Introduction
Almost 90% of triggers for atrial fibrillation (AF) are believed to originate from the pulmonary veins,1 and pulmonary vein isolation is the mainstay of therapy for patients with paroxysmal AF.2 Patients who demonstrate recurrent AF despite pulmonary vein isolation highlight the challenge and need of identifying non–pulmonary vein triggers. Reproducibility and inherent risk of the location of these foci may limit the success.
Case report
We present a 51-year-old woman with recurrent symptomatic paroxysmal AF despite antiarrhythmic therapy and 2 ablation attempts. The patient had a medical history of gastroesophageal reflux disease, Schatzki ring, and Barrett’s esophagus. She was initially diagnosed with paroxysmal AF 2 years prior, and underwent radiofrequency ablation for pulmonary vein isolation under general anesthesia. Dobutamine infusion, programmed stimulation, and adenosine infusion post isolation did not induce any triggers or atrial arrhythmias. Her postprocedural course was complicated by pericarditis and episodes of atrial tachycardia, which were felt to be related to inflammation. She was started on flecainide. She had ongoing palpitations and an event monitor 5 months post ablation demonstrated recurrence of AF. The patient then underwent redo ablation, at which time all 4 veins were found to be isolated from the prior procedure. High-dose dobutamine infusion, programmed stimulation, and adenosine infusion again failed to trigger atrial ectopy. Empiric posterior wall isolation, cavotricuspid isthmus ablation, and superior vena cava isolation was performed. The following day in the hospital the patient again had episodes of atrial tachycardia. The patient continued to have AF after the blanking period and thus a third ablation was discussed, with a focus on non–pulmonary vein triggers while awake.
Her third procedure was performed with no sedation, and it was only at this study that she had reproducible premature atrial contractions and atrial tachycardia with dobutamine infusion. Earliest activation was found at the mid crista terminalis using the Biosense CARTO 3 system. Unfortunately, she demonstrated phrenic nerve capture at the site of early activation, and after brief and unsuccessful radiofrequency applications it was decided to try cryoablation. A 6 mm cryocatheter (Freezor® Xtra; Medtronic, Minneapolis, MN) was advanced into the right atrium and visualized with the Biosense CARTO 3 system (Biosense Webster, Johnson & Johnson, Diamond Bar, CA). A quadripolar catheter was advanced into the right subclavian vein until reliable phrenic capture was achieved from the distal poles. Successful ablation was performed with the cryocatheter with continuous phrenic nerve pacing from the subclavian. Five total 4-minute applications of cryoablation were performed to the area, and the atrial tachycardia terminated with the first application (Figure 1). After a 40-minute waiting period, there was no further evidence of atrial tachyarrhythmias. There was diaphragmatic contraction noted throughout the duration of cryoablation. In follow-up the patient has been free of AF and atrial tachycardia for 9 months based on symptoms and event monitoring.
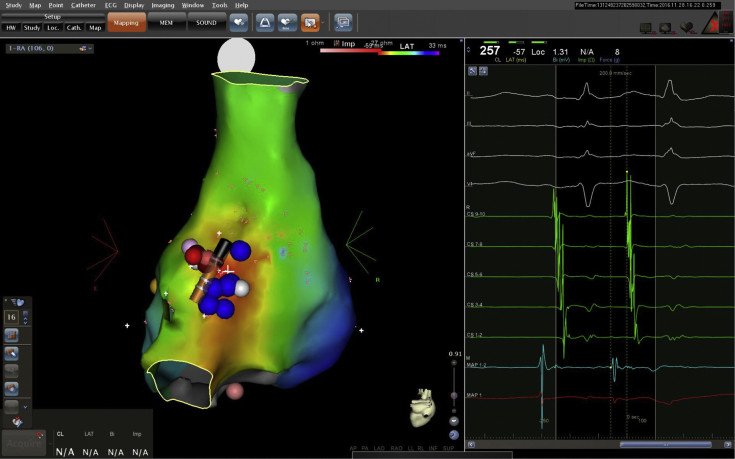
Figure 1.
Posterior view of the right atrium. On left panel, blue dots represent areas of phrenic capture as well as eventual cryoablation lesions. The catheter tip shown is the cryocatheter. Pink and red lesions represent initial failed radiofrequency ablations, which were slightly adjacent to the area of early activation and phrenic capture. Right panel demonstrates activation point of the earliest atrial premature contraction with pre-P wave on ablation distal (MAP1, 2) with QS on unipolar (MAP1).
