Abstract
Systemic lupus erythematosus (SLE) is associated with a variety of inflammatory processes that can affect the lymph nodes, brain, kidneys, and spleen. We present two patients with SLE in whom SLE-associated conditions complicated interpretation of 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) imaging of the lymph nodes and the spleen. The imaging findings mimicked lymphoma, but histopathological evaluation showed benign processes including reactive follicular hyperplasia in the lymph nodes, Kikuchi-Fujimoto disease in perisplenic lymph nodes, and inflammatory changes and lymphoid hyperplasia in the spleen.
Keywords: Systemic lupus erythematosus, SLE, Lymphoma, Kikuchi-Fujimoto disease, Spleen, PET/CT
Introduction
Systemic lupus erythematosus (SLE) is an immune complex disease of unknown etiology, which causes excessive production of autoantibodies to components of the cell nucleus. The formation and deposition of immune complexes leads to inflammatory responses that are significant contributors to the pathogenic mechanisms resulting in tissue damage, specifically in the lymph nodes, brain, kidneys and spleen [1]. Lymph nodes are commonly enlarged in patients with active SLE. On histological evaluation, the involved lymph nodes show varying degrees of coagulative necrosis with hematoxyphil bodies or reactive follicular hyperplasia associated with increased vascularity [2–4]. Kikuchi-Fujimoto disease (KFD) is a histiocytic necrotizing lymphadenitis that is found mainly in young women and may be a self-limiting form of SLE, based on similarities in microscopic features. About 35 cases of KFD associated with SLE have been reported in the literature so far [5, 6].
18F-Fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) imaging has been described only in a few SLE patients in the literature. Increased 18F-FDG uptake in lymph nodes affected by SLE has been described and is a known potential mimic of lymphoma [7]. Although 18F-FDG uptake in lymph nodes affected by KFD has been described in a few patients [8, 9], abnormal 18F-FDG uptake in an SLE patient with KFD has not been previously described. Abnormal 18F-FDG uptake in the spleen of SLE patients has also not been described in the literature. We present 18F-FDG PET/CT imaging findings in two SLE patients.
Case reports
Patient 1
A 26-year-old woman presented with a 3-month history of intense fatigue, diffuse myalgia, headaches, palpable cervical lymph nodes and pancytopenia. A bone marrow biopsy did not show any abnormalities. She was referred for an 18F-FDG PET/CT scan for the evaluation of possible lymphoma. Maximum intensity projection (MIP) images showed diffuse mildly 18F-FDG-avid lymphadenopathy throughout the body. The most metabolically active foci were a 1-cm node (5) in the aortopulmonary window with a maximum standardized uptake value (SUVmax) of 4.3, a 0.8-cm right paratracheal node (4R) with SUVmax 3.6 and a 1-cm subcarinal lymph node (7) with SUVmax 3.1. There were multiple foci of more intense 18F-FDG uptake in the splenic hilum (SUVmax 6.2) which were concerning for lymphoma. There was also diffusely increased uptake in the spleen with SUVmax 4.6, which was also concerning for malignant involvement (Figs. 1 and 2). The patient had a mediastinoscopy, and histological evaluation of a right lower paratracheal node (4R) and a subcarinal node (7) showed anthracosis, follicular hyperplasia and sinus histiocytosis compatible with SLE, and no evidence of malignancy.
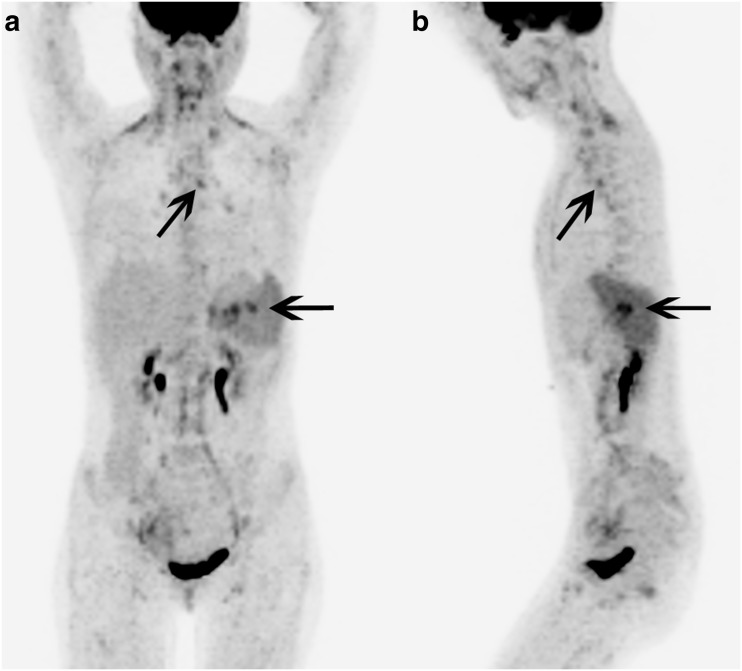
Fig. 1.
