Abstract
Abstract
Early embryo development and endometrial differentiation are initially independent processes, and synchronization, imposed by a limited window of implantation, is critical for reproductive success. A putative negative regulator of endometrial receptivity is LEFTY2, a member of the transforming growth factor (TGF)-β family. LEFTY2 is highly expressed in decidualizing human endometrial stromal cells (HESCs) during the late luteal phase of the menstrual cycle, coinciding with the closure of the window of implantation. Here, we show that flushing of the uterine lumen in mice with recombinant LEFTY2 inhibits the expression of key receptivity genes, including Cox2, Bmp2, and Wnt4, and blocks embryo implantation. In Ishikawa cells, a human endometrial epithelial cell line, LEFTY2 downregulated the expression of calcium release-activated calcium channel protein 1, encoded by ORAI1, and inhibited store-operated Ca2+ entry (SOCE). Furthermore, LEFTY2 and the Orai1 blockers 2-APB, MRS-1845, as well as YM-58483, inhibited, whereas the Ca2+ ionophore, ionomycin, strongly upregulated COX2, BMP2 and WNT4 expression in decidualizing HESCs. These findings suggest that LEFTY2 closes the implantation window, at least in part, by downregulating Orai1, which in turn limits SOCE and antagonizes expression of Ca2+-sensitive receptivity genes.
Key messages
•Endometrial receptivity is negatively regulated by LEFTY2.
•LEFTY2 inhibits the expression of key murine receptivity genes, including Cox2, Bmp2 and Wnt4, and blocks embryo implantation.
•LEFTY2 downregulates the expression of Orai1 and inhibits SOCE.
•LEFTY2 and the Orai1 blockers 2-APB, MRS-1845, and YM-58483 inhibit COX2, BMP2, and WNT4 expression in endometrial cells.
•Targeting LEFTY2 and Orai1 may represent a novel approach for treating unexplained infertility.
Electronic supplementary material
The online version of this article (10.1007/s00109-017-1610-9) contains supplementary material, which is available to authorized users.
Keywords: Implantation, LEFTY2, ORAI1, SOCE, Calcium, Pregnancy, Miscarriage
Introduction
A transient window of uterine receptivity ensures that embryos implant in an optimal endometrial environment [1]. Failure to establish [2] or premature closure [1, 3, 4] of the implantation window is thought to be a major cause of infertility, which affects ~ 15% of couples. Conversely, prolonged receptivity may lead to out-of-phase implantation and miscarriage [3, 4] or recurrent pregnancy loss [1, 4, 5]. The stromal signals responsible for terminating the window of implantation are not well characterized. A putative candidate is Left-Right Determination Factor 2 (LEFTY2), a cytokine highly expressed by decidualizing stromal cells during the late secretory phase of the menstrual cycle [6], i.e., following closure of the window of implantation and prior to menstruation [7]. LEFTY2, initially designated endometrial bleeding associated factor (EBAF), is a secreted ligand of the transforming growth factor-beta (β) superfamily of proteins [7, 8]. Following proteolytic processing of the secreted precursor, LEFTY2 acts as an antagonist of the Nodal signaling pathway by interfering both with the binding of NODAL to the activin receptor and the formation of a receptor complex [9]. During the late luteal phase of the menstrual cycle, strong LEFTY2 immunoreactivity was found in the stroma and to a lesser extent in the endometrial glands [10]. Furthermore, LEFTY2 has also been detected in the endometrial fluid of fertile women, indicating that LEFTY2 is secreted into the lumen of the uterus. Interestingly, induction of the proprotein convertase (PC) 5/6, which processes LEFTY2, is triggered in the mouse uterus in response to artificial decidualization with oil or upon mechanical injury [11]. Endometrial LEFTY2 is elevated during the receptive phase in some patients with “unexplained infertility” suggesting that dysregulation of LEFTY2 contributes to infertility [12, 13]. Further, in vivo gene transfer of Lefty2 in the mouse uterus leads to implantation failure [14]. However, the mechanisms underlying the negative impact of LEFTY2 on endometrial receptivity remain largely unknown.
Several implantation events, including blastocyst-endometrium adhesion [15], regulation of growth factor signaling [16], transcription factor activity [17], epithelial tight junctions [18], protease activity [19], cyclooxygenase 2 (COX2)-dependent prostaglandin production [20, 21], and epithelial transport [22, 23] are regulated by Ca2+ signaling. Embryo-derived tryptic serine proteases have emerged as important implantation signals, capable of inducing COX2-dependent prostaglandin E2 (PGE2) production in response to cytosolic Ca2+([Ca2+]i) oscillations [21]. [Ca2+]i is tightly regulated by several mechanisms, including Ca2+ release from intracellular stores and subsequent activation of store-operated Ca2+ entry (SOCE) [24]. SOCE is initiated by the Ca2+ sensor proteins, stromal interaction molecule 1 (STIM1) and STIM2, that are located within the endoplasmic reticulum (ER) [25]. Following store depletion, STIMs cluster and trap the plasma membrane (PM) proteins ORAI1–3 into ER-PM junctions [24]. These regions become sites of highly selective Ca2+ entry, predominantly through ORAI assembled channels [26, 27]. Prolonged and disordered Ca2+ oscillations in decidualizing stromal cells in response to serine proteases secreted by low-quality human embryos are thought to trigger ER- stress, which in turn facilitates early maternal rejection of a non-viable conceptus [28]. Recently, we reported that Orai1 is expressed in the secretory phase endometrium as well as in endometrial carcinoma cells [29]. Both Orai1 expression and function are upregulated by TGFß1, an effect presumably participating in the regulation of endometrial regeneration [29]. The role of Orai1 in implantation remains to be determined.
The mechanisms regulating [Ca2+]i levels in endometrial cells remain incompletely understood but presumably involve Ca2+ release from intracellular stores with subsequent activation of SOCE [27, 30–34]. Here, we show that LEFTY2 inhibits the expression of Ca2+ responsive receptivity genes by downregulating Orai1 expression and limiting SOCE. Our findings provide new insights into the molecular mechanisms that define a limited window of implantation in the human endometrium.
