Abstract
Positron Emission tomography Response Criteria In Solid Tumors (PERCIST) version 1.0 was introduced in 2009 for objective assessment of tumor metabolic response using 18F-FDG PET/CT. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0 was published in 2016 to review and clarify some of the issues with the PERCIST. In this article, we reflect on the benefits and challenges of implementing PERCIST, and speculate on topics that could be discussed in PERCIST 1.1 in the future.
Introduction
We could ask ourselves before arguing for or against the routine utilization of Positron Emission tomography Response Criteria In Solid Tumors (PERCIST) [1, 2], “Is there an immediate need for quantitative positron emission tomography (PET) response criteria?”. After all, we have been using 18F–fluorodeoxyglucose (18F–FDG) PET/computed tomography (PET/CT) for monitoring response to therapy in various tumor types for years now, and visual assessment seems sufficient in many cases. And there are the European Organization for Research and Treatment of Cancer (EORTC) recommendations from 1999 [3]. Our short answer is yes, there is a need. We may have been using 18F–FDG PET/CT for years to assess treatment response, but we are far from being able to present a clear answer to the referring physician in a consistent manner. One complaint heard not uncommonly in the clinic is that depending on who is reading the 18F–FDG PET/CT study, the responses are somewhat different, as visual assessment of tumor response can and does lack sufficient inter-reader agreement (Fig. 1). And simple categorization of the 18F–FDG PET/CT study results into two groups of responders or non-responders fails to utilize the rich data 18F–FDG PET/CT contains. Quantitative PET assessment presented as percent change in FDG uptake would retain the inherently continuous nature of the data, and have high inter-observer reproducibility [4]. The EORTC recommendations were a great place to start standardization of PET assessment, but they did not clearly state which tumor, or tumors, to measure, and do not detail which PET parameter to utilize. To be able to present an objective and reliable assessment of response to therapy, a detailed and straightforward description of how to perform quantitative PET response that could be employed regularly in the reading rooms appears essential.
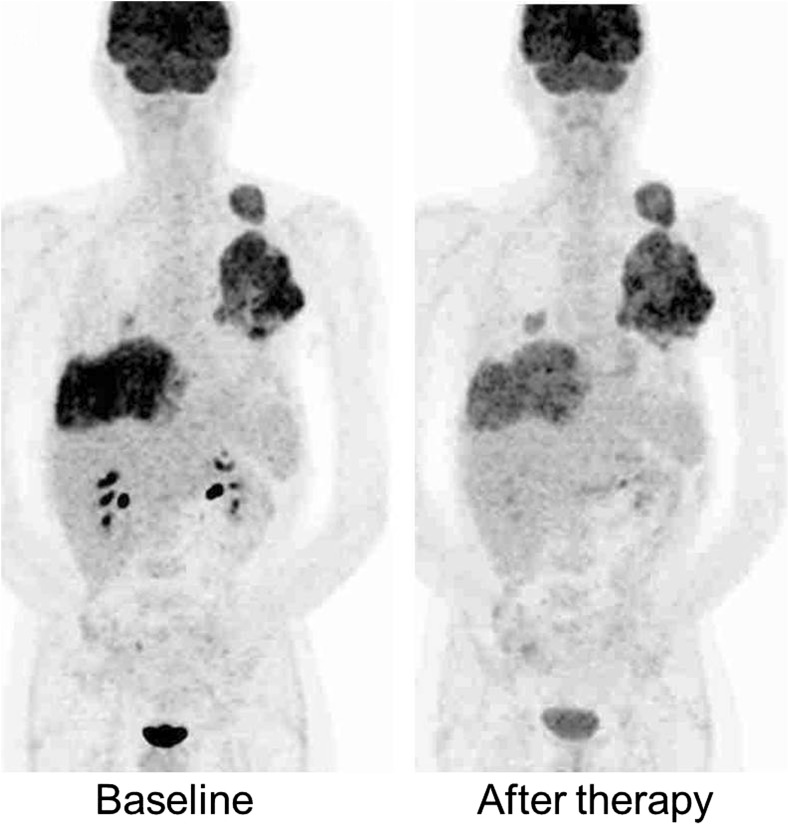
Fig. 1.
