Abstract
Background
Monocyte to high-density-lipoprotein cholesterol ratio (MHR) simply reflects proatherogenic and antiatherogenic balance and high level of this ratio is associated with severity of coronary atherosclerosis and cardiac events. We investigated the association between MHR and coronary artery disease severity using SYNTAX score and SYNTAX score II (SSII) in ST-elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention (pPCI).
Methods
A total of 315 consecutive patients with STEMI who underwent pPCI from January 2014 to January 2016 were enrolled. After exclusion 264 patients remained in the study population. Patients were divided into 2 groups according to median SSII [SSII ≤ 34.2 as low group (n = 132) and > 34.2 as high group (n = 132)].
Results
Median value of MHR was 10.5 in SSII low group and 16.1 in SSII high group (p < 0.001). There was a strong correlation between MHR and SSII (r = 0.580, p < 0.001). Diabetes mellitus [odds ratio (OR): 8.604; 95% confidence interval (CI): 2.469-29.978], glomerular filtration rate (OR: 0.961; 95% CI: 0.939-0.983), infarct related artery of left anterior descending (LAD) (OR: 7.325; 95% CI: 2.262-23.723), SYNTAX score (OR: 1.422; 95% CI: 1.275-1.585), neutrophil to lymphocyte ratio (NLR) (OR: 1.156; 95% CI: 1.058-1.264) and MHR (OR: 1.027; 95% CI: 1.013-1.041) were independent predictors of SSII > 34.2 in multivariate analysis.
Conclusions
MHR could be a better parameter than NLR and C-reactive protein at predicting severity of coronary artery disease in STEMI patients treated with pPCI.
Keywords: Monocyte to high density cholesterol ratio, ST elevation myocardial infarction, SYNTAX score, SYNTAX score II
INTRODUCTION
Inflammation plays an important role in the progression and destabilization of atherosclerosis and cardiovascular diseases. Monocytes are one of the most important components of the inflammatory process in atherosclerosis, and a high monocyte count is associated with the progression of atherosclerotic plaque.1,2 In contrast to monocytes, high-density lipoprotein cholesterol (HDL-C) has many antiatherogenic properties that are mainly associated with reverse cholesterol transport.3 The monocyte to high-density lipoprotein cholesterol ratio (MHR) simply reflects proatherogenic and antiatherogenic balance, and a high ratio is associated with the severity of coronary atherosclerosis and cardiac events.4-6 The SYNTAX score (SS) is a widely adopted anatomical scoring system used to grade the severity of coronary artery disease (CAD) according to the number of lesions and their functional impact, location, and complexity.7 The SYNTAX score II (SSII) includes clinical variables determined by applying a Cox proportional hazards model to the results of the SYNTAX trial.8 In a study investigating the predictive performance of the SSII compared to the SS, the SSII was found to have better prognostic accuracy for patients with CAD.9 Furthermore, the SS and SSII have been studied in patients with ST elevation myocardial infarction (STEMI), and both were found to be associated with long-term mortality and major adverse cardiac events.10,11 In the present study, we investigated the association between the MHR and severity of CAD using the SS and SSII in patients with STEMI treated with primary percutaneous coronary intervention (pPCI). To the best of our knowledge, this is the first study to investigate the association between the MHR and SSII.
METHOD
Study population
A total of 315 consecutive patients with STEMI who underwent pPCI from January 2014 to January 2016 were enrolled in this study. ST-segment elevation myocardial infarction was defined based on the following criteria: ongoing ischemic symptoms (within 12 hours), typical rise or fall in cardiac biomarkers and a new ST elevation in two or more contiguous leads with leads V1, V2, and V3 measuring at least 0.2 mV or at least 0.1 mV in the remaining leads, or a newly developed left bundle-branch block pattern.12 Patients with a history of CAD (32 patients), chronic inflammatory and infectious diseases (3 patients), renal replacement therapy (2 patients), hepatic failure (1 patients), cardiogenic shock (7 patients) and failure of reperfusion therapy (6 patients) were excluded. The remaining 264 patients were enrolled as the study population. The clinical findings and medical history including previous drug use of the patients were recorded. All patients received dual antiplatelet therapy, statins, beta blockers and spironolactone, and angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were given to all patients without contraindications. All patients were monitored at our coronary care unit for at least 24 hours after the pPCI. The study protocol was reviewed and approved by the Local Ethics Committee of our university, and it was conducted in accordance with the Declaration of Helsinki.
