Abstract
Provitamin A (PVA) bio-fortification of crops offers a sustainable strategy to prevent the prevalence of vitamin A deficiency (VAD), one of the world’s major public health problems. The present work aimed to enhance PVA accumulation in cottonseed, the main by-product in the production of cotton fibers and the third largest source of edible plant oil in the world. On the basis of comprehensive identification of carotenoid synthase genes and their expression levels in various cotton tissues, we selected phytoene synthase as the target for manipulating carotenoid biosynthesis in the developing cottonseeds. After functional verification in transgenic tobacco, a cotton phytoene synthase gene (GhPSY2D) driven by a seed-specific promoter was transformed into cotton. The transgenic cottonseeds showed golden appearance and contained over 6-fold higher carotenoid contents in the extracted oil than the non-transgenic control. Thin layer chromatograph analysis indicated that the main PVA carotenoid β-carotene was predominant in the transgenic cottonseeds, but undetectable in the wild-type control. By simultaneously providing economically valuable fibers and edible oils, the transgenic cottons bio-fortified with β-carotene in seeds may be a new powerful tool against VAD in low-income regions.
Introduction
Vitamin A (retinol) is an essential nutrient for vertebrates, including humans. Vitamin A deficiency (VAD) may result in a series of disorders in animals, including impaired growth, reproduction, epithelial integrity, and disease resistance1. In human, this nutrient deficiency causes xerophthalmia, including night blindness, and increases the risk of infant morbidity and mortality from measles and diarrhoea in children2. VAD has been one of major human health problems for a long time. Although the overall prevalence of worldwide VAD has significantly decreased in the last two decades, this deficiency is still unacceptably serious in some regions, such as south Asia and sub-Sahara Africa3,4. This may be largely attributed to insufficient dietary diversification, unsuccessful food fortification, and the restricted vitamin A capsule delivery in these regions3. Compared to the supply of vitamin A capsule and vitamin A-rich animal-source foods, growing and consuming crops with high PVA level (PVA biofortified crops) is more sustainable and effective to alleviate VAD prevalence, especially in the low-income regions5,6. Several PVA biofortified crops have been reported to prevent the VAD prevalence effectively in various populations7,8.
PVA include a group of carotenoids containing at least one non-substituted β-ring, such as β-carotene, α-carotene, and β-cryptoxanthin, which are synthesized via a complex carotenoid pathway (Supplementary Fig. S1)6,9–11. In higher plants, carotenoids are synthesized in the plastids from geranylgeranyl pyrophosphate (GGPP), which is the precursor for multiple pathways and contains four molecules of isopentenyl pyrophosphate originating from glyceraldehyde phosphate and pyruvate (Supplementary Fig. S1). Phytoene synthase (PSY) catalyzes the first committed reaction of carotenoid pathway to synthesize phytoene from two GGPP molecules. Accumulating evidence indicates that PSY is the key regulatory enzyme in the biosynthesis of carotenoids6,11–13. Biotechnological strategies have been successfully employed to increase PVA level (especially β-carotene) in a wealth of crops, resulting in golden canola14, rice15–17, wheat18, sorghum19, corn20,21, cassava22, potato23, sweet potato24, tomato25, soybean26 and banana5. These works mainly adopted two strategies, i.e. to up-regulate PSY solely5,14 or with other synthases5,15–20 to promote carotenoid biosynthesis, and to express an Orange gene to enhance carotenoid accumulation15,21,24,27. Recently, it was reported that the Orange protein might promote carotenoid biosynthesis via stabilizing the key synthase PSY28,29.
Cotton (Gossypium) is the leading natural fiber crop in the world, and is one of the major economic drivers in developing countries. In addition to fiber, cottonseed is an important source of edible oil (ranking 3rd in the world) and high-quality proteins30,31. Therefore, cotton is a potential supporting crop for poor regions to improve the economic and nutrient status simultaneously. PVA bio-fortified cotton and cottonseed oil may be a powerful tool against VAD prevalence in low-income cotton-growing regions, for example in south Asia and sub-Sahara Africa3,32,33. Our work aimed to increase PVA content of cottonseed and the resultant cottonseed oil. To this end, a functional PSY gene predominantly expressed in cotton was cloned, and upregulated specifically in the developing seeds. The transgenic cottons, with normal growth and development, produced golden cottonseeds and cottonseed oil fortified with β-carotene. This work significantly improved the nutritional value of cottonseeds, which provided this cash crop with the potential to prevent VAD prevalence.
