Introduction
Atrial standstill (AS), a rare arrhythmogenic condition, is defined by (1) the absence of P waves in surface and intracavitary electrocardiograms (ECGs), (2) the absence of A waves in jugular venous pulse and right atrial pressure tracings, (3) the presence of a supraventricular type QRS complex, (4) the immobility of the atria on fluoroscopy, and (5) the inability to stimulate the atria electrically.1 Several etiologies have been identified including metabolic derangements, ischemia, drug intoxication, amyloidosis, and muscular dystrophies.1 The discovery of familial AS leads to the identification of channelopathies involved, particularly SCN5A mutations leading to Nav1.5 sodium channel dysfunction.1 AS is rarely reported in pediatric patients. An 11-year-old with permanent AS was found to have an SCN5A mutation; a 3-year-old developed AS transiently after dexmedetomidine infusion; and systemic illness was implicated in an 8-year-old with thiamine-responsive megaloblastic anemia.1, 2, 3 Here we present a unique case of AS, possibly due to novel caveolin-3 (CAV3) mutation, in a pediatric patient whose clinical presentation and progression were unusual.
Case report
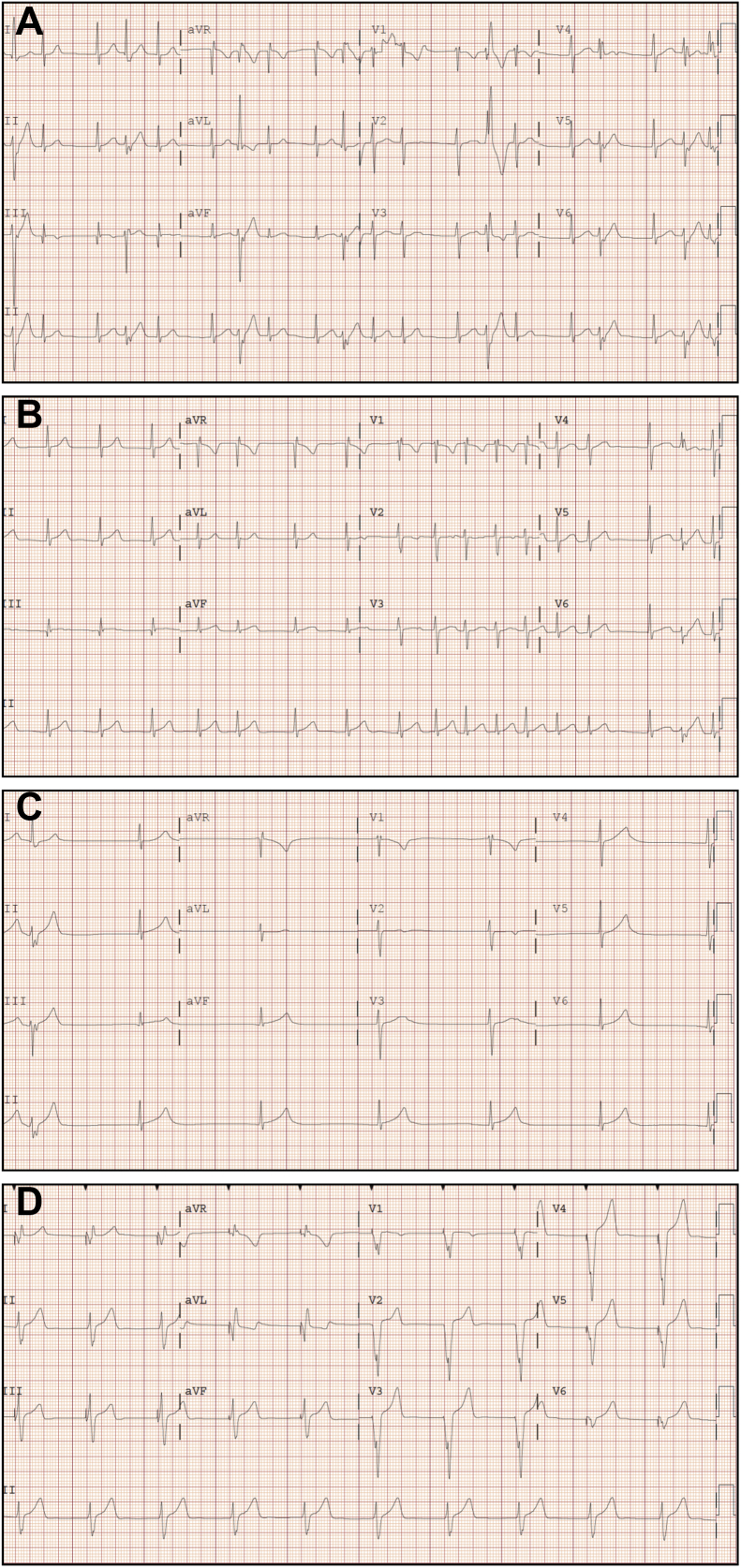
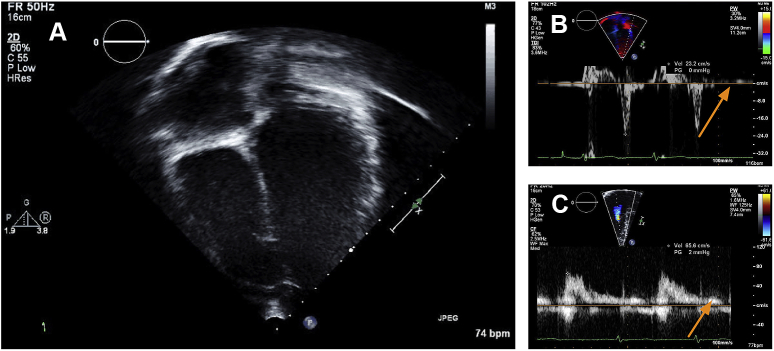
An African American male patient presented with palpitations and syncope at the age of 12 years. His ECG initially revealed accelerated junctional rhythm with intermittent isolated ventricular ectopy (Figure 1A). With metoprolol, he had improved ectopy but Holter monitoring and subsequent ECG showed continued junctional rhythm and episodes of nonsustained atrial tachycardia alternating with junctional bradycardia with 3-second pauses (Figure 1B). Further evaluation with echocardiography showed normal biatrial size with no atrial contraction (Figure 2). Cardiac magnetic resonance imaging showed normal morphology, function, and no delayed myocardial enhancement. Subsequent exercise testing revealed no sinus node activity and no sinus P waves. The rhythm was predominantly junctional with a maximum rate of 