Abstract
The use of colors in food industry is essential for the creation of new products or their improvement. As an important pigment group, anthocyanin could be used as a natural coloring pigment in foods. This study aims at exploring strategies that result in color stability of anthocyanin in pear‐shaped variety of blueberry (Cornus mas–Macrocarpa). In this study, the effects of different pH values (1, 2, 3, 4) as well as various concentrations (0, 120, 240, 480, 960 mg/L) of five copigments, including tannic, caffeic, benzoic, and coumaric acids, on anthocyanin copigment complexes (ratio 1:1) were investigated. The studied copigments were tannic, caffeic, benzoic, and coumaric acids. Anthocyanin was influenced by the highest concentration of 960 mg/L copigment in the presence of different pHs. Five groups were considered, one of which contained anthocyanin without copigment and the rest consisted of copigments. To evaluate the response of copigmentation through spectrophotometer, absorbance from samples was measured after 30 min of adding copigment to anthocyanin in the range of 450–600 nm wavelengths. The results showed that caffeic acid possessed the greatest anthocyanin stability compared to other copigments and it was the best copigment. An increase in the concentrations of copigments led to a higher level of anthocyanin stability and changes in hyperchromic and bathochromic. Moreover, the results revealed that the strongest hyperchromic effect for all organic acids was observed in pH 2, and the strongest bathochromic changes were observed in pH 4.
Keywords: Anthocyanin, blueberry, color, copigment, pH
1. INTRODUCTION
Pear‐shaped variety of blueberry (Cornus mas–Macrocarpa) belongs to the Cornaceae family; a shrub to a height of about 5–8 m with tiny leaves that grows in the vast region of Europe and Asia especially Arasbaran (Kaleibar) forests(Hassanpour, Hamidoghli, & Samizadeh, 2012, 2013). Blueberry contains tannins and organic acids, anthocyanins, carotenoids, and antioxidant melatonin. Besides, this fruit's extract is antidiabetic, antibacterial, anticancer, anti‐ pain and fever, and antioxidant while it has counter‐oxidative properties due to its ascorbic acid (Mirbadalzadeh & Shirdel, 2012; Tsuda, 2012).
Anthocyanins are a large subgroup of flavonoids responsible for red, purple, and blue colors in flowers, fruits, and vegetables (Chandrasekhar, Madhusudhan, & Raghavarao, 2012; Malaj, de Simone, Quartarolo, & Russo, 2013). They play an important role in the treatment of high blood pressure, liver disorders, diarrhea, and urinary problems such as kidney stones, urinary tract infections, and the common cold (Ercisli et al., 2011; Li et al., 2016; West, Ma, Deng, Jensen, & Su, 2012). Anthocyanins as a natural food coloring are an excellent option for food industry, but their very low stability in food matrix has caused some limitations (Jing et al., 2012; Pineda‐Vadillo et al., 2017) .Increasing the concentration of anthocyanins leads to greater stability and increased color intensity (several times) (Su et al., 2016).
Solvents, storage temperature, pH, concentration, structure of anthocyanins, copigments, oxygen, light, the use of polyphenols, presence of enzymes, and other materials connected to them significantly affect the stability of anthocyanin (Chung, Rojanasasithara, Mutilangi, & McClements, 2016; Jiao et al., 2016). Anthocyanins are more stable in acidic environments with lower pH compared to alkaline solvents with higher pH and are mainly found in the form of red falvylum. Adding copigment results in a significant color maintenance in anthocyanins which is due to copigment role in preventing the formation of a colorless falvylum cation (Andersen & Markham, 2005). Gauche, Malagoli, and Bordignon Luiz (2010) studied the effects of pH on the stability of anthocyanins in combination with organic acids. In this study, anthocyanins extracted from grapes (Vitis vinifera L.) were combined with four organic acids (tannic, caffeic, ferulic and gallic acid) and their stability was measured at different pHs (1, 2, 3, 3.3, 3.5, 3.7, 4, and 4.5). They found that most stability in all acids were at pH 3.3.