Results
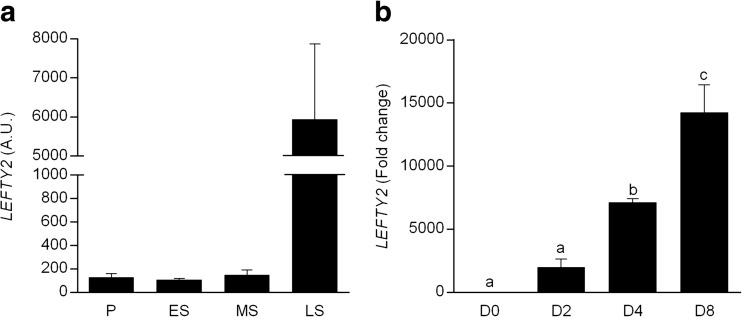
Mining of publicly available microarray data (Gene Expression Omnibus accession number: 24460960) demonstrated a marked increase (30-fold) in LEFTY2 mRNA levels in whole endometrial biopsies upon transition from mid-secretory (MS; receptive) to late secretory (LS; refractory) phase of the menstrual cycle (Fig. 1a). In culture, decidual transformation of HESCs increased LEFTY2 expression. After 8 days of differentiation, LEFTY2 mRNA levels increased approximately 14,000-fold (Fig. 1b).
Fig. 1.
Expression of LEFTY2 in human endometrium. a Arithmetic means ± SEM of LEFTY2 transcripts expressed in arbitrary units (a.u.) in whole endometrial biopsies throughout the menstrual cycle. Expression levels were derived from in silico analysis of publicly available microarray data (GEO profile LEFTY2: ID 24460960). The phases of the cycle are indicated as follows: P proliferative, ES early-secretory, MS mid secretory, and LS late secretory. b Induction of LEFTY2 in decidualizing HESCs. Arithmetic means ± SEM (n = 3) of transcript levels of LEFTY2 measured in undifferentiated HESCs and cells decidualized with 8-br-cAMP and MPA for 2, 4 or 8 days. The data are expressed relative to that in undifferentiated cells (D0). Different letters above the error bars indicate that those groups are significantly different from each other at *P < 0.05 using the Kruskal-Wallis test
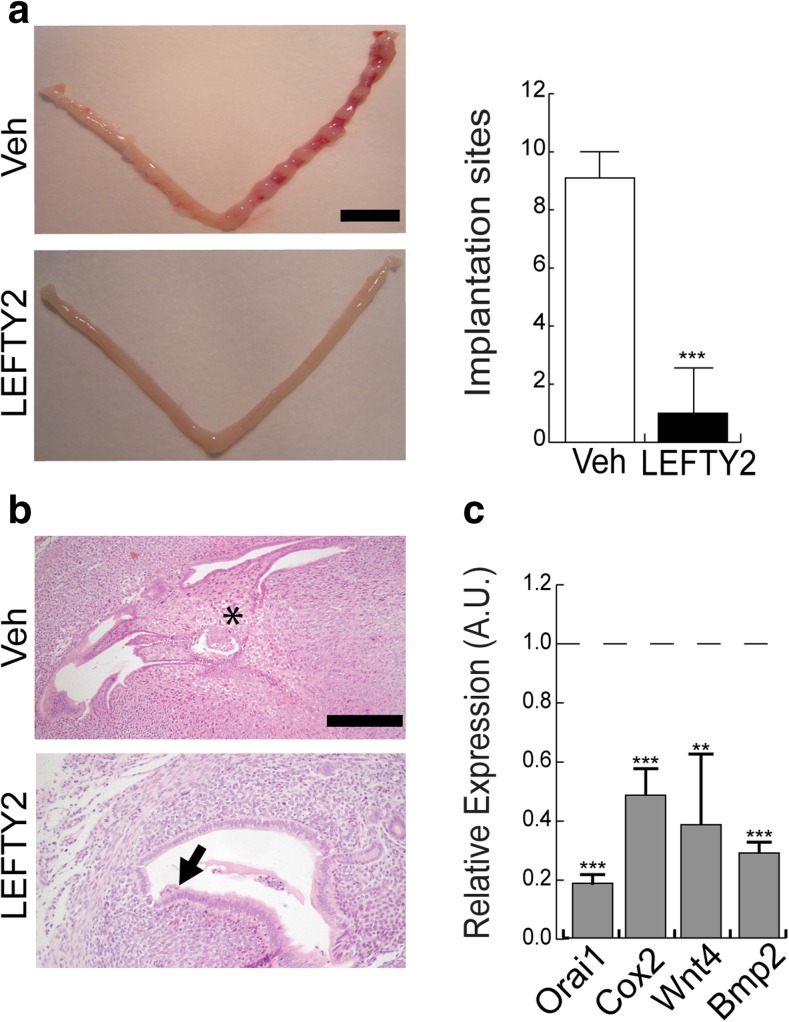
In order to explore the functional significance of increased LEFTY2 expression, we examined the effect of LEFTY2 on embryo implantation in the mouse uterus. Embryo implantation in mice is a stepwise process that starts with attachment of the blastocysts to the endometrial luminal epithelial layer between 3.5 to 4.5 days postcoitus (dpc), triggering decidualization of the underlying stromal cells [35] and closure of the uterine lumen [36].To confirm that LEFTY2 renders the endometrium refractory to implantation [14], murine uterine flushing experiments were performed in C57BL/6 female mice mated with vasectomized males. The uterine lumen of pseudopregnant female mice was injected with recombinant LEFTY2 before transfer of 10 cultured blastocysts equivalent of 3.5 dpc into a single uterine horn. Phosphate-buffered saline (PBS; vehicle) was flushed through the uterus prior to embryo transfer in control animals. The number of implantation sites was determined 72 h later. Out of 50 embryos transferred in each group, 43 (86%) embryos failed to implant in uteri treated with recombinant LEFTY2. By contrast, only 5 (10%) embryos failed to implant following flushing of the uterine lumen with PBS (Fig. 2a). Viable embryos were observed in the implantation sites of control (Veh) animals (Fig. 2b). The few established implantation sites following LEFTY2 exposure appeared smaller and the presence of collapsed blastocysts suggested imminent demise (Fig. 2b). To exclude a possible effect of LEFTY2 on the blastocysts, we performed further flushing experiments. C57BL/6 female mice were mated with fertile males to induce a normal pregnancy. At day 3.0 dpc, the uterus was flushed once either with PBS or LEFTY2. In this natural mating model, the blastocysts are still within the oviduct at day 3.0 dpc and are therefore not exposed to recombinant LEFTY2. Our results show again a significant decrease of implantation sites in LEFTY2 flushed mice (Supplementary Fig. 1), further suggesting that LEFTY2 blocks implantation by acting directly on the endometrium.
Fig. 2.
