111/anec.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 3.de Azevedo JC, Reis BC, Barreto NM, et al. BNP was associated with ischemic myocardial scintigraphy and death in patients at chest pain unit. Arq Bras Cardiol. 2015;104:16–23. doi: 10.5935/abc.20140175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;354:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 5.Onoue Y, Izumiya Y, Hanatani S, et al. Fragmented QRS complex is a diagnostic tool in patients with left ventricular diastolic dysfunction. Heart Vessels. 2016;31:563–567. doi: 10.1007/s00380-015-0651-7. [DOI] [PubMed] [Google Scholar]
- 6.Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 7.Omland T, Persson A, Ng L, et al. N-terminal Pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 8.Cakmak HA, Aslan S, Gul M, et al. Assessment of the relationship between a narrow fragmented QRS complex and coronary slow flow. Cardiol J. 2015;22:428–436. doi: 10.5603/CJ.a2015.0007. [DOI] [PubMed] [Google Scholar]
- 9.Uslu N, Gul M, Cakmak HA, et al. The assessment of relationship between fragmented QRS complex and left ventricular wall motion score index in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Ann Noninvasive Electrocardiol. 2015;20:148–157. doi: 10.1111/anec.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–1637. doi: 10.1016/j.amjcard.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Nomura A, Konno T, Fujita T, et al. Fragmented QRS predicts heart failure progression in patients with hypertrophic cardiomyopathy. Circ J. 2015;79:136–143. doi: 10.1253/circj.CJ-14-0822. [DOI] [PubMed] [Google Scholar]
- 12.Stavileci B, Cimci M, Ikitimur B, et al. Significance and usefulness of narrow fragmented QRS complex on 12-lead electrocardiogram in acute ST-segment elevation myocardial infarction for prediction of early mortality and morbidity. Ann Noninvasive Electrocardiol. 2014;19:338–344. doi: 10.1111/anec.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tigen K, Sunbul M, Ozen G, et al. Regional myocardial dysfunction assessed by two-dimensional speckle tracking echocardiography in systemic sclerosis patients with fragmented QRS complexes. J Electrocardiol. 2014;47:677–683. doi: 10.1016/j.jelectrocard.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Yang XW, Hua W, Wang J, et al. Regression of fragmented QRS complex: a marker of electrical reverse remodeling in cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2015;20:18–27. doi: 10.1111/anec.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalkan AK, Cakmak HA, Kalkan ME, et al. The predictive value of admission fragmented QRS complex for in-hospital cardiovascular mortality of patients with type 1 acute aortic dissection. Ann Noninvasive Electrocardiol. 2015;20:454–463. doi: 10.1111/anec.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdem FH, Tavil Y, Yazici H, et al. Association of fragmented QRS complex with myocardial reperfusion in acute ST-elevated myocardial infarction. Ann Noninvasive Electrocardiol. 2013;18:69–74. doi: 10.1111/anec.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terho HK, Tikkanen JT, Junttila JM, et al. Prevalence and prognostic significance of fragmented QRS complex in middle-aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014;114:141–147. doi: 10.1016/j.amjcard.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Pu M, Rodriguez D, et al. Ischemic and viable myocardium in patients with non-Q-wave or Q-wave myocardial infarction and left ventricular dysfunction: a clinical study using positron emission tomography, echocardiography, and electrocardiography. J Am Coll Cardiol. 2004;43:592–598. doi: 10.1016/j.jacc.2003.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Kang KW, Janardhan AH, Jung KT, et al. Fragmented QRS as a candidate marker for high-risk assessment in hypertrophic cardiomyopathy. Heart Rhythm. 2014;11:1433–1440. doi: 10.1016/j.hrthm.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Lorgis L, Jourda F, Hachet O, et al. Prognostic value of fragmented QRS on a 12-lead ECG in patients with acute myocardial infarction. Heart Lung. 2013;42:326–331. doi: 10.1016/j.hrtlng.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 22.Mahenthiran J, Khan BR, Sawada SG, Das MK. Fragmented QRS complexes not typical of a bundle branch block: a marker of greater myocardial perfusion tomography abnormalities in coronary artery disease. J Nucl Cardiol. 2007;14:347–353. doi: 10.1016/j.nuclcard.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Basaran Y, Tigen K, Karaahmet T, et al. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–68. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 24.Smit B, Spoelstra-de Man AM, Girbes AR, de Waard MC. NT-proBNP in cardiopulmonary resuscitated patients treated with mild therapeutic hypothermia is not independently associated with mortality: a retrospective observational study. BMC Anesthesiol. 2015;15:48. doi: 10.1186/s12871-015-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JH, Cheng PY, Hsu CH, et al. Effects of acetaminophen on left atrial contractility. Acta Cardiol Sin. 2016;32:485–490. doi: 10.6515/ACS20150823A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G, Sou SM, Twerenbold R, et al. B-type natriuretic peptide and clinical judgment in the detection of exercise-induced myocardial ischemia. Am J Med. 2014;127:427–435. doi: 10.1016/j.amjmed.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim KS, Mayyas FA, Kheirallah K, et al. Is left atrial size a predictor of mortality after coronary artery bypass surgery? A single center study. Acta Cardiol Sin. 2017;33:195–203. doi: 10.6515/ACS20160621A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards AM, Nicholls MG, Yandle TG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 29.Silva FB, Romero WG, Carvalho AL, et al. Hormone therapy with tamoxifen reduces plasma levels of NT-B-type natriuretic peptide but does not change ventricular ejection fraction after chemotherapy in women with breast cancer. Braz J Med Biol Res. 2015;48:154–160. doi: 10.1590/1414-431X20144189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen SJ, Gong Z, Duan QL. Evaluation of heart function with impedance cardiography in acute myocardial infarction patients. Int J Clin Exp Med. 2014;7:719–727. [PMC free article] [PubMed] [Google Scholar]
- 31.Tsao HM, Hu WC, Tsai PH, et al. Functional remodeling of both atria is associated with occurrence of stroke in patients with paroxysmal and persistent atrial fibrillation. Acta Cardiol Sin. 2017;33:50–57. doi: 10.6515/ACS20160411A. [DOI] [PMC free article] [PubMed] [Google Scholar]