Which response category would you designate this patient? Many readers hesitate between partial metabolic response and stable metabolic disease. Using quantitative method such as PERCIST, the peak SUL change from 9.0 to 5.4 and thus 40% decrease could easily be reproduced across sites and readers
Performing PERCIST
One barrier to routine use of PERCIST is the perceived tedium associated with performing PERCIST. The reader should note that only two short extra steps are needed to perform PERCIST. First, the reader is required to measure the hepatic 18F–FDG activity. In order to perform PERCIST, the reader needs to see if the target tumor lesion shows sufficiently high glucose uptake for reliable assessment using 18F–FDG PET/CT. PERCIST has a minimum threshold for measurability, defined as 1.5 × (mean value of normal liver) + 2 × (standard deviation of liver) or greater at baseline. The hepatic 18F–FDG activity can be measured quickly with a fixed 3-cm diameter spherical volume of interest in most of the current commercial reading software. The other required extra step is conversion of the quantitative PET parameter from standardized uptake value (SUV) to SUV corrected for lean body mass (SUL). This conversion can be made with one or two clicks in the reading software as long as the patient’s height and weight are known, or the reader could opt to set SUL as the default display unit. The current commercial software packages use slightly different methods to compute the peak SUL, and future cooperative efforts from vendors are required to unify the peak SUL computation method. Also, the Janmahasatian formula was recommended for estimation of lean body mass in modern PET scanners [5], but this method is currently not widely incorporated into the commercial workstations.
PERCIST asks the reader to measure the single hottest tumor in the patient at each time point for assessment. Selecting the hottest uptake lesion may require additional time and care when there are multiple tumors. It is possibly worth mentioning that the single hottest voxel which shows the maximum value may not be contained within the 1-cm3 sphere with the peak SUL. The time and effort it takes to select the single hottest tumor site could be saved with wide distribution of automated software such as Auto-PERCIST™ [6], which is already available to readers for research purposes.
The single hottest tumor becomes the target lesion in PERCIST, though up to five lesions have been suggested for measurement. One recent paper looked at the number of lesions measured in metastatic breast cancer, and found that the number did not have a major impact on the prognostic value using PERCIST [7]. We have found similar findings of high correlation for single lesion measurement and up to five hottest lesions in breast cancer, sarcoma and lymphoma studies [8]. How many lesions should be assessed and how to compute the change for multiple measurements require further studies in various different clinical settings. The rationale for measuring a single lesion is to clearly identify lesions which might be the “worst” behaving, especially when tumor resistance to therapy begins to evolve. For now, PERCIST can be performed with one target lesion, a pro that will not be lost to the readers.
Routine size measurement is not necessary for PERCIST, but measurement could be made when a lesion demonstrates noticeable increase in size even though the FDG uptake is decreased. Which would be the best method to express such discordant changes between the PET and CT images? PERCIST puts greater emphasis on the metabolic change, as does the widely used Deauville criteria for malignant lymphoma [9]. Several exploratory parameters mentioned in PERCIST look at both the SUL and the volume of the tumor, but these parameters require further validation. Total lesion glycolysis, the product of the mean SUL and the metabolic volume, showed predictive or prognostic values in cancers of the head and neck [10], lung [11], and uterus [12] to list just some, and in pancreas neuroendocrine tumors [13]. Simple product of volume and metabolic activity may not be the most accurate solution, and recently, a new metric was proposed that incorporates the commonly utilized metrics of SUV, metabolic tumor volume, and total lesion glycolysis into a single parameter [14]. Various textural features and parameters representing tumor heterogeneity have shown much potential for predicting disease progression [15, 16]. How to best represent the volumetric and SUL data, and possibly such features as intratumoral metabolic heterogeneity, is a challenge that needs to be further investigated.
In PERCIST, decrease greater than or equal to 30% in peak SUL is considered partial metabolic response, and peak SUL rise greater than or equal to 30%, when coupled with an absolute change in SUL of at least 0.8, is considered as progressive metabolic disease. PERCIST 1.0 does not suggest different thresholds according to therapeutic regimen or number of chemotherapy cycles performed prior to assessment. This topic could be considered after additional data are collected, possibly in PERCIST 1.1.
The advantages of peak compared to maximum SUV, especially in noisy 18F–FDG PET/CT studies, should hold true for studies performed in patients with benign diseases as well. Quantification of inflammatory conditions can be advantageous for monitoring therapy, in such diseases as sarcoidosis or arthritis.
Comparison of PERCIST with RECIST
Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 is currently the standard imaging method in clinical trials. As RECIST and PERCIST measure two different features of a tumor, and as 18F–FDG PET/CT can detect more metastatic lesions including bone metastases than enhanced CT, the two criteria can be expected to yield different responses not uncommonly. There are not many studies that directly compare the prognostic values of PERCIST and the revised RECIST 1.1. In 20 patients with metastatic breast cancer, tumor response by PERCIST was a superior predictor of progression-free survival (PFS) than response by RECIST 1.1 [17]. PERCIST showed difference in PFS between the patients with partial metabolic response and stable metabolic disease, while the PFS did not significantly differ between the partial remission and stable disease groups according to RECIST 1.1 after chemotherapy in 35 patients with non-small-cell lung cancer (NSCLC) [18]. There are additional studies that showed superior predictive value of PERCIST compared to RECIST 1.0 in esophageal cancer, NSCLC, and Ewing sarcoma [19–21]. PERCIST could lead to more accurate response assessment by noninvasive imaging in this age of precision medicine, but many more studies are required to validate this possibility. Future studies need to compare the predictive and prognostic values of response according to PERCIST and RECIST 1.1 in various tumors for various therapeutic options.