184 and episodes of nonsustained atrial tachycardia during exercise and recovery. He also had a 3-second pause during exercise with associated dizziness. Because of these findings and history of fatigue and frequent presyncopal episodes, he was taken off metoprolol, causing improvement in his symptomatic bradycardia but worsening of symptomatic tachyarrhythmia.
Figure 1.
Progressive electrocardiograms (ECGs) demonstrating rhythm over time. A: Initial ECG showing junctional rhythm with isolated ectopic beats. B: ECG from presentation showing junctional rhythm with episodes of nonsustained atrial tachycardia. C: ECG after ablation showing junctional bradycardia and no evidence of atrial activity approximately 2 weeks after the first ECG. D: Last available ECG obtained 5 months after ablation with no evidence of atrial activity.
Figure 2.
Echocardiograms demonstrating (A) lack of atrial contraction, (B) absence of the a′ wave, and (C) absence of the a. wave.
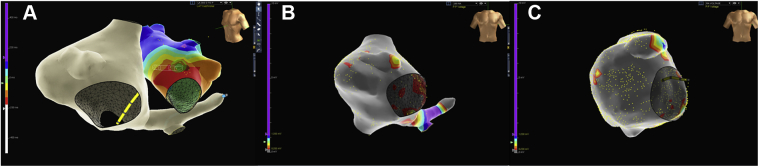
An electrophysiology study showed inducible left anterior mitral valve annulus atrial tachycardia, which was successfully ablated (Figure 3A). The left atrium appeared healthy without evidence of electrical scar. The right atrium showed small to no atrial activity with no atrial capture at high outputs. Voltage mapping showed significant right atrial scar (Figure 3B). After ablation, his symptomatic atrial tachycardia resolved but he remained in junctional rhythm with worsened fatigue and exercise intolerance. A repeat ECG showed marked junctional bradycardia without evidence of atrial activity (Figure 1C). Repeat Holter monitoring showed junctional bradycardia with more frequent and longer pauses, up to 5.1 seconds, and episodes of junctional tachycardia.
Figure 3.
A: Voltage mapping from the first electrophysiology study showing focus of left atrial tachycardia near the anterior mitral annulus, which was subsequently ablated. B and C: Voltage mapping from the first (panel B) and second (panel C) electrophysiology studies demonstrating progressive right atrial scar.
