Abstract
Purpose
Herein, we report characteristics of 18F–fluorodeoxyglucose (FDG) uptake in abdominal aortic aneurysms (AAAs) during a long-term follow-up. In addition, we investigated the association between FDG uptake and the physician decision to perform an intervention.
Methods
We performed a retrospective review of 42 patients with AAAs who underwent FDG positron emission tomography (PET)/computed tomography (CT). The size of the AAA was measured in serial CT or PET/CT images. The long-term growth rate of AAAs was calculated by linear regression of the size change. Maximal SUV of the AAA (SUVAAA) and mean SUV of the blood pool (SUVBlood) were measured in PET/CT fusion images. To assess the FDG uptake of AAAs, the target-to-background ratio (TBR) was defined as the ratio of SUVAAA to SUVBlood. We compared FDG uptake of AAAs with the long-term growth rate of AAAs and clinical data.
Results
TBR was not significantly different between patients with and without significant growth (1.55 ± 0.20 vs. 1.57 ± 0.14; P = 0.5599). However, in patients with significant growth, TBR exhibited a significant positive correlation with the growth rate (r 2 = 0.2601, P = 0.0306). TBR also exhibited a significant difference between patients with and without intervention (P = 0.0228).
Conclusion
FDG uptake of AAA is associated with long-term growth of AAAs in a specified group that exhibits growth. FDG PET/CT may only be effective in predicting the long-term growth of AAAs in specific subgroups of patients. It is also suggested that FDG PET is potentially related to the clinical conditions of AAA patients who need surgical or interventional treatment.
Keywords: FDG, PET/CT, Abdominal aortic aneurysm, Intervention
Introduction
Abdominal aortic aneurysm (AAA) is one of the most common vascular diseases and can result in the fatal consequence of aortic rupture. The prevalence of AAA is estimated to be approximately 4–9% in the general population [1]. Although surgical and intravascular interventions are curative treatment methods, it was reported that the perioperative mortality of endovascular aorta repair (EVAR) is 1.6% and that of aorto-femoral bypass graft surgery (AFB) is 5.2% [2]. Thus, it is of critical importance to determine an optimal indication for surgical or interventional treatment of AAA. Several criteria have been suggested for predicting the growth of AAA and selecting patients who need treatment. Currently, the most widely used criteria are size larger than 5.5 cm or rapid expansion with a growth rate of more than 1 cm/year in case of asymptomatic AAA [3].
Computed tomography (CT) is the most commonly used imaging modality to assess AAA. CT provides high resolution for vascular structures with high image contrast, and three-dimensional reconstruction of CT visualizes the detailed structure of AAAs. Therefore, serial CT examination is essential in AAA to evaluate size change and to determine the need of elective surgery. In addition, there have been several attempts to evaluate aortic wall stress or strain using specific three-dimensional reconstruction methods for predicting AAA expansion on contrast-enhanced CT [4–6]. However, size-based criteria are of limited performance to predict sudden aortic rupture [7].
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is an imaging modality for metabolic activity, and it can be used not only in malignant but also in inflammatory diseases [8]. FDG PET has been reportedly useful in diagnosing various vascular diseases caused by inflammation [9–11]. Because inflammation is one of the key processes in the expansion of AAA [12], the relationship between FDG uptake and AAA has been investigated in several studies. However, the results from these studies are not consistent; while it was reported that increased FDG uptake was associated with expansion of AAAs [13], contrarily FDG uptake being lower in the group with size-increasing AAAs was also reported [14]. In terms of intervention, there was no difference in FDG uptake between repaired and non-repaired AAAs [15].
The purpose of this study was to investigate characteristics of FDG uptake in AAA patients under long-term follow-up to evaluate the efficacy of FDG PET in the management of AAA. Additionally, FDG PET/CT findings were analyzed with regard to the clinically made decisions of surgical or interventional treatment for AAAs.
Materials and Methods
Patients
Among patients who underwent FDG PET/CT for cancer evaluation at our institution between March 2006 and December 2015, those who were diagnosed with AAA at the time of FDG PET/CT or during follow-up were retrospectively retrieved. The inclusion criteria were (1) clinically confirmed diagnosis of AAA, (2) no surgical treatment for AAA such as AFB or EVAR before FDG PET/CT, and (3) follow-up after FDG PET/CT longer than 1 month, with contrast-enhanced CT covering the abdominal aorta more than one time. Medical records regarding AAA were reviewed on the medical information system.