Copigmentation is an available natural tool to enhance anthocyanin color food of products; a color that can be stabilized and increased by intensity after adding different plant extracts rich of copigments (Ko, Lee, Sop Nam, & Gyu Lee, 2016; Zozio, Pallet, & Dornier, 2011). Similar to other anthocyanins reactions, copigmentation reactions are also affected by pH, temperature, concentration, and molecular structure (Sari, 2015; Wang, Shen, & Chen, 2013; Weber, Boch, & Schieber, 2017). Copigments are individually colorless, but they get an increased color intensity and solvent stability when added to the anthocyanin solvent (Heras‐Roger, Alonso‐Alonso, Gallo‐Montesdeoca, Díaz‐Romero, & Darias‐Martín, 2016). Copigments are an electron‐rich system able to communicate with falvylum ion that is electron poor (Weber et al., 2017). The association protects the water falvylum ions from nucleophilic attack (Gris, Ferreira, Falcão, & Bordignon Luiz, 2007; Pina, 2014). Most studied group from copigments is flavonoids. Besides, phenolic acids such as hydrosynamic acids and hydroxy benzoic acids are thoroughly investigated in copigments phenomenon and has great effect on the stability of anthocyanins (Castaneda‐Ovando, De Lourdes Pacheco‐Hernández, Páez‐Hernández, Rodríguez, & Galán‐Vidal, 2009; Willstatter & Zollinger, 2008). Zhang, Ma, Zhao, and Mu (2009) studied the effect of citric acid copigmentation on potato peel anthocyanins through thermodynamic measurements and found that citric acid enhances anthocyanin stability.
With reference to the findings from similar studies in the literature, it was found out that the colors were fortified and became stronger in the copigmentation of phenolic acids with anthocyanins.(Pineda‐Vadillo et al., 2017; Sun, Cao, Liao, & Hu, 2010; Tierno & Ruiz De Galarreta, 2016).
Color is a crucial criterion of a food product for clients. When manufacturing situations had been taken into consideration, in the course of storage, color bleaching and/or losses in ingredients could occur. For that reason, food‐coloring additives are used to augment coloration losses or enhance coloration. The most vital issue of concern among natural colorants is anthocyanin. However, distinctly low stability of anthocyanins in comparison to artificial dyes has restricted their use as natural colorants. Copigments are a group of colorless or pale substances which result in reinforcement of anthocyanins by making complex and thereby increasing the tone of anthocyanins color. Even though they are typically colorless, they may be delivered to solutions containing anthocyanins. Their color turns out to be more potent and the balance of the color increases their trade potentials. For these reasons, the purpose of this study was to stabilize the color of anthocyanin pigments through copigmentation methods. In this research, the effect of various pH values and different concentrations of copigments on the stability of anthocyanins in pear‐shaped variety of blueberry (C. mas–Macrocarpa) were investigated.
2. MATERIALS AND METHODS
2.1. Sample preparation and anthocyanin extraction
Agriculture Research Center of Kaleybar in East Azerbayjan province in Iran supplied the main sample of C. mas–Macrocarpa in September 2015. Department of Botany and Herbal Medicine at Urmia University, Urmia, Iran was in charge of pharmacognostic verification of the plant. Finally, the voucher specimens were deposited at the institute herbarium and received the code no: 4560.
The method consisted of extracting the anthocyanin using 96% ethanol and 0.1% HCl (Wrolstad et al., 2005). Then, 100 g of the specimen was mixed with 100 ml of extraction solvent to an extent that it was completely crushed and homogenous. Next, it was stirred with a magnetic stirrer for 4 hr. Later, the solvent was filtered by Buchner funnel and Whatman No.1 filter and diluted it to 500 ml. Afterwards, the resultant solvent was centrifuged at 8,000 g for 30 min and then, upper colored and clear solvent was gathered for the experimentations. At the end, the samples were kept in sealed vials for a period of 1 day at 4°C temperature (Skrede, Wrolstad, & Durst, 2000).