He underwent a second electrophysiology study 4 months after the initial procedure during planned implantation for a dual-chamber pacemaker. This study showed progressive right atrial disease (Figure 3C) and lack of right atrial sensing or capture with transvenous atrial lead placement attempts, so a single-chamber ventricular pacemaker was placed. This clinical course and findings were consistent with AS.
Since device placement, serial ECGs over 2 years continue to show junctional rhythm and follow-up device checks show chronic ventricular pacing with increasing dependence at 69% with heart rate set at 50 beats/min suggesting disease progression (Figure 1D). His family history is reassuring, but his asymptomatic parents refuse evaluation including ECGs and genetic testing. Because of his unusual course, he underwent genetic testing with comprehensive arrhythmia panel through GeneDx (Gaithersburg, MD), which analyzed 30 genes known to cause arrhythmia syndromes. Of those 30 genes, 2 have been associated with AS—RYR2 and SCN5A. Our patient’s testing was negative for RYR2 and SCN5A but did reveal mutation in CAV3. His mutation p.Leu84Pro (L84P) is a semiconservative amino acid substitution. It was initially classified as a likely pathogenic mutation, but given that there are no published reports on this variant, no functional studies, and no segregation data, it was reclassified as a variant of unknown significance. This mutation has not been reported as a benign polymorphism and was not observed in 6500 individuals in the NHLBI GO Exome Sequencing Project.
Discussion
AS with associated tachyarrhythmia and a progressive course is an unusual presentation. Many reports of AS in the literature are in patients with structurally abnormal hearts. In the adult literature, there are reports of structural abnormality related to cardiomyopathies secondary to systemic illnesses as well as related to muscular dystrophies.1 In the pediatric literature, reports typically describe children with congenital heart disease including atrial septal defects, tetralogy of Fallot, and Ebstein anomaly.1, 2, 3 In contrast, our patient had no structural abnormalities. To date, only one other pediatric case of AS without structural abnormalities has been reported in a patient with SCN5A mutation.1
Prior cases of AS have been reported in the adult literature with tachyarrhythmias in Brugada syndrome due to SCN5A mutation and catecholeminergic polymorphic ventricular tachycardia associated with RYR2 mutation.4, 5 Jorat et al6 reported a case of isolated right AS with left atrial tachycardia in a 32-year-old female patient. Like our patient, she presented with chronic fatigue and dyspnea suggestive of bradycardia and a progressive course. She was diagnosed with AS, but no genetic testing was performed. This case, and ours, differs from those in the pediatric literature because of the associated atrial tachycardia and rapidly progressive clinical course.
Cardiac channelopathies have also been implicated as a cause of AS. SCN5A have been identified for some time as causing AS, among other rhythm issues.1, 7 More recently, familial RYR2 has been associated with AS.4 Bhuiyan et al4 described 2 unrelated family pedigrees with RYR2 mutations. In each family, 1 individual developed AS including a 14-year-old female patient. Functional studies have not been performed with the specific mutation found in these patients with AS.4 Other channelopathies have not been reported in relation to AS.
Our patient had neither RYR2 nor SCN5A mutation but was found to have a novel mutation in CAV3. The CAV3 gene codes for CAV3, a protein essential in the formation of caveolae needed for endocytosis and cell signal transduction on muscle cell membranes. Mutations in CAV3 are associated with a variety of human muscular diseases. In skeletal muscles, CAV3 deficiency can cause limb-girdle muscular dystrophy (LGMD), isolated hyperCKemia, rippling muscle disease, and distal myopathy.8 CAV3 is particularly concentrated in cardiomyocytes and plays a large role in cardiac physiology.9 It is therefore unsurprising that mutations in CAV3 are also associated with cardiac disease including hypertrophic cardiomyopathy, long QT syndrome, and SIDS.8, 10, 11, 12
Numerous associations have been identified between CAV3 and ion channels and exchangers, particularly sodium channels and exchangers. Bossuyt et al9 demonstrated that CAV3 coprecipitates with the cardiac sodium-calcium exchanger, NCX1. NCX1 is involved in the regulation of myocardial contractility; therefore, this association emphasizes the role CAV3 plays in contractility. In addition, the human cardiac voltage-gated sodium channel hNav1.5, coded by SCN5A, has been localized to caveolae. Specifically, it has been suggested that CAV3 and hNav1.5 may be part of the same macromolecular protein complex.11 Necessary for fast influx of sodium, the hNav1.5 channel allows depolarization of the cell, leading to the cardiac action potential. The interaction between hNav1.5 and CAV3 again highlights the role CAV3 plays in cardiac contractility and function.
