Introduction
Junctional ectopic tachycardia (JET) is one of the rarest forms of fetal tachycardia. When associated with maternal anti-SSA (Sjogren) antibodies,1, 2 JET is intermittent, with variable fetal heart rate (FHR) and decreases in frequency as gestation progresses. In the absence of anti-SSA antibodies, JET can also present as an incessant tachycardia with minimal heart rate variability, 1:1 ventriculoatrial (VA) conduction, or VA dissociation and discordant atrial and ventricular rates.3, 4, 5, 6 Incessant JET with 1:1 VA conduction can mimic sinus tachycardia, a slow atrioventricular reentrant tachycardia (AVRT), accelerated ventricular rhythm, or slow ventricular tachycardia by fetal echocardiography/Doppler. Given the slow tachycardia rate, it can be overlooked as the etiology for hydrops fetalis, which occurs in >50% of fetal JET cases, even when rates are minimally elevated.3, 4, 5, 6 Even when it is correctly identified, treatment of incessant fetal JET is notoriously difficult: in most cases, tachycardia rate can be controlled but infants are frequently delivered prematurely.3, 4, 5, 6 We report the first case of incessant fetal JET successfully treated in utero after arrhythmia confirmation by fetal magnetocardiography (fMCG). To demonstrate the benefits of fMCG, we compare the management of the successfully treated fetus with a second fetus with JET evaluated only by fetal echocardiography.
Case report
Case 1
A healthy secunda gravida 23-year-old woman was referred at 20-3/7 weeks gestational age (GA) with fetal tachycardia (FHR 175–180 beats per minute [bpm]) and hydrops fetalis. The fetal heart was structurally normal, with normal systolic function and a regular cardiac rhythm with a 1:1 AV conduction but minimal heart rate variability. Increased retrograde flow during atrial systole was noted by spectral Doppler in the ductus venosus and the hepatic and pulmonary veins, and ventricular filling was monophasic. Ascites and a small pericardial effusion were also present. The differential diagnosis included incessant atrial ectopic or sinus tachycardia, accelerated ventricular rhythm, ventricular tachycardia, or JET. Maternal antithyroglobulin antibodies and anti-SSA/SSB antibodies were negative; fetal anemia and infection were excluded by ultrasound and lab titers, respectively. An fMCG using a 21-channel biomagnetometer (Tristan 624 Biomagnetometer, Tristan Technologies, Inc, San Diego, CA) in a magnetically shielded room was performed at 21-5/7 weeks GA.7 The dominant rhythm was an incessant narrow QRS tachycardia with a cycle length of 333 ms (181 bpm) (Figure 1A–C). There was predominantly 1:1 retrograde conduction (VA = 77 ms) and brief episodes of VA dissociation (Figure 1B) and sinus capture beats consistent with JET.
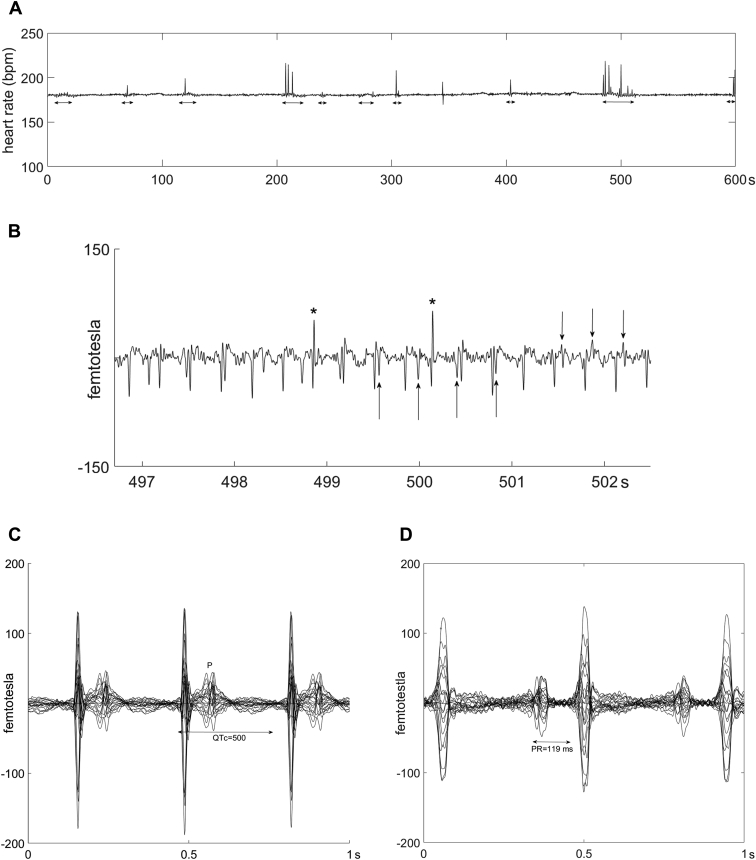
Figure 1.