Blood sampling
Peripheral blood samples were obtained from all patients on admission to determine complete blood count, biochemical parameters and cardiac biomarkers. Hematological parameters were measured using a Horiba Pentra DX 120 automated blood cell counter (Horiba Medical, Montpellier, France), and biochemical parameters were measured using a Roche Cobas C501 autoanalyzer system (Roche Diagnostics, Indianapolis, IN, USA). The glomerular filtration rate was estimated using the Cockroft-Gault formula. The MHR and neutrophil to lymphocyte ratio (NLR) were calculated from parameters that were obtained from the admission blood sample.
Angiographic analysis
All patients underwent selective coronary angiography using the Judkins percutaneous trans-femoral technique. All patients received, on a routine basis, 300 mg acetylsalicylic acid and a 600 mg loading dose of clopidogrel before the intervention and unfractionated heparin during the intervention. The decision as to whether to use tirofiban was made at the discretion of the operator. Culprit lesions were treated with stent implantation and balloon angioplasty if necessary. Coronary angiograms were recorded on digital media for quantitative analysis (Dicom-viewer; MedCom GmbH, Darmstadt, Germany). Digital angiograms were analyzed by two independent and experienced interventional cardiologists who were blinded to all data. In cases of disagreement, the final decision was made by consensus.
Coronary blood flow patterns before and after primary PCI were thoroughly evaluated on the basis of thrombolysis in myocardial infarction (TIMI) flow grade using grades 0, 1, 2, and 3.13 Myocardial blush grade (MBG) was assessed according to the technique defined by Van’t Hof et al.14 Thrombus burden was assessed according to TIMI thrombus grading scale ranging from grade 0 (no thrombus) to grade 5 (very large thrombus causing vessel occlusion). Patients with grade 5 thrombus were re-classified from grade 0 to grade 4 after recanalization with a guidewire or small balloon.15 We defined angiographic no-reflow as a coronary TIMI flow grade ≤ 2 after a vessel had been re-canalized or TIMI flow grade 3 together with a final MBG ≤ 2 as previously described.16
Calculation of Syntax score and Syntax score II
Each lesion ≥ 1.5 mm in diameter and with ≥ 50% stenosis was scored using the online SS Calculator, version 2.1 (www.syntaxscore.com). Because patients with STEMI were excluded from the initial SS algorithm, we defined an occluded infarct-related artery as an occluded artery of < 3 months duration, as reported in previous studies of STEMI patients.10 The SSII was also calculated using the online calculator (www.syntaxscore. com), and included two anatomical variables [anatomical SS and unprotected left main coronary artery disease] and six clinical variables [age, creatinine clearance (CrCl), left ventricular ejection fraction (LVEF), sex, chronic obstructive pulmonary disease (COPD), and peripheral arterial disease (PAD)].8
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, Illinois). Normality of data distribution was analyzed using the Kolmogorov-Smirnov test. Numeric variables with a normal distribution were presented as mean ± standard deviation (SD), whereas those without a normal distribution were presented as median and interquartile range. Categorical variables were presented as number and percentage (%). Continuous variables were compared between the two groups using the Student’s t test or Mann Whitney U test. Categorical data were compared using the chi-square or Fisher’s exact test. Correlations between different variables were assessed using the Pearson correlation test for continuous variables and Spearman test for non-continuous variables. Statistical significance was defined as a p value < 0.05. Multivariate logistic regression analysis (backward elimination) was performed to identify the independent predictors of a high SSII using variables showing marginal association in univariate analysis. Receiver operating characteristic (ROC) curve analysis was used to detect the cutoff values of MHR and NLR in predicting SSII > 34.2 and SS > 22. De Longs test was used to compare ROC curves of MHR and NLR in the prediction of the SSII and SS.
RESULTS
The study population consisted of 264 STEMI patients (mean age: 63 ± 10 years; 31.8% females) who underwent pPCI. Because there is currently no definite classification of SSII, the patients were divided into two groups according to median SSII [SSII ≤ 34.2 for the low group (n = 132) and > 34.2 for the high group (n = 132)]. The high SSII group had higher C-reactive protein (CRP), neutrophil and monocyte count, and lower glomerular filtration rate, HDL-C, and lymphocyte count. The high SSII group had more infarct-related left anterior descending (LAD) arteries, proximal lesions, a longer lesion length, higher syntax score, and higher rates of 3-vessel disease, pre-procedural TIMI 0, TIMI thrombus grade 3-4 and no reflow. The baseline characteristics, clinical, angiographic and laboratory findings of the whole study population and two groups are summarized in Table 1.