Recombinant LEFTY2 blocks embryo implantation. Vas mated C57BL/6 female mice were subjected to laparotomy and both uterine horns gently flushed with either vehicle (PBS) or recombinant LEFTY2 (500 ng/ml). Ten embryos (equivalent to stage 3.5 dpc) were then transferred to the right horn. Each treatment group consisted of five mice. a The gross morphological appearance of the uteri and implantation sites at 9.5 dpc (scale bar, 1 cm); the right panel shows arithmetic means ± SEM (n = 5) of the number of implantation sites following uterine flushing with PBS (Veh) or recombinant LEFTY2. b Corresponding histological appearance of implantation sites. The upper panel (Veh; control) shows a normal blastocyst. The lower panel shows fetal demise following uterine LEFTY2 exposure prior to embryo transfer (scale bar, 100 μm). c Arithmetic means ± SEM (n = 5) of the relative expression of murine Orai1, Cox2 Wnt4 and Bmp2, transcript levels, normalized to Cyclo (housekeeping) mRNA and expressed in arbitrary units (a.u.), following uterine flushing with LEFTY2 or vehicle (PBS, dotted line). **P < 0.01 and ***P < 0.001 indicate statistically significant difference to absence of LEFTY2 (Student’s t test)
We speculated that failed implantation following LEFTY2 exposure could reflect inhibition of key murine implantation genes. In agreement, murine levels of Orai1, Bmp2, Cox2 and Wnt4 were significantly downregulated in LEFTY2-treated uteri when compared to control mice (Fig. 2c). Other cardinal murine implantation genes, including Lif, Ihh, Hoxa10 and Hbegf were unaffected by LEFTY2 (Supplementary Fig. 2).
To determine the relevance of these observations to the human endometrium, we decidualized HESCs with 8-Br-cAMP and MPA (medroxyprogesterone acetate, a progestin) for 6 days in the presence or absence of LEFTY2. As was the case in mice, recombinant LEFTY2 also attenuated the expression of WNT4, BMP2, and COX2 at protein and mRNA level in decidualizing HESCs (Fig. 3 and Supplementary Fig. 3). By contrast, endometrial WNT4, BMP2 and COX2 expression was markedly upregulated in response to the Ca2+ ionophore, ionomycin (Fig. 3), indicating that the expression of these implantation factors is responsive to increase in [Ca2+]i. Interestingly, LEFTY2 abrogated the induction of COX2 and WNT4 in response to Ionomycin, although it failed to reverse the induction of BMP2. Ionomycin treatment had no impact on LIF, IHH, HOXA10, or HBEGF mRNA levels in decidualizing HESCs (Supplementary Fig. 4).
Fig. 3.
Differential regulation of Ca2+-dependent receptivity factors in response to LEFTY2 and Ionomycin. Primary HESC cultures were treated with 8-Br-cAMP and MPA for 6 days with or without LEFTY2 (25 ng/ml) or Ionomycin (1 μM) or in combination. a Original Western blots showing COX2, WNT4, BMP2 and GAPDH. b – d The abundance of COX2, WNT4 and BMP2 was quantified by ImageJ analysis of six Western blots and normalized to GAPDH. The data shows arithmetic means ± SEM. *P < 0.05, **P < 0.01 indicate significant difference to absence of LEFTY2 (Student’s t test)
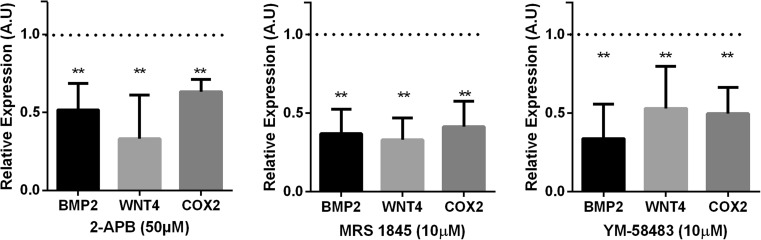
In order to determine if the inhibition of Ca2+-responsive receptivity genes following LEFTY2 treatment was a consequence of SOCE inactivation, primary HESCs were decidualized with 8-Br-cAMP and MPA for 6 days in the absence or presence of Orai1 inhibitors 2-aminoethoxydiphenyl borate (2-APB) [29], YM-58483 [37] or MRS-1845 [38]. Induction of IGFBP1 and PRL transcript levels confirmed that the cells mounted a decidual response (data not shown). As shown in Fig. 4, exposure of decidualizing HESC Orai1 inhibitors 2-APB, YM-58483 and MRS-1845 significantly decreased COX2, BMP2 and WNT4 transcript levels.
Fig. 4.
Orai1 inhibitors 2-APB, MRS-1845, and YM-58483 inhibit Ca2+-responsive implantation genes. Arithmetic means ± SEM (n = 6) of BMP2, WNT4, and COX2 transcript levels relative to that of L19 (housekeeping) in primary HESC cultures that were treated with 8-Br-cAMP and MPA for 6 days in the absence (dotted line) or in presence of the Orai-1 inhibitors 2-APB (50 μM), MRS-1845 (10 μM), or YM-58483 (10 μM); **P < 0.01 indicates significant difference from the control using Student’s t test
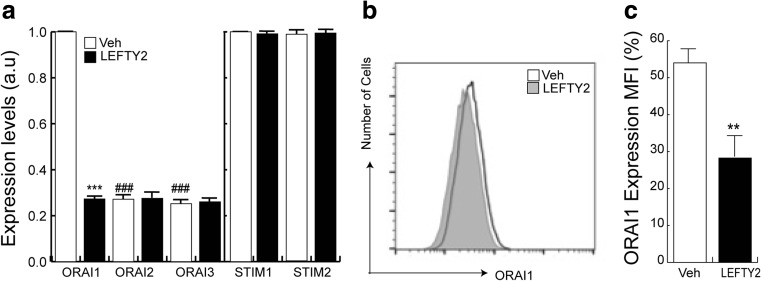
We hypothesized that LEFTY2 may similarly impair SOCE in luminal endometrial epithelial cells at implantation. To test this hypothesis, we first determined the transcript levels of the ORAI1–3 and STIM1–2 isoforms in endometrial Ishikawa cells treated with or without LEFTY2. Ishikawa cells are a commonly used cell line to study human implantation events [28, 39, 40]. As illustrated in Fig. 5a, LEFTY2 selectively decreased the transcript levels of ORAI1, the dominant ORAI isoform in these cells. The transcript levels for ORAI2-3 were low in Ishikawa cells, irrespective of LEFTY2 treatment. Similarly, STIM1-2 expression did not change in response to LEFTY2 treatment. The decrease of ORAI1 transcript levels following LEFTY2 treatment was paralleled by a decrease of ORAI1 at the protein level. Flow cytometry showed a significant reduction in ORAI1 in response to LEFTY2 treatment (Fig. 5b–c).