Case 1: Fetal magnetocardiography (fMCG) recordings during and after junctional ectopic tachycardia (JET). A: MCG fetal heart rate (FHR) trend recording over 10 minutes at 20 weeks gestational age (GA) during incessant JET (181 beats per minute). Low FHR variability is seen, except during ventriculoatrial (VA) dissociation with resultant sinus capture (horizontal arrows). Periods of VA dissociation are associated with increased beat-to-beat heart rate variability and spikes in FHR owing to shortened R-R intervals from conducted beats. B: fMCG rhythm tracing at 20 weeks GA during JET. The sinus P waves are indicated by upward arrows. Retrograde P waves are indicated by downward arrows. The 2 early beats (asterisks) are preceded by a P wave and have different QRS morphology, consistent with conducted sinus capture beats. These support that antegrade atrioventricular node conduction is still present. C: Signal-averaged butterfly (20 weeks GA) showing retrograde bifid P waves occurring within the lower-amplitude T waves. QTc is prolonged. Cardiac intervals are as follows: R-R = 333 ms, VA = 77 ms, QRS = 33 ms, and QTc = 513 ms. D: Signal-averaged butterfly plot at 25 weeks GA during sinus rhythm showing tall P waves and P-R prolongation.
Based on the fMCG results, the fetus was treated with transplacental digoxin and sotalol was added (maximum dose 480 mg/day). The FHR decreased to 170 bpm, but hydrops did not improve. At 28-4/7 weeks GA, sotalol and digoxin were discontinued and amiodarone was given to the mother (600 mg orally every 8 hours × 24 hours, followed by 300 mg orally every 12 hours). After 2 weeks of amiodarone treatment a second fMCG was performed. The rhythm had converted to sinus rhythm (Figure 1D). Hydrops fetalis and the increased retrograde flow in the pulmonary and hepatic veins resolved, but ventricular filling remained monophasic despite sustained sinus rhythm. At 38-1/7 weeks GA a vigorous 2.654 kg female infant (Apgars 9 and 9 at 1 and 5 minutes) was delivered by repeat C-section. The initial 12-lead electrocardiograms (ECGs) showed junctional rhythm (rate 115 bpm) and left bundle branch block (QRS duration 102 ms). Brief periods of 2:1 AV block occurred during sleep (Figure 2A and B). The infant was treated with propranolol, 2 mg/kg/day. Over the next 2 days, intermittent episodes of JET at 150–170 bpm were noted and the QRS remained prolonged (Figure 2C), but by day of life (DOL) 2, the QRS duration had normalized to 58 ms and the infant was in sustained sinus rhythm (Figure 2D). The transient left bundle branch block and prolonged QRS duration may have been due to the dual effects of the amiodarone and sotalol on the His-Purkinje system. Even though the sotalol had been stopped weeks before birth, sotalol concentrates in amniotic fluid and can be reabsorbed through fetal swallowing. The infant’s postnatal course was complicated by moderate pulmonary hypertension, which resolved on oxygen. The infant was discharged to home on DOL 5; now 3 months of age, she remains in sinus rhythm.
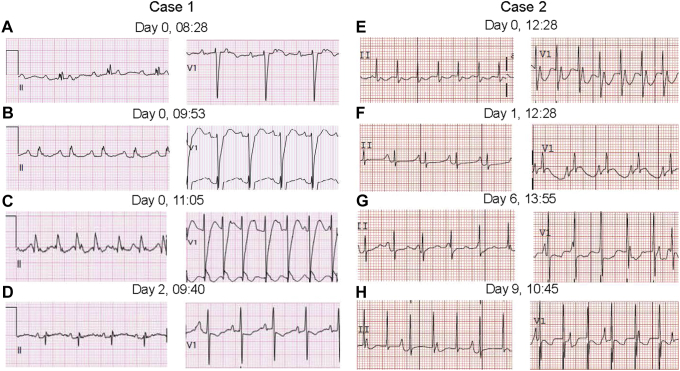
Figure 2.
