Abstract
Background
Epicardial catheter ablation has been shown to be an effective strategy for treating ventricular arrhythmias (VA). We investigated the efficacy and safety from a tertiary referral center in Taiwan.
Methods
From 2010 to 2016, patients undergoing epicardial ablation for VAs were consecutively enrolled. The clinical characteristics, disease entity, electrophysiological studies, and ablation outcome were extracted for further analysis.
Results
A total of 80 patients were eligible, including 34 patients for arrhythmogenic right ventricular cardiomyopathy (ARVC), 16 for Brugada syndrome (BrS), 13 for idiopathic VAs, 11 for idiopathic dilated cardiomyopathy (IDCM), 2 for ischemic cardiomyopathy, and 4 for other nonischemic cardiomyopathies (NICM). Epicardial ablation rendering VAs non-inducible was achieved in 78 patients (97.5%). There were no procedure-related deaths. Major complications were reported in 8 (10.0%) patients, including an acute hemopericardium in 5 (6.3%), delayed tamponade in 1 (1.3%), hemothorax in 1 (1.3%), and major pericardial reaction in 1 (1.3%). Two (2.7%) patients died due to causes other than procedure-related deaths. After a mean follow-up of 31 ± 15 months, 20 patients (25.0%) presented with VA recurrences, including 13 with ARVC, 1 with BrS, 1 with idiopathic VAs, 4 with IDCM, and 1 with other NICM.
Conclusions
In this tertiary referral center’s experience, the complication rate of an epicardial approach was acceptable. Patients with NICM displayed a growing trend for a referral for epicardial ablation. The long-term follow-up demonstrated that an epicardial ablation for idiopathic VAs and BrS was associated with a better prognosis than that for the other etiologies.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Brugada syndrome, Complication, Epicardial ablation, Idiopathic dilated cardiomyopathy, Ventricular arrhythmia
INTRODUCTION
Since Sosa and colleagues first described the use of epicardial ablation to treat ventricular arrhythmias (VAs) in Chagas disease,1 the use of catheter ablation through a percutaneous pericardial access has been expanded to treat Vas in other diseases.2 The epicardial approach is supported by the fact that a combined endo-epicardial approach has been reported to yield a better prognosis for the ablation of ventricular tachycardia (VT) in patients with non-ischemic cardiomyopathy (NICM) and certain VAs occurring after a myocardial infarction.3-6 Furthermore, epicardial mapping and ablation have been proven to improve the acute and long-term outcomes with an acceptable risk of procedure-related complications in experienced and high-volume centers.7 However, the technical difficulties of a percutaneous pericardial access and ablation of surrounding epicardial vasculature structures or nerves, especially for unskilled operators, may contribute to serious and detrimental complications. Previous studies have reported an incidence rate of major complications ranging from 4.1-8.8%, including the common adverse event of a hemopericardium, followed by intraabdominal bleeding and epicardial vascular and phrenic nerve injuries.7-9
A multicenter study reported the safety and efficacy of a percutaneous pericardial access for radiofrequency catheter ablation (RFCA),9 however the findings may have been confounded by the experience of operators from different hospitals and diverse disease entities. The evolution of epicardial VA ablation and the associated outcomes have not previously been systemically investigated in Taiwan. Thus, the purpose of this study was to elucidate advances in epicardial VA ablation, associated disease entities, feasibility and safety of the epicardial procedures, and ablation outcomes during long-term follow-up in an experienced referral center in Taiwan.
METHODS
Patient selection
The present analysis included patients with drug-refractory VAs undergoing a percutaneous pericardial access and epicardial ablation for the secondary prevention of VAs from June 2010 to June 2016 at an experienced tertiary referral center in Taiwan. Structural heart diseases were assessed by echocardiography, cardiac magnetic resonance imaging (CMR), and invasive ventriculography and/or coronary artery angiography before the electrophysiology study. Both the right ventricular (RV) and left ventricular (LV) ejection fractions (EFs) were obtained from CMR and/or echocardiography. Complete 12-lead electrocardiography (ECG) and 24-hour Holter monitoring were performed prior to the ablation. An epicardial approach was performed for patients with failed endocardial ablation, those with electrocardiographic evidence supporting an epicardial origin,10-12 those with a disease entity favoring an epicardial substrate, and those with electroanatomic mapping supporting the existence of a diseased epicardial substrate.13-15 All percutaneous epicardial procedures were performed under general anesthesia. This study was conducted at the Taipei Veterans General Hospital in Taiwan, and was approved by the Institutional Review Board of the Taipei Veterans General Hospital (VGH-IRB No. 2014-10-004BC) and Department of Health, Taiwan. Written informed consent was obtained from all patients.