Table 1. The baseline clinical and laboratory characteristics of the patients according to median SS II.
| All study population (N = 264) | SS2 low group (SSII ≤ 34.2; n = 132) | SS2 high group (SSII > 34.2; n = 132) | p value | |
| Age, years | 63 ± 10 | 60 ± 10 | 65 ± 10 | 0.001 |
| Female sex, % (n) | 31.8 (84) | 30.3 (40) | 33.3 (44) | 0.597 |
| Hypertension, % (n) | 47 (124) | 45.5 (60) | 48.5 (64) | 0.696 |
| Diabetes mellitus, % (n) | 35.2 (93) | 27.3 (36) | 43.2 (57) | 0.007 |
| Dyslipidemia, % (n) | 40.2 (106) | 36.4 (48) | 43.9 (58) | 0.209 |
| Smoking, % (n) | 51.1 (135) | 47 (62) | 55.3 (73) | 0.176 |
| Family history, % (n) | 22 (58) | 19.7 (26) | 24.2 (32) | 0.372 |
| COPD, % (n) | 28.8 (76) | 18.2 (24) | 39.4 (52) | < 0.001 |
| PAD, % (n) | 23.5 (62) | 9.1 (12) | 37.9 (50) | < 0.001 |
| Antihypertansive drug use, % (n) | 42.4 (112) | 40.2 (53) | 44.7 (59) | 0.455 |
| ASA use, % (n) | 43.2 (114) | 34.1 (45) | 52.3 (69) | 0.003 |
| Antihyperlipidemic drug use, % (n) | 29.9 (79) | 22 (29) | 37.9 (50) | 0.005 |
| Oral antidiabetic drug use, % (n) | 30.8 (80) | 19.7 (26) | 40.9 (54) | < 0.001 |
| Insulin use, % (n) | 7.2 (19) | 3 (4) | 11.4 (15) | 0.009 |
| Heart rate, per minute | 71 (64-81) | 70 (62-78) | 73 (64-81) | 0.069 |
| Systolic blood pressure, mmHg | 141 ± 20 | 137 ± 22 | 143 ± 18 | 0.062 |
| FGL, mg/dl | 106 (95-125) | 102 (92-118) | 110 (96-126) | 0.006 |
| Creatinine, mg/dl | 0.87 ± 0.17 | 0.86 ± 0.16 | 0.87 ± 17 | 0.715 |
| GFR, ml/min | 96 (85-114) | 101 (86-116) | 88 (76-113) | 0.008 |
| Hemoglobin, g/dl | 14.9 ± 1.7 | 15 ± 1.3 | 14.6 ± 1.9 | 0.96 |
| Platelet, 103/mm3 | 189 (170-235) | 189 (165-226) | 191 (171-246) | 0.128 |
| White blood cell, 103/μl | 11.5 ± 3.3 | 11 ± 3.3 | 11.8 ± 3.1 | 0.054 |
| Neutrophil, 103/μl | 9.2 ± 3.4 | 8.6 ± 3.5. | 9.7 ± 3.1 | 0.014 |
| Lymphocyte, 103/μl | 1.41 (1.02-2.08) | 1.71 (1.18-2.09) | 1.27 (0.98-1.79) | 0.006 |
| Monocyte, /μl | 411 (340-483) | 369 (311-441) | 461 (361-580) | < 0.001 |
| Total cholesterol, mg/dl | 169 (149-186) | 175 (163-189) | 155 (142-177) | < 0.001 |
| LDL-C, mg/dl | 107 (97-128) | 112 (103-125) | 103 (91-132) | 0.013 |
| HDL-C, mg/dl | 33 (29-39) | 35 (31-43) | 31 (26-36) | 0 |
| Triglyceride, mg/dl | 110 (80-152) | 117 (89-154) | 108 (71-142) | 0.042 |
| CRP, mg/dl | 1.12 (0.7-1.602) | 0.762 (0.473-1.147) | 1.498 (1.038-1.946) | < 0.001 |
| NLR | 7.1 (3.7-10.5) | 5.4 (3.3-8.8) | 7.9 (4.5-11.4) | 0.001 |
| MHR | 12.6 (9.9-16.3) | 10.5 (8.5-12.9) | 16.1 (12.2-20) | < 0.001 |
| Symptom to ballon time, hours | 3 ± 1 | 2.9 ± 1.1 | 3.1 ± 0.8 | 0.084 |