Cases 1 and 2 postnatal electrocardiograms (ECGs) (leads II and V1). Case 1: At age < 1 day (A–C) and age 2 days (D). A: Second-degree atrioventricular (AV) block, left bundle branch block (LBBB). B: Sinus rhythm with first-degree AV block, LBBB. C: Junctional ectopic tachycardia (JET) demonstrating probable drug-related LBBB. D: Sinus rhythm; the LBBB has resolved. Case 2: At age < 1 day (E, F) and age 6 and 9 days (G, H). All ECGs are recorded at an amplitude of 0.1 mV/mm and at 25 mm/s. E: JET, 1:1 retrograde ventriculoatrial (VA) conduction. F: JET, VA dissociation possibly secondary to flecainide. G: JET with VA dissociation, the sinus capture beat in V1 suggests intact AV conduction.
Case 2
A 27-year-old mother with a normal medical, family, and obstetric history was referred at 31-6/7 weeks GA in her third pregnancy for evaluation of fetal tachycardia (FHR 185–190 bpm). As in case 1, the fetus was hydropic with a structurally and functionally normal fetal heart. A 1:1 VA relationship with simultaneous atrial and ventricular contractions was seen by spectral Doppler in the ascending aorta and superior vena cava (Figure 3A). No VA dissociation was seen but rare sinus capture beats were observed (Figure 3B). Significant flow reversal during atrial systole was noted in the pulmonary veins and ductus venosus (Figure 3C). The peak prograde flow velocity during early diastole was about half normal (0.37 vs 0.7 cm/s).8 We suspected either JET or ventricular tachycardia, and admitted the mother for antiarrhythmic treatment with flecainide and digoxin. As the fetus was viable, dexamethasone was also given to promote lung maturity. Maternal Sjogren antibodies and antithyroid antibodies were negative. Twenty-four hours after treatment, the FHR slowed to the 150s, but the fetus remained in JET, slowing to the 120s with treatment, but no change in the retrograde hepatic and pulmonary venous flow occurred. As in the first case, ventricular filling was monophasic despite FHRs in the 120s.
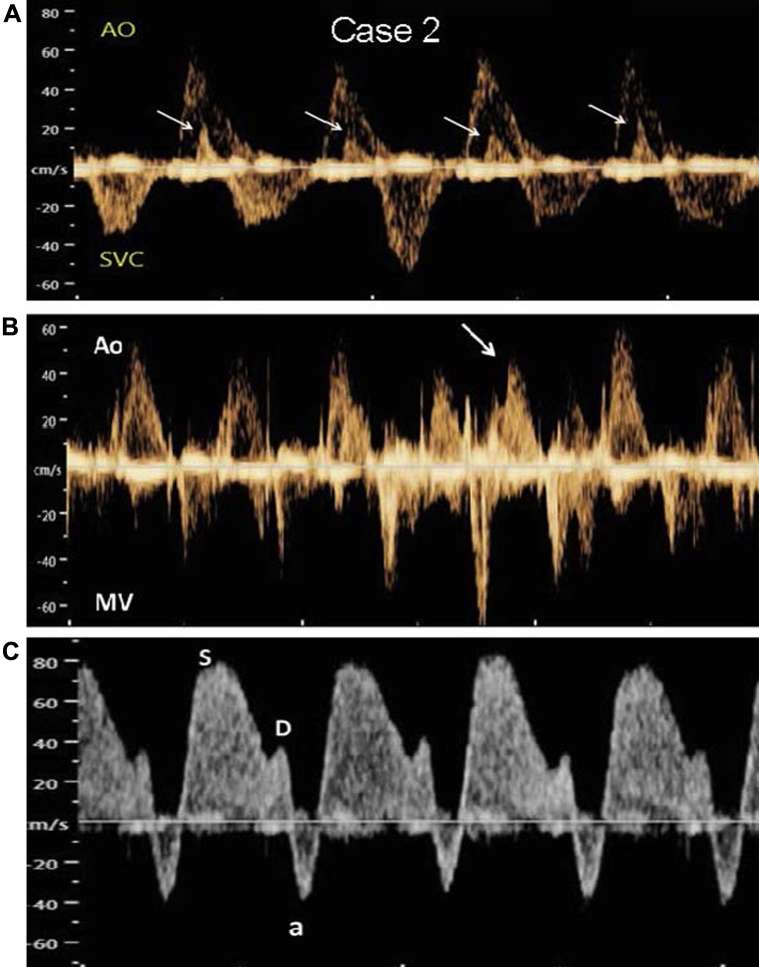
Figure 3.
Case 2. Spectral Doppler images of junctional ectopic tachycardia (JET). A: Ascending aorta (Ao) and superior vena cava (SVC) waveforms. Atrial systole (seen as retrograde SVC flow velocities [arrows]) occurs simultaneously with ventricular systole (antegrade Ao flow velocity). B: A sinus capture beat (arrow) is seen from simultaneous mitral E inflow (MV) and Ao outflow. C: Increased retrograde flow during atrial systole (atrial wave [a] below baseline) is seen in the ductus venosus. Flow toward the heart is biphasic (S and D waves).