Patient 1. 18F-FDG PET/CT maximum intensity projection (a) anterior and (b) left lateral images show diffuse mildly 18F-FDG avid lymphadenopathy with several foci of 18F-FDG uptake in the AP window (diagonal arrows), and the right lower paratracheal and subcarinal regions, as well as several foci of intense 18F-FDG uptake in the region of the splenic hilum (left-pointing arrows). Histological evaluation of these areas confirmed Kikuchi-Fujimoto disease. There is also diffusely increased 18F-FDG uptake in the entire spleen
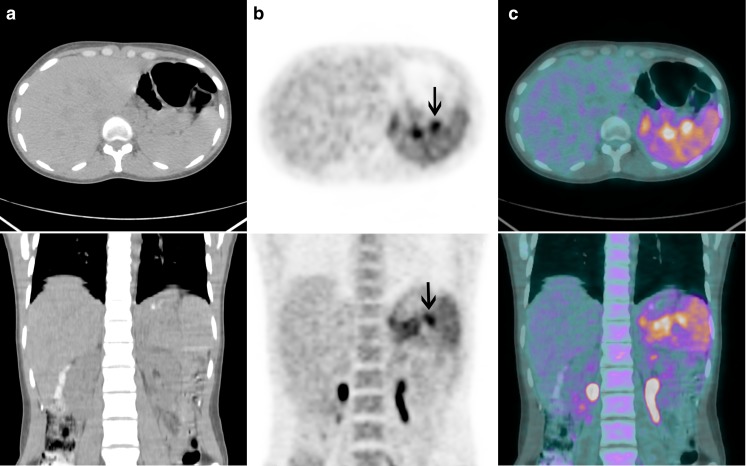
Fig. 2.
Patient 1. Transaxial and coronal (a) CT, (b) PET and (c) PET/CT fusion images show intensely 18F-FDG-avid lymph nodes in the splenic hilum (arrows) and diffusely increased 18F-FDG uptake in the spleen
The patient underwent splenectomy 2 weeks following the PET/CT scan. Sectioning of four splenic hilar lymph nodes revealed a partly distorted architecture. There were large areas of coagulative necrosis with abundant karyorrhexic debris and basophilic substance having the appearance of hematoxyphil bodies surrounded by a variety of cells including histiocytes, plasmacytoid monocytes, immunoblasts and some small and large lymphocytes. A few residual hyperplastic lymphoid follicles were seen. The findings were consistent with KFD (Fig. 3). Sectioning of the spleen showed splenic tissue whose architecture was partly altered by a variable expansion of white pulp with small to medium sized cells with mildly irregular nuclei and mixed with scattered large cells. The splenic sinuses were dilated and contained macrophages, some of whose cytoplasm was filled with leukocytes having pyknotic and karyorrhexic nuclei. Mildly hyperplastic germinal centers were identified. Multiple blood vessels showed infiltration by lymphocytes and plasma cells producing vasculitis-like changes with mildly irregular cells in the subendothelium.