Results
Identification and expression analysis of carotenoid synthase genes in Gossypium
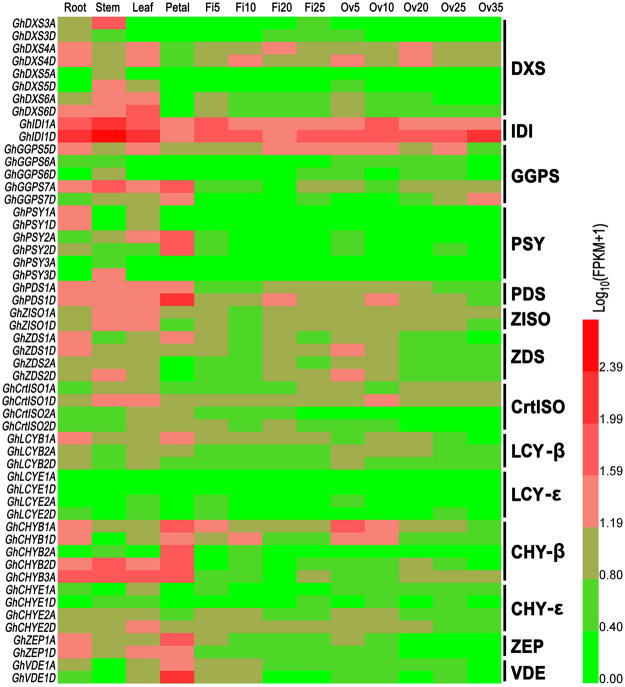
To characterize intrinsic carotenoid biosynthesis in various cotton tissues, especially in the developing seeds, we compared transcript levels of carotenoid related genes using transcriptomic data34. Firstly, we identified 36, 29 and 54 carotenoid synthase genes from the assembled cotton genomes of G. raimondii, G. arboreum and G. hirsutum, respectively (Table 1)34–36. These genes encoded all the enzymes catalyzing violaxanthin synthesis from GGPP and 3 key enzymes upstream to GGPP. As shown in Fig. 1, the transcript levels of the investigated carotenoid synthase genes varied with tissues and development stages, indicating that the carotenoid biosynthesis in cotton was developmentally regulated at transcription level. Generally, the transcript levels of carotenoid synthase genes were lower in the developing fibers and ovules compared to those in the roots, stems, leaves and petals. In the developing ovules, the PSY and LYC-ε genes had only a trace of transcription, while genes coding the rest carotenoid synthases had low-to-moderate expression. The transcript profile of carotenoid synthase genes suggested that the transcription of PSY genes may be a limiting factor of carotenoid biosynthesis in cottonseeds.
Table 1.
The coding genes of carotenoid synthases identified in cottons. Enzymes are abbreviated as in Supplementary Fig. S1. Genes are named alphabetically according to their IDs in G. raimondii, and homeologs annotated in different genomes (D5, A2, Dt1 and At1) are list in the same line.
| Enzymes | Genes | G. raimondii (D5) | G. arboreum (A2) | G. hirsutum-Dt1 | G. hirsutum-At1 |
|---|---|---|---|---|---|
| DXS | GoDXS1 | Gorai.004G030400 | Cotton_A_06764 | — | — |
| GoDXS2 | Gorai.004G030800 | Cotton_A_06768 | — | — | |
| GoDXS3 | Gorai.004G030900 | Cotton_A_06769 | Gh_D08G0270 | Gh_A08G0193 | |
| GoDXS4 | Gorai.004G111100 | Cotton_A_21777 | Gh_D08G1005 | Gh_A08G2461 | |
| GoDXS5 | Gorai.008G093600 | Cotton_A_31168 | Gh_D12G2793 | Gh_A12G0784 | |
| GoDXS6 | Gorai.011G184200 | Cotton_A_39687 | Gh_D10G1640 | Gh_A10G2292 | |
| IDI | GoIDI1 | Gorai.004G262800 | Cotton_A_05787 | Gh_D08G2391 | Gh_A08G1997 |
| GGPS | GoGGPS1 | Gorai.002G251300 | Cotton_A_00433 | Gh_D01G2110 | Gh_A01G1866 |
| GoGGPS2 | Gorai.002G251400 | — | — | — | |
| GoGGPS3 | Gorai.005G054800 | — | — | — | |
| GoGGPS4 | Gorai.005G120800 | — | — | — | |
| GoGGPS5 | Gorai.007G051300 | Cotton_A_02023 | Gh_D11G0475 | — | |
| GoGGPS6 | Gorai.007G206700 | Cotton_A_32196 | Gh_D11G1883 | Gh_A11G1725 | |
| GoGGPS7 | Gorai.011G102700 | Cotton_A_17013 | Gh_D10G0907 | Gh_A10G0844 | |
| GoGGPS8 | Gorai.011G102800 | — | — | — | |
| GoGGPS9 | Gorai.011G103000 | — | — | — | |
| GoGGPS10 | Gorai.011G133100 | — | — | — | |
| PSY | GoPSY1 | Gorai.001G083700 | Cotton_A_12202 | Gh_D07G0746 | Gh_A07G0668 |
| GoPSY2 | Gorai.006G009400 | Cotton_A_14731 | Gh_D09G0078 | Gh_A09G0080 | |