The effusions resolved over the next week. Because we could not confirm the diagnosis (JET vs ventricular tachycardia) or a normal fetal QT interval and because the fetus had reassuring biophysical profile scores, we did not change treatment.
At 37 weeks GA, a 3.0 kg female infant was delivered by C-section (1- and 5-minute Apgars, 9 and 9). The maternal/infant flecainide levels were 0.4/0.3 μg/mL, respectively. The 12-lead ECG revealed a narrow complex tachycardia (343 ms) with predominantly 1:1 VA conduction but occasional VA dissociation and intermittent sinus capture beats (Figure 2E). Because of systemic hypotension and pulmonary hypertension, the infant was intubated and received an infusion of intravenous vasopressors and calcium for the first 72 hours of life. Telemetry revealed brief periods of sinus rhythm with first-degree AV block (PR 188 ms) and a prolonged QTc (534 ms) interval (Figure 2F). The infant was treated with flecainide and propranolol, which controlled the JET rate to ∼140–160 bpm (Figure 2G and H). She was discharged on DOL 13, still predominantly in JET (Figure 2H). At 1 month of age, she remains in JET at 130–160 bpm.
Discussion
These cases demonstrate the value of fMCG in the diagnosis and management of incessant fetal tachycardia with hydrops fetalis. Confirming the mechanism as JET by fMCG was instrumental in the successful treatment, elective term delivery, and short hospitalization of the infant in case 1. In contrast, even though with treatment hydrops resolved, the tachycardia rate was controlled, and the infant delivered at 37 weeks, the infant in case 2 required intubation and pressor support and was hospitalized for 2 weeks.
When JET presents antenatally, the difficulty in making an accurate diagnosis and providing appropriate treatment, and the relatively high incidence of hydrops, often result in premature delivery of a hydropic infant.4, 5, 6 In the largest series of 94 pediatric JET patients, 44 of 94 patients presented at <6 months of age and 16 presented during fetal life. All 4 deaths among the 94 subjects occurred in patients presenting at <6 months. Two of the 4 deaths occurred during the first week of life after premature deliveries secondary to incessant fetal tachycardia and hydrops.6 With or without good tachycardia rate control, fetuses with JET should be diagnosed, treated, and delivered at centers of excellence in pediatric arrhythmia and neonatal care. By fetal echocardiography, the VA conduction may appear as simultaneous atrial and ventricular contraction, an unusual finding. Other helpful echocardiography findings include VA dissociation (however, as seen here, this is far rarer than seen postnatally) and marked flow reversal in the venous system.
One of the unique features of JET is the high occurrence of hydrops fetalis despite relatively low tachycardia rates. Causes for this finding may be multifactorial. First, one characteristic of JET is its incessant nature. For AVRT, early GA and longer duration of tachycardia, not tachycardia rate, correlated with hydrops.9 Second, monophasic AV valve inflow patterns and increased reverse flow in the ductus venosus (Figure 3) suggested abnormal diastolic filling and increased right ventricular end-diastolic pressure, both of which may have contributed to the pulmonary hypertension seen after birth. Third, we speculate that retrograde systemic venous flow is exacerbated in JET compared to AVRT because atrial contractions are more coordinated in JET, since they arise from the AV node rather than through an accessory connection.10 This is evidenced by the observation that the P waves in Figure 1C are substantially larger than those typically seen in AVRT.11
The findings of QTc prolongation, as seen prenatally in case 1 and postnatally in case 2, have not been previously reported in JET. We speculate that some patients with JET may also have inherited abnormalities of calcium regulation. Evidence for this speculation is as follows: first, there is a recent finding of a mutation in TNNI3 in a family with conduction system disease and JET.12 TNNI3 encodes for an inhibitory troponin kinase, which regulates myocardial contraction and relaxation.10 Second, a Brugada ECG pattern was recently reported in a febrile 5-year-old child with a history of congenital JET.13 Third, sudden infant death has been reported with JET.6 Finally, 20%–50% of JET recurs in families, which implies a genetic etiology.4, 6, 14 Whether disorders of calcium regulation or channelopathies may be causative in some cases of perinatal JET will require further investigation.
Conclusion
These cases demonstrate the value of fMCG in making a definitive diagnosis of a rare fetal tachycardia and in guiding aggressive treatment while monitoring for fetal proarrhythmia. Even with rate control, fetal JET has significant morbidity, and diagnosis, treatment, and delivery should be planned in a cardiac center of excellence.
Key Teaching Points.