Fig. 5.
LEFTY2 downregulates endometrial Orai1 expression. a Endometrial Ishikawa cells were treated with or without LEFTY2 (25 ng/ml) for 6 days, and ORAI1, ORAI2, ORAI3, STIM1 and STIM2 mRNA levels were measured. The results show arithmetic means ± SEM (n = 6). Expression levels of the ORAI2 and ORAI3 isoforms are relative to the ORAI1 transcript level in the absence of recombinant LEFTY2 treatment. b and c Original FACS plot and bar graph of ORAI1 expression in parallel cultures treated with and without LEFTY2. **P < 0.01, ***P < 0.001 indicates statistically significant difference to absence of LEFTY2. ### P < 0.001 indicates statistically significant difference from Control-Orai1 transcripts (Student’s t test)
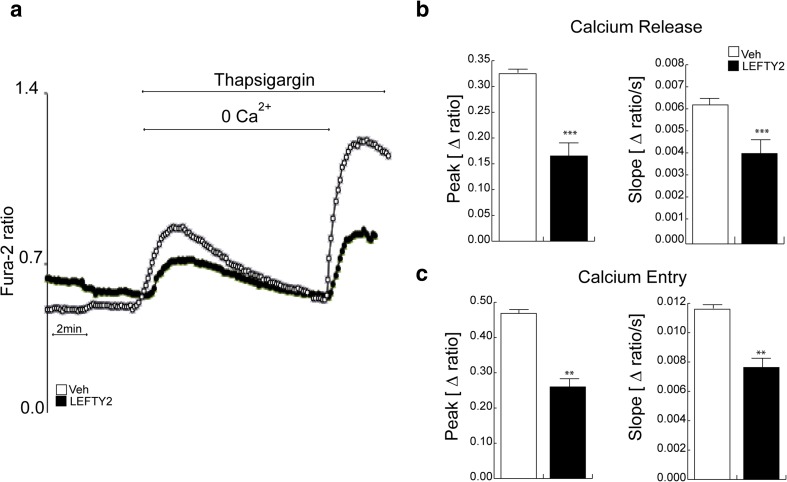
In order to explore whether LEFTY2 impacts on Ca2+ signaling, Ishikawa cells were treated with thapsigargin, a potent inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), in the presence or absence of recombinant LEFTY2. Treatment with thapsigargin in the absence of extracellular Ca2+elicited a transient increase in [Ca2+]i(Δ[Ca2+]i) (Fig. 6a). Both peak and slope of Δ[Ca2+]i were significantly blunted in response to LEFTY2 treatment. Subsequent addition of extracellular Ca2+sharply increased [Ca2+]i, reflecting SOCE. Peak and slope of Ca2+entry were significantly decreased by LEFTY2 (Fig. 6b–c). Basal Ca2+ levels were not significantly modified by LEFTY2 (Supplementary Fig. 5). Taken together, the data show that LEFTY2 blunts intracellular Ca2+release and SOCE.
Fig. 6.
LEFTY2 reduces SOCE. a Original tracing of Fura-2 fluorescence-ratio in fluorescence spectrometry during and after Ca2+ depletion (1 μM thapsigargin) in LEFTY2 treated (black circles) and vehicle treated (PBS; white circles) Ishikawa cells. b – c Arithmetic means ± SEM (n = 6, each experiment 15–30 cells) of the peaks (left panels) and slopes (right panels) of b Ca2+ release and c Ca2+ entry, respectively **P < 0.01, ***P < 0.001 indicates significant difference from absence of LEFTY2 (Student’s t test)
Discussion
LEFTY2, a member of the TGF-β superfamily, is implicated in embryo development and stem cell differentiation through its antagonistic action on the TGF-β/Smad signaling pathway [41–43]. LEFTY2 is also expressed in the human endometrium, most prominently during the late secretory phase of the menstrual cycle [10, 44, 45]. Previous studies reported that overexpression of LEFTY2 is associated with unexplained infertility [46] and irregular menstrual bleeding [8], and in vivo gene transfer studies in mice have also shown inhibition of implantation [14]. Furthermore, auto/paracrine LEFTY signaling attenuates decidual transformation of HESCs [47, 48], although the underlying mechanisms are incompletely understood.
In this study, we demonstrate that LEFTY2 renders the endometrium refractory to implantation, an effect paralleled by downregulation of ORAI1 expression and of SOCE in endometrial epithelial cells. By attenuating [Ca2+]i signaling, LEFTY2 decreases expression of Ca2+-responsive implantation genes such as COX2, WNT4 and BMP2. Other key implantation genes, including LIF, IHH, HOXA10 and HBEGF transcripts [12, 49], were not affected by LEFTY2 or by Ionomycin treatment.
The pathways that couples LEFTY2 to transcriptional repression of ORAI1 and the mechanisms linking ORAI1 function and increases of [Ca2+]i to expression of receptivity genes requires further elucidation. The present observations uncover a novel function of Orai1 and store-operated Ca2+ entry. SOCE is known to trigger Ca2+ oscillations [27, 50], which stimulate several cellular functions [51–55] including entering into the S and the M phase of the cell cycle [56, 57] as well as cell survival [58, 59]. In contrast, sustained increase of cytosolic Ca2+ activity leads to apoptosis [53, 55, 60–68]. By mediating SOCE and oscillations of cytosolic Ca2+ activity, Orai1-3 and their regulators STIM 1–2 are decisive for cell survival [69–72]. It is noteworthy that ORAI1/STIM1 is upregulated by the serum and glucocorticoid inducible kinase (SGK1) and TGF-β in endometrial cancer cells [29], but downregulated by the AMP activated kinase (AMPK) [73]. Although as yet untested, downregulation of Orai1/STIM1 in response to AMPK activation potentially plays a role in preventing implantation of an embryo into an energy-deficient endometrium.
