Abstract
Objectives
The aim of this study was to analyse baseline characteristics and outcome of patients with heart failure and mid-range left ventricular ejection fraction (HFmrEF, left ventricular ejection fraction (LVEF) 40%–49%) and the effect of 1-year change in LVEF in this group.
Setting
Multicentre prospective observational study of ambulatory patients with HF followed up at four university hospitals with dedicated HF units.
Participants
Fourteen per cent (n=504) of the 3580 patients included had HFmrEF.
Interventions
Baseline characteristics, 1-year LVEF and outcomes were collected. All-cause death, HF hospitalisation and the composite end-point were the primary outcomes.
Results
Median follow-up was 3.66 (1.69–6.04) years. All-cause death, HF hospitalisation and the composite end-point were 47%, 35% and 59%, respectively. Outcomes were worse in HF with preserved ejection fraction (HFpEF) (LVEF>50%), without differences between HF with reduced ejection fraction (HFrEF) (LVEF<40%) and HFmrEF (all-cause mortality 52.6% vs 45.8% and 43.8%, respectively, P=0.001). After multivariable Cox regression analyses, no differences in all-cause death and the composite end-point were seen between the three groups. HF hospitalisation and cardiovascular death were not statistically different between patients with HFmrEF and HFrEF. At 1-year follow-up, 62% of patients with HFmrEF had LVEF measured: 24% had LVEF<40%, 43% maintained LVEF 40%–49% and 33% had LVEF>50%. While change in LVEF as continuous variable was not associated with better outcomes, those patients who evolved from HFmrEF to HFpEF did have a better outcome. Those who remained in the HFmrEF and HFrEF groups had higher all-cause mortality after adjustment for age, sex and baseline LVEF (HR 1.96 (95% CI 1.08 to 3.54, P=0.027) and HR 2.01 (95% CI 1.04 to 3.86, P=0.037), respectively).
Conclusions
Patients with HFmrEF have a clinical profile in-between HFpEF and HFrEF, without differences in all-cause mortality and the composite end-point between the three groups. At 1 year, patients with HFmrEF exhibited the greatest variability in LVEF and this change was associated with survival.
Keywords: heart failure, echocardiography, ejection fraction, prognosis, recovered
Strengths and limitations of this study.
Cohort study of patients with heart failure (HF) followed up at four hospitals with a dedicated HF unit, thereby reflecting different clinical practice within guidelines recommendations.
The hospitals varied from community-oriented hospitals to reference centres with transplant and ventricular assist devices programmes. The inclusion of hospitals with different levels of complexity determined that baseline characteristics of patients were different among the four hospitals, which allows an easier generalisation of the results.
Not all patients had an echocardiogram during follow-up, which might have resulted in a bias and affected the results.
Introduction
Despite the improvement in knowledge and treatment of heart failure (HF) in the last decades, HF is still a prevalent disease with a bad prognosis1 and to which considerable healthcare resources are dedicated.2 Much of the research to date has focused on patients with HF and reduced ejection fraction (HFrEF) and so far pharmacological and invasive treatments have only shown to be useful in this group of patients.3 Furthermore, definition of HF with preserved ejection fraction (HFpEF) varies widely in registries and in randomised controlled trials (from left ventricular ejection fraction (LVEF)>40% to >55%)4 and hence a grey zone of patients with LVEF ranging 40%–50% has hardly been studied. For this reason, the last 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic HF included this new mid-range group in the classification of HF in order to stimulate research in this subpopulation of patients.3 Previous studies have shown that patients with HF and mid-range left ventricle ejection fraction (HFmrEF) have a baseline profile in-between of HFrEF and HFpEF, with some characteristics closer to HFrEF (predominant ischaemic aetiology) and others to HFpEF (higher prevalence of women and elderly patients). Moreover, differences in outcomes have also been described between groups. Given the differences in baseline characteristics and prognosis in the three groups, some authors suggest that HFmrEF has a phenotype closer to HFpEF5–8 whereas other authors consider it closer to HFrEF.9–11 In patients with HFrEF (LVEF<40%) an improvement in LVEF has been associated with better outcomes.12 Whether this is also true in patients with mid-range EF (HFmrEF) is unknown.