Image Acquisition
FDG PET/CT scans were performed as previously described [16]. Briefly, a patient fasted at least 6 h, and FDG (5.18 MBq/kg) was injected intravenously. Sixty minutes after injection, PET/CT images were acquired using dedicated PET/CT scanners (Biograph 40, Siemens Healthcare, Knoxvillle, TN, USA, or Gemini, Philips Healthcare, Cleveland, OH, USA) from the skull base to the proximal thigh levels. CT images for attenuation correction and lesion localization were obtained first, and PET images were obtained subsequently. PET images were reconstructed using iterative algorithms (ordered subsets-expectation maximization; 2 iterations), with matrix size of 200 × 200 and voxel size of 2.6 × 2.6 × 2.5 mm3.
Image Analysis
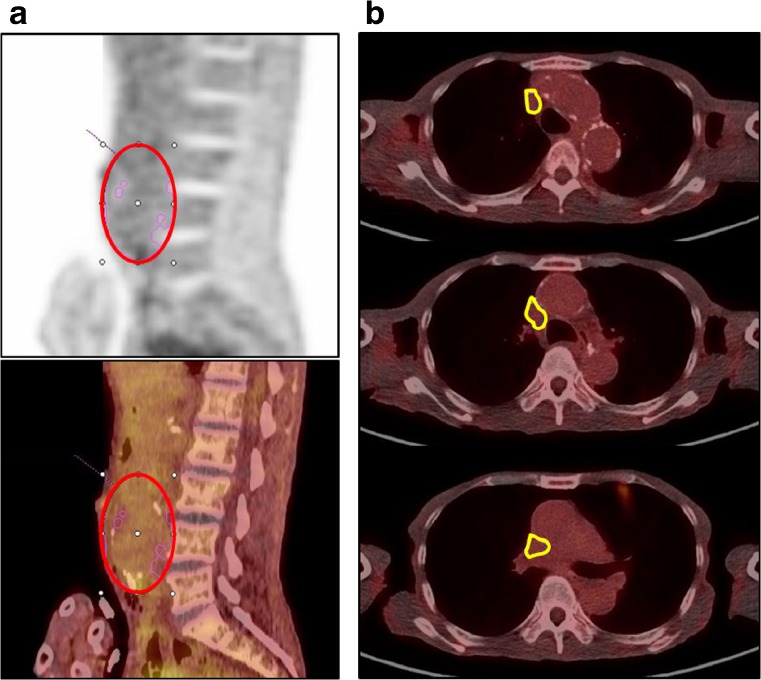
On FDG PET/CT images, an ellipsoidal volume of interest (VOI) was placed on the abdominal aorta to encircle the entire extent of AAAs. The standardized uptake value (SUV) was measured in the VOI by using a commercial software package (Syngo.via, Siemens Healthcare, Knoxvillle, TN, USA) (Fig. 1a). Blood pool activity was measured in the superior vena cava as background activity; a region of interest (ROI) was manually drawn for the superior vena cava on each slice of PET/CT fusion images from the root of the brachiocephalic vein to the cavo-atrial junction levels. The ROIs were stacked to make a VOI in which the mean SUV was measured (Fig. 1b). A target-to-background uptake ratio (TBR) of an AAA was defined as the ratio between the maximal SUV of AAAs and mean SUV of the superior vena cava. When two or more FDG PET/CT scans were performed in a patient, the first PET/CT that demonstrated the AAA was used for analysis.
Fig. 1.
a Measurement method for AAA. An ellipsoidal VOI was placed over the abdominal aorta including all lesions of the AAA, excluding the anatomical structures of physiological uptake (red circle). Maximal SUV of the AAA was obtained from this VOI. b Measurement method for the SVC. ROIs were drawn manually on each slice of the PET/CT image to cover the SVC from the root of the brachiocephalic vein to the cavo-atrial junction to make a stacked VOI (yellow contour). Mean SUV was obtained from this VOI
Follow-Up and Clinical Outcome
On FDG PET/CT or contrast-enhanced CT, the maximal diameter of an AAA was measured perpendicularly to the long axis of the AAA. The average growth rate of an AAA was calculated by linear regression of sizes measured on serial FDG PET/CT or contrast CT; the slope of linear regression was defined as the average growth rate of the AAA. In case a significant positive correlation was determined on the linear regression of sizes, it was defined as a significant growth of the AAA.