Electrophysiological study, mapping, and radiofrequency catheter ablation
After obtaining informed consent, we performed a standardized electrophysiological study with the patients in a fasting state under general anesthesia. All antiarrhythmic drugs were discontinued for a minimum of five half-lives before the RFCA (except for amiodarone). In the absence of any spontaneous VAs, rapid ventricular pacing and/or programmed stimulation of up to three extra-stimuli was performed from the RV apex and/or RV outflow tract (RVOT). If the VAs were not inducible, intravenous isoprenaline at 1-5 μg/min was infused to achieve at least a 20% increase in the heart rate. If spontaneous VAs were not inducible during pharmacological provocation, the induction protocol was repeated. The QRS morphologies of spontaneous and/or induced VAs were compared with the clinically documented VAs.
The localization of any arrhythmogenic foci or critical isthmuses was performed using a 3D mapping system (EnSiteNavXTM, St Jude Inc., St Paul, MN, USA or CARTO 3 MEM with UDM module, Biosense Webster, Diamond Bar, CA, USA). For idiopathic VAs, activation mapping, defined as the earliest local electrograms, and/or pace mapping were performed by comparing the 12-lead QRS morphology of paced premature ventricular complexes with the clinical VAs, aiming for a match of at least 11 of the 12 leads. For hemodynamically stable substrate VAs, activation mapping and entrainment mapping were performed to localize the critical isthmus within a scar zone, whereas a pace mapping and/or substrate-based modification strategy targeting late and fractionated electrograms within scar/low voltage zones during sinus rhythm or ventricular pacing, was used for unstable VAs.
Indications for an epicardial approach
In the present study, the patients were selected for an epicardial approach according to the features of the VAs, disease entity, substrate characteristics of the endocardial mapping, and the presence of intramural scarring by CMR.10,16-22 In brief, the epicardial approach was considered for patients with failed idiopathic VAs or those with an adequate endocardial approach for substrate VAs. In addition, the epicardial approach was performed for most patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) or Brugada syndrome (BrS) in whom arrhythmogenic substrates were predominantly located within the epicardium.
Percutaneous pericardial access: technique and management
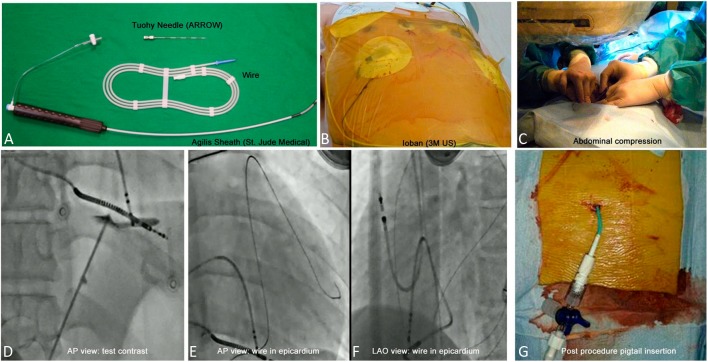
Pre-procedure subxiphoid echocardiography was performed routinely to avoid liver or gastric injuries. A subxiphoid puncture was performed in all cases according to the technique described by Sosa et al. (Figure 1).1 Access to the pericardium was achieved by using an 18 G Tuohy Needle (Arrow International, Inc., Reading, PA, USA) through the subxiphoid process with simultaneous epigastric compression of up to 3 to 4 cm deep. The anteroposterior projection was used to direct the access in the anterior/posterior plane, while the left anterior oblique (LAO) 60° projection was used to guide the needle leftward tangentially to the cardiac silhouette to prevent an RV puncture. After passing through the diaphragm, 1-2 cm3 of contrast was injected between the diaphragm and pericardium to observe tenting of the pericardial puncture. After posterior pericardial entry, a 0.032 guidewire was advanced to the left heart border in the LAO projection, and 10 cm3 of contrast was injected into the pericardial space through a soft-tip 5F dilator to allow for visualization of any adhesions. An 8-Fr Cordis Sheath was then inserted into the pericardial space. An Agilis Steerable Sheath (St. Jude Medical) was used at the discretion of the operator. Epicardial bleeding of > 80 cm3 from the initial puncture until the removal of the pericardial drain, intra-thoracic bleeding, intra-abdominal bleeding, major pericardial reaction, or delayed tamponade requiring further drainage were classified as major complications.8,9 After the procedure, the epicardial sheath was exchanged for a pigtail. Pericardial injections of hydrocortisone 100 mg and ketorolac tromethamine 30 mg were given immediately and 24 hours after the epicardial procedure through the pigtail to prevent any future epicardial adhesions or the development of pericarditis.