The present observations point to a new role of ORAI1-dependent SOCE in the regulation of endometrial receptivity genes, and hence the likelihood of successful embryo implantation. However, our findings do not rule out the involvement of additional mechanisms of Ca2+ release and/or entry during implantation. For example, evidence has emerged to implicate voltage-gated Ca2+ channels in Ca2+ entry in endometrial cells exposed to embryonic proteases [21]. Moreover, progesterone-dependent induction of phospholipase C (PLC)-related catalytically inactive protein 1 (PRIP-1) in decidual cells blocks Ca2+ release from the ER by inhibiting inositol 1,4,5-trisphosphate (IP3) signaling [74]. Most importantly, knockout of Orai1 on the specific Institute of Cancer Research (ICR) genetic background mice did not abrogate female fertility [75]. Unlike mice lacking Orai1 on a C57/DBA/129 background, the Orai1-deficient ICR mice did not suffer from high incidence of perinatal lethality [75] and may have partially replaced the function of Orai1 by other mechanisms. Further experiments are required to identify those mechanisms and explore their LEFTY2 sensitivity.
Our findings provide a novel mechanistic explanation for the clinical observation that elevated LEFTY2 levels are associated with implantation failure and infertility [13]. Taken together, our observations provide further evidence that coordinated temporal and spatial regulation of Ca2+ signaling in the endometrium across the window of receptivity is critical for reproductive success.
Materials and methods
Patient selection and sample collection
The study was approved by the NHS National Research Ethics–Hammersmith and Queen Charlotte’s & Chelsea Research Ethics Committee (1997/5065). The biopsies were timed between 6 and 10 days after the pre-ovulatory luteinizing hormone (LH) surge. None of the subjects were on hormonal treatments for at least 3 months prior to the procedure. Written informed consent was obtained from all participants in accordance with the guidelines in The Declaration of Helsinki 2000.
Cell culture
Human endometrial stromal cells (HESCs) were isolated from endometrial tissues as described previously [76]. Purified HESCs were expanded in maintenance medium of DMEM/F-12 (Invitrogen, Schwerte, Germany) containing 10% dextran-coated charcoal-treated fetal bovine serum (DCC-FBS; Invitrogen, UK) and 1% antibiotic-antimycotic solution (Invitrogen). Confluent monolayers were decidualized in DMEM/F-12 containing 2% DCC-FBS with 0.5 mM 8-bromo-cAMP (8-Br-cAMP; Sigma, Munich, Germany) with or without 10−6 M medroxyprogesterone acetate (MPA; Sigma) to induce a differentiated phenotype. Where indicated, the cells were treated with recombinant LEFTY2 (25 ng/ml; R&D Systems, Germany) as described previously [77]. Ionomycin was used at 1 μM (Sigma) and the Orai inhibitors: 2-APB, YM-58483, and MRS-1845(TOCRIS, Germany). Ishikawa cells, an endometrial epithelial-like cell line (ECACC 99040201) [28, 29], were maintained in DMEM/F12 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin (Invitrogen). All cells were incubated at 37 °C in a humid atmosphere maintained at 5% (vol/vol) CO2, and routinely tested for mycoplasma infection.
Animal experiments
C57BL/6 mice were purchased from Charles River Ltd. (Margate, UK). All experiments were carried out in accordance with the UK Home Office regulations (PPL70/6867). Mice had free access to food and water ad libitum, and were kept under constant humidity (55 ± 10%), temperature (22 ± 2 °C), and 12 h light-dark cycle conditions. To assess implantation, C57BL/6 female mice were mated by vasectomized males and the day of the appearance of the vaginal plug designated as day 1.0 dpc. Laparotomy was performed at 3.0 dpc. Both uterine horns were injected with 100 μL PBS or LEFTY2 dissolved in PBS (500 ng/ml). After 10 min, 10 cultured blastocysts (equivalent of 3.5 dpc) were transferred to a single treated uterine horn. The uteri were harvested 72 h following surgery, implantation sites counted, and tissues fixed in formalin or snap frozen for further analysis.
Natural mating model
We conducted timed matings by placing wild-type C57BL/6 female mice with fertile wild-type males to induce pregnancy. The day when a vaginal plug was apparent was designated as 1.0 dpc, and mice were anesthetized at 3.0 dpc and subjected to laparotomy to expose the uterus [12]. We used two groups of mice: a control group flushed with PBS (Veh) and a study group flushed with LEFTY2 (as described above). The incision was then closed to allow the mice to recover. The uteri were then harvested at 9.5 dpc and the implantation sites counted.
Western blot analysis
Whole cell protein extracts were prepared by lysing cells in RIPA buffer. Protein yield was quantified using the Bio-Rad DC protein assay kit (Bio-Rad). Equal amounts of protein were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) before wet-transfer onto a PVDF membrane (Amersham Biosciences, UK). Nonspecific binding sites were blocked by overnight incubation with 5% nonfat dry milk in Tris-buffered saline with 1% Tween (TBS-T; 130 mmol/L NaCl, 20 mmol/L Tris, pH 7.6, and 1% Tween) as previously described [76]. The following primary antibodies were used: anti-BMP2 (♯sc6895, Santa Cruz Biotechnology Inc., Texas, USA), anti-WNT4 (♯ sc376279, Santa Cruz Biotechnology Inc), anti-Cox2 (♯15191; Abcam, Cambridge, UK), and anti-GAPDH (♯21185; Cell Signaling, Leiden, The Netherlands). All primary antibodies were diluted 1:1000. Protein complexes were visualized with a chemiluminescent detection kit (WesternBright™ ECL, Advansta, CA, USA).
Real-time quantitative (qRT)-PCR
Total RNA was extracted from cell cultures or from snap frozen whole uteri using Trizol (Invitrogen) based on a phenol-chloroform extraction protocol [78]. Equal amounts of total RNA (2 μg) were reverse transcribed using the Superscript III First-Strand synthesis system for RT-PCR (Invitrogen) with oligo dT priming. The resulting first-strand cDNA was diluted and used as a template in qRT-PCR analysis. L19 and Cyclophilin A (Cyclo), representing non-regulated human and murine housekeeping genes, respectively, were used to normalize for variances in input cDNA. Detection of gene expression was performed with KappaFast-SYBR Green (Peqlab, Erlangen, Germany), and qRT-PCR was performed on a BioRad iCycler iQ™ Real-Time PCR Detection System (Bio-Rad Laboratories, Munich, Germany). The non-template control (NTC) reactions (cDNA was substituted with DNase/RNase free water) and reverse transcriptase (RT) controls were included in each PCR reaction. The PCR products were not detected in NTC or RT control reactions (data not shown). Transcript levels were determined by the ΔΔCt method [79] and expressed in arbitrary units. All measurements were performed in triplicate. Melting curve analysis and agarose gel electrophoresis confirmed amplification specificity. Primer sequences are provided on request.