2.2. The preparation of anthocyanin‐copigment complex and pH treatment
Four types of organic acids were used comprising benzoic, tannic, caffeic, and coumaric acids for this study. The effect of the type of copigment on anthocyanin stability was evaluated by the same concentration (960 mg/L) and copigment‐anthocyanin ratio (1:1) for all copigments. Five groups were formed consisting of the control group (Ι) that got no copigment and treatment groups (ΙΙ–IV) whom we administered various kinds of copigments. Solvents’ absorbance was measured half an hour after the addition. Organic acids tannic, coumaric, caffeic, and benzoic acids were added and studied to the anthocyanins at a ratio of 1:1. The optical absorption rate was measured to assess copigmentation reactions spectrophotometerically at wavelengths of 450 and 650 nm (Biowave, S2100, UK) (Gauche et al., 2010; Giusti & Wrolstad, 2003). Five varying concentrations of each copigment (0, 120, 240, 480, and 960 mg/L) with the ratio of 1:1 to anthocyanin were used (Gauche et al., 2010). Next, the mixture of copigments and anthocyanin was prepared and after 30 min, the absorption level was measured at pH 3.5. Finally, the effects of various pH values, namely 1, 2, 3, and 4, were evaluated on the copigmentation reaction with the dissimilar concentrations of each copigment as well (Lambert, Asenstorfer, Williamson, Iland, & Jones, 2011; Wang & Xu, 2007).
2.3. Statistical analysis
All experimentations were performed in triplicate. The results were obtained in the form of mean and standard deviation (SD) of the means. One‐way ANOVA analysis and Tukey's test with the probability of p ≤ .05 was utilized to assess the difference between the treatments. SPSS 21 and Microsoft Excel were used to plot the diagrams and analyze the statistics.
3. RESULTS AND DISCUSSION
Among the factors affecting the copigmentation reactions is copigmental intensity that is added to anthocyanin. Copigmentation intensity depends on the molar ratio of anthocyanin to copigment (Jamei & Babaloo, 2017; Sari, 2015). The study revealed that the concentration of anthocyanin and copigment both affect copigmentation. Increasing the concentration of anthocyanins enhances bathchromic absorption. Increase in absorption depends on the molar ratio of anthocyanins to copigment (Jamei & Babaloo, 2017; Sari, 2015). Anthocyanin concentration was considered stable in the present research and by changing the concentration of copigment, the results were evaluated based on changes at the concentration of copigment. Due to the fact that anthocyanins were stable, it could be deduced that the ratio of bathochromic shift and hyperchromic effect depends on the concentration of copigment. Copigment used in this study consisted of coumaric, tannic, caffeic, and benzoic acids. In the current research, effects of five different concentrations of the aforementioned copigments (0, 120, 240, 480, and 960 mg/L) were assessed at stable pH value of 3.5 (Gauche et al., 2010; Jamei & Babaloo, 2017). and the ratio of 1:1 was used for each concentration of anthocyanins. The results were as the following graphs (Figure 1).
Figure 1.
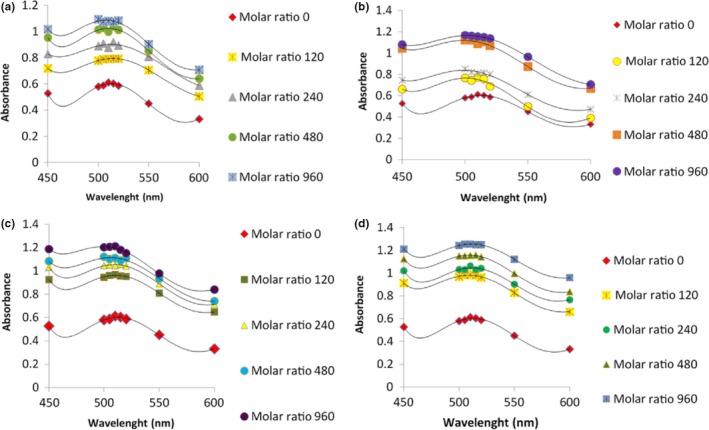
Absorption spectra of various anthocyanins with different concentrations at pH 3.5 for pear‐shaped variety of blueberry. (a) Tannic acid, (b) benzoic acid, (c) coumaric acid, (d) caffeic acid. The data are the mean value for three replications ± standard deviation
The results showed that the most stability and impact of concentration is observed in 960 mg/L and increasing concentrations of copigments, enhances absorption and stability of anthocyanins along with hyperchromic and bathochromic changes. Anthocyanin‐copigment complex showed a higher level of stability compared to anthocyanin individually and adding materials as copigments results in durability and stability of anthocyanins in bad conditions and it also is an inhibiting factor of anthocyanin pigments’ destruction. In pear‐shaped variety of blueberry (C. mas–Macrocarpa), caffeic acid at a concentration of 960 mg/L caused greater stability in anthocyanins compared to other copigments and benzoic acid at a concentration of 960 mg/L has less impact on stability (Figure 2).
Figure 2.