Figure 1.
An example of a percutaneous pericardial access and post-procedural drainage. (A) Epicardial puncture set; (B) 3M Ioban Incise Drapes on the subxiphoid area; (C) Abdominal compression was performed to avoid gastric or liver injury; (D) A slight contrast injection for testing under fluoroscopic guidance (AP view); (E) A 0.032” guidewire was inserted smoothly into the epicardial space under fluoroscopic monitoring; (F) A pigtail was inserted with an intrapericardial injection of steroid and non-steroidal anti-inflammatory drugs.
Follow-up
The patients were followed up at our cardiology outpatient clinic with 12-lead ECGs, 24-hour Holter recordings, and echocardiography after the RFCA every 3 months for the first year, and then 6 months thereafter. The patients who could not attend outpatient follow-up visits at our institution were contacted by telephone for any recurrent symptoms and recurrent arrhythmias. We also advised these patients to visit our affiliated institutions to complete follow-up screening, and then obtained their medical reports from these affiliated institutions. Recurrence was defined as the recurrence of any sustained VT or VF, non-sustained VT by implantable cardioverter defibrillator (ICD) interrogation, and the recurrence of premature ventricular complexes based on the findings of 24-hour Holter monitoring.23
Statistical analysis
All analyses were performed using SPSS statistical software, version 20.0 (IBM Corporation, Armonk, NY). The baseline patient characteristics were expressed as the mean ± standard deviation for continuous variables and as percentages for categorical variables. Continuous variables were analyzed using a two-tailed t-test, and discrete variables were compared using a chi-square test. Kaplan-Meier cumulative recurrence curves were plotted, and survival curves were compared using the log-rank test. Statistical significances was set at p < 0.05.
RESULTS
Study population
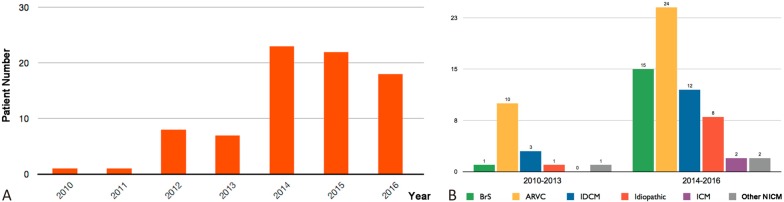
Data obtained from 80 of 989 patients (60 men; age, 45 ± 14 years; 8.1% of all VA ablation procedures) undergoing VA ablation procedures with a percutaneous pericardial access at Taipei Veterans General Hospital were extracted for further analysis (Table 1). Of the 80 patients, 34 (42.5%) had ARVC, 16 (20.0%) had BrS, 13 (16.3%) had idiopathic VAs, 11 (13.8%) had idiopathic dilated cardiomyopathy (IDCM), 2 (2.5%) had ischemic cardiomyopathy (ICM), and 4 (5.0%) had other NICM, including hypertrophic cardiomyopathy, post-pericarditis VT, congenital heart disease, and amyloidosis-related heart disease (Figure 2). The averaged LVEF was 52 ± 11%, however, 6 (7.5%) patients had an LVEF of < 30%. Sixty (75.0%) patients had an ICD. Figure 3A shows the annual distribution of the patients receiving epicardial procedures from 2010 to 2016. A significantly greater increase in the need for an epicardial approach was observed from 2014 to 2016 compared to that from 2010 to 2013 (53 vs. 17, p < 0.001, Figure 3B).
Table 1. Basic characteristics of patients undergoing epicardial approaches (N = 80).
| Clinical characteristics | |
| Age | 45 ± 14 |
| Sex (male, %) | 60 (75%) |
| Underlying diseases | |
| Hypertension | 18 (22.5%) |
| Diabetes mellitus | 7 (8.8%) |
| Heart failure | 18 (24.7%) |
| Dyslipidemia | 10 (12.5%) |
| Thyroid dysfunction | 2 (%) |
| ICD implantation | 60 (75.0%) |
| Structural assessment | |
| Left ventricular ejection fraction (%) | 52 ± 11 |
| Right ventricular ejection fraction (%) | 42 ± 10 |
| Clinical manifestations | |
| Syncope | 36 (45.0%) |
| Palpitation | 66 (82.5%) |
| Dyspnea | 28 (35.0%) |
| ICD shock or sudden cardiac arrest | 43 (53.8%) |
| Hemodynamic support during the procedure | 3 (3.8%) |
| Previous failed endocardial procedure | 42 (52.5%) |
ICD, implantable cardioverter defibrillator.