In our institution, intervention for an AAA was determined based on the risk of abdominal aortic aneurysm [17]. EVAR was preferred to AFB for patients at high risk for open surgery such as elderly patients or those with relatively lower risk of rupture based on the surgeon’s perspective. The treatment option was chosen by the surgeons, irrespective of the FDG PET/CT findings. The medical history of the interventions for AAAs was obtained from our medical information system.
Statistical Analysis
Correlation of the average growth rate with the TBR was evaluated using the linear regression coefficient. The Mann-Whitney test was used to evaluate the significance of differences between the two groups. A P-value less than 0.05 was considered statistically significant. Statistical analysis was performed using a statistical software package (Graphpad Prism Version 5, San Diego, CA, USA).
Results
Patients
Of 69,757 patients who underwent FDG PET/CT for oncological evaluation during the study period, 42 were diagnosed with AAA. Among them, 5 patients were excluded because of the short follow-up period, and finally 37 patients were included in the analysis. Demographic characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of patients and lesions
| Characteristics | Value |
|---|---|
| Total number of patients | 37 |
| Gender, n | |
| Female | 6 (16%) |
| Male | 31 (84%) |
| Age at first diagnosis, years (range) | 72.19 ± 8.58 (42–82) |
| Treatment, n | |
| Observation | 26 (70%) |
| AFB | 4 (11%) |
| EVAR | 7 (19%) |
| Follow-up period, days (range) | 1243 ± 789 (176–3348) |
| FDG PET/CT, times (range) | 2.1 ± 1.1 (1–5) |
| Contrast CT, times (range) | 3.2 ± 1.8 (1–8) |
| TBR (range) | 1.61 ± 0.27 (1.14–2.23) |
AFB: Aorto-femoral bypass graft surgery
EVAR: Endovascular aorta repair
TBR: Target-to-background uptake ratio
Correlation between FDG Uptake and Growth of the AAA