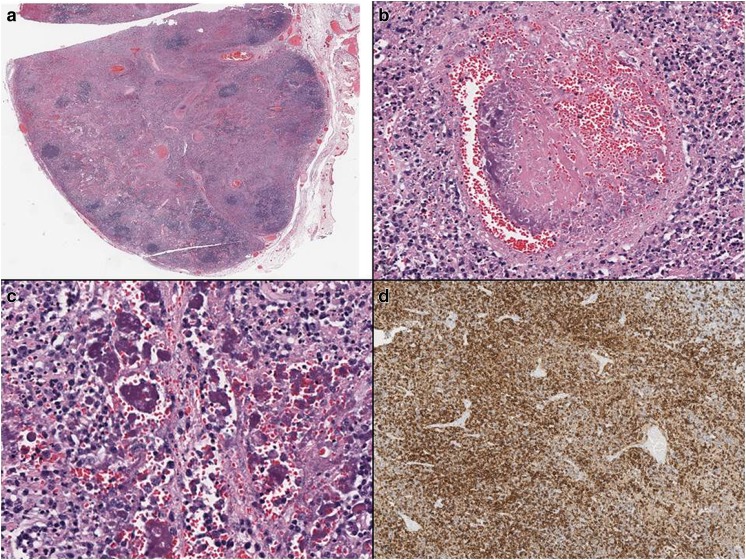
Fig. 3.
Patient 1. a Immunohistochemical evaluation of a splenic hilar lymph node showing overall preserved architecture and multiple well-circumscribed areas of necrosis. b Necrosis of a small blood vessel with hematoxyphil bodies in the blood vessel wall. c Multiple hematoxyphil bodies in the sinuses and abundant karyorrhexis. d CD8 positivity in a majority of the lymphocytes. The findings were consistent with Kikuchi-Fujimoto disease
The splenic findings were identified as atypical lymphoid hyperplasia with vascular (predominantly subendothelial) infiltration by lymphoid cells, most consistent with an immune disorder and with SLE. There was no evidence of lymphoma in the histology. The patient’s serum laboratory values obtained at the time of the PET/CT scan also corresponded to a diagnosis of SLE: parvovirus B19 IgG-positive (IgM-negative), antihistone antibody-positive, antinuclear antibody (ANA) screen positive at 1:160 dilution, anti-DNA antibody >1,000 kIU/L (normal <30 kIU/L). The serum was negative for anti-SSA, anti-SSB, anti-Smith, anti-RNP, anti-Jo-1, anti-SCL-70, anti-smooth muscle, anti-mitochondrial cell, anti-parietal cell, anti-cardiolipin, HIV, hepatitis B and C, and cytomegalovirus (CMV).
Patient 2
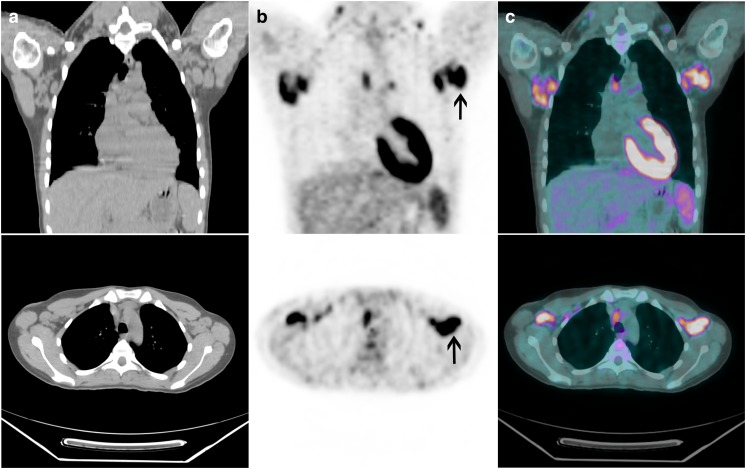
A 24-year-old woman with a 4-year history of SLE originally diagnosed after presenting with malar rash, severe polyarthritis, palpable lower extremity purpura and positive antinuclear and anti-DNA antibodies, now presented with recurrent high fever (up to 39°C), myalgia, and enlarged lymph nodes (cervical, axillary, and inguinal). She was referred for an 18F-FDG PET/CT scan to rule out lymphoma. MIP images showed diffuse and extensive intensely 18F-FDG-avid lymphadenopathy throughout the body with the most intense 18F-FDG uptake in a 3.1-cm left axillary lymph node with SUVmax 6.8, which was concerning for lymphoma. There was also diffusely increased uptake in the spleen with SUVmax 4.1, which was concerning for malignant involvement (Figs. 4 and 5).