Figure 2.
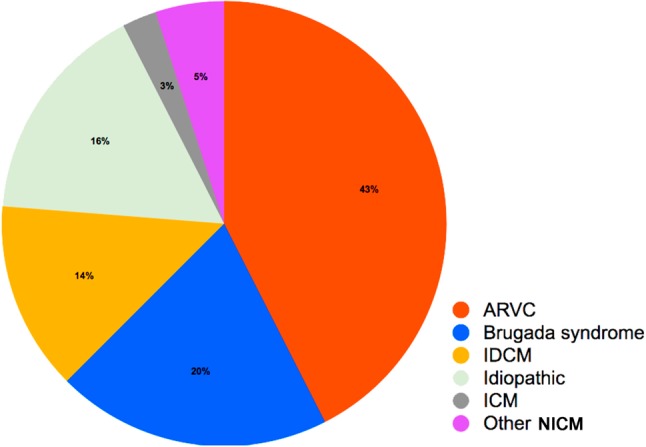
Disease entities in patients receiving an epicardial approach. The pie chart demonstrates the disease entities of the patients undergoing an epicardial procedure in the present study. Most of the patients in this study had ARVC. ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; ICM, ischemic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; NICM, non-ischemic cardiomyopathy; VA, ventricular arrhythmia.
Figure 3.
Annual distribution of epicardial procedures. (A) The annual distribution of the total number of patients undergoing epicardial procedures for VAs from 2010-2016. (B) The cumulative cases regarding different disease entities from 2010-2013 and 2014-2016. The abbreviations are as in Figure 2.
Ventricular arrhythmias, epicardial mapping, and ablation
Forty-three of the 80 patients (53.8%) received appropriate ICD interventions or an aborted sudden cardiac arrest. Forty-six (57.5%) patients presented with sustained VT, and ventricular fibrillation (VF) was documented by an ICD in 18 (22.5%) patients. The characteristics of the VAs are summarized in Figure 4.
Figure 4.
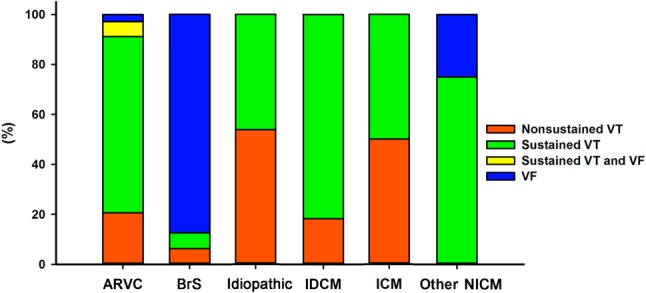
Characteristics of the ventricular arrhythmias. The clinical manifestations of the VAs, including ventricular fibrillation, sustained VT/VF, and non-sustained VT in the patients undergoing epicardial procedures. VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
The epicardial approach was attempted during the index procedure in 38 (47.5%) patients (Table 2), including 12 for BrS, 16 for ARVC, 1 for an idiopathic VA, 6 for IDCM, and 3 for other NICM. Epicardial ablation was not performed in 3 patients due to an inappropriate target based on the activation map or protected foci by adjacent coronary arteries. For idiopathic VAs, the arrhythmogenic targets were determined by both pace mapping and activation mapping in 8 patients (61.5%), activation only in 4 patients (30.8%), and pace mapping only in 1 patient (7.7%). For substrate VAs, activation mapping was achieved in 42 patients (62.7%), pace mapping in 53 patients (79.1%), and entrainment mapping in 40 patients (59.7%). All of the patients also received additional substrate modification after targeting the clinical VAs.
Table 2. Indication of epicardial ablation and procedure approach.
| Heart disease | Number | Previous failed endocardial ablation | Reasons for limited epicardial ablation | |
| BrS | 16 | 4 (25.0%) | 0 (0.0%) | |
| ARVC | 34 | 18 (52.9%) | 1 (2.9%) | Adjacent to RCA |
| Idiopathic | 13 | 12 (92.3%) | 2 (15.4%) | 1: No appropriate target by activation map |
| 1: Adjacent to LCA | ||||
| IDCM | 11 | 5 (45.5%) | 0 (0.0%) | |
| ICM | 2 | 2 (100.0%) | 0 (0.0%) | |
| Others | 4 | 1 (25.0%) | 0 (0.0%) | |
| Total | 80 | 42 (52.5%) | 3 (3.8%) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; ICM, ischemic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LCA, left coronary artery; NICM, nonischemic cardiomyopathy; RCA, right coronary artery.