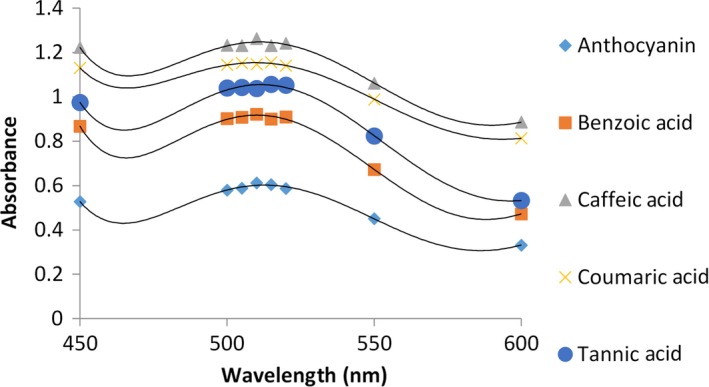
Comparison of absorption spectra of anthocyanin with different copigments (960 mg/L) and without copigment at pH 3.5 for pear‐shaped variety of blueberry. The data are the mean value for three replications ± standard deviation
Another factor affecting copigmentation is pH. This article aimed at investigating the effect of 1, 2, 3, and 4 pHs by adding tannic, caffeic, benzoic and coumaric acid at the ratio of 1:1. Anthocyanins are more stable in acidic environments with lower pH compared to alkaline solvents. The findings of this study revealed that pH increase causes a high level of destruction of anthocyanins in the samples. The present research also explored the effect of pH on anthocyanin‐copigment complex. The findings indicated that the highest anthocyanins absorption rate and also the highest stability of anthocyanin‐copigment complex occur in the lowest pH value (Figure 3). Table 1 reports the level of bathochromic and hyperchromic after the addition of organic acids to anthocyanin solvent in maximum absorption of anthocyanins in four differing pH of blueberry (C. mas–Macrocarpa).
Figure 3.
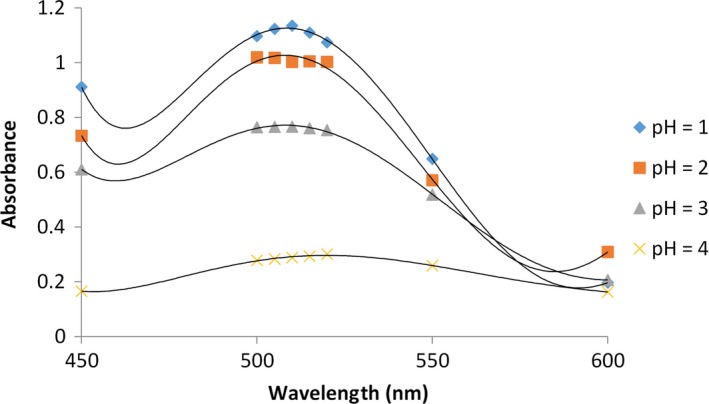
Diagram of the effects of different pH values on anthocyanin in blueberry; the data are the mean value for three replications ± standard deviation
Table 1.
The level of bathochromic and hyperchromic in maximum absorption of anthocyanins of pear‐shaped variety of blueberry (Cornus mas–Macrocarpa) of tannic, coumaric, caffeic, and benzoic acids in four differing pH
| pH | Anthocyanin | Tannic acid | Coumaric acid | Caffeic acid | Benzoic acid | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ∆A | ∆λ (nm) | ∆A | ∆λ (nm) | ∆A | ∆λ (nm) | ∆A | ∆λ (nm) | ∆A | ∆λ (nm) | |
| 1 | 135/1 | 510 | 482/0 | 0 | 484/0 | 5 | 675/0 | 5 | 655/0 | 5 |
| 2 | 118/1 | 505 | 562/0 | 5 | 49/0 | 0 | 721/0 | 0 | 432/0 | 5 |
| 3 | 667/0 | 51 | 27/0 | 5 | 207/0 | 0 | 035/0 | 0 | 015/0 | 5 |
| 4 | 201/0 | 520 | 09/0 | 10 | 067/0 | 10 | 001/0 | 5 | 088/0 | 10 |
∆A=0.721
∆A=0.001.