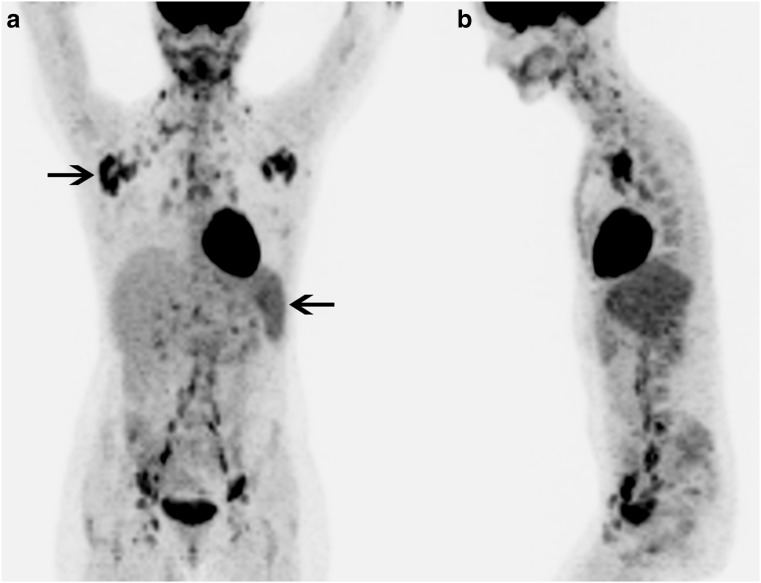
Fig. 4.
Patient 2. 18F-FDG PET/CT MIP (a) anterior and (b) left lateral images show diffuse intensely 18F-FDG-avid lymphadenopathy throughout the body, with the most intense 18F-FDG avidity in the axillae (right-pointing arrow). There is also diffusely increased 18F-FDG uptake in the entire spleen (left-pointing arrow)
Fig. 5.
Patient 2. Coronal and transaxial (a) CT, (b) PET and (c) PET/CT fusion images show intensely 18F-FDG avid axillary lymph nodes (arrows). Histological evaluation of these nodes confirmed reactive follicular hyperplasia
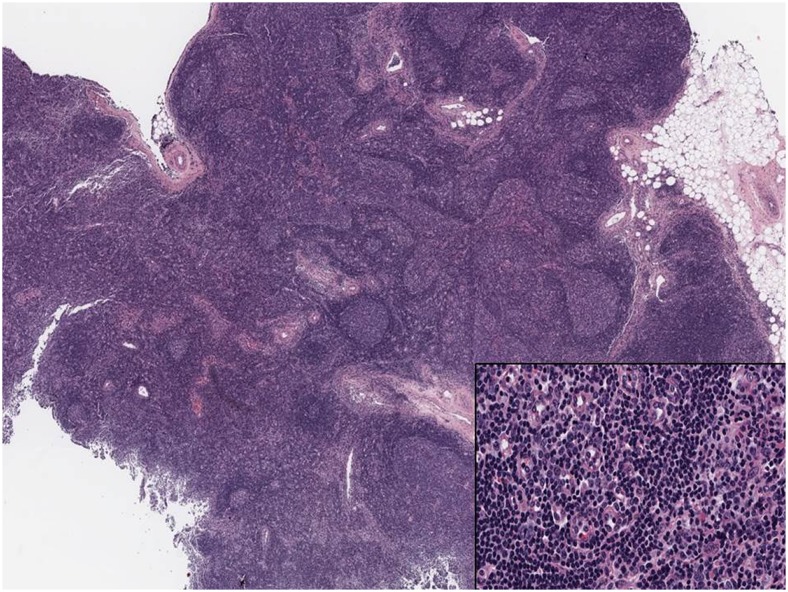
Biopsy and histological evaluation of the 3.1-cm left axillary node showed a reactive lymphocyte population identified as reactive follicular hyperplasia, with no evidence of lymphoma (Fig. 6). Biopsy and histological evaluation of the spleen showed lymphoid hyperplasia and inflammatory changes consistent with SLE, with no evidence of lymphoma. Serum laboratory tests done at the time of the PET/CT scan showed positivity for anti-DNA antibody 623 kIU/L (normal <30 kIU/L), ANA speckled at 1:160 dilution, anti-cardiolipin IgM 35 MPL U/mL (normal range 5–15 MPL U/mL; IgG was 9 U/mL and within the normal range), anti-SSA 165 U/mL (normal range 0–20 U/mL), anti-SSB 117 U/mL, anti-Smith 190 U/mL, anti-RNP 228 U/mL, erythrocyte sedimentation rate 56 mm/h (normal range 0–10 mm/h), and C-reactive protein 4.5 mg/L (normal range 0–5.0 mg/L).