Epicardial ablation acutely eliminated the VAs in all patients with idiopathic VAs, while acute procedural success was achieved in 65 (97.0%) patients with substrate VAs and partial success in 2 patients (3.0%). The details of the procedural parameters of the patients with ARVC and BrS are provided in Supplemental Table 1.
Supplemental Table 1. Procedure parameters of ARVC and BrS patients.
| ARVC (N = 34) | BrS (N = 16) | |
| Ablation time (min) | 34 ± 17 | 29 ± 12 |
| Ablation area (cm2) | ||
| Endocardial | 11 ± 7 | 2 ± 2 |
| Epicardial | 23 ± 16 | 16 ± 6 |
| Ablation site* | ||
| Endocardium | ||
| RVOT | 24 (70.6%) | 26 (76.5%) |
| TVA-superior | 5 (14.7%) | 9 (26.5%) |
| TVA-inferior | 5 (14.7%) | 11 (32.4%) |
| Basal-superior | 12 (35.3%) | 19 (55.9%) |
| Basal-inferior | 14 (41.2%) | 17 (50.0%) |
| Epicardium | ||
| RVOT | 7 (43.8%) | 16 (100.0%) |
| TVA-superior | 0 (0.0%) | 1 (6.3%) |
| TVA-inferior | 0 (0.0%) | 0 (0.0%) |
| Basal-superior | 0 (0.0%) | 1 (6.3%) |
| Basal-inferior | 0 (0.0%) | 0 (0.0%) |
* Right ventricle was segmented into distinct anatomic segments based on a previously described model.S1
ARVC, arrhythmogenic ventricular cardiomyopathy; BrS, Brugada syndrome; RVOT, right ventricular outflow tract; TVA, tricuspid valve annulus.
Reference
S1. Van Herendael H, Garcia F, Lin D, et al. Idiopathic right ventricular arrhythmias not arising from the outflow tract: prevalence, electrocardiographic characteristics, and outcome of catheter ablation. Heart Rhythm 2011;8:511-8.
Complications
There were no procedure-related deaths. Major peri-procedural complications were identified in 8 (10.0%) patients, including 5 with intrapericardial bleeding (> 80 cm3), 1 with intra-thoracic bleeding, 1 with a delayed tamponade requiring drainage, and 1 with a major pericardial reaction. All of the patients fully recovered within 72 hours after appropriate management. Intolerable chest pain immediately after the epicardial procedure requiring aggressive pain control was reported in 1 patient. Fourteen (17.5%) patients had minor complications, including an RV puncture in 6 patients (7.5%), subxiphoid hematoma requiring manual compression in 1 patients, prolonged chest pain for more than 3 days in 4 patients, and transient ST elevation without any evidence of coronary stenosis during the application of radiofrequency energy within the epicardium in 3 patients. Table 3 shows the summary of the major and minor periprocedural complications related to the percutaneous pericardial access.
Table 3. Acute and delayed major and minor complications related to epicardial approaches.
| Major complications | Numbers |
| Acute | |
| Intrapericardial bleeding (> 80 cm3) | 5 (6.3%) |
| Intra-thoracic bleeding | 1 (1.3%) |
| Intra-abdominal bleeding | 0 (0.0%) |
| Delayed (> 48 h) | |
| Major pericardial reaction | 1 (1.3%) |
| Delayed tamponade | 1 (1.3%) |
| Myocardial infarction | 0 (0.0%) |
| Total | 8 (10.0%) |
| Minor complications | |
| RV puncture without consequence | 6 (7.5%) |
| Subxyphoid hematoma | 1 (1.3%) |
| Prolonged pleuritic pain > 3 days | 4 (5.0%) |
| Transient acute coronary syndrome | 3 (3.8%) |
| Total | 14 (17.5%) |
RV, right ventricle.