According to the obtained values in pear‐shaped variety of blueberry (C. mas–Macrocarpa), the highest level of hyperchromic of all organic acids was observed in pH 2 (∆A = 0.721), but these variations were not associated with changes bathochromic and most changes in bathochromic was observed of pH 4. The least level of effect of hyperchromic was observed in anthocyanin‐caffeic acid complex (∆A = 0.001). Bathochromic changes were not found in pHs 3 and 2, however, most changes were observed in bathochromic of pH 4. In pear shaped variety of pHs 1 and 2, the most stable copigment belonged to anthocyanin‐caffeic acid complex. In pH 4, the most stable copigment was anthocyanin‐tannic acid complex in which the destruction of anthocyanin increased and the color of anthocyanin‐copigment complex was severely weakened. The results revealed that highest stability of anthocyanin–copigment complex was observed in pH 1 and by increasing pH from 1 to 4, absorption is decreased due to the decrease in cation of falvylum crude extract and more anthocyanins are destructed. In highly acidic solvents (pHs 3 and 4), lots of hemiketals appear colorless and chalcone forms could change into flavylum cations that are quinoid through the formation of copigmentation complexes and as a result, produce significant color, besides, in these solvents, all anthocyanins are in flavylum colored form (Pina, 2014; Weber et al., 2017).
Gras, Bogner, Carle, and Schweiggert (2016) analyzed the coloration depth and stability of black carrot anthocyanins by way of intermolecular copigmentation with the addition of chlorogenic acid, and an aqueous phenolic‐rich green coffee bean extract at numerous anthocyanin copigment ratios on heating. They found out that the hyperchromic copigmentation effect fixated the concentration of delivered co‐pigments, which was followed by an absorption increase in over one fifth. After adding anthocyanin copigments, heat stability substantially improved (Gras et al., 2016).
Recently, Jamei & Babaloo (2017) investigated anthocyanin stability of C. mas–Yulyush by means of pHs and copigment treatments. They proved that in higher pH value degradation of anthocyanin and in comparison to other copigments, caffeic acid had a profound preventive impact and benzoic acid had the lowest hyperchromic and bathochromic changes.
(Malaj et al., 2013) revealed that anthocyanin–copigment leads to an increase in bath‐ and hyperchromic effect (Malaj et al., 2013).
4. CONCLUSION
The findings of the current article on pear‐shaped variety of blueberry (C. mas–Macrocarpa) showed that most hyperchromic effect all organic acids was seen of pH 2. These changes were not associated with changes of bathochromic and the most changes were observed in pH 4. The least effect of hyperchromic was recorded in anthocyanin‐caffeic acid complex. Bathchromic changes were not significant in pHs 2 and 3, but it was the highest in pH 4. In pear‐shaped variety of pHs 1 and 2, the most stable copigment was anthocyanin‐caffeic acid complex. In pH 4, the most stable copigment was anthocyanin‐tannic acid complex in which the level of anthocyanins destruction increased and anthocyanin‐copigment complex was severely weakened in color. The results of this research may be beneficial for improving anthocyanin stability from the point of color and could be utilized in food and pharmaceutical industries considering the necessity to use nonsynthetic colors and the significance of natural colors in meals productions.
CONFLICT OF INTEREST
None declared.