| GoPSY3 | Gorai.010G126900 | Cotton_A_36110 | Gh_D06G1167 | Gh_A06G0932 | |
| GoPSY4 | Gorai.012G039100 | Cotton_A_29856 | Gh_D04G0308 | Gh_A05G3296 | |
| PDS | GoPDS1 | Gorai.011G149900 | Cotton_A_36228 | Gh_D10G1327 | Gh_A10G1167 |
| ZISO | GoZISO1 | Gorai.005G080900 | Cotton_A_34612 | Gh_D02G0721 | Gh_A02G0673 |
| ZDS | GoZDS1 | Gorai.007G284900 | Cotton_A_14988 | Gh_D11G2616 | Gh_A11G2306 |
| GoZDS2 | Gorai.013G201600 | Cotton_A_12871 | Gh_D13G1826 | Gh_A13G1497 | |
| CrtISO | GoCrtISO1 | Gorai.002G153800 | Cotton_A_34115 | Gh_D01G1197 | Gh_A01G1122 |
| GoCrtISO2 | Gorai.002G218000 | Cotton_A_27012 | Gh_D01G1808 | Gh_A01G1557 | |
| LCY-β | GoLCYB1 | Gorai.006G113300 | Cotton_A_26267 | Gh_D09G0946 | Gh_A09G0916 |
| GoLCYB2 | Gorai.013G002400 | Cotton_A_20533 | Gh_D13G0026 | Gh_A13G0010 | |
| LCY-ε | GoLCYE1 | Gorai.001G012900 | Cotton_A_05719 | Gh_D07G2366 | Gh_A07G0117 |
| GoLCYE2 | Gorai.009G032900 | Cotton_A_09351 | Gh_D05G0313 | Gh_A05G0229 | |
| CHY-β | GoCHYB1 | Gorai.006G199400 | Cotton_A_27296 | Gh_D09G1728 | Gh_A09G1634 |
| GoCHYB2 | Gorai.008G249600 | Cotton_A_32712 | Gh_D12G2438 | Gh_A12G2678 | |
| GoCHYB3 | Gorai.008G274800 | Cotton_A_01286 | — | Gh_A12G2302 | |
| CHY-ε | GoCHYE1 | Gorai.007G372200 | Cotton_A_05513 | Gh_D11G3263 | Gh_A11G2878 |
| GoCHYE2 | Gorai.013G180200 | Cotton_A_26341 | Gh_D13G1644 | Gh_A13G1336 | |
| ZEP | GoZEP1 | Gorai.007G213500 | Cotton_A_24363 | Gh_D11G3469 | Gh_A11G1774 |
| VDE | GoVDE1 | Gorai.007G268500 | Cotton_A_18131 | Gh_D11G2473 | Gh_A11G2173 |
Figure 1.
Transcript levels of carotenoid synthase genes in various upland cotton tissues. The gene expression levels (FPKM) in root, stem, leaf, petal, and fibers (Fi5–25) and ovules (Ov5–35) of various days post anthesis (DPA) are converted to Log10(FPKM + 1) and illustrated as seven classes in the heat map. Genes are named as the abbreviation of species and enzyme plus code plus A or D to indicate the subgenome origin. Enzymes are abbreviated as in Supplementary Fig. S1. Transcript levels are inferred from the transcriptomic data34. The genes with very low transcript level (total FPKM < 2) are omitted.
Cloning and functional analysis of the GhPSY2 gene
To manipulate carotenoid biosynthesis in developing cottonseeds, we firstly cloned the coding sequences of the predominantly-expressed cotton PSY2 genes (Fig. 1) and analyzed their biological functions in transgenic tobacco. The coding regions of GhPSY2A and GhPSY2D were amplified from the leaf cDNA of upland cotton line T586, and their sequences were identical to those in the assembled TM-1 genome. Both GhPSY2A and GhPSY2D encoded proteins of 398 aa. GhPSY2A/2D were highly similar to plant group I PSYs, and distantly related to group II and III (Supplementary Fig. S2A). Multiple sequence alignment indicated that both GhPSY2 proteins had conserved DxxxD motifs, substrate binding pocket, catalytic residues and active site lid motifs (Supplementary Fig. S2B). Along with the expression data, these results suggested that GhPSY2A/2D might encode biologically functional PSYs.