Patient follow-up and recurrence of ventricular arrhythmias
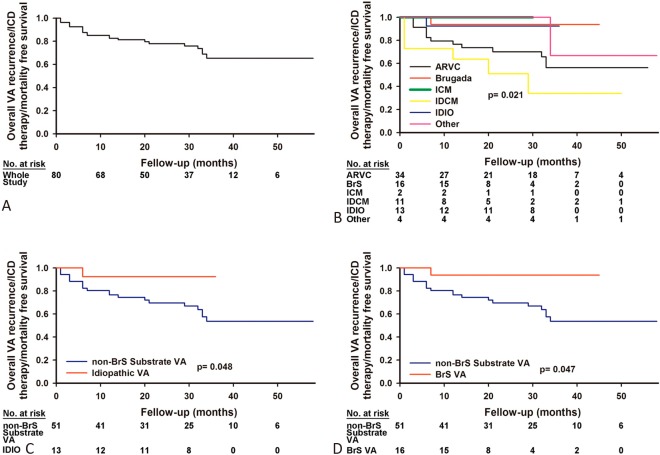
During a mean follow-up period of 31 ± 15 months (1-79 months), the recurrence of VAs and/or appropriate ICD therapy was documented in 20 (25.0%) patients, including 13 (38.3%) with ARVC, 1 (12.5%) with BrS, 1 (7.7%) with idiopathic VA, 4 (36.4%) with IDCM, and 1 (25.0%) with other NICM. Two patients with substrate VAs died due to non-cardiac diseases, 1 of whom due to a brain stem stroke secondary to atrial fibrillation, and the other due to a progressive hematologic disease despite standard chemotherapy. Figure 5A and 5B demonstrate Kaplan-Meier analysis of the endpoints, consisting of overall VA recurrence, ICD therapy, and mortality between the different disease entities. When comparing the endpoints of the substrate VAs rather than BrS, epicardial ablation of idiopathic VAs and BrS was associated with a better prognosis (log-rank p < 0.05, Figure 5C and 5D). There were no significant differences in the endpoints between the substrate VAs and BrS.
Figure 5.
Recurrence of VAs after epicardial procedures. (A) Kaplan-Meier survival curve for the events (overall VA recurrence/ICD therapy/mortality) in the total study population receiving an epicardial approach. (B) Kaplan-Meier survival curve for the events regarding different disease entities after an epicardial approach. (C) Kaplan-Meier survival curve for the events in the patients with idiopathic VAs and substrate VAs rather than BrS after epicardial ablation. (D) Kaplan-Meier survival curve for overall VA recurrence/ICD therapy/mortality: BrS vs. non-BrS substrate Vas. The abbreviations are as in Figure 2 and 4.
DISCUSSION
Major findings
The present study has several important findings. First, we reported our experience of epicardial RFCA for VAs from one tertiary referral center in Taiwan. There was a trend of a gradually increasing need for an epicardial approach for both idiopathic and substrate VAs. Second, the patients with ARVC and BrS had the most common diseases requiring an epicardial approach, in contrast to previous Western reports. Third, epicardial ablation provided an effective strategy with acceptable safety for VAs refractory to an endocardial approach. In addition, the endpoints were significantly lower in the patients with idiopathic VAs and BrS than in those with other substrate VAs.
The application of epicardial VA ablation
Since Sosa et al. first introduced the application of a percutaneous pericardial access for epicardial substrates in patients with Chagas disease in 1996,1 the application of an epicardial approach has expanded to other diseases. With the improvements in clinical outcomes, epicardial RFCA of VAs has been advocated as a first-line strategy in selected patients, such as those with ARVC, IDCM, ICM with transmural scarring, myocarditis, and BrS.3,7,17,19,24,25 However, even though epicardial study and ablation has promising ablation efficacy and has provided a better understanding of the arrhythmogenesis of diseased substrates,26-28 the procedure still carries a certain risk of catastrophic complications, even in experienced and high-volume centers. Therefore, the application of the epicardial procedure should be balanced between the benefits of procedural success and the risk of procedure-related complications. To the best of our knowledge, the present study is the first to report comparable efficacy and safety profiles in a relatively large sample to a multicenter study regarding the application of percutaneous pericardial access in an experienced tertiary referral center in Taiwan.9 In addition, idiopathic VAs that failed endocardial ablation and substrate VAs of BrS and ARVC comprised a significant proportion of the patients who received an epicardial approach. In contrast to the present findings, a previous European multicenter study demonstrated that ICM and IDCM were the most common diseases requiring epicardial ablation.7 These findings reflect a distinct pattern in the evolution of VA ablation and non-uniform disease characteristics between different ethnicities as previously reported.23
Regarding the ARVC group, a previous study reported that epicardial ablation resulted in freedom from the recurrence of VT in 64% and 45% of the patients at 1 and 5 years follow-up, respectively, which is consistent with our findings.21 In this study, epicardial mapping was performed only in the patients who were refractory to previous endocardial ablation. Furthermore, in our previous study, 42.5% (34 of 80) of the patients with ARVC required epicardial ablation to achieve non-inducibility of ventricular tachyarrhythmias, which is also consistent with a previous study.18 The overall acute success rate of catheter ablation for secondary prevention in a recent report of VT/VF in ARVC was 71-100%.29 Further studies to evaluate the efficacy of catheter ablation for primary or secondary prevention are warranted.