| Killip class ≥ 2 | 6.8 (18) | 4.5 (6) | 9.1 (12) | 0,143 |
| IRA of LAD, % (n) | 42.4 (112) | 21.2 (28) | 63.6 (84) | < 0.001 |
| 3 vessel disease, % (n) | 12.5 (33) | 1.5 (2) | 23.5 (31) | < 0.001 |
| Proximal lesion, % (n) | 56.1 (148) | 48.5 (64) | 63.6 (84) | 0.013 |
| Preprocedural TIMI 0, % (n) | 68.2 (180) | 51.5 (68) | 84.8 (112) | < 0.001 |
| Thrombus grade ≥ 3, % (n) | 68.2 (180) | 51.5 (68) | 84.8 (112) | < 0.001 |
| Stent length, mm | 27 (23-28) | 23 (18-28) | 28 (25-33) | < 0.001 |
| SYNTAX score | 18.4 ± 7.8 | 12.9 ± 4.3 | 23.8 ± 6.5 | < 0.001 |
| SYNTAX score II | 34.2 (25.1-44.4) | 25.1 (21-28.1) | 44.4 (38.6-51.7) | < 0.001 |
| No-reflow, % (n) | 54.4 (144) | 39.4 (52) | 69.7 (92) | < 0.001 |
| Peak CK-MB, mg/dl | 207 (115-312) | 140 (95-208) | 278 (206-384) | < 0.001 |
| LVEF, % | 46.5 (40-52) | 49 (47-52) | 40 (34-46) | < 0.001 |
ASA, acetylsalicylic acid; CK-MB, creatine kinase myocardial band; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FGL, fasting glucose level; GFR, glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; IRA, infarct related artery; LAD, left anterior descending; LDL-C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MHR, monocyte to HDL-C ratio; NLR, neutrophil to lymphocyte ratio; PAD, peripheral arterial disease; SYNTAX, SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery.
The median MHR value was 10.5 in the low SSII group and 16.1 in the high SSII group (p < 0.001). Moreover, NLR and CRP levels were significantly higher in the high SSII group than in the low SSII group (p = 0.001 and p < 0.001, respectively).
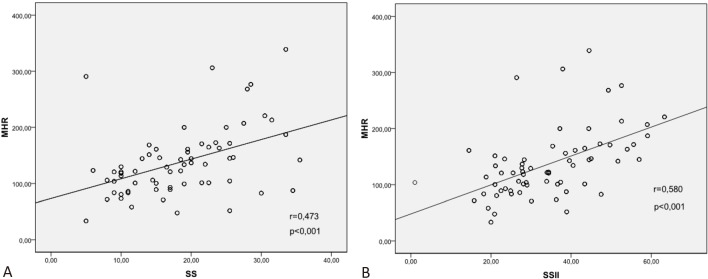
There was a moderate correlation between MHR and SS (r = 0.473, p < 0.001; Figure 1A) and a strong correlation between MHR and SSII (r = 0.580, p < 0.001; Figure 1B). The patients were divided into LAD and non-LAD groups according to the infarct related artery (IRA). There was a strong correlation between MHR and SS (r = 0.624, p < 0.001) and MHR and SSII (r = 0.640, p < 0.001) in the IRA-LAD group. However, the correlation coefficients (r value) were lower in the non-LAD group (MHR-SS; r = 0.249, p < 0.002 and MHR-SSII; r = 0.459, p < 0.001). The same pattern was observed in correlations between CRP level, NLR, SS and SSII in all patients and the IRA-LAD and non-LAD groups, however, the correlation coefficients were lower than MHR. Furthermore, there were no correlations between NLR and SS in all patients and the IRA-non-LAD group. The results of the correlation analysis are listed in Table 2.
Figure 1.