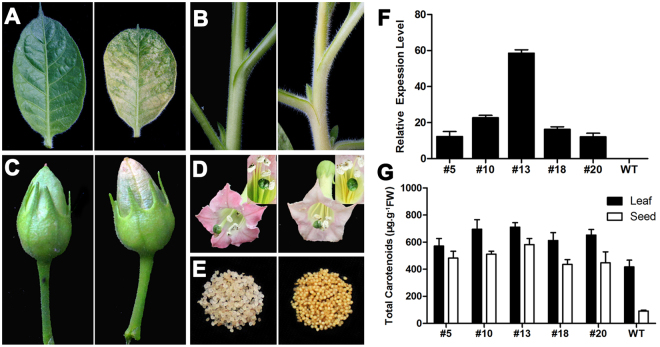
To further elucidate the biological functions of the cloned PSY genes, GhPSY2D was overexpressed in tobaccos. Compared with the wild-type control, the GhPSY2D over-expressers accumulated higher levels of carotenoids, and had golden appearance in several organs, such as leaves, stems, filaments, and developing seeds (Fig. 2A-E). Meanwhile, the over-expressers showed retarded growth and dwarf phenotype (Supplementary Fig. S3) as reported in tomatoes37. These results indicated that GhPSY2D was biologically functional to promote carotenoid synthesis in plants.
Figure 2.
Overexpression of GhPSY2D promotes carotenoid biosynthesis in tobacco. (A–E) Phenotypic comparison of GhPSY2D over-expresser (transformant #13, right) and the wild-type control (a null segregant of transformant #13, left); (A) Leaves; (B) Stems; (C) Capsules; (D) Flower and stamens; (E), 12-DPA seeds; (F) Transcript levels of GhPSY2D in wild-type control (WT) and GhPSY2D over-expressers (#5, #10, #13, #18 and #20); (G) Carotenoid quantification in leaves and 12-DPA seeds. Error bars indicate standard deviations of 3 biological replicates.
Golden cottonseed from specific upregulation of GhPSY2D
To increase the carotenoid content of cottonseeds, GhPSY2D was constructed downstream to a seed-specific promoter pV38,39, and transformed into upland cotton. We finally obtained 3 transformants (#1, #2 and #3) with GhPSY2D highly expressed in developing seeds (Fig. 3). As expected, GhPSY2D transcript (Fig. 3B) and carotenoid (Fig. 3C) levels in the transgenic embryos dramatically increased at the mid-to-late stage (after 25 DPA) and the embryos turned golden accordingly (Fig. 3A). The mature pV:GhPSY2D cottonseeds had golden kernel and the carotenoid contents in the extracted oil of transgenic cottonseeds were significantly higher (over 6-fold) than that from the wild type (Fig. 4A–D). Importantly, thin layer chromatography (TLC) analysis indicated that the major carotenoid increased in the transgenic cottonseeds was β-carotene (Fig. 4E), which was the main plant PVA carotenoid. Moreover, the pV:GhPSY2D cottons showed no obvious defect in plant growth and fiber development compared to the wild-type control (Supplementary Fig. S4), although the germination rates of transgenic cottonseeds decreased as reported in Arabidopsis (Supplementary Fig. S5)40. These results demonstrated that the transgenic cottonseeds were successfully bio-fortified for PVA, rendering the transgenic cotton a potential tool against VAD in cotton-growing regions including south Asia and sub-Sahara Africa32,33.
Figure 3.
GhPSY2D expression and carotenoid accumulation in the developing cottonseeds. Colors (A), GhPSY2D transcript levels (B) and carotenoid contents (C) in the developing embryos (20–35 DPA) of the transformants #1, #2 and #3, and the wild-type control (WT, a null segregant of transformant #1) are indicated.
Figure 4.
Carotenoids in mature cottonseeds. (A) Transverse view of mature seed kernels; (B) Seed kernel powder; (C) Cottonseed oils; (D) Total carotenoid content in cottonseed oils; (E) TLC analysis of carotenoid components in cottonseed oils. Standards β-carotene is separated along with samples.