In the patient group, the follow-up period was 1243 ± 789 days (range 176–3348 days) after the FDG PET/CT. Of 37 patients, 26 were observationally managed without any intervention until the last follow-up, whereas 11 patients underwent interventions for AAA (AFB in 4 patients and EVAR in 7). FDG PET/CT was performed 2.1 ± 1.1 times (range 1–5 times), and abdominal contrast-enhanced CT was performed 3.2 ± 1.8 times (range 1–8 times) for a patient. The TBR of AAA was 1.61 ± 0.27 (range 1.14–2.23). Detailed information on AAA lesions is summarized in Table 1.
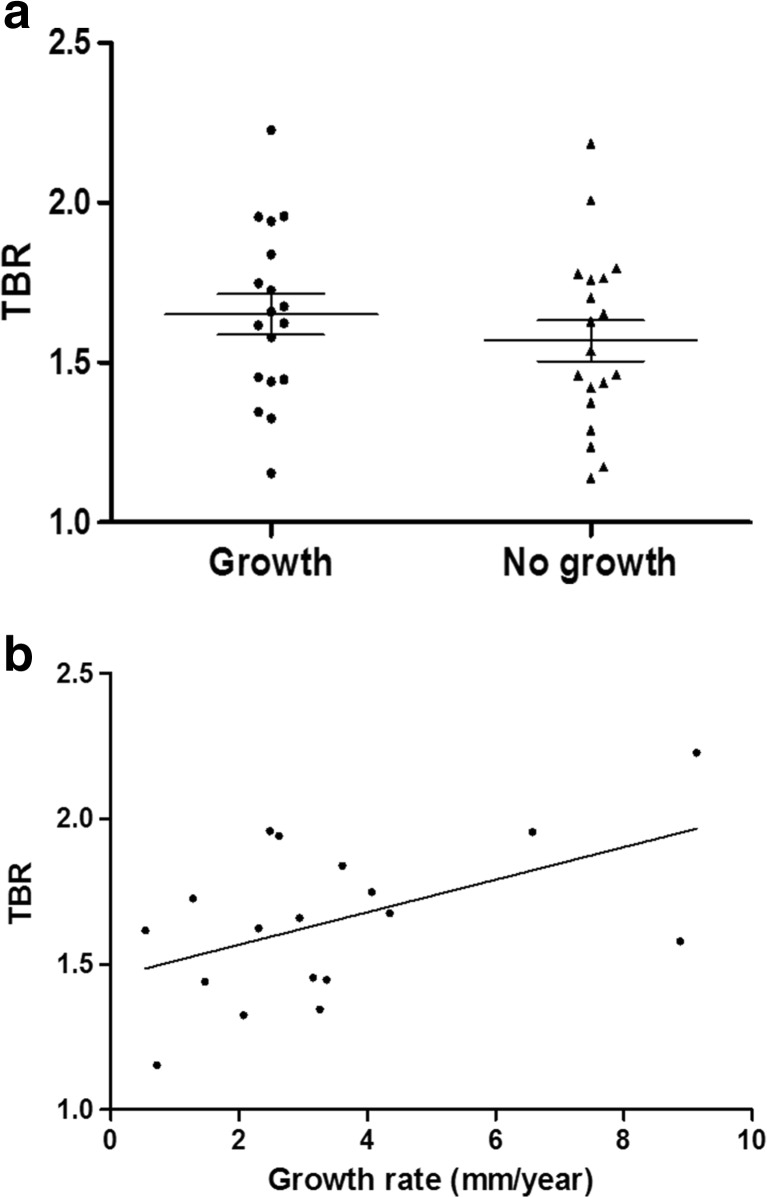
Of 37 cases, 19 patients did not exhibit significant AAA growth during the follow-up period, whereas growth was observed in the other 18 patients. In these 18 patients, the average growth rate of AAA was 3.49 ± 2.45 mm/year (range 0.54–9.13 mm/year). TBR was not significantly different between patients with and without growth (1.55 ± 0.20 vs. 1.57 ± 0.14; P = 0.5599; Fig. 2a). However, in 18 patients with significant growth of AAA, TBR exhibited a significant correlation with the growth rate (r 2 = 0.2601, P = 0.0306; Fig. 2b).
Fig. 2.
a TBR according to growth of the AAA. In overall patients, TBR was not significantly different between patients with and without significant growth (1.55 ± 0.20 vs. 1.57 ± 0.14; P = 0.5599). b Correlation between TBR and growth rate of AAA in patients with significant growth. In this group, TBR exhibited a significant positive correlation with the long-term growth rate of the AAA (r 2 = 0.2601, P = 0.0306)
FDG Uptake and Clinical Outcome
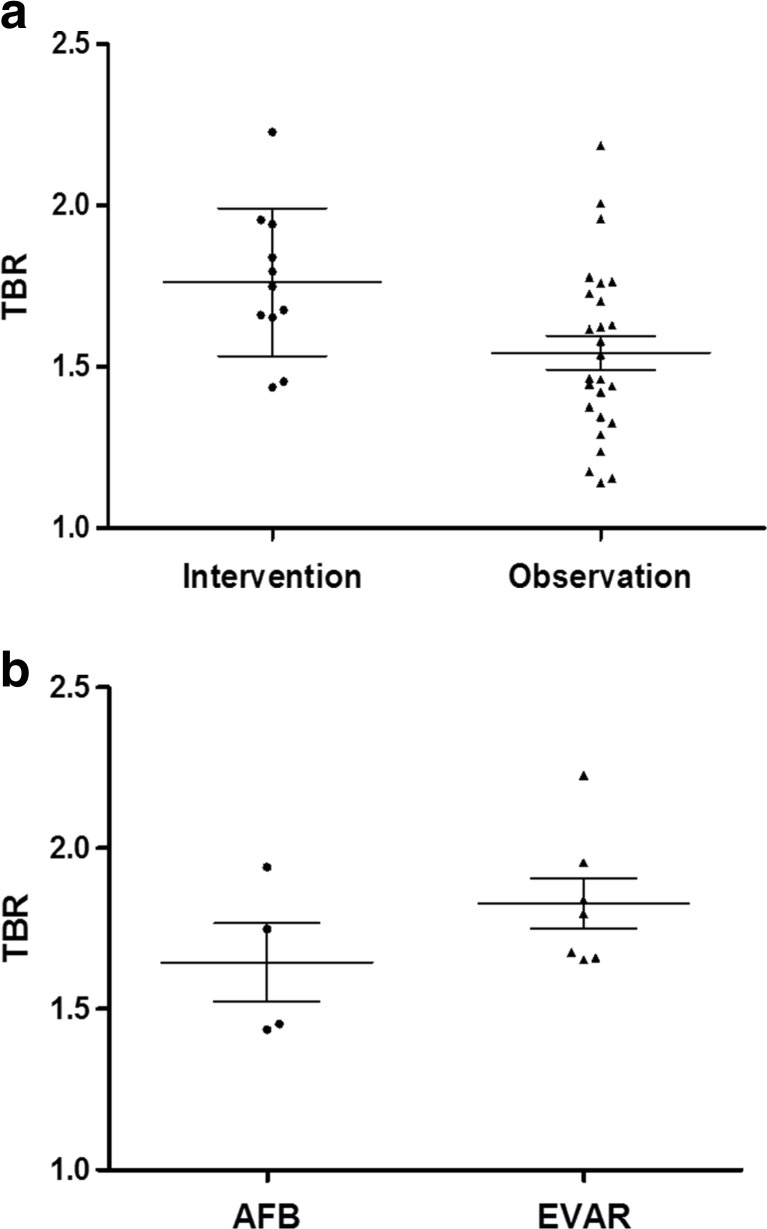
The TBR of patients who received interventions for the AAA was significantly higher than that of patients who did not (1.76 ± 0.23 vs. 1.54 ± 0.27; P = 0.0228), although there was a considerable overlap of values between the two groups (Fig. 3a). In a subgroup analysis, TBR was higher in patients who received EVAR than in those who received AFB, but without clinical significance (1.83 ± 0.21 vs. 1.65 ± 0.24; P = 0.3152; Fig. 3b). FDG PET/CT images of representative cases are demonstrated in Fig. 4.
Fig. 3.