Hence, the aim of this study was to analyse the baseline characteristics and outcome of patients with HFmrEF compared with patients with HFpEF (LVEF >50%) and HFrEF and to analyse the effect of 1 year change in LVEF in patients with HFmrEF on outcome.
Methods
Study population
This was a prospective observational study of patients with HF followed at four university hospitals with a dedicated Heart Failure Unit. Patients were consecutively enrolled from August 2001 to June 2015 and HF was diagnosed following the ESC guidelines.3 Baseline demographic, clinical and echocardiographic data were collected. Patients were classified into three groups according to the new ESC Guidelines for the diagnosis and treatment of acute and chronic HF: HFrEF, HFmrEF and HFpEF.3 Changes in medical therapy over time were not collected.
Data on 1-year LVEF were also collected when available. Follow-up echocardiograms were performed as per each institutional protocol. All patients were followed up at regular intervals. Those who failed to attend the clinic appointment were contacted by telephone, and hospital and primary care records were reviewed in order to assess vital status and HF hospitalisations. The main outcome was recorded as death from all causes, HF hospitalisation and a composite end-point of all-cause death and HF hospitalisation. Cardiovascular (CV) death was also analysed. A death was considered of CV origin if it was caused by HF, sudden death, acute myocardial infarction, stroke, CV procedures or other CV causes.
All patients gave written informed consent during the initial visit.
Statistical analyses
Continuous variables were expressed as mean±SD or median and IQR, depending on whether data distribution was normal (assessed by normal Q–Q plots); categorical variables were expressed as percentages. A comparative analysis between variables was carried out using χ² test (categorical variables) and Student’s t-test or Mann–Whitney U-test, one-way analysis of variance or Kruskal-Wallis test for continuous variables. P value adjustment for multiple testing was performed by Tukey (normal-distribution) or Benjamini and Hochberg method (otherwise). Kaplan-Meier survival curves were compared by the log-rank test. Multivariate analysis was performed using Cox proportional hazard regression (Cox) including centre as strata.
In all analyses involving CV death and HF hospitalisation, a competing risks strategy by the Gray method was adopted,13 considering non-CV death as the competing event for CV death and all-cause death for HF hospitalisation. HFrEF was used as the reference category. The analyses were performed with R (V.3.3.2) R: A Language and Environment for Statistical Computing (Vienna, Austria). We considered P values <0.05 from two-sided tests to indicate statistical significance.
In all analyses involving CV, HF-related and sudden death, competing risk strategy by the Gray method was adopted, considering non-CV death as the competing event for CV death and other CV death and non-CV death for HF-related and sudden death. The category ‘HF-recovered’ was used as a reference. Survival curves for all-cause death and cumulative incidence curves for the composite primary endpoint, and CV, HF-related and sudden death were plotted. P-values were obtained using log-rank and Gray method, respectively.
Results
Baseline clinical characteristics
Baseline clinical characteristics and treatment were categorised according to LVEF and are summarised in table 1. Fourteen per cent (n=504) of the 3580 patients included in the study had HFmrEF, 62% had HFrEF and 24% HFpEF. In the whole cohort, mean age was 68±13 years, 62% were men and mean LVEF was 38%±16%. Baseline characteristics of patients with HFmrEF were in-between those of HFpEF and HFrEF. Use of neurohormonal treatment was higher in patients with HFrEF and HFmrEF, whereas the use of loop diuretics was highest in the HFpEF group. The four cohorts were clinically different (see online supplementary material, table S1, describing the baseline characteristics according to hospital).
Table 1.