Discussion
The objective of the present work is to generate PVA bio-fortified cottonseeds, which may be useful tool against VAD prevalence in low-income regions. On the basis of comprehensive identification of carotenoid synthase genes in the assembled cotton genomes, we compared the transcript levels of these genes in various cotton tissues, including developing ovules, and demonstrated that the transcription of PSY genes might be the limiting factor for carotenoid biosynthesis and accumulation in cottonseeds. Next, we cloned the GhPSY2 genes, and confirmed their biological functions by over-expression in tobacco. Finally, the GhPSY2D gene driven by a seed-specific promoter pV was transformed into upland cotton, and significantly expressed in the mid-to-late embryos. The resultant transgenic cottonseeds had golden kernel, and the extracted oil contained significantly higher level of carotenoids, especially β-carotene (the major active PVA). Given cottons are widely grown as cash crop in south Asia and sub-Sahara Africa32,33, the PVA bio-fortified transgenic cottonseeds may be quite potential to prevent VAD prevalence in these regions.
To prevent VAD prevalence, a wealth of crops were bred or engineered to contain high levels of carotenoids or PVAs6,24. Compared with the previously reported PVA bio-fortified crops, cotton has significant advantages in the battle against VAD prevalence. Firstly, cottonseeds are lipid-rich and PVAs are easily extracted with cottonseed oil (Fig. 4)30,31,41. As reported, fat in diet significantly increase the β-carotene bioavailability42,43, implying that enhanced PVA in cottonseed and cottonseed oil should be more easily utilized by human compared with these in starch-rich crops. Secondly, human malnutrition, including VAD, generally occurs in developing regions, where the demands on economic development and nutrient improvement, somewhat mutually dependent and contradictory, are both pressing. As one of the most important cash crops in developing regions including south Asia and sub-Sahara Africa32,33, cotton plays a crucial role in economic development and poverty reduction. PVA bio-fortified transgenic cottons can simultaneously meet the demands on PVA supply and income increments, therefore may become a powerful tool in the battle against poverty and VAD prevalence. Notably, our method is easy to combine with other tactics, such as specific inhibition of gossypol synthesis via RNA interfering31, to further enhance the nutrient value and utilization of cottonseeds.
Carotenoid biosynthesis in plants involves a multi-step complex pathway (Fig. 1)6,9–11. The final carotenoid level and profile in a certain tissue are collectively determined by the substrate accessibility and enzyme activities catalyzing these synthesis steps. For example, the golden rice from upregulation of PSY and CrtI mainly accumulates β-carotene, instead of lycopene, attributing to the constitutively expressed intrinsic carotenoid synthases44. Before the sequenced genomes and comprehensive expression data available, designing strategies to engineer carotenoid and other secondary metabolites largely depended on experience and generally multiple enzymes were simultaneously targeted, which substantially added difficulty in gene manipulation5,15,26,45. In this work, we designed the transgenic strategy for PVA bio-fortification on the basis of comprehensive identification and expression analysis of carotenoid synthase genes using assembled genomes and transcriptomic data (Table 1 and Fig. 1), and obtained the golden cottonseed by specifically upregulating a single gene (GhPSY2D). Consistent with the very low transcript levels of the encoding genes of LCY-ε, ZEP and VDE, and moderate expressions of PDS, ZISO, ZDS, crtISO, LCY-β and CHY-β genes in the late-stage cottonseeds (35 DPA, Fig. 1), GhPSY2D upregulation in cottonseeds mainly promoted the accumulation of β-carotene and another carotenoid, probably zeaxathin (Fig. 4E). These results indicated that comprehensive evaluation of all the genes involved in a certain pathway may be a useful foundation for manipulation of secondary metabolites in plants.
Methods
Identification and expression analysis of carotenoid synthase genes in cotton
To identify the carotenoid synthase genes in cottons, Arabidopsis proteins were employed as probe to query homologous sequences from G. raimondii genome in phytozome (https://phytozome.jgi.doe.gov/)46. The resultant G. raimondii sequences were aligned with all the annotated proteins of certain synthase, and subjected to construct a neighbor-joining tree with 1000 replicates of bootstrap test in MEGA6.047. The homologs clustered with certain Arabidopsis carotenoid synthase were regarded as carotenoid synthases in G. raimondii. The corresponding orthologous genes in G. arboreum and G. hirsutum were identified with a standalone BLAST software using G. raimondii genes as probe48.
The transcript levels of all predicted G. hirsutum genes were evaluated routinely using public available transcriptomic data34 released by Zhang’s lab (http://mascotton.njau.edu.cn). The transcript levels (in FPKM, fragment per kilobase per million) of identified carotenoid synthase genes in various G. hirsutum tissues were depicted as heat map using the program HemI 1.049.