Babaloo F, Jamei R. Anthocyanin pigment stability of Cornus mas–Macrocarpa under treatment with pH and some organic acids. Food Sci Nutr. 2018;6:168–173. https://doi.org/10.1002/fsn3.542
REFERENCES
- Andersen, O. M. , & Markham, K. R. (2005). Flavonoids: Chemistry, biochemistry and applications. Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487‐2742 United States of America: CRC Press. [Google Scholar]
- Castaneda‐Ovando, A. , De Lourdes Pacheco‐Hernández, M. , Páez‐Hernández, M. E. , Rodríguez, J. A. , & Galán‐Vidal, C. A. (2009). Chemical studies of anthocyanins: A review. Food chemistry, 113(4), 859–871. [Google Scholar]
- Chandrasekhar, J. , Madhusudhan, M. , & Raghavarao, K. (2012). Extraction of anthocyanins from red cabbage and purification using adsorption. Food and Bioproducts Processing, 90(4), 615–623. [Google Scholar]
- Chung, C. , Rojanasasithara, T. , Mutilangi, W. , & McClements, D. J. (2016). Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chemistry, 212, 596–603. [DOI] [PubMed] [Google Scholar]
- Ercisli, S. , Yilmaz, S. O. , Gadze, J. , Dzubur, A. , Hadziabulic, S. , & Aliman, Y. (2011). Some fruit characteristics of Cornelian Cherries (Cornus mas L.). Notulae Botanicae Horti Agrobotanici Cluj‐Napoca, 39(1), 255. [Google Scholar]
- Gauche, C. , Malagoli, E. D. S. , & Bordignon Luiz, M. T. (2010). Effect of pH on the copigmentation of anthocyanins from Cabernet Sauvignon grape extracts with organic acids. Scientia Agricola, 67(1), 41–46. [Google Scholar]
- Giusti, M. M. , & Wrolstad, R. E. (2003). Acylated anthocyanins from edible sources and their applications in food systems. Biochemical Engineering Journal, 14(3), 217–225. [Google Scholar]
- Gras, C. C. , Bogner, H. , Carle, R. , & Schweiggert, R. M. (2016). Effect of genuine non‐anthocyanin phenolics and chlorogenic acid on color and stability of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) anthocyanins. Food Research International, 85, 291–300. [DOI] [PubMed] [Google Scholar]
- Gris, E. F. , Ferreira, E. A. , Falcão, L. D. , & Bordignon Luiz, M. T. (2007). Influence of ferulic acid on stability of anthocyanins from Cabernet Sauvignon grapes in a model system and a yogurt system. International journal of food science & technology, 42(8), 992–998. [Google Scholar]
- Hassanpour, H. , Hamidoghli, Y. , & Samizadeh, H. (2012). Some fruit characteristics of Iranian cornelian cherries (Cornus mas L.). Notulae Botanicae Horti Agrobotanici Cluj‐Napoca, 40(1), 247. [Google Scholar]
- Hassanpour, H. , Hamidoghli, Y. , & Samizadeh, H. (2013). Estimation of genetic diversity in some Iranian cornelian cherries (Cornus mas L.) accessions using ISSR markers. Biochemical Systematics and Ecology, 48, 257–262. [Google Scholar]
- Heras‐Roger, J. , Alonso‐Alonso, O. , Gallo‐Montesdeoca, A. , Díaz‐Romero, C. , & Darias‐Martín, J. (2016). Influence of copigmentation and phenolic composition on wine color. Journal of food science and technology, 53(6), 2540–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamei, R. , & Babaloo, F. (2017). Stability of blueberry (Cornus mas–Yulyush) anthocyanin pigment under pH and co‐pigment treatments. International Journal of Food Properties, 20(9), 2128–2133. [Google Scholar]
- Jiao, Y. , Zhang, Y. , He, Z. , Zhai, W. , Gong, H. , & Yang, Z. (2016). Effect of ferulic acid on the formation of pyranoanthocyanins from purple corn (Zea mays L.) cob in a model system and their effects on color. International Journal of Food Properties, 19(4), 847–858. [Google Scholar]
- Jing, P. , Zhao, S.‐J. , Ruan, S.‐Y. , Xie, Z.‐H. , Dong, Y. , & Yu, L. L. (2012). Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chemistry, 133(4), 1569–1576. [Google Scholar]
- Ko, A. , Lee, J. S. , Sop Nam, H. , & Gyu Lee, H. (2016). Stabilization of black soybean anthocyanin by chitosan nanoencapsulation and copigmentation. Journal of Food Biochemistry, 41(2), https://doi.org/10.1111/jfbc.12316 . [Google Scholar]
- Lambert, S. G. , Asenstorfer, R. E. , Williamson, N. M. , Iland, P. G. , & Jones, G. P. (2011). Copigmentation between malvidin‐3‐glucoside and some wine constituents and its importance to colour expression in red wine. Food Chemistry, 125(1), 106–115. [Google Scholar]