Flow cytometry
Orai1 expression was analyzed by flow cytometry. Cultured cells were detached, washed three times with phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde for 15 min on ice. The cells were then incubated for 60 min (37 °C) with anti-Orai1 monoclonal primary antibody (1:200, Abcam) washed once in PBS, and stained in 1:250 diluted CF™ 488A-labeled anti-rabbit secondary antibody (Sigma) for 30 min (37 °C). Samples were immediately analyzed on a FACSCalibur flow cytometer (BD Biosciences, Heidelberg, Germany). Data were analyzed using the FlowJo software (FlowJo LLC, Ashland, Oregon, USA).
Calcium measurements
Fura-2 fluorescence was utilized to determine [Ca2+]i [80]. Cells were loaded with Fura-2/AM (2 μM; Invitrogen) for 20 min at 37 °C. Cells were excited alternatively at 340 and 380 nm through an objective (Fluor ×40/1.30 oil) built in an inverted phase-contrast microscope (Axiovert 100, Zeiss, Oberkochen, Germany). Emitted fluorescence intensity was recorded at 505 nm. Data were acquired using specialized computer software (Metafluor, Universal Imaging, Downingtown, USA). Cytosolic Ca2+ activity was estimated from the 340 nm/380 nm ratio. SOCE was determined by extracellular Ca2+ removal in the presence of sarcoendoplasmatic Ca2+ ATPase inhibitor thapsigargin (1 μM, Invitrogen) and subsequent Ca2+ re-addition [81]. For quantification of Ca2+ entry, the slope (delta ratio) and peak (delta ratio) were calculated following re-addition of Ca2+ [80, 82]. Experiments were performed with Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1.2 MgSO4, 2 CaCl2, 2 Na2HPO4, 32 HEPES, 5 glucose, and pH 7.4. To reach nominally Ca2+-free conditions, experiments were performed using Ca2+-free Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1.2 MgSO4, 2 Na2HPO4, 32 HEPES, 0.5 EGTA, 5 glucose, and pH 7.4. For calibration purposes, ionomycin (10 μM, Sigma) was applied at the end of each experiment.
Histology
For histological assessment, uterine horns were formalin-fixed and embedded in paraffin, cut in 5-μm sections, and stained with H&E as previously described [12].
Statistical analysis
Data were analyzed with the statistical package GraphPad Prism (GraphPad software Inc., CA, USA). Student’s t test or Kruskal-Wallis test was used when appropriate. Statistical significance was assumed when P < 0.05.
Electronic supplementary material
(PDF 217 kb)
Acknowledgements
We are grateful to all the women who participated in this study. This work was supported the Biomedical Research Unit in Reproductive Health, a joint initiative between the University Hospitals Coventry and Warwickshire NHS Trust and Warwick Medical School, to M.S.S the EMBO Long-Term Fellowship (ALTF 20-2013), the Zukunftskonzept award (Deutsche Forschungsgemeinschaft; ZUK63), and the Fortüne-Programme award (2426-0-0).To F.L the Deutsche Forschungsgemeinschaft (GRK 1302, SFB 773 B4/A1, La 315/13-3) and the Open Access Publishing Fund of Tuebingen University. This work is dedicated to Dr Jennifer H. Steel, who lost her battle with cancer shortly after the acceptance of this manuscript. She will be missed.
Compliance with ethical standards
The study was approved by the NHS National Research Ethics–Hammersmith and Queen Charlotte’s & Chelsea Research Ethics Committee (1997/5065). Written informed consent was obtained from all participants in accordance with the guidelines in The Declaration of Helsinki 2000. All experiments were carried out in accordance with the UK Home Office regulations (PPL70/6867).
Conflict of interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00109-017-1610-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jan J. Brosens, Phone: 44 (0) 24 7696 8704, Email: J.J.Brosens@warwick.ac.uk
Florian Lang, Phone: +49 7071 29 72194, Email: florian.lang@uni-tuebingen.de.
References
- 1.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 2.Evers JL. Female subfertility. Lancet. 2002;360:151–159. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 3.Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salker MS, Nautiyal J, Steel JH, Webster Z, Sucurovic S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW, et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One. 2012;7:e52252. doi: 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RM, Chen Y, Ezashi T, Walker AM. Interferons and the maternal-conceptus dialog in mammals. Semin Cell Dev Biol. 2008;19:170–177. doi: 10.1016/j.semcdb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 7.Tabibzadeh S (2005) Role of EBAF/Lefty in implantation and uterine bleeding. Ernst Schering Research Foundation workshop DOI:159–189 [DOI] [PubMed]
- 8.Kothapalli R, Buyuksal I, Wu SQ, Chegini N, Tabibzadeh S. Detection of ebaf, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding. J Clin Invest. 1997;99:2342–2350. doi: 10.1172/JCI119415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 10.Tabibzadeh S, Lessey B, Satyaswaroop PG. Temporal and site-specific expression of transforming growth factor-beta4 in human endometrium. Mol Hum Reprod. 1998;4:595–602. doi: 10.1093/molehr/4.6.595. [DOI] [PubMed] [Google Scholar]
- 11.Tang M, Mikhailik A, Pauli I, Giudice LC, Fazelabas AT, Tulac S, Carson DD, Kaufman DG, Barbier C, Creemers JW, et al. Decidual differentiation of stromal cells promotes Proprotein Convertase 5/6 expression and lefty processing. Endocrinology. 2005;146:5313–5320. doi: 10.1210/en.2005-0684. [DOI] [PubMed] [Google Scholar]
- 12.Salker MS, Christian M, Steel JH, Nautiyal J, Lavery S, Trew G, Webster Z, Al-Sabbagh M, Puchchakayala G, Foller M, et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17:1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- 13.Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B. Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab. 2000;85:2526–2536. doi: 10.1210/jcem.85.7.6674. [DOI] [PubMed] [Google Scholar]
- 14.Tang M, Taylor HS, Tabibzadeh S. In vivo gene transfer of lefty leads to implantation failure in mice. Hum Reprod. 2005;20:1772–1778. doi: 10.1093/humrep/deh849. [DOI] [PubMed] [Google Scholar]
- 15.Thie M, Denker HW. In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs. 2002;172:237–252. doi: 10.1159/000066963. [DOI] [PubMed] [Google Scholar]