While CAV3 mutations have not previously been associated with AS, SCN5A mutations are among the most commonly genetically identified causes of AS.7 It is therefore plausible that a mutation in CAV3 could inherently prevent normal function of hNav1.5 if the CAV3 protein is necessary for the normal presence of hNav1.5 on cell membranes in caveolae. With this assumption, it may be extrapolated that a mutation in CAV3 could lead to the same phenotypic disease of a mutation in SCN5A by preventing the function of its encoded protein, without having that mutation present.
Beyond the functional relationship between CAV3 and hNav1.5, it is notable that the genes which encode both proteins are relatively near each other in the genome. SCN5A and CAV3 are both found on the third chromosome. An event in genetic replication that mutates one of these genes could feasibly alter the other as well, as they are genetic “neighbors.” Given that CAV3 mutations are implicated in LGMD, which is in itself associated with AS, it is possible that coinheritance of genetic polymorphisms determines whether patients with LGMD have AS.8 If CAV3 mutations become a more recognized etiology of AS, the opposite could be true as well—that polymorphisms determine whether patients with AS manifest LGMD when CAV3 is responsible. Incomplete penetrance in AS due to SCN5A mutations has been attributed to coinherited genetic polymorphisms, so this could also be true with CAV3 mutations and various clinical presentations of the mutation.7
The association between CAV3 and hNav1.5 is well established, as is the dysfunction of hNav1.5 channels in AS, as demonstrated by the commonality of SCN5A mutations in patients with AS. What is novel about this case is the fact that his AS cannot be attributed to a previously associated genetic mutation and the fact that his CAV3 mutation and AS are not accompanied by simultaneous skeletal muscle disease. It is possible that he may develop LGMD in the future and that his illness simply presented with AS rather than other symptoms, though even this would be unusual for the natural history of LGMD. Hong et al noted that “neuromuscular symptoms precede cardiological symptoms in nearly all patients with LGMD1B.”13 It is also possible that the degree to which our patient’s mutation affects his muscles is only clinically relevant in his heart, as suggested by Hayashi et al10 in cases of CAV3 mutation–associated cardiomyopathy without associated skeletal muscle disease. They explain that continuous beating of the heart might make it vulnerable to even minor disturbances whereas the voluntary nature of skeletal muscle contraction could mean that it is not affected by small functional changes.
Lastly, embryological differences between the left and right atria could make the tissues differentially susceptible to genetic mutation such as CAV3. The distribution of connexins and orientation of fibers differs throughout the heart and affect conduction propagation.14 Christoffels and Moorman15 comment in their 2009 article that under pathological conditions such as gene defects, the embryonic origin of certain tissues within the myocardium could play a role in the clinical manifestation of conductive issues. In addition, they highlight the importance of differentiation of the embryonic heart tube via specific regulatory pathways that give rise to the formation and function of the conduction system and associated “conducting” cardiac myocytes based on anatomic location.15 This suggests that there could be differential effects of CAV3 mutations in different parts of the heart based on their embryonic origin.
While there does seem to be evidence in the literature that a CAV3 mutation could lead to AS, it is important to note that this mutation remains classified as a variant of unknown significance and is not definitively disease causing. We are limited in our ability to fully understand this case because of the lack of functional testing as well as the refusal of genetic testing by his family members. With time, it is possible that CAV3 mutations could be associated with other cases of AS, lending support to the hypotheses we have presented here, but at present we are certainly limited in our full understanding of the implications of this patient’s CAV3 mutation and its relationship with his disease.
Conclusion
We have presented a unique, unusual case of pediatric AS with associated focal ectopic atrial tachycardia and progressive clinical course in a patient who has a novel mutation in CAV3.
Key Teaching Points.
-
•
Atrial standstill is a rare arrhythmogenic disorder that has previously been associated with systemic disease and specific channelopathies, particularly SCN5A mutations.
-
•
Reports of atrial standstill are rare in the pediatric literature and are most commonly associated with structural heart disease.
-
•
Caveolin-3 mutations have been previously associated with systemic diseases but have not been previously reported in association with atrial standstill.