a TBR according to treatment. TBR of patients who underwent intervention was significantly higher than that of patients who were observationally followed up (P = 0.0228). b TBR according to options of interventional treatment. TBR of patients who received AFB was not significantly different from that of patients who received EVAR
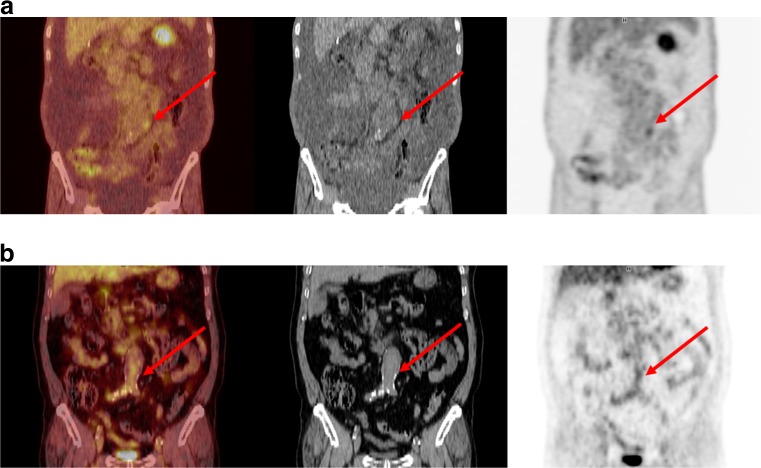
Fig. 4.
a FDG PET/CT image of a 69-year-old male patient with AAA. Focally increased FDG uptake was demonstrated on PET/CT (arrow). TBR of his AAA was 1.96, and the long-term growth rate was 6.57 mm/year. The diameter of the AAA was less than 5.5 cm, and its growth rate was less than 10 mm/year. However, he underwent EVAR for this lesion. b FDG PET/CT image of a 75-year-old male patient with AAA. TBR of his AAA was 1.25, and the long-term growth rate was 0.72 mm/year. He did not receive any intervention during the follow-up period
Discussion
In the present study, no significant difference was observed in FDG uptake between AAAs with or without growth during follow-up in the overall group. However, in the subgroup that exhibited growth of AAA, the degree of FDG uptake exhibited a significant correlation with the growth rate of AAA. Additionally, FDG uptake was higher in patients who received surgical or interventional treatment than in those who did not.
The progression and growth of AAA closely relate to vascular wall inflammation. In a previous study, tissues of AAA exhibited higher expression of cytokines, transcription factors, and cellular responses related to general inflammation than tissues of atherosclerotic disorders [11]. Also, several studies have reported the inflammatory activity of aortic aneurysms is correlated with aneurysmal development and growth [18, 19]. FDG PET is a well-known imaging method targeting inflammation, because glucose metabolism is enhanced in active inflammatory cells. The efficacy of FDG PET in various inflammatory conditions including vascular diseases has been reported in many studies [8–10, 20–22].