RNA Extraction and qRT-PCR
Total RNAs were extracted from approximately 100 mg of plant tissues using a rapid plant RNA extraction kit (Aidlab, Beijing, China). The first-stranded cDNAs were synthesized from 1 µg total RNA using a reverse transcriptase kit (TaKaRa, Dalian, China). Quantitative PCR was performed on a CFX96 real-time PCR detection system using SYBR Green Supermix (Bio-Rad, CA, USA) and gene-specific primers (Table S1). The thermocycling parameters were as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, 57 °C for 20 s, and a standard melting curve to monitor PCR specificity. The actin50 and histone3 (AF024716)51 gene were amplified as internal control in tobacco and cotton, respectively. The analyses included three biological replicates and data were analyzed using the software Bio-Rad CFX Manager 2.0 provided by the manufacturer.
Cloning and bioinformatics analysis of GhPSY2
A pair of primers (GhPSY2-U and GhPSY2-D, Supplementary Table S2) encompassing the full-length ORF, designed according to the GhPSY2 sequences identified in G. hirsutum, was employed to amplify the cDNA coding sequences from leaves. The reaction included 1 μl first-stranded cDNA, 0.2 μM each primer, and 1 × PrimeSTAR max Premix (TaKaRa, Dalian, China), and amplified for 35 cycles of 98 °C for 10 s, 56 °C for 15 s and 72 °C for 40 s. The PCR products were cloned into pGEM-T easy vector (Promega, Shanghai, China), sequenced in BGI (Shenzhen, China), and further compared to the GhPSY2 genes identified in the assembled G. hirsutum genome. GhPSY2 proteins were aligned with Arabidopsis, rice and maize PSYs from Phytozome (https://phytozome.jgi.doe. gov/)46 using ClustalW, and the NJ tree was constructed with 1000 replicates of bootstrap test in MEGA6.047.
Vector construction and plant transformation
A modified pBI121 vector p5 (pBI121-GN), containing selection marker NPTΙΙ and GUS genes, was used to construct the plant expression vectors52. The cDNA sequences of GhPSY2D ORF was excised from the cloning vector pGEM-T easy using BamHΙ and EcoRΙ, and inserted downstream to a CaMV35S promoter in the p5 vector restricted by the same enzymes, resulting in the overexpression vector. To construct seed-specific expression vectors, the promoter of Phaseolus vulgaris β-type phaseolin storage protein gene (pV, GenBank accession no. J01263.1)39, were amplified with restriction sites of HindΙΙΙ and BamHΙ (Table S1). The promoter was constructed upstream to GhPSY2D by replacing the CaMV35S promoter in the overexpression vector using HindΙΙΙ and BamHΙ sites. All these expression vectors were transferred into Agrobacterium tumefaciens strains (LBA4404), and the resulting Agrobacterium strain was used for tobacco and cotton (Jimian No. 14) transformation as previously described50,52.
Carotenoid extraction and analysis
Carotenoids in fresh tobacco tissues and developing cottonseeds were extracted according Fuentes’ method53 with some modification. In brief, approximately 500 mg of fresh tissues were ground to fine powder in liquid nitrogen, extracted for 15 min in 3 ml hexane/acetone/ethanol (2:1:1 v/v/v) with shacking. Two to three successive extractions were performed to remove carotenoids until the tissues were colorless. The extracts were combined, dried with nitrogen, and re-suspended in 1 ml acetone. Total carotenoids were measured spectrophotometrically at 474 nm, and quantified according to a standard curve of β-carotene. The extractions were repeated in three biological replicates.
Carotenoids in dry mature cottonseeds were extracted along with oil with Soxhlet extractor (Buchi B-811, Switzerland) with ether. The resultant cottonseed oil were 20-fold diluted in acetone and subjected to spectrophotometric quantification of total carotenoids as mentioned above and TLC analysis54. Ten microliter of diluted cottonseed oil were loaded and separated on Silica gel plate (0.2 mm-thick, Jiangyou Silica Gel Co., Yantai, Shangdong, China) along with 5 μg β-carotene standards. The plate was developed in hexane:ether:acetone (60:30:20, v/v) and photographed directly.
Electronic supplementary material
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (30971713 and 31571582 to Y.X.) and by the Genetically Modified Organisms Breeding Major Project of China (2016ZX08005005–001 to Y.X.).