- Li, X. , Xu, J. , Tang, X. , Liu, Y. , Yu, X. , Wang, Z. , & Liu, W. (2016). Anthocyanins inhibit trastuzumab‐resistant breast cancer in vitro and in vivo. Molecular Medicine Reports, 13(5), 4007–4013. [DOI] [PubMed] [Google Scholar]
- Malaj, N. , de Simone, B. C. , Quartarolo, A. D. , & Russo, N. (2013). Spectrophotometric study of the copigmentation of malvidin 3‐O‐glucoside with p‐coumaric, vanillic and syringic acids. Food chemistry, 141(4), 3614–3620. [DOI] [PubMed] [Google Scholar]
- Mirbadalzadeh, R. , & Shirdel, Z. (2012). Antihyperglycemic and antihyperlipidemic effects of Cornus mas extract in diabetic rats compared with glibenclamide. Elixir (Hormo. & Signal), 47, 8969–8972. [Google Scholar]
- Pina, F. (2014). Chemical applications of anthocyanins and related compounds. A source of bioinspiration. Journal of Agricultural and Food Chemistry, 62(29), 6885–6897. [DOI] [PubMed] [Google Scholar]
- Pineda‐Vadillo, C. , Nau, F. , Guerin‐Dubiard, C. , Jardin, J. , Lechevalier, V. , Sanz‐Buenhombre, M. , … Hingyi, H. (2017). The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food chemistry, 214, 486–496. [DOI] [PubMed] [Google Scholar]
- Sari, F. (2015). The copigmentation effect of different phenolic acids on Berberis crataegina anthocyanins. Journal of Food Processing and Preservation, 40(3), 422–430. [Google Scholar]
- Skrede, G. , Wrolstad, R. , & Durst, R. (2000). Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). Journal of Food Science, 65(2), 357–364. [Google Scholar]
- Su, G. , Zhu, S. , Xu, M. , Ramaswamy, H. S. , Lin, Y. , & Yu, Y. (2016). Pressure degradation kinetics of anthocyanin pigment and visual color of chinese bayberry juice. International Journal of Food Properties, 19(2), 443–453. [Google Scholar]
- Sun, J. , Cao, X. , Liao, X. , & Hu, X. (2010). Comparative analyses of copigmentation of cyanidin 3‐glucoside and cyanidin 3‐sophoroside from red raspberry fruits. Food Chemistry, 120(4), 1131–1137. [Google Scholar]
- Tierno, R. , & Ruiz De Galarreta, J. I. (2016). Influence of selected factors on anthocyanin stability in colored potato extracts. Journal of Food Processing and Preservation, 40(5), 1020–1026. [Google Scholar]
- Tsuda, T. (2012). Dietary anthocyanin‐rich plants: Biochemical basis and recent progress in health benefits studies. Molecular nutrition & food research, 56(1), 159–170. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Shen, X. , & Chen, Y. (2013). Effect of pH, temperature and iron on the stability of anthocyanins from black‐skinned peanuts (Arachis hypogaea L.). African Journal of Agricultural Research, 8(18), 2044–2047. [Google Scholar]
- Wang, W.‐D. , & Xu, S.‐Y. (2007). Degradation kinetics of anthocyanins in blackberry juice and concentrate. Journal of food engineering, 82(3), 271–275. [Google Scholar]
- Weber, F. , Boch, K. , & Schieber, A. (2017). Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT‐Food Science and Technology, 75, 72–77. [Google Scholar]
- West, B. J. , Ma, D.‐L. , Deng, S. , Jensen, C. J. , & Su, C. X. (2012). Bioactivities and iridoid determination of a beverage containing noni, cornelian cherries and olive leaf extract. Advance Journal of Food Science and Technology, 4(2), 91–96. [Google Scholar]
- Willstatter, R. , & Zollinger, E. H. (2008). Anthocyanins of the grape and of the billberry. Journal of the Chemical Society, 112, 47–48. [Google Scholar]
- Wrolstad, R. E. , Acree, T. E. , Decker, E. A. , Penner, M. H. , Reid, D. S. , Schwartz, S. J. , … Sporns, P. (2005). Handbook of food analytical chemistry, volume 1: Water, proteins, enzymes, lipids, and carbohydrates. Hoboken, New Jersey: Jon Wiely & Sons, Inc. [Google Scholar]
- Zhang, C. , Ma, Y. , Zhao, X. , & Mu, J. (2009). Influence of copigmentation on stability of anthocyanins from purple potato peel in both liquid state and solid state. Journal of Agricultural and Food Chemistry, 57(20), 9503–9508. [DOI] [PubMed] [Google Scholar]
- Zozio, S. , Pallet, D. , & Dornier, M. (2011). Evaluation of anthocyanin stability during storage of a coloured drink made from extracts of the Andean blackberry (Rubus glaucus Benth.), açai (Euterpe oleracea Mart.) and black carrot (Daucus carota L.). Fruits, 66(3), 203–215. [Google Scholar]