There have been several attempts to investigate the effectiveness of FDG PET/CT in the diagnosis and prognosis prediction of AAA. However, results of these studies were inconsistent with each other regarding the relationship between FDG uptake and growth of AAA [23]. In some studies, no significant correlation was observed between FDG uptake and the growth of AAA [6, 24, 25], and in some other studies, even negative correlations were observed [13, 26]. It is speculated that an ambivalent feature of inflammation is related to the results. In inflammation, two polarized subtypes of macrophages, M1 and M2, are activated and play different roles; M1 is usually proinflammatory and induces destruction of pathogens or tissues, whereas M2 induces repair and recovery of tissues. Although M1 macrophages accumulate FDG more preferentially, both M1 and M2 can induce increased FDG uptake in inflammatory tissue [27]. Thus, increased FDG uptake in AAA may represent activation of either M1 or M2 macrophages, which results in different consequences: growth of AAA with tissue destruction or tissue repair. In our study, polarization and activation of different subtypes of macrophages may have been a cause for the result of no difference in FDG uptake between AAAs with or without growth. In contrast, a significant correlation between the FDG uptake and growth rate in the growing AAA group suggests that the degree of macrophage activation relates to growth of AAAs in a subgroup where a specific subtype of inflammation is dominant.
In addition to inflammation, other demographic and clinical factors such as age, sex, hypertension, and smoking history are suspected to be related to growth of AAAs [28]. However, none of them was related to growth of AAAs in our study (data not shown), partly because of the small case number. In recent studies, other factors directly related to the pathophysiological process of AAA growth have been tried as imaging targets: matrix metalloproteinase, neoangiogenesis, and tissue elastin [29]. It is expected that the correlation between these factors and AAA growth will be more clearly proven in future studies.
Similar to the relationship between FDG uptake and growth of AAAs, inconsistent results have been reported in previous studies regarding the relationship between FDG uptake and the clinical decision on treatment. It was reported that there is no difference in FDG uptake between finally treated and non-treated patients [15], whereas another group reported that some cases with increased FDG uptake required operation [13]. In the present study, there was a significant difference in FDG uptake between observed and treated patients. At present, there is no guidance for using FDG PET for determining the surgical or interventional treatment of AAA. The decision for treatment is usually made by size criteria in which an AAA that exceeds 5.5 cm or grows faster than 1 cm/year is recommended to undergo treatment. However, some patients are treated despite the small size or slow growth rate of the AAA based on clinical conditions. In our hospital, surgical or interventional treatment of AAAs is performed even if size criteria are unmet in case of ominous findings such as a large extent, progression of a penetrating ulcer, and involvement of the common iliac artery. In the present study, among the 11 patients who received treatment, 3 patients met the size criteria, 1 with an AAA larger than 5.5 cm and 2 with a growth rate faster than 1 cm/year. Thus, it is an intriguing finding that FDG uptake is related to the decision on treatment, because it suggests that ominous findings for clinical decisions may be related to increased FDG uptake. Further study is required for the relationship between FDG uptake and those findings.
In the present study, we used TBR instead of SUV. Maximal SUV is a reproducible and effective parameter to evaluate the metabolic activity of a target area in most oncological PET imaging. However, the target lesion in case of AAA is not a solid tumor but a thin tissue abutting the blood activity. Thus, TBR appears to be a more appropriate parameter than SUV because TBR is a parameter corrected by blood pool activity. Additionally, the long-term influence of FDG uptake was analyzed in the present study based on long-term follow-up data. Because AAA exhibits slow growth and progression in many patients, it can be speculated that underlying chronic inflammatory activity was assessed in this study. It would be intriguing to investigate correlations between the FDG uptake and chronic wall stress of AAAs that can be measured using three-dimensional reconstruction of CT angiography and hydraulic simulation.
There are some limitations in the present study. First, this study was based on retrospective review; thus, the intervals of FDG PET, CT, and clinical follow-up were not regularly performed. The decision on AAA treatment may have been affected by cancer conditions. Instead, the follow-up period was relatively long for evaluating the long-term influence of FDG uptake in AAA. Second, the case number was relatively small to exhibit statistically robust results. Third, the growth rate of AAAs was computed by linear regression analysis, although there are other models for growth of AAAs such as a bi-exponential growth model, and it is well known that growth of AAAs is often not continuous but intermittent, depending on activation of inflammation. However, linear regression was performed to define an index for long-term growth of AAAs, and the growth pattern of AAAs demonstrated good fitting with the linear regression model in most cases.