- 16.Armant DR, Wang J, Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal-embryonic dialogue. Semin Reprod Med. 2000;18:273–287. doi: 10.1055/s-2000-12565. [DOI] [PubMed] [Google Scholar]
- 17.Sales KJ, Grant V, Cook IH, Maldonado-Perez D, Anderson RA, Williams AR, Jabbour HN. Interleukin-11 in endometrial adenocarcinoma is regulated by prostaglandin F2alpha-F-prostanoid receptor interaction via the calcium-calcineurin-nuclear factor of activated T cells pathway and negatively regulated by the regulator of calcineurin-1. Am J Pathol. 2010;176:435–445. doi: 10.2353/ajpath.2010.090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Phys. 1998;274:F1–F9. doi: 10.1152/ajpcell.1998.274.1.C1. [DOI] [PubMed] [Google Scholar]
- 19.Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- 20.Burns PD, Hayes SH, Silvia WJ. Cellular mechanisms by which oxytocin mediates uterine prostaglandin F2 alpha synthesis in bovine endometrium: role of calcium. Domest Anim Endocrinol. 1998;15:477–487. doi: 10.1016/S0739-7240(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 21.Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH et al (2012) Activation of the epithelial Na(+) channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med DOI. 10.1038/nm.2771 [DOI] [PubMed]
- 22.Chan HC, Chen H, Ruan Y, Sun T. Physiology and pathophysiology of the epithelial barrier of the female reproductive tract: Role of ion channels. Adv Exp Med Biol. 2012;763:193–217. doi: 10.1007/978-1-4614-4711-5_10. [DOI] [PubMed] [Google Scholar]
- 23.Chan HC, Liu CQ, Fong SK, Law SH, Wu LJ, So E, Chung YW, Ko WH, Wong PY. Regulation of Cl- secretion by extracellular ATP in cultured mouse endometrial epithelium. J Membr Biol. 1997;156:45–52. doi: 10.1007/s002329900186. [DOI] [PubMed] [Google Scholar]
- 24.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen WW, Frieden M, Demaurex N. Remodelling of the endoplasmic reticulum during store-operated calcium entry. Biol Cell. 2011;103:365–380. doi: 10.1042/BC20100152. [DOI] [PubMed] [Google Scholar]
- 26.Niemeyer BA. Changing calcium: CRAC channel (STIM and Orai) expression, splicing, and posttranslational modifiers. Am J Physiol Cell Physiol. 2016;310:C701–C709. doi: 10.1152/ajpcell.00034.2016. [DOI] [PubMed] [Google Scholar]
- 27.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan YW, Boomsma CM, et al. Uterine selection of human embryos at implantation. Sci Rep. 2014;4:3894. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt S, Schneider S, Yang W, Liu G, Schmidt EM, Schmid E, Mia S, Brucker S, Stournaras C, Wallwiener D, et al. TGFbeta1 and SGK1-sensitive store-operated Ca2+ entry and Orai1 expression in endometrial Ishikawa cells. Mol Hum Reprod. 2014;20:139–147. doi: 10.1093/molehr/gat066. [DOI] [PubMed] [Google Scholar]
- 30.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogeski I, Al-Ansary D, Qu B, Niemeyer BA, Hoth M, Peinelt C. Pharmacology of ORAI channels as a tool to understand their physiological functions. Expert Rev Clin Pharmacol. 2010;3:291–303. doi: 10.1586/ecp.10.23. [DOI] [PubMed] [Google Scholar]
- 32.Dynes JL, Amcheslavsky A, Cahalan MD. Genetically targeted single-channel optical recording reveals multiple Orai1 gating states and oscillations in calcium influx. Proc Natl Acad Sci U S A. 2016;113:440–445. doi: 10.1073/pnas.1523410113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putney JW. Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1. Curr Top Membr. 2013;71:109–123. doi: 10.1016/B978-0-12-407870-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 35.Aplin JD, Kimber SJ. Trophoblast-uterine interactions at implantation. Reprod Biol Endocrinol. 2004;2:48. doi: 10.1186/1477-7827-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Asp Med. 2013;34:939–980. doi: 10.1016/j.mam.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin H, Kent P, Isales CM, Parker PM, Wilson MV, Bollag WB. The role of calcium influx pathways in phospholipase D activation in bovine adrenal glomerulosa cells. J Endocrinol. 2009;202:77–86. doi: 10.1677/JOE-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlinguer-Palmini R, Masi A, Narducci R, Cavone L, Maratea D, Cozzi A, Sili M, Moroni F, Mannaioni G. GPR35 activation reduces Ca2+ transients and contributes to the kynurenic acid-dependent reduction of synaptic activity at CA3-CA1 synapses. PLoS One. 2013;8:e82180. doi: 10.1371/journal.pone.0082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YJ, Lees M, Matthews LC, Kimber SJ, Forbes K, Aplin JD. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 2015;128:804–814. doi: 10.1242/jcs.164004. [DOI] [PubMed] [Google Scholar]
- 40.Singh H, Nardo L, Kimber SJ, Aplin JD. Early stages of implantation as revealed by an in vitro model. Reproduction. 2010;139:905–914. doi: 10.1530/REP-09-0271. [DOI] [PubMed] [Google Scholar]
- 41.Tabibzadeh S, Hemmati-Brivanlou A. Lefty at the crossroads of “stemness” and differentiative events. Stem Cells. 2006;24:1998–2006. doi: 10.1634/stemcells.2006-0075. [DOI] [PubMed] [Google Scholar]
- 42.Ulloa L, Tabibzadeh S. Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. J Biol Chem. 2001;276:21397–21404. doi: 10.1074/jbc.M010783200. [DOI] [PubMed] [Google Scholar]
- 43.Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature. 1996;381:151–155. doi: 10.1038/381151a0. [DOI] [PubMed] [Google Scholar]
- 44.Papageorgiou I, Nicholls PK, Wang F, Lackmann M, Makanji Y, Salamonsen LA, Robertson DM, Harrison CA. Expression of nodal signalling components in cycling human endometrium and in endometrial cancer. Reprod Biol Endocrinol. 2009;7:122. doi: 10.1186/1477-7827-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornet PB, Picquet C, Lemoine P, Osteen KG, Bruner-Tran KL, Tabibzadeh S, Courtoy PJ, Eeckhout Y, Marbaix E, Henriet P. Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloprotineases. J Biol Chem. 2002;277:42496–42504. doi: 10.1074/jbc.M201793200. [DOI] [PubMed] [Google Scholar]
- 46.Tabibzadeh S. Isolation, characterization, and function of EBAF/LEFTY B: role in infertility. Ann N Y Acad Sci. 2011;1221:98–102. doi: 10.1111/j.1749-6632.2010.05944.x. [DOI] [PubMed] [Google Scholar]