Fig. 6.
Patient 2. Immunohistochemical evaluation of a left axillary lymph node showing follicular hyperplasia with enlarged germinal centers, intact mantle zones and follicles of variable size and shape. Inset: High-power view of the germinal center with multiple tingible body macrophages and apoptotic bodies. There was no evidence of lymphoma
Discussion
SLE is a multisystem disorder that is associated with localized or generalized lymphadenopathy at some stage in the evolution of the disease in up to 60% of patients. SLE patients also have an increased risk of developing non-Hodgkin lymphoma [2]. SLE lymph nodes contain varying degrees of coagulative necrosis with hematoxyphil bodies, or reactive follicular hyperplasia. Reactive follicular hyperplasia was considered to be a nonspecific change and received little attention in the literature, although recent studies have shown a rather wide variety of identifiable histological patterns, one of which is similar to Castleman disease [3, 4]. The differences between the histological subtypes of SLE reactive follicular hyperplasia may affect the 18F-FDG avidity of the affected lymph nodes. A few patients with increased 18F-FDG uptake in SLE-associated lymphadenopathy have been described in the literature [7], although the role of 18F-FDG PET/CT imaging in SLE patients has never been investigated. In our case series, patient 1 showed mild 18F-FDG uptake in her SLE-associated lymphadenopathy while patient 2 showed much more intense 18F-FDG uptake in her SLE-associated lymphadenopathy.
KFD is a histiocytic necrotizing lymphadenitis first described in Japanese women by Kikuchi [5] and Fujimoto et al. [6] in 1972, but later described worldwide. Patients usually present with enlarged cervical lymph nodes and fever. The course of KFD is almost always benign with excellent outcomes and spontaneous resolution between 1 and 4 months. The etiology of KFD is unknown, but several infectious agents have been proposed as the causative agents of the disease that initiate a hyperimmune response of T cells and histiocytes including Epstein-Barr virus, HTLV-1, HHV-6, toxoplasma, parvovirus B19, CMV, Brucella, Yersinia enterocolitica, and parainfluenza virus. An autoimmune origin and association with SLE has also been suggested due to a number of patients in whom SLE was diagnosed before, simultaneously with, or after KFD. Approximately 35 patients with KFD associated with SLE have been reported in the literature [10–15]. Increased 18F-FDG uptake in lymph nodes involved by KFD has been found in a few patients [8, 9], however abnormal 18F-FDG uptake in SLE patients with KFD has not been previously reported.
SLE can cause numerous inflammatory changes as well as lymphoid hyperplasia within the spleen [16]. Abnormal diffusely increased 18F-FDG uptake in the spleen of SLE patients (as seen in both of our patients) has not been previously reported in the literature, and is a potential pitfall in the evaluation of malignancy such as lymphoma, especially since diffusely increased 18F-FDG uptake in the spleen typically represents disease involvement in lymphoma [17]. The etiology of the increased 18F-FDG uptake in the spleen of our patients, whether due to inflammatory changes or lymphoid hyperplasia typical of SLE, remains unknown. The abnormal 18F-FDG uptake seen in the lymph nodes and spleen of both of our SLE patients represents a significant challenge in the evaluation and interpretation of PET/CT scans for the presence of a malignancy such as lymphoma and further investigation of PET/CT findings in SLE patients and their clinical significance is warranted.
Compliance with Ethical Standards
Conflict of interest
William Makis, Anthony Ciarallo, Milene Gonzalez-Verdecia and Stephan Probst declare that they have no conflict of interest.