Conclusion
In conclusion, FDG PET/CT may only be effective for predicting the long-term growth of AAAs in a specific subgroup of patients. It is also suggested that FDG PET is potentially related to the clinical conditions of AAA patients who need surgical or interventional treatment. Further study is required regarding the role of FDG PET in AAAs with higher subject numbers and a prospective study design.
Compliance with Ethical Standards
Conflict of Interest
Hyunjong Lee, Jin Chul Paeng, Kyung Hwan Kim, Gi Jeong Cheon, Dong Soo Lee, June-Key Chung, and Keon Wook Kang declare that they have no conflict of interest. This study was supported by the Korean National Research Foundation (NRF-2015R1D1A1A02062323).
Ethical Approval
All procedures performed in studies involving human participants were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. This manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Informed Consent
The study design of the retrospective analysis and exemption of informed consent were approved by the Institutional Review Board of the Seoul National University Hospital (H-1611-106-809).
Contributor Information
Hyunjong Lee, Email: hyunjong88@hotmail.com.
Jin Chul Paeng, Phone: 82-2-2072-3341, Email: paengjc@snu.ac.kr.
Kyung Hwan Kim, Email: kkh726@gmail.com.
Gi Jeong Cheon, Email: larrycheon@gmail.com.
Dong Soo Lee, Email: dsl@snu.ac.kr.
June-Key Chung, Email: jkchung@snu.ac.kr.
Keon Wook Kang, Email: kangkw@snu.ac.kr.
References
- 1.Saucy F, Deglise S, Holzer T, Briner L, Benezit M, Delay C, et al. Abdominal aortic aneurysm: what about screening? Curr Pharm Des. 2015;21:4084–4087. doi: 10.2174/1381612821666150826095148. [DOI] [PubMed] [Google Scholar]
- 2.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med. 2015;373:328–338. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard DJ, Filardo G, Fowkes G, Powell JT. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD001835.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Tanios F, Gee M, Pelisek J, Kehl S, Biehler J, Grabher-Meier V, et al. Interaction of biomechanics with extracellular matrix components in abdominal aortic aneurysm wall. Eur J Vasc Endovasc Surg. 2015;50:167–174. doi: 10.1016/j.ejvs.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Larsson E, Labruto F, Gasser TC, Swedenborg J, Hultgren R. Analysis of aortic wall stress and rupture risk in patients with abdominal aortic aneurysm with a gender perspective. J Vasc Surg. 2011;54:295–299. doi: 10.1016/j.jvs.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Reeps C, Gee M, Maier A, Gurdan M, Eckstein H-H, Wall WA. The impact of model assumptions on results of computational mechanics in abdominal aortic aneurysm. J Vasc Surg. 2010;51:679–688. doi: 10.1016/j.jvs.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls SC, Gardner JB, Meissner MH, Johansen KH. Rupture in small abdominal aortic aneurysms. J Vasc Surg. 1998;28:884–888. doi: 10.1016/S0741-5214(98)70065-5. [DOI] [PubMed] [Google Scholar]
- 8.Vaidyanathan S, Patel C, Scarsbrook A, Chowdhury F. FDG PET/CT in infection and inflammation—current and emerging clinical applications. Clin Radiol. 2015;70:787–800. doi: 10.1016/j.crad.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O, et al. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine. 2015;94:e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39:344–353. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 11.Cocker MS, Mc Ardle B, Spence JD, Lum C, Hammond RR, Ongaro DC, et al. Imaging atherosclerosis with hybrid [18F] fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol. 2012;19:1211–1225. doi: 10.1007/s12350-012-9631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindeman J, Abdul-Hussien H, Schaapherder A, Roelen D, Kleemann R. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6-and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci. 2008;114:687–697. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
- 13.Sakalihasan N, Van Damme H, Gomez P, Rigo P, Lapiere C, Nusgens B, et al. Positron emission tomography (PET) evaluation of abdominal aortic aneurysm (AAA) Eur J Vasc Endovasc Surg. 2002;23:431–436. doi: 10.1053/ejvs.2002.1646. [DOI] [PubMed] [Google Scholar]