Conclusion
Future studies directed for input and validation of quantified response using 18F–FDG PET/CT could address the current limitations of PERCIST 1.0 and propel the routine use of 18F–FDG PET/CT and PERCIST for response assessment in various diseases.
Compliance with Ethical Standards
Conflict of Interest
Joo Hyun O and Richard Wahl declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
References
- 1.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O JH, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280(2):576-84. [DOI] [PMC free article] [PubMed]
- 3.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of cancer (EORTC) PET study group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 4.Jacene HA, Leboulleux S, Baba S, Chatzifotiadis D, Goudarzi B, Teytelbaum O, et al. Assessment of interobserver reproducibility in quantitative 18F-FDG PET and CT measurements of tumor response to therapy. J Nucl Med. 2009;50:1760–1769. doi: 10.2967/jnumed.109.063321. [DOI] [PubMed] [Google Scholar]
- 5.Tahari AK, Chien D, Azadi JR, Wahl RL. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med. 2014;55:1481–1484. doi: 10.2967/jnumed.113.136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal J, Wahl R. Auto-PERCIST™: Semi-automated quantitative assessment of FDG-PET based on the PERCIST criteria. Radiological Society of North America 2013 Scientific Assembly and Annual Meeting, Chicago, 2013.
- 7.Pinker K, Riedl CC, Ong L, Jochelson M, Ulaner GA, McArthur H, et al. The impact that number of analyzed metastatic breast cancer lesions has on response assessment by 18F-FDG PET/CT using PERCIST. J Nucl Med. 2016;57:1102–1104. doi: 10.2967/jnumed.115.166629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oo JH, Jacene H, Stearns V, Wahl R. How many lesions to measure for response assessment? Comparing SULpeak changes in a single lesion and the sum of the 5 hottest lesions in 3 types of cancer and 3 types of therapy. J Nucl Med. 2013;54:229. doi: 10.2967/jnumed.112.109603. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Nishio M, Nakanishi H, Hanai N, Hirakawa H, Kodaira T, et al. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol Lett. 2016;12:1493–1500. doi: 10.3892/ol.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho KC, Fang YD, Chung HW, Liu YC, Chang JW, Hou MM, et al. TLG-S criteria are superior to both EORTC and PERCIST for predicting outcomes in patients with metastatic lung adenocarcinoma treated with erlotinib. Eur J Nucl Med Mol Imaging. 2016;43(12):2155-2165. [DOI] [PubMed]
- 12.Lee JW, Heo EJ, Moon SH, Lee H, Cheon GJ, Lee M, et al. Prognostic value of total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with uterine carcinosarcoma. Eur Radiol. 2016;26(11):4148-4154 [DOI] [PubMed]
- 13.Kim HS, Choi JY, Choi DW, Lim HY, Lee JH, Hong SP, et al. Prognostic value of volume-based metabolic parameters measured by (18)F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. 2014;48:180–186. doi: 10.1007/s13139-013-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahmim A, Schmidtlein C, Bak-Fredslund K, Subramaniam R, Morsing A, Keiding S, et al. Application of novel PET metric to quantify liver metastases for enhanced prognostication of clinical outcome. J Nucl Med. 2016;57:352. [Google Scholar]
- 15.Kang SR, Song HC, Byun BH, Oh JR, Kim HS, Hong SP, et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent Chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Nucl Med Mol Imaging. 2014;48:16–25. doi: 10.1007/s13139-013-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Jung JH, Son SH, Kim CY, Hong CM, Oh JR, et al. Prognostic significance of Intratumoral metabolic heterogeneity on 18F-FDG PET/CT in pathological N0 non-small cell lung cancer. Clin Nucl Med. 2015;40:708–714. doi: 10.1097/RLU.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 17.Riedl CC, Pinker K, Ulaner GA, Ong LT, Baltzer P, Jochelson MS, et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1428–1437. doi: 10.1007/s00259-017-3703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang J, Ling X, Zhang L, Tang Y, Xiao Z, Cheng Y, et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43:1945–1953. doi: 10.1007/s00259-016-3420-7. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa M, Tatsumi M, Miyata H, Morii E, Tomiyama N, Watabe T, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med. 2012;53:872–880. doi: 10.2967/jnumed.111.098699. [DOI] [PubMed] [Google Scholar]
- 20.Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, et al. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST) J Thorac Dis. 2014;6:677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshkin VS, Bolejack V, Schwartz LH, Wahl RL, Chugh R, Reinke DK, et al. Assessment of imaging modalities and response metrics in Ewing sarcoma: correlation with survival. J Clin Oncol 2016;34(30):3680–3685. [DOI] [PMC free article] [PubMed]