- 47.Tang M, Naidu D, Hearing P, Handwerger S, Tabibzadeh S. LEFTY, a member of the transforming growth factor-beta superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role. Endocrinology. 2010;151:1320–1330. doi: 10.1210/en.2009-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Li H, Bai L, Yu H. Lefty inhibits in vitro decidualization by regulating P57 and cyclin D1 expressions. Cell Biochem Funct. 2014;32:657–664. doi: 10.1002/cbf.3069. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 50.Lang F, Friedrich F, Kahn E, Woll E, Hammerer M, Waldegger S, Maly K, Grunicke H. Bradykinin-induced oscillations of cell membrane potential in cells expressing the Ha-ras oncogene. J Biol Chem. 1991;266:4938–4942. [PubMed] [Google Scholar]
- 51.Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 52.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 53.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 54.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 55.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 56.Steinhardt RA, Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature. 1988;332:364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- 57.Taylor JT, Zeng XB, Pottle JE, Lee K, Wang AR, Yi SG, Scruggs JA, Sikka SS, Li M. Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol. 2008;14:4984–4991. doi: 10.3748/wjg.14.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heise N, Palme D, Misovic M, Koka S, Rudner J, Lang F, Salih HR, Huber SM, Henke G. Non-selective cation channel-mediated Ca2+-entry and activation of Ca2+/calmodulin-dependent kinase II contribute to G2/M cell cycle arrest and survival of irradiated leukemia cells. Cell Physiol Biochem. 2010;26:597–608. doi: 10.1159/000322327. [DOI] [PubMed] [Google Scholar]
- 59.Parkash J, Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87:587–595. doi: 10.1016/j.lfs.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damm TB, Egli M. Calcium's role in mechanotransduction during muscle development. Cell Physiol Biochem. 2014;33:249–272. doi: 10.1159/000356667. [DOI] [PubMed] [Google Scholar]
- 61.Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104:1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- 62.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 63.Lang F, Hoffmann EK. Role of ion transport in control of apoptotic cell death. Compr Physiol. 2012;2:2037–2061. doi: 10.1002/cphy.c110046. [DOI] [PubMed] [Google Scholar]
- 64.Liu XH, Kirschenbaum A, Yu K, Yao S, Levine AC. Cyclooxygenase-2 suppresses hypoxia-induced apoptosis via a combination of direct and indirect inhibition of p53 activity in a human prostate cancer cell line. J Biol Chem. 2005;280:3817–3823. doi: 10.1074/jbc.M406577200. [DOI] [PubMed] [Google Scholar]
- 65.Shaik N, Zbidah M, Lang F. Inhibition of Ca(2+) entry and suicidal erythrocyte death by naringin. Cell Physiol Biochem. 2012;30:678–686. doi: 10.1159/000341448. [DOI] [PubMed] [Google Scholar]
- 66.Spassova MA, Soboloff J, He LP, Hewavitharana T, Xu W, Venkatachalam K, van Rossum DB, Patterson RL, Gill DL. Calcium entry mediated by SOCs and TRP channels: variations and enigma. Biochim Biophys Acta. 2004;1742:9–20. doi: 10.1016/j.bbamcr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Svoboda N, Pruetting S, Grissmer S, Kerschbaum HH. cAMP-dependent chloride conductance evokes ammonia-induced blebbing in the microglial cell line, BV-2. Cell Physiol Biochem. 2009;24:53–64. doi: 10.1159/000227813. [DOI] [PubMed] [Google Scholar]
- 68.Towhid ST, Schmidt EM, Tolios A, Munzer P, Schmid E, Borst O, Gawaz M, Stegmann E, Lang F. Stimulation of platelet death by vancomycin. Cell Physiol Biochem. 2013;31:102–112. doi: 10.1159/000343353. [DOI] [PubMed] [Google Scholar]
- 69.Bergmeier W, Weidinger C, Zee I, Feske S (2013) Emerging roles of store-operated Ca ( 2+) entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels (Austin) 7 [DOI] [PMC free article] [PubMed]
- 70.Courjaret R, Machaca K. STIM and Orai in cellular proliferation and division. Front Biosci (Elite Ed) 2012;4:331–341. doi: 10.2741/e380. [DOI] [PubMed] [Google Scholar]
- 71.Qu B, Al-Ansary D, Kummerow C, Hoth M, Schwarz EC. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium. 2011;50:261–269. doi: 10.1016/j.ceca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lang F, Eylenstein A, Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell Calcium. 2012;52:347–354. doi: 10.1016/j.ceca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Muter J, Brighton PJ, Lucas ES, Lacey L, Shmygol A, Quenby S, Blanks AM, Brosens JJ. Progesterone-dependent induction of phospholipase C-related catalytically inactive protein 1 (PRIP-1) in decidualizing human endometrial stromal cells. Endocrinology. 2016;157:2883–2893. doi: 10.1210/en.2015-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D'Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, et al. Essential role of Orai1 store-operated calcium channels in lactation. Proc Natl Acad Sci U S A. 2015;112:5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- 77.Salker MS, Zhou Y, Singh Y, Brosens J, Lang F. LeftyA sensitive cytosolic pH regulation and glycolytic flux in Ishikawa human endometrial cancer cells. Biochem Biophys Res Commun. 2015;460:845–849. doi: 10.1016/j.bbrc.2015.03.120. [DOI] [PubMed] [Google Scholar]
- 78.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 79.Singh Y, Garden OA, Lang F, Cobb BS. MicroRNA-15b/16 enhances the induction of regulatory T cells by regulating the expression of Rictor and mTOR. J Immunol. 2015;195:5667–5677. doi: 10.4049/jimmunol.1401875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhavsar SK, Schmidt S, Bobbala D, Nurbaeva MK, Hosseinzadeh Z, Merches K, Fajol A, Wilmes J, Lang F. AMPKalpha1-sensitivity of Orai1 and Ca(2+) entry in T - lymphocytes. Cell Physiol Biochem. 2013;32:687–698. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 81.Bird GS, DeHaven WI, Smyth JT, Putney JW., Jr Methods for studying store-operated calcium entry. Methods. 2008;46:204–212. doi: 10.1016/j.ymeth.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang W, Nurbaeva MK, Schmid E, Russo A, Almilaji A, Szteyn K, Yan J, Faggio C, Shumilina E, Lang F. Akt2- and ETS1-dependent IP3 receptor 2 expression in dendritic cell migration. Cell Physiol Biochem. 2014;33:222–236. doi: 10.1159/000356664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 217 kb)