-
•
Caveolin-3 mutation is possibly responsible for atrial standstill in the patient reported here.
References
- 1.Baskar S., Ackerman M., Clements D., Mayuga K., Aziz P. Compound heterozygous mutations in the SCN5A-encoded Nav1.5 cardiac sodium channel resulting in atrial standstill and His-Purkinje system disease. J Pediatr. 2014;165:1050–1052. doi: 10.1016/j.jpeds.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Shepard S., Tejman-Yarden S., Khanna S., Davis C.K., Batra A.S. Dexmedetomidine-related atrial standstill and loss of capture in a pediatric patient after congenital heart surgery. Crit Care Med. 2011;39:187–189. doi: 10.1097/CCM.0b013e3181feb4b3. [DOI] [PubMed] [Google Scholar]
- 3.Doğan V., Senocak F., Orün U.A., Ceylan O. Heart failure after transvenous closure of atrial septal defect associated with atrial standstill and thiamine-responsive megaloblastic anemia. Turk Kardiyol Dern Ars. 2013;41:638–641. doi: 10.5543/tkda.2013.63295. [DOI] [PubMed] [Google Scholar]
- 4.Bhuiyan Z.A., van den Berg M.P., van Tintelen P., Bink-Boelkens M.T.E., Wiesfeld A.C.P., Alders M., Postma A.V., van Langen I., Mannens M., Wilde A.A.M. Expanding spectrum of human RYR2-related disease. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 5.Takehara N., Makita N., Kawabe J., Sato N., Kawamura Y., Kitabatake A., Kikuchi K. A cardiac sodium channel mutation identified in Brugada syndrome associated with atrial standstill. J Intern Med. 2004;255:137–142. doi: 10.1046/j.0954-6820.2003.01247.x. [DOI] [PubMed] [Google Scholar]
- 6.Jorat M.V., Nikoo M.H., Yousefi A. Persistent isolated right atrial standstill associated with left atrial tachycardia. Res Cardiovasc Med. 2014;3:e25173. doi: 10.5812/cardiovascmed.25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makita N., Sasaki K., Groenewegen W.A., Yokota T., Yokoshiki H., Murakami T., Tsutsui H. Congenital atrial standstill associated with coinheritance of a novel SCN5A mutation and connexin 40 polymorphisms. Heart Rhythm. 2005;2:1128–1134. doi: 10.1016/j.hrthm.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Gazzero E., Sotgia F., Bruno C., Lisanti M., Minetti C. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossuyt J., Taylor B.E., James-Kracke M., Hale C.C. Evidence for cardiac sodium-calcium exchanger association with caveolin-3. FEBS Lett. 2002;511:113–117. doi: 10.1016/s0014-5793(01)03323-3. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T., Arimura T., Ueda K., Shibata H., Hohda S., Takahashi M., Hori H., Koga Y., Oka N., Imaizumi T., Yasunami M., Kiumura A. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2004;313:178–184. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- 11.Vatta M., Ackerman M., Ye B., Mekielski J.C., Ughanze E.E., Taylor E.W., Tester D.J., Balijepalli R.C., Foell J.D., Li Z., Kamp T.J., Towbin J.A. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 12.Aravamudan B., Volonte D., Ramani R., Gursoy E., Lisanti M.P., London B., Galbiati F. Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 2013;12:2777–2788. doi: 10.1093/hmg/ddg313. [DOI] [PubMed] [Google Scholar]
- 13.Hong J., Ki C., Kim J., Suh Y., Kim J.S., Baek K.K., Kim B.J., Ahn K.J., Kim D. Cardiac dysrhythmias, cardiomyopathy and muscular dystrophy in patients with Emergy-Dreifuss muscular dystrophy and limb-girdle muscular dystrophy type 1B. J Korean Med Sci. 2005;20:283–290. doi: 10.3346/jkms.2005.20.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirzoyev S., McLeod C., Asirvatham S. Embryology of the conduction system for the electrophysiologist. Indian Pacing Electrophysiol J. 2010;10:329–338. [PMC free article] [PubMed] [Google Scholar]
- 15.Christoffels V., Moorman A. Development of the cardiac conduction system: why are some regions of the heart more arrhythmogenic than others? Circ Arrhythm Electrophysiol. 2009;2:195–207. doi: 10.1161/CIRCEP.108.829341. [DOI] [PubMed] [Google Scholar]