Ethical statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco R, McLaren B, Davis B, Steele P, Smith R. Systemic lupus erythematosus-associated lymphoproliferative disorder: report of a case and discussion in light of the literature. Hum Pathol. 1997;28:980–985. doi: 10.1016/S0046-8177(97)90015-0. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Nakamura S, Morishita Y, Itoh H, Yoshida K, Ohno Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: a clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000;50:304–312. doi: 10.1046/j.1440-1827.2000.01052.x. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Motoori T, Asano S, Nakamura S. Histological diversity of reactive and atypical proliferative lymph node lesions in systemic lupus erythematosus patients. Pathol Res Pract. 2007;203:423–431. doi: 10.1016/j.prp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes. Acta Hematol Jpn. 1972;35:379–380. [Google Scholar]
- 6.Fujimoto Y, Kojima Y, Yamaguchi K. Cervical subacute necrotizing lymphadenitis. Naika. 1972;20:920–927. [Google Scholar]
- 7.Curiel R, Akin EA, Beaulieu G, Depalma L, Hashefi M. PET/CT imaging in systemic lupus erythematosus. Ann NY Acad Sci. 2011;1228:71–80. doi: 10.1111/j.1749-6632.2011.06076.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Hyun OJ, Yoo IR, Kim SH, Sohn HS, Chung SK. Kikuchi disease mimicking malignant lymphoma on FDG PET/CT. Clin Nucl Med. 2007;32:711–712. doi: 10.1097/RLU.0b013e3181250346. [DOI] [PubMed] [Google Scholar]
- 9.Tsujikawa T, Tsuchida T, Imamura Y, Kobayashi M, Asahi S, Shimizu K, et al. Kikuchi-Fujimoto disease. PET/CT assessment of a rare cause of cervical lymphadenopathy. Clin Nucl Med. 2011;36:661–664. doi: 10.1097/RLU.0b013e31821a2878. [DOI] [PubMed] [Google Scholar]
- 10.Quintas-Cardama A, Fraga M, Cozzi SN, Caparrini A, Maceiras F, Forteza J. Fatal Kikuchi-Fujimoto disease: the lupus connection. Ann Hematol. 2003;82:186–188. doi: 10.1007/s00277-003-0773-3. [DOI] [PubMed] [Google Scholar]
- 11.el-Ramahi KM, Karrar A, Ali MA. Kikuchi disease and its association with systemic lupus erythematosus. Lupus. 1994;3:409–411. doi: 10.1177/096120339400300508. [DOI] [PubMed] [Google Scholar]
- 12.Santana A, Lessa B, Galrao L, Lima I, Santiago M. Kikuchi-Fujimoto’s disease associated with systemic lupus erythematosus: case report and review of the literature. Clin Rheumatol. 2005;24:60–63. doi: 10.1007/s10067-004-0923-6. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Kuo TT, Hong HS. Lupus lymphadenitis simulating Kikuchi’s lymphadenitis in patients with systemic lupus erythematosus: a clinicopathological analysis of six cases and review of literature. Pathol Int. 2003;53:221–226. doi: 10.1046/j.1320-5463.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 14.Lopez C, Oliver M, Olavarria R, Sarabia MA, Chopite M. Kikuchi-Fujimoto necrotizing lymphadenitis associated with cutaneous lupus erythematosus: a case report. Am J Dermatopathol. 2000;22:328–333. doi: 10.1097/00000372-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Vazquez C, Hughes G, Bordon J, Alonso-Alonso J, Anibarro-Garcia A, Martinez E, et al. Histiocytic necrotizing lymphadenitis, Kikuchi-Fujimoto’s disease, associated with systemic lupus erythematosus. QJM. 1997;90:531–533. doi: 10.1093/qjmed/90.8.531. [DOI] [PubMed] [Google Scholar]
- 16.Burnett R, Ravel G, Descotes J. Clinical and histopathological progression of lesions in lupus-prone (NZB x NZW) F1 mice. Exp Toxicol Pathol. 2004;56:37–44. doi: 10.1016/j.etp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Salaun PY, Gastinne T, Bodet-Milin C, Campion L, Cambefort P, Moreau A, et al. Analysis of 18F-FDG PET diffuse bone marrow uptake and splenic uptake in staging of Hodgkin’s lymphoma: a reflection of disease infiltration or just inflammation? Eur J Nucl Med Mol Imaging. 2009;36:1813–1821. doi: 10.1007/s00259-009-1183-0. [DOI] [PubMed] [Google Scholar]