Author Contributions
D.Y. and Y.X. conceived the experiments and wrote the paper, D.Y., Y.W., Q.L. and X.O. conducted the experiments, D.Y., Y.W., Q.L., Y.L. and Y.X. analyzed the data, C.W., L.D., L.H. and M.L. contributed reagents/materials/analysis tools. All authors reviewed manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Dan Yao and Yi Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19866-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Underwood BA. Vitamin A deficiency disorders: international efforts to control a preventable “pox”. The Journal of nutrition. 2004;134:231S–236S. doi: 10.1093/jn/134.1.231S. [DOI] [PubMed] [Google Scholar]
- 2.Harjes CE, et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science (New York, N.Y.) 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens GA, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. The Lancet Global Health. 2015;3:e528–e536. doi: 10.1016/S2214-109X(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 4.Hamer DH, Keusch GT. Vitamin A deficiency: slow progress towards elimination. The Lancet Global Health. 2015;3:e502–e503. doi: 10.1016/S2214-109X(15)00096-0. [DOI] [PubMed] [Google Scholar]
- 5.Paul JY, et al. Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol J. 2017;15:520–532. doi: 10.1111/pbi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano GP. A biofortification of crop plants: a gold rush with many miners. Current opinion in biotechnology. 2017;44:169–180. doi: 10.1016/j.copbio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Girard AW, et al. Promotion of Orange-Fleshed Sweet Potato Increased Vitamin A Intakes and Reduced the Odds of Low Retinol-Binding Protein among Postpartum Kenyan Women. The Journal of nutrition. 2017;147:955–963. doi: 10.3945/jn.116.236406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Moura FF, et al. Biofortified beta-carotene rice improves vitamin A intake and reduces the prevalence of inadequacy among women and young children in a simulated analysis in Bangladesh, Indonesia, and the Philippines. The American journal of clinical nutrition. 2016;104:769–775. doi: 10.3945/ajcn.115.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enfissi EM, Nogueira M, Bramley PM, Fraser PD. The regulation of carotenoid formation in tomato fruit. The Plant journal: for cell and molecular biology. 2017;89:774–788. doi: 10.1111/tpj.13428. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Sola, M. Á. & Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. The Arabidopsis Book, e0158, doi:10.1199/tab.0158 (2012). [DOI] [PMC free article] [PubMed]
- 11.Nisar N, Li L, Lu S, Khin NC, Pogson BJ. Carotenoid metabolism in plants. Molecular plant. 2015;8:68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Toledo-Ortiz G, Huq E, Rodriguez-Concepcion M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11626–11631. doi: 10.1073/pnas.0914428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsch R, et al. Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell. 2010;22:3348–3356. doi: 10.1105/tpc.110.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. The Plant journal: for cell and molecular biology. 1999;20:401–412X. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 15.Bai C, et al. Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotechnol J. 2016;14:195–205. doi: 10.1111/pbi.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, et al. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science (New York, N.Y.) 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 17.Paine, J. A. et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotech23, 482-487, http://www.nature.com/nbt/journal/v23/n4/suppinfo/nbt1082_S1.html (2005). [DOI] [PubMed]
- 18.Zeng J, et al. Metabolic Engineering of Wheat Provitamin A by Simultaneously Overexpressing CrtB and Silencing Carotenoid Hydroxylase (TaHYD) Journal of agricultural and food chemistry. 2015;63:9083–9092. doi: 10.1021/acs.jafc.5b04279. [DOI] [PubMed] [Google Scholar]
- 19.Che P, et al. Elevated vitamin E content improves all-trans beta-carotene accumulation and stability in biofortified sorghum. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:11040–11045. doi: 10.1073/pnas.1605689113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqvi S, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7762–7767. doi: 10.1073/pnas.0901412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman J, et al. The Arabidopsis ORANGE (AtOR) gene promotes carotenoid accumulation in transgenic corn hybrids derived from parental lines with limited carotenoid pools. Plant cell reports. 2017;36:933–945. doi: 10.1007/s00299-017-2126-z. [DOI] [PubMed] [Google Scholar]
- 22.Sayre R, et al. The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annual review of plant biology. 2011;62:251–272. doi: 10.1146/annurev-arplant-042110-103751. [DOI] [PubMed] [Google Scholar]
- 23.Diretto G, et al. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC plant biology. 2006;6:13. doi: 10.1186/1471-2229-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang L, et al. Metabolic engineering of carotenoids in transgenic sweetpotato. Breeding science. 2017;67:27–34. doi: 10.1270/jsbbs.16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosati C, et al. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. The Plant journal: for cell and molecular biology. 2000;24:413–419. doi: 10.1046/j.1365-313x.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, et al. Genetic modification of the soybean to enhance the beta-carotene content through seed-specific expression. PLoS One. 2012;7:e48287. doi: 10.1371/journal.pone.0048287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diretto G, et al. Metabolic Engineering of Potato Carotenoid Content through Tuber-Specific Overexpression of a Bacterial Mini-Pathway. PLoS ONE. 2007;2:e350. doi: 10.1371/journal.pone.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S, et al. Orange protein has a role in phytoene synthase stabilization in sweetpotato. Scientific reports. 2016;6:33563. doi: 10.1038/srep33563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, et al. Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3558–3563. doi: 10.1073/pnas.1420831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan D, et al. The genome sequence of Sea-Island cotton (Gossypium barbadense) provides insights into the allopolyploidization and development of superior spinnable fibres. Scientific reports. 2015;5:17662. doi: 10.1038/srep17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunilkumar G, Campbell LM, Puckhaber L, Stipanovic RD, Rathore KS. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18054–18059. doi: 10.1073/pnas.0605389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Statista. World cotton production by country 2016, https://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/ (2017).