Correlation graphics between MHR and SS (A), and MHR and SSII (B). MHR, monocyte to HDL-C ratio; SS, SYNTAX score; SSII, SYNTAX score II.
Table 2. Correlation between MHR, NLR, CRP, SS and SSII.
| Syntax score | Syntax score 2 | |||||
| All patients | IRA LAD | IRA non-LAD | All patients | IRA LAD | IRA non-LAD | |
| MHR (r; p) | 0.473; < 0.001 | 0.624; < 0.001 | 0.249; 0.002 | 0.580; < 0.001 | 0.640; < 0.001 | 0.459; < 0.001 |
| NLR (r; p) | 0.097; 0.114 | 0.230; 0.015 | -0.086; 0.294 | 0.292; < 0.001 | 0.315; 0.001 | 0.190; 0.019 |
| CRP (r; p) | 0.383; < 0.001 | 0.305; 0.001 | 0.189; 0.02 | 0.461; < 0.001 | 0.385; < 0.001 | 0.278; 0.001 |
Abbreviations are in Table 1.
Multivariate logistic regression analysis was used to determine the independent predictors of SSII > 34.2. Univariate analysis showed that age, a history of diabetes mellitus (DM), COPD, PAD, acetylsalicylic acid, antihyperlipidemic drugs, oral antidiabetic drugs and insulin, IRA of LAD, fasting glucose level (FGL), glomerular filtration rate, total cholesterol, LDL-C, triglycerides, CRP level, MHR, NLR and SS were significantly correlated with SSII. However, in multivariate analysis, DM [odds ratio (OR): 8.604; 95% confidence interval (CI): 2.469-29.978; p = 0.001], glomerular filtration rate (OR: 0.961; 95% CI: 0.939-0.983; p = 0.001), IRA of LAD (OR: 7.325; 95% CI: 2.262-23.723; p = 0.001), SYNTAX score (OR: 1.422; 95% CI: 1.275-1.585; p < 0.001), NLR (OR: 1.156; 95% CI: 1.058-1.264; p = 0.001) and MHR (OR: 1.027 95% CI: 1.013-1.041; p < 0.001) were independent predictors of SSII > 34.2 (Table 3). ROC analysis was performed to determine the cutoff value of MHR to predict SSII > 34.2 and SS > 22 (Figure 2A, 2B) and the cutoff value of NLR to predict SSII > 34.2 (Figure 2A). The cutoff values of MHR and NLR to predict SSII > 34.2 were 12.2 [with a sensitivity of 75% and specificity of 70% (area under the curve (AUC): 0.766; p < 0.001)] and 6.8 [with a sensitivity of 67% and specificity of 61% (AUC: 0.618; p = 0.001)], respectively. The MHR ROC curve was significantly higher than that for NLR (difference between areas: 0.168; 95% CI: 0.087-0.248; p < 0.001). The cutoff value of MHR to predict SS > 22 was 13.9 with a sensitivity of 76% and specificity of 74% (AUC: 0.786; p < 0.001).
Table 3. Independent predictors of SS II > 34.2 in multivariate logistic regression analysis.
| Univariate (p value) | Univariate OR, 95% CI | Multivariate (p value) | Multivariate OR, 95% CI | |||||
| Diabetes mellitus % (n) | 0.007 | 2.027 | 1.211 | 3.392 | 0.001 | 8.604 | 2.469 | 29.978 |
| GFR, ml/min | 0.022 | 0.986 | 0.975 | 0.998 | 0.001 | 0.961 | 0.939 | 0.983 |
| IRA of LAD % (n) | < 0.001 | 6.500 | 3.759 | 11.239 | 0.001 | 7.325 | 2.262 | 23.723 |
| SYNTAX score | < 0.001 | 1.399 | 1.294 | 1.513 | < 0.001 | 1.422 | 1.275 | 1.585 |
| MHR | < 0.001 | 1.021 | 1.015 | 1.028 | < 0.001 | 1.027 | 1.013 | 1.041 |
| NLR | 0.001 | 1.092 | 1.036 | 1.151 | 0.001 | 1.156 | 1.058 | 1.264 |
CI, confidence interval; OR, odds ratio. Abbreviations are in Table 1.
Figure 2.
ROC graphics to detect the best cutoff value of MHR and NLR in the prediction of SSII > 34.2 (A) and MHR in prediction of SS > 22 (B). MHR, monocyte to HDL-C ratio; ROC, receiver-operating characteristic; SS, SYNTAX score; SSII, SYNTAX score II.