The requirement of an epicardial approach for ventricular tachyarrhythmias
The clinical clues used to identify the need for an epicardial approach rely on the features of the VAs, disease entity, substrate characteristics of the endocardial mapping, and presence of intramural scarring in CMR. In the past decade, the surface ECG of Vas has been used to provide initial information regarding the potential exit of the VT or extension of epicardial scarring.10,16-18,20,30 The need for and benefits of epicardial ablation have also been shown in certain diseases including ARVC and BrS.19,21,22 Therefore, an initial epicardial or combined endo-epicardial approach as a first-line strategy might be reasonable for ARVC and BrS, and this may also explain the distinct distribution of the patient population in the present study. Furthermore, recent studies have also demonstrated the predictive value of endocardial unipolar voltage mapping with electroanatomic mapping for the detection of epicardial diseased substrates,13,31 and this was also correlated with the scarring identified in CMR in an experimental model.32 This implies that incorporating unipolar endocardial electroanatomic mapping and late gadolinium enhancement may help in the delineation of the presence of epicardial arrhythmogenic substrates.
Periprocedural complications of an epicardial approach
In the present study, the incidence of epicardial procedure-related major complications was 10.0%, and that for minor complications was 17.5%, which is similar to prior single and multicenter studies.7,8 This supports the safety of the epicardial approach in our institute. However, it is important to note that these results were obtained from centers with an established program of epicardial VT ablation and in the presence of surgical backup to rapidly treat any complications. Epicardial mapping and ablation still carry the potential risk of cardiac and extra-cardiac side effects, and therefore it should be used with caution. This is supported by the reports of major complications of abdominal bleeding due to diaphragmatic vessel damage and non-Q-wave myocardial infarctions owing to ablation.33 Another possible cause of abdominal bleeding is a liver perforation. Thus, detailed preoperative evaluations by ECG, especially for patients with hepatomegaly or a congestive liver, may prevent the occurrence of any life-threatening complications.
Apart from these factors, a dry RV puncture has been reported to be a minor complication with an incidence of 4.5~7.5%,9,33 however this can be dramatically reduced after an appropriate learning curve. Post-procedural precordial pain was observed in every case in the present study. However, prolonged and intolerable chest pain were rare, which maybe due to the routine administration of intrapericardial steroids and non-steroidal anti-inflammatory drugs. In addition, phrenic nerve injuries and coronary artery damage can be avoided by high-intensity pacing and pre-ablation angiography.34,35 Evaluation of the distances between the ablation target and major branches of the coronary arteriesis also recommended. In spite of the detailed evaluations before attempting energy delivery, transient ST segment changes were noted in 3 patients during epicardial ablation, reflecting the complex reaction of ablation to coronary flow. The occurrence of a delayed tamponade re-emphasized the importance of sequential follow-up with ECG after each epicardial procedure.
Limitations
There are several limitations to the present study. First, the recurrence of idiopathic VAs and substrate VAs in patients without ICD implantation was assessed by Holter monitoring, and thus the recurrence of VAs may have been underestimated. Second, in spite of the bet-ter prognosis in the patients with BrS and idiopathic VAs requiring epicardial ablation, the clinical implication of an epicardial approach for different disease entities should be validated in larger cohort studies.
CONCLUSIONS
The need for an epicardial approach for VA ablation displayed a gradually increasing trend. Our results demonstrate the effectiveness of epicardial RFCA for idiopathic VAs that failed an endocardial approach for substrates of VAs with recurrence and/or appropriate ICD interventions with acceptable safety in an experienced referral center.
CONFLICTS AND INTEREST
None.