- 33.Staritz, C. & Tröster, B. Cotton-based development in Sub-Saharan Africa? Global commodity chains, national market structure and development outcomes in Burkina Faso, Mozambique and Tanzania. Working Papers (2015).
- 34.Zhang T, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nature biotechnology. 2015;33:531–537. doi: 10.1038/nbt.3207. [DOI] [PubMed] [Google Scholar]
- 35.Li F, et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nature genetics. 2014;46:567–572. doi: 10.1038/ng.2987. [DOI] [PubMed] [Google Scholar]
- 36.Paterson AH, et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature. 2012;492:423–427. doi: 10.1038/nature11798. [DOI] [PubMed] [Google Scholar]
- 37.Fray RG, et al. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. The Plant Journal. 1995;8:693–701. doi: 10.1046/j.1365-313X.1995.08050693.x. [DOI] [Google Scholar]
- 38.Chen ZL, Pan NS, Beachy RN. A DNA sequence element that confers seed-specific enhancement to a constitutive promoter. The EMBO journal. 1988;7:297–302. doi: 10.1002/j.1460-2075.1988.tb02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slightom JL, Sun SM, Hall TC. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindgren LO, Stalberg KG, Hoglund AS. Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant physiology. 2003;132:779–785. doi: 10.1104/pp.102.017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukonge E, Labuschagne MT, Hugo A. The evaluation of oil and fatty acid composition in seed of cotton accessions from various countries. Journal of the Science of Food and Agriculture. 2007;87:340–347. doi: 10.1002/jsfa.2731. [DOI] [Google Scholar]
- 42.La Frano MR, de Moura FF, Boy E, Lonnerdal B, Burri BJ. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutrition reviews. 2014;72:289–307. doi: 10.1111/nure.12108. [DOI] [PubMed] [Google Scholar]
- 43.Lipkie TE, et al. Bioaccessibility of carotenoids from transgenic provitamin A biofortified sorghum. Journal of agricultural and food chemistry. 2013;61:5764–5771. doi: 10.1021/jf305361s. [DOI] [PubMed] [Google Scholar]
- 44.Schaub P, Al-Babili S, Drake R, Beyer P. Why Is Golden Rice Golden (Yellow) Instead of Red? Plant Physiology. 2005;138:441–450. doi: 10.1104/pp.104.057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka Y, Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Current opinion in biotechnology. 2008;19:190–197. doi: 10.1016/j.copbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Goodstein DM, et al. Phytozome: a comparative platform for green plant genomics. Nucleic acids research. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 49.Deng W, Wang Y, Liu Z, Cheng H, Xue Y. HemI: a toolkit for illustrating heatmaps. PLoS One. 2014;9:e111988. doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo K, et al. Leaf senescence is delayed in tobacco plants expressing the maize knotted1 gene under the control of a wound-inducible promoter. Plant cell reports. 2006;25:1246–1254. doi: 10.1007/s00299-006-0178-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhu YQ, Xu KX, Luo B, Wang JW, Chen XY. An ATP-binding cassette transporter GhWBC1 from elongating cotton fibers. Plant physiology. 2003;133:580–588. doi: 10.1104/pp.103.027052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo M, et al. GhDET2, a steroid 5α-reductase, plays an important role in cotton fiber cell initiation and elongation. The Plant Journal. 2007;51:419–430. doi: 10.1111/j.1365-313X.2007.03144.x. [DOI] [PubMed] [Google Scholar]
- 53.Fuentes P, et al. Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant molecular biology. 2012;79:47–59. doi: 10.1007/s11103-012-9893-2. [DOI] [PubMed] [Google Scholar]
- 54.Angaman D, et al. Precursor uptake assays and metabolic analyses in isolated tomato fruit chromoplasts. Plant Methods. 2012;8:1. doi: 10.1186/1746-4811-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.