DISCUSSION
In this study, MHR was significantly associated with SS and SSII and it was an independent predictor for SSII > 34.2. Furthermore, MHR was correlated with SS and SSII, and this correlation was stronger in the IRA-LAD group than in the non-LAD group.
Previous studies have shown that inflammatory responses play an important role in the progression and destabilization of atherosclerosis and cardiovascular diseases. In patients with active inflammation, increased concentrations of mediators or markers of inflammation can be used to predict subsequent atherosclerotic cardiovascular disease.17 Monocytes and HDL particles are important keystones in the development and progression of atherosclerosis. During atherosclerotic plaque formation, blood monocytes are recruited into the intima and differentiate into foam cells by taking up oxidized LDL and other lipids.18 The circulating monocyte count has been reported to be a predictor of new plaque development1 and high plaque volume.2 In the current study, CRP level, and neutrophil and monocyte count were significantly higher in the high SSII group than in the low SSII group. HDL-C particles have been shown to have antiatherogenic properties by removing cholesterol from macrophages, exhibiting an antioxidant effect, protecting against thrombosis, and maintaining endothelial function and low blood viscosity through red blood cell deformability.3,19-21 Consistent with previous studies,3,22 we found that HDL-C levels were significantly lower in the high SSII group than in the low SSII group. In addition, levels of total cholesterol, LDL-C and triglycerides were significantly lower in the high SSII group, suggesting a high frequency of antihyperlipidemic drug use in the high SSII group.
The MHR is a newly developed inflammatory marker that is calculated as the ratio of monocyte count to HDL-C level. Some researchers have hypothesized that the MHR may be superior to monocyte count or HDL-C level alone in predicting the development and progression of atherosclerosis and hence coronary events. Kanbay et al. reported that a higher MHR was an independent predictor of major cardiovascular events during follow-up in patients with chronic kidney disease.4 In addition, Kundi et al. reported that MHR was independently and significantly related to SS in patients with stable CAD.5 Moreover, in a recent study, MHR was found to be independently and significantly related to short- and long-term mortality in STEMI patients treated with pPCI.6 In the present study, MHR was strongly correlated with SS and SSII, and there was a stronger correlation between MHR-SSII than MHR-SS. This finding is consistent with previous studies in which old age,23 COPD,24 PAD,25 heart failure,26 high SS27,28 and low CrCl29 were associated with a high inflammatory status and biomarkers. Correlations between MHR, SS and SSII were stronger in the IRA-LAD group than in the non-LAD group, possibly due to a high inflammatory status and large infarct size.30
We also investigated the associations between SS, SSII, NLR and CRP level, all of which were associated with the severity of CAD.27,28 We also observed that NLR but not CRP level was an independent predictors of high SSII. However, NLR was not correlated with SS, and the ROC curve for MHR was larger than that for NLR to predict SSII.
Limitations
The present study has several limitations. First, it has a cross-sectional design, and did not include prognostic information. Second, the number of patients was limited. Third, it focused on the association between MHR and severity of CAD as evaluated by only visual assessment of coronary angiograms. In addition, it did not include any further information about coronary atherosclerosis such as lumen area, plaque size, distribution, and composition. Further studies are needed to confirm whether MHR is superior to CRP level and NLR in predicting high SSII.