REFERENCES
- 1.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyle NG, Shivkumar K. Epicardial interventions in electrophysiology. Circulation. 2012;126:1752–1769. doi: 10.1161/CIRCULATIONAHA.111.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosa E, Scanavacca M, d’Avila A, et al. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35:1442–1449. doi: 10.1016/s0735-1097(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 4.Maury P, Escourrou G, Guilbeau C, et al. Histopathologic effects of endocardial and epicardial percutaneous radiofrequency catheter ablation in dilated nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2008;31:1218–1222. doi: 10.1111/j.1540-8159.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 5.Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Santangeli P, Zado ES, Supple GE, et al. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–1421. doi: 10.1161/CIRCEP.115.003562. [DOI] [PubMed] [Google Scholar]
- 7.Della Bella P, Brugada J, Zeppenfeld K, et al. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011;4:653–659. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 8.Tung R, Michowitz Y, Yu R, et al. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013;10:490–498. doi: 10.1016/j.hrthm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 10.Valles E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 11.Daniels DV, Lu YY, Morton JB, et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006;113:1659–1666. doi: 10.1161/CIRCULATIONAHA.105.611640. [DOI] [PubMed] [Google Scholar]
- 12.Lin CY, Chung FP, Lin YJ, et al. Radiofrequency catheter ablation of ventricular arrhythmias originating from the continuum between the aortic sinus of Valsalva and the left ventricular summit: electrocardiographic characteristics and correlative anatomy. Heart Rhythm. 2016;13:111–121. doi: 10.1016/j.hrthm.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:49–55. doi: 10.1161/CIRCEP.110.959957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 15.Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 16.Oloriz T, Silberbauer J, Maccabelli G, et al. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol. 2014;7:414–423. doi: 10.1161/CIRCEP.114.001568. [DOI] [PubMed] [Google Scholar]
- 17.Maccabelli G, Tsiachris D, Silberbauer J, et al. Imaging and epicardial substrate ablation of ventricular tachycardia in patients late after myocarditis. Europace. 2014;16:1363–1372. doi: 10.1093/europace/euu017. [DOI] [PubMed] [Google Scholar]
- 18.Lin CY, Lin YJ, Li CH, et al. Heterogeneous distribution of substrates between the endocardium and epicardium promotes ventricular fibrillation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Europace. 2017;[Epub ahead of print] doi: 10.1093/europace/euw393. [DOI] [PubMed] [Google Scholar]
- 19.Chung FP, Raharjo SB, Lin YJ, et al. A novel method to enhance phenotype, epicardial functional substrates, and ventricular tachyarrhythmias in Brugada syndrome. Heart Rhythm. 2017;14:508–517. doi: 10.1016/j.hrthm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Berruezo A, Mont L, Nava S, et al. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 21.Philips B, Madhavan S, James C, et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505. doi: 10.1161/CIRCEP.111.968677. [DOI] [PubMed] [Google Scholar]
- 22.Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4:478–485. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 23.Chung FP, Lin YJ, Chang SL, et al. Long-term follow-up of catheter ablation of ventricular arrhythmias: experiences from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2015;31:8–17. doi: 10.6515/ACS20140721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchlinski FE, Zado E, Dixit S, et al. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293–2298. doi: 10.1161/01.CIR.0000145154.02436.90. [DOI] [PubMed] [Google Scholar]
- 25.Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. Journal of the American College of Cardiology. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Ozturk MT, Ebinc FA, Okyay GU, Kutlugun AA. Epicardial adiposity is associated with microalbuminuria in patients with essential hypertension. Acta Cardiol Sin. 2017;33:74–80. doi: 10.6515/ACS20160418A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin E, Altin C, Sakallioglu O, et al. Epicardial adipose tissue thickness and carotid intima-media thickness in hemodialysis patients. Acta Cardiol Sin. 2017;33:266–272. doi: 10.6515/ACS20161023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramazan Oncel C, Kucuk M. The value of epicardial adipose tissue thickness for cardiovascular risk stratification in hypertensive patients. Acta Cardiol Sin. 2017;33:559. doi: 10.6515/ACS20170220C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrado D, Wichter T, Link MS, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan V, Bala R, Garcia FC, et al. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132–1139. doi: 10.1016/j.hrthm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Chung FP, Lin YJ, Chang SL, et al. Current and state of the art on the electrophysiologic characteristics and catheter ablation of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiol. 2015;65:441–450. doi: 10.1016/j.jjcc.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Tung R, Kim S, Yagishita D, et al. Scar voltage threshold determination using ex vivo magnetic resonance imaging integration in a porcine infarct model: influence of interelectrode distances and three-dimensional spatial effects of scar. Heart Rhythm. 2016;13:1993–2002. doi: 10.1016/j.hrthm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Sosa E, Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. J Cardiovasc Electrophysiol. 2005;16:449–452. doi: 10.1046/j.1540-8167.2005.40710.x. [DOI] [PubMed] [Google Scholar]
- 34.Bai R, Patel D, Di Biase L, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–948. doi: 10.1111/j.1540-8167.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 35.Roberts-Thomson KC, Steven D, Seiler J, et al. Coronary artery injury due to catheter ablation in adults: presentations and outcomes. Circulation. 2009;120:1465–1473. doi: 10.1161/CIRCULATIONAHA.109.870790. [DOI] [PubMed] [Google Scholar]