Abstract
The dose-limiting salivary gland toxicity of 225Ac-labelled PSMA for treatment of metastatic, castration-resistant prostate cancer remains unresolved. Suppressing the metabolism of the gland by intraparenchymal injections of botulinum toxin appears to be a promising method to reduce off-target uptake. A 68Ga-PSMA PET/CT scan performed 45 days after injection of 80 units of botulinum toxin A into the right parotid gland in a 63-year-old patient showed a decrease in the SUVmean in the right parotid gland of up to 64% as compared with baseline. This approach could be a significant breakthrough for radioprotection of the salivary glands during PSMA radioligand therapy.
Keywords: Prostate cancer, PSMA radioligands, Salivary glands, Positron emission tomography, Botulinum toxin, Xerostomia
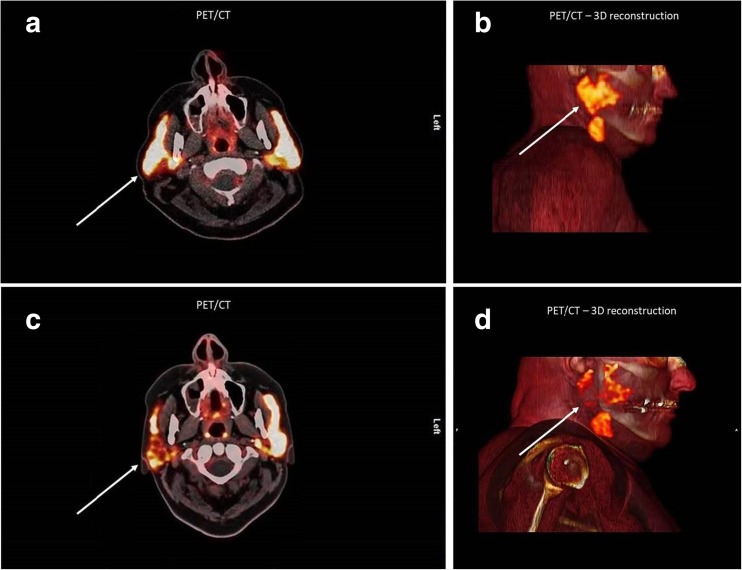
A 63-year-old patient with advanced metastatic castration-resistant prostate cancer underwent 68Ga-PSMA PET/CT imaging before and 45 days after receiving multifocal, ultrasound-guided injections of a total of 80 units botulinum toxin A into the right parotid gland (Fig. 1) according to a clinically approved method used in patients who have suffered from sialorrhoea for over two decades [1–3]. The SUVmean of the radioligand in the injected parotid gland showed a highly significant decrease of up to 60% compared with the left side, especially in the pars profunda of the gland, and a decrease of up to 64%, but no significant change in the left parotid gland, compared with the baseline PET/CT study. At the time of this report, after a follow-up of 3 months, the patient had not reported any adverse effects of the injections. The impressive decrease in PSMA radioligand uptake in the salivary glands, which is described here for the first time, could be a significant breakthrough for salivary gland protection, which is highly needed in the context of targeted alpha therapy using 213Bi-PSMA and 225Ac-PSMA [4, 5].
Fig. 1.
Axial fused (a, c) and sagittal reconstructed 3D (b, d) 68Ga-PSMA PET/CT images in a 63-year-old patient with advanced metastatic castration-resistant prostate cancer before (a, b) and 45 days after (c, d) multifocal, ultrasound-guided injections of a total of 80 units botulinum toxin A into the right parotid gland. The SUVmean of the radioligand in the injected parotid gland shows a highly significant decrease of up to 60% compared with the left side (c, d white arrows), especially in the pars profunda of the gland, and a decrease of up to 64%, but no significant change in the left parotid gland, compared with the baseline PET/CT study (a, b)
Acknowledgements
We thank Dr. Anthony Chang, Rethink Imaging, USA, for providing 3D reconstructed PET/CT images. We are also grateful to Dr. Guillaume Chaussé, Nuclear Medicine, McGill University, Montreal, Quebec, Canada, for his remarks on pharmacological and cellular mechanisms in the salivary glands after injection of botulinum toxin.
Compliance with Ethical Standards
Conflict of Interest
Richard P. Baum, Thomas Langbein, Aviral Singh, Mostafa Shahinfar, Christiane Schuchardt, Gerd Fabian Volk, Harshad Kulkarni declare that they have no conflict of interest.
Ethical Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from the patient who was the subject of the study.
Footnotes
Richard P. Baum and Thomas Langbein contributed equally to this work.
References
- 1.Rodwell K, Edwards P, Ware RS, Boyd R. Salivary gland botulinum toxin injections for drooling in children with cerebral palsy and neurodevelopmental disability: a systematic review. Dev Med Child Neurol. 2012;54:977–987. doi: 10.1111/j.1469-8749.2012.04370.x. [DOI] [PubMed] [Google Scholar]
- 2.Reddihough D, Erasmus CE, Johnson H, McKellar GMW, Jongerius PH. Botulinum toxin assessment, intervention and aftercare for paediatric and adult drooling: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):109–121. doi: 10.1111/j.1468-1331.2010.03131.x. [DOI] [PubMed] [Google Scholar]
- 3.Teymoortash A, Pfestroff A, Wittig A, Franke N, Hoch S, Harnisch S, et al. Safety and efficacy of Botulinum toxin to preserve gland function after radiotherapy in patients with head and neck cancer: a prospective, randomized, placebo-controlled, double-blinded phase I clinical trial. PLoS One. 2016;11:e0151316. doi: 10.1371/journal.pone.0151316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 5.Kratochwil C, Schmidt K, Afshar-Oromieh A, Bruchertseifer F, Rathke H, Morgenstern A, et al. Targeted alpha therapy of mCRPC: dosimetry estimate of (213)bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45:31–37. doi: 10.1007/s00259-017-3817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]