Discussion
Our case demonstrates the importance of monitoring diaphragmatic stimulation during radiofrequency ablation or cryoablation of non–pulmonary vein triggers in proximity to the phrenic nerve. Our method was extrapolated from our experience with cryoballoon ablation (Medtronic Arctic Front Advance), which is established as an effective means for pulmonary vein isolation in drug-refractory AF.3 Phrenic nerve injury (PNI) is a well-documented complication of cryoablation procedures, specifically when ablating the right-sided pulmonary veins,4 even being described in as many as 10.8% of patients undergoing the procedure.5 One of the strategies designed to minimize PNI during cryoablation involves constant diaphragmatic phrenic nerve pacing through a catheter in the superior vena cava or subclavian vein, and monitoring diaphragmatic contraction.6 Early signs of diaphragmatic injury should be met with immediate termination of cryoablation. Although diaphragmatic pacing has been well studied in cryoablation and isolation of the right-sided pulmonary veins, its use in the focal ablation of non–pulmonary vein triggers with either radiofrequency ablation or cryoablation is less defined.
Although close to 90% of AF originates at the level of the pulmonary veins, non–pulmonary vein triggers for AF are well described. Potential anatomic locations for these triggers include the coronary sinus, the left atrial appendage, the superior vena cava, the crista terminalis, and the ligament of Marshall.7 When standard pulmonary vein isolation fails to eliminate AF, one must investigate these other sites for potential triggers.
Our patient was given dobutamine (with heart rate elevations > 100% of baseline) and adenosine, and had programmed stimulation during her first 2 ablations without inducing atrial ectopy. Despite literature suggesting that the presence of anesthesia does not affect inducibility of triggers,8 it was thought that general anesthesia may have been a factor and bringing the patient back while awake facilitated induction of atrial ectopy.
In addition to keeping the patient awake, we were able to use the Biosense CARTO 3 mapping system to visualize the cryocatheter. This was accomplished by creating a catheter in the Biosense CARTO 3 system with similar size, electrode spacing, and tip electrode length to the cryocatheter. The cryocatheter can then interface with the Biosense CARTO 3 patient interface unit and the catheter can then be visualized once a visualization matrix is created. Owing to interference between the cryocatheter and the Biosense CARTO 3 system, the distal pin of the cryocatheter has to be unplugged from the patient interface unit before the cryo lesion is performed. Finally, the ablation lesions cannot be tracked on Biosense with the cryocatheter, but screenshots of the catheter can be made.
The right phrenic nerve originates in the cervical spine and courses downward through the thoracic cavity to innervate the diaphragm. As it travels downward, it runs rightward and anteriorly to the right pulmonary veins, in closer proximity to the right superior pulmonary vein (1.5–2.5 mm) than the right inferior pulmonary vein (10–15 mm). It also runs in close proximity to the cavoatrial junction (5–8 mm).9 Owing to this close proximity, one runs the risk of phrenic nerve injury in both left-sided and right-sided atrial ablations.
There have been various methods described to avoid phrenic nerve injury during ablations. Some of these methods include preprocedural identification of the phrenic nerve by means of imaging or mapping.10, 11 Avoiding antral catheter positioning during ablation has been described as well. Methods to monitor phrenic nerve function periprocedurally include continuous esophageal temperature monitoring, monitoring of the diaphragmatic compound motor action potentials, and manual diaphragmatic stimulation with continuous observation for loss of diaphragmatic contraction.12 Despite the advent of these methods, PNI remains a complication during catheter ablation.
Because of the imperfection of many of these methods to avoid PNI, manual isolation of the phrenic nerve from the epicardial surface has been described as the best strategy to avoid PNI.13 This is generally accomplished by injecting air or saline into the pericardial space or with balloon inflation, which creates a physical separation between the nerve and the epicardial surface. Although this method of manual separation has been shown to be the most effective way to avoid PNI, the invasive nature of this strategy makes it less desirable as an option.