CONCLUSIONS
The present study showed that a high MHR was associated with increased severity of CAD, and that MHR and NLR were independent predictors of high SSII in STEMI patients treated with pPCI. Even though CRP level and NLR were higher in the SSII group, correlations between CRP level, NLR, SS and SSII were weaker than those between MHR, SS and SSII, and CRP was not an independent predictor of high SSII. In addition, NLR was not correlated with SS, and the ROC curve of MHR was greater than that of NLR. According to these results, we suggest that MHR could be a better parameter than NLR and CRP level in predicting the severity of CAD in STEMI patients treated with pPCI.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1.Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. 2012;217:476–482. doi: 10.1016/j.imbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Nozawa N, Hibi K, Endo M, et al. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J. 2010;74:1384–1391. doi: 10.1253/circj.cj-09-0779. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990;86:379. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46:1619–1625. doi: 10.1007/s11255-014-0730-1. [DOI] [PubMed] [Google Scholar]
- 5.Kundi H, Kiziltunc E, Cetin M, et al. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz. 2016;41:523–529. doi: 10.1007/s00059-015-4393-1. [DOI] [PubMed] [Google Scholar]
- 6.Çiçek G, Kundi H, Bozbay M, et al. The relationship between admission monocyte HDL-C ratio with short-term and long-term mortality among STEMI patients treated with successful primary PCI. Coronary Artery Dis. 2016;27:176–184. doi: 10.1097/MCA.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 8.Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 9.Campos CM, van Klaveren D, Iqbal J, et al. Predictive performance of SYNTAX score II in patients with left main and multivessel coronary artery disease. Circ J. 2014;78:1942–1949. doi: 10.1253/circj.cj-14-0204. [DOI] [PubMed] [Google Scholar]
- 10.Magro M, Nauta S, Simsek C, et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: The MI SYNTAX score study. Am Heart J. 2011;161:771–781. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Wang C, Zhang Y, et al. Usefulness of the SYNTAX score II to predict 1-year outcome in patients with primary percutaneous coronary intervention. Coron Artery Dis. 2016;27:483–489. doi: 10.1097/MCA.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 12.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 13.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 14.Van’t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiograhically evident thrombus in acute myocardial infarction – a TIMI 14 substudy. Circulation. 2001;103:2550–2554. doi: 10.1161/01.cir.103.21.2550. [DOI] [PubMed] [Google Scholar]
- 16.Schröder R. Prognostic impact of early ST-segment resolution in acute ST elevation myocardial infarction. Circulation. 2004;110:506–510. doi: 10.1161/01.CIR.0000147778.05979.E6. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 18.Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541–1551. doi: 10.1016/j.jacc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Rosenson RS, Brewer HB, Jr., Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamos TD, Rosenson RS. Low high density lipoprotein levels are associated with an elevated blood viscosity. Atherosclerosis. 1999;146:161. doi: 10.1016/s0021-9150(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 21.Shah PK, Kaul S, Nilsson J, Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part I. Circulation. 2001;104:2376–2383. doi: 10.1161/hc4401.098467. [DOI] [PubMed] [Google Scholar]
- 22.Aksakal E, Tanboga IH, Kurt M, et al. Predictors of coronary lesions complexity in patients with stable coronary artery disease. Angiology. 2013;64:304–309. doi: 10.1177/0003319712464815. [DOI] [PubMed] [Google Scholar]
- 23.Anuurad E, Enkhmaa B, Gungor Z, et al. Age as a modulator of inflammatory cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2151–2156. doi: 10.1161/ATVBAHA.111.232348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul T King. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med. 2015;4:26. doi: 10.1186/s40169-015-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen IC, Yu CC, Wu YH, Chao TH. Elevated neutrophil-to-lymphocyte ratio predicts intermediate-term outcomes in patients who have advanced chronic kidney disease with peripheral artery disease receiving percutaneous transluminal angioplasty. Acta Cardiol Sin. 2016;32:532–541. doi: 10.6515/ACS20150731D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueland T, Gullestad L, Nymo SH, et al. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta. 2015;443:71–77. doi: 10.1016/j.cca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi H, Momiyama Y, Ohmori R, et al. Associations of plasma C-reactive protein levels with the presence and extent of coronary stenosis in patients with stable coronary artery disease. Atherosclerosis. 2005;178:173–177. doi: 10.1016/j.atherosclerosis.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Ateş AH, Aytemir K, Koçyiğit D, et al. Association of neutrophil-to-lymphocyte ratio with the severity and morphology of coronary atherosclerotic plaques detected by multidetector computerized tomography. Acta Cardiol Sin. 2016;32:676–683. doi: 10.6515/ACS20160225A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shu HS, Tai YY, Chang KT, et al. Plasma high-sensitivity C-reactive protein level is associated with impaired estimated glomerular filtration rate in hypertensives. Acta Cardiol Sin. 2015;31:91–97. doi: 10.6515/ACS20140630C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim DS, Ahn Y, Kim YH, et al. The relationship among N-terminal Pro-B-type natriuretic peptide, high-sensitivity C-reactive protein and infarct size in patients with acute ST-elevation myocardial infarction. Korean Circ J. 2015;45:285–293. doi: 10.4070/kcj.2015.45.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]