- 14.Morel O, Mandry D, Micard E, Kauffmann C, Lamiral Z, Verger A, et al. Evidence of cyclic changes in the metabolism of abdominal aortic aneurysms during growth phases: a FDG-PET sequential observational study. J Nucl Med. 2015;56:1030–1035. doi: 10.2967/jnumed.114.146415. [DOI] [PubMed] [Google Scholar]
- 15.Barwick TD, Lyons O, Mikhaeel N, Waltham M, O’Doherty M. 18F-FDG PET-CT uptake is a feature of both normal diameter and aneurysmal aortic wall and is not related to aneurysm size. Eur J Nucl Med Mol Imaging. 2014;41:2310–2318. doi: 10.1007/s00259-014-2865-9. [DOI] [PubMed] [Google Scholar]
- 16.Choi E-S, Ha S-G, Kim H-S, Ha JH, Paeng JC, Han I. Total lesion glycolysis by 18F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur J Nucl Med Mol Imaging. 2013;40:1836–1842. doi: 10.1007/s00259-013-2511-y. [DOI] [PubMed] [Google Scholar]
- 17.Brewstera DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 18.Lu H-Y, Huang C-Y, Shih C-M, Lin Y-W, Tsai C-S, Lin F-Y, et al. A potential contribution of dipeptidyl peptidase-4 by the mediation of monocyte differentiation in the development and progression of abdominal aortic aneurysms. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2016.05.093. [DOI] [PubMed] [Google Scholar]
- 19.Vorkapic E, Dugic E, Vikingsson S, Roy J, Mäyränpää MI, Eriksson P, et al. Imatinib treatment attenuates growth and inflammation of angiotensin II induced abdominal aortic aneurysm. Atherosclerosis. 2016;249:101–109. doi: 10.1016/j.atherosclerosis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Lee SJ, Lee D-J, Kwon SH, Jo K-S, An Y-S, et al. Carotid artery FDG uptake may serve as a biomarker for cardiovascular risk stratification in asymptomatic adults. Nucl Med Mol Imaging. 2014;48:196–202. doi: 10.1007/s13139-014-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özmen Ö, Gökçek A, Tatcı E, Biner İ, Akkalyoncu B. Integration of PET/CT in current diagnostic and response evaluation methods in patients with tuberculosis. Nucl Med Mol Imaging. 2014;48:75–78. doi: 10.1007/s13139-013-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YH, Yu CM, Kim ES, Jung JO, Seo HS, Lee JH, et al. Monitoring therapeutic response in a case of extrapulmonary tuberculosis by serial F-18 FDG PET/CT. Nucl Med Mol Imaging. 2012;46:69–72. doi: 10.1007/s13139-011-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalalzadeh H, Indrakusuma R, Planken R, Legemate D, Koelemay M, Balm R. Inflammation as a predictor of abdominal aortic aneurysm growth and rupture: a systematic review of imaging biomarkers. Eur J Vasc Endovasc Surg. 2016;52:333–342. doi: 10.1016/j.ejvs.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Nchimi A, Cheramy-Bien J-P, Gasser TC, Namur G, Gomez P, Seidel L, et al. Multifactorial relationship between 18F-fluoro-deoxy-glucose positron emission tomography signaling and biomechanical properties in unruptured aortic aneurysms. Circ Cardiovasc Imaging. 2014;7:82–91. doi: 10.1161/CIRCIMAGING.112.000415. [DOI] [PubMed] [Google Scholar]
- 25.Kotze C, Menezes L, Endozo R, Groves A, Ell P, Yusuf S. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) Eur J Vasc Endovasc Surg. 2009;38:93–99. doi: 10.1016/j.ejvs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Kotze CW, Rudd JH, Ganeshan B, Menezes LJ, Brookes J, Agu O, et al. CT signal heterogeneity of abdominal aortic aneurysm as a possible predictive biomarker for expansion. Atherosclerosis. 2014;233:510–517. doi: 10.1016/j.atherosclerosis.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Satomi T, Ogawa M, Mori I, Ishino S, Kubo K, Magata Y, et al. Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J Nucl Med. 2013;54:999–1004. doi: 10.2967/jnumed.112.110551. [DOI] [PubMed] [Google Scholar]
- 28.Brady AR, Thompson SG, Fowkes FGR, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 29.Golestani R, Sadeghi MM. Emergence of molecular imaging of aortic aneurysm: implications for risk stratification and management. J Nucl Cardiol. 2014;21:251–267. doi: 10.1007/s12350-013-9845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]