-
•
Fetal magnetocardiography can diagnose incessant congenital junctional ectopic tachycardia (JET), which has unique fetal characteristics, such as predominance of 1:1 ventriculoatrial (VA) conduction, relatively slow rates, and QTc prolongation.
-
•
Despite the relatively slow rate during JET, development of hydrops fetalis is common, as is diastolic dysfunction, and JET should be treated pharmacologically even when fetal heart rates during tachycardia are less than 200 beats per minute. Drugs used to treat fetal JET (flecainide, sotalol, and amiodarone) may produce transient left bundle branch block in the newborn.
-
•
Owing to the complexity of electrophysiologic responses, these fetuses should be cared for in pediatric cardiac centers of excellence.
Footnotes
This work was supported in part by a grant from the National Institutes of Health (RO1HL063174; PI R.T. Wakai, PhD).
References
- 1.Zhao H., Cuneo B.F., Strasburger J.F., Huhta J.C., Gotteiner N.L., Wakai R.T. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51:77–84. doi: 10.1016/j.jacc.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubin A.M., Cuneo B.F., Strasburger J.F., Wakai R.T., Van Hare G.F., Rosenthal D.N. Congenital junctional ectopic tachycardia and congenital complete atrioventricular block: a shared etiology? Heart Rhythm. 2005;2:313–315. doi: 10.1016/j.hrthm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Fouron J., Fournier A., Proulx F., Lamarche J., Bigras J.L., Boutin C., Brassard M., Gamache S. Management of fetal tachyarrhythmia based on superior vena cava/aorta Doppler flow recordings. Heart. 2003;89:1211–1216. doi: 10.1136/heart.89.10.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupoglazoff J.M., Denjoy I., Luton D., Magnier S., Azancot A. Prenatal diagnosis of a familial form of junctional ectopic tachycardia. Prenat Diagn. 1999;19:767–770. doi: 10.1002/(sici)1097-0223(199908)19:8<767::aid-pd617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Villazon E., Fouron J.-C., Fournier A., Proulx F. Prenatal diagnosis of junctional ectopic tachycardia. Pediatr Cardiol. 2001;22:160–162. doi: 10.1007/s002460010186. [DOI] [PubMed] [Google Scholar]
- 6.Collins K.K., Van Hare G.F., Kertesz N.J. Pediatric nonpost-operative junctional ectopic tachycardia: medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690–697. doi: 10.1016/j.jacc.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins D.L., Strasburger J.F., Gotteiner N.L., Cuneo B., Wakai R.T. Magnetophysiologic and echocardiographic comparison of blocked atrial bigeminy and 2:1 atrioventricular block in the fetus. Heart Rhythm. 2013;10:1192–1198. doi: 10.1016/j.hrthm.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecher K., Campbell S., Snijders R., Nicolaides K. Reference ranges for fetal venous and atrioventricular blood flow parameters. Ultrasound Obstet Gynecol. 1994;4:381–390. doi: 10.1046/j.1469-0705.1994.04050381.x. [DOI] [PubMed] [Google Scholar]
- 9.Strasburger J.F., Duffy C.E., Gidding S.S. Abnormal systemic venous doppler flow patterns in atrial tachycardia in infants. Am J Cardiol. 1997;80:640–643. doi: 10.1016/s0002-9149(97)00440-2. [DOI] [PubMed] [Google Scholar]
- 10.Naheed Z.J., Strasburger J.F., Deal B.J., Benson D.W., Gidding S.S. Fetal tachycardia: mechanisms and predictors of hydrops fetalis. J Am Coll Cardiol. 1996;27:1736–1740. doi: 10.1016/0735-1097(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 11.Wakai R.T., Strasburger J.F., Li Z., Deal B.J., Gotteiner N.L. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation. 2003;107:307–312. doi: 10.1161/01.cir.0000043801.92580.79. [DOI] [PubMed] [Google Scholar]
- 12.Xi Y., Honeywell C., Zhang D. Whole exome sequencing identifies the TNNI3K gene as a cause of familial conduction system disease and congenital junctional ectopic tachycardia. Int J Cardiol. 2015;185:114–116. doi: 10.1016/j.ijcard.2015.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crea P, Oreto L, Andò G. Junctional ectopic tachycardia and type 1 Brugada ECG in a pediatric patient: casuality or causality? [published online ahead of print] Cor Vasa. http://dx.doi.org/10.1016/j.crvasa.2016.12.005.
- 14.Villain E., Vetter V.L., Garcia J.M., Herre J., Cifarelli A., Garson A. Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation. 1990;81:1544–1549. doi: 10.1161/01.cir.81.5.1544. [DOI] [PubMed] [Google Scholar]