Although these methods have been studied largely in AF cryoablation, they have not been as well studied in the ablation of non–pulmonary vein triggers. In our case, owing to the atrial focus being in close proximity to the phrenic nerve, we needed to strategize a way to avoid PNI. We hypothesized that those methods devised for PNI protection in left-sided AF ablations would be just as effective for right-sided ablations located anatomically close to the phrenic nerve. We found that constant, manual pacing of the phrenic nerve throughout the procedure with a catheter in the subclavian vein while monitoring for the loss of diaphragmatic contraction was just as effective in preventing PNI as in left-sided ablations. Although we decided to use cryoablation, it is possible that we could have safely ablated with radiofrequency ablation. Given that both cryoablation and radiofrequency ablation can cause transmural lesions, monitoring of phrenic capture is likely more important than the ablation method used. Further studies are needed to demonstrate whether these methods are effective in preventing PNI for non–pulmonary vein ablations at rates similar to those in pulmonary vein isolations. In addition, despite literature suggesting that general anesthesia does not have a significant impact on suppression of triggers,8 our experience suggests the role for limited anesthesia in patients coming for repeat procedures.
Conclusion
Our patient highlights the importance of non–pulmonary vein triggers and the challenges that they may present with ablation. Minimizing anesthesia during repeat procedures allowed finding of a non–pulmonary vein trigger, and visualizing the cryocatheter on the Biosense CARTO 3 system facilitated accurate ablation in the region of early activation. Finally, our experience with constant diaphragmatic pacing, which has been shown to be effective in minimizing PNI risk during pulmonary vein isolation and ablation, provided a technique that allowed for safe ablation in the region of the phrenic nerve and led to a successful outcome.
Key Teaching Points.
-
•
Non–pulmonary vein triggers for atrial fibrillation are often difficult to detect and sometimes even more difficult to treat.
-
•
Strategies to induce non–pulmonary vein triggers for atrial fibrillation include pharmacologic therapies, programmed stimulation, and minimizing anesthesia.
-
•
The strategies to protect surrounding anatomic structures for cryoablation with pulmonary vein isolation can be applied to cryoablation in more unique anatomic locations.
-
•
Constant diaphragmatic pacing is a key to avoiding phrenic nerve injury, and can be applied to both radiofrequency ablation and cryoablation.
References
- 1.Haïssaguerre M., Jaïs P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3.Van Belle Y., Janse P., Rivero-Ayerza M.J. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28:2231–2237. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 4.Kuck K.H., Fürnkranz A. Cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1427–1431. doi: 10.1111/j.1540-8167.2010.01944.x. [DOI] [PubMed] [Google Scholar]
- 5.Guhl E.N., Siddoway D., Adelstein E. Incidence and predictors of complications during cryoballoon pulmonary vein isolation for atrial fibrillation. J Am Heart Assoc. 2016;5:e003724. doi: 10.1161/JAHA.116.003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalski M., Ellenbogen K.A., Koneru J.N. Prevention of phrenic nerve injury during interventional electrophysiologic procedures. Heart Rhythm. 2014;11:1839–1844. doi: 10.1016/j.hrthm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Romero J., Gianni C., Di Biase L., Natale A. Catheter ablation for long-standing persistent atrial fibrillation. Methodist Debakey Cardiovasc J. 2015;11:87–93. doi: 10.14797/mdcj-11-2-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mountantonakis S.E., Elkassabany N., Kondapalli L., Marchlinski F.E., Mandel J.E., Hutchinson M.D. Provocation of atrial fibrillation triggers during ablation: does the use of general anesthesia affect inducibility? J Cardiovasc Electrophysiol. 2015;26:16–20. doi: 10.1111/jce.12512. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Quintana D., Cabrera J.A., Climent V., Farré J., Weiglein A., Ho S.Y. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16:309–313. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
- 10.Horton R., Di Biase L., Reddy V. Locating the right phrenic nerve by imaging the right pericardiophrenic artery with computerized tomographic angiography: implications for balloon-based procedures. Heart Rhythm. 2010;7:937–941. doi: 10.1016/j.hrthm.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt B., Chun K.R., Ouyang F., Metzner A., Antz M., Kuck K.H. Three-dimensional reconstruction of the anatomic course of the right phrenic nerve in humans by pace mapping. Heart Rhythm. 2008;5:1120–1126. doi: 10.1016/j.hrthm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Kühne M., Knecht S., Altmann D. Phrenic nerve palsy during ablation of atrial fibrillation using a 28-mm cryoballoon catheter: predictors and prevention. J Interv Card Electrophysiol. 2013;36:47–54. doi: 10.1007/s10840-012-9740-z. discussion 54. [DOI] [PubMed] [Google Scholar]
- 13.Di Biase L., Burkhardt J.D., Pelargonio G. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957–961. doi: 10.1016/j.hrthm.2009.03.022. [DOI] [PubMed] [Google Scholar]