Baseline clinical characteristics and treatment categorised according to LVEF
| All n=3580 | HFrEF n=2232(62%) | HFmrEF n=504 (14%) | HFpEF n=844 (24%) | P value* | P value** | P value*** | N | |
| Male | 2397 (67.0) | 1689 (75.7) | 337 (66.9) | 371 (44.0) | <0.001 | <0.001 | <0.001 | 3580 |
| Age | 68.2±12.7 | 66.2±12.5 | 68.1±12.9 | 73.5±11.4 | <0.001 | 0.003 | <0.001 | 3580 |
| LVEF | 38.3±16.0 | 28.0±6.9 | 43.5±2.9 | 62.5±8.3 | <0.001 | <0.001 | <0.001 | 3580 |
| Aetiology: | <0.001 | <0.001 | <0.001 | 3579 | ||||
| Ischaemic | 1600 (44.7) | 1174 (52.6) | 261 (51.8) | 165 (19.5) | ||||
| Dilated | 552 (15.4) | 450 (20.2) | 58 (11.5) | 44 (5.21) | ||||
| Hypertensive | 592 (16.5) | 169 (7.58) | 72 (14.3) | 351 (41.6) | ||||
| Valvular | 321 (8.97) | 131 (5.87) | 45 (8.93) | 145 (17.2) | ||||
| Other | 514 (14.4) | 307 (13.8) | 68 (13.5) | 139 (16.5) | ||||
| Heart rate | 72.7±14.8 | 72.8±14.9 | 70.8±14.3 | 73.8±14.6 | 0.001 | 0.005 | <0.001 | 3577 |
| Hypertension | 2485 (69.4) | 1434 (64.2) | 366 (72.6) | 685 (81.2) | <0.001 | <0.001 | <0.001 | 3580 |
| Diabetes mellitus | 1547 (43.2) | 956 (42.8) | 210 (41.7) | 381 (45.1) | 0.386 | 0.669 | 0.235 | 3580 |
| COPD | 721 (20.1) | 427 (19.1) | 103 (20.4) | 191 (22.6) | 0.096 | 0.544 | 0.381 | 3580 |
| Dyslipidaemia | 1843 (51.5) | 1158 (51.9) | 263 (52.2) | 422 (50.0) | 0.611 | 0.942 | 0.472 | 3580 |
| Atrial fibrillation | 999 (27.9) | 435 (19.5) | 158 (31.3) | 406 (48.2) | <0.001 | <0.001 | <0.001 | 3579 |
| BMI, kg/m2 | 27.8±5.30 | 27.3±4.93 | 28.2±5.30 | 28.9±6.01 | <0.001 | <0.001 | 0.045 | 3528 |
| Sodium, mmol/L | 139±4.33 | 139±4.67 | 140±3.50 | 140±3.63 | <0.001 | <0.001 | 0.730 | 3556 |
| Anaemia | 1231 (34.9) | 656 (29.9) | 184 (37.1) | 391 (46.8) | <0.001 | 0.002 | 0.001 | 3580 |
| NT-proBNP, ng/L | 1638 (697;3937) | 1898 (769;4465) | 1484 (532;3866) | 1320 (635;2818) | <0.001 | <0.001 | 0.518 | 2705 |
| eGFR, mL/min/1.73 m2 | 60.4±25.4 | 62.4±25.4 | 60.4±26.5 | 55.2±23.8 | <0.001 | 0.125 | <0.001 | 3562 |
| NYHA class III–IV | 1293 (36.1) | 746 (33.4) | 172 (34.1) | 375 (44.4) | <0.001 | 0.808 | <0.001 | 3579 |
| Treatment: | ||||||||
| ACEI or ARB | 2992 (83.9) | 1992 (89.5) | 412 (82.1) | 588 (70.1) | <0.001 | <0.001 | <0.001 | 3567 |
| Beta-blockers | 3094 (86.4) | 2040 (91.4) | 448 (88.9) | 606 (71.8) | <0.001 | 0.092 | <0.001 | 3580 |
| MRA | 1890 (52.8) | 1447 (64.8) | 219 (43.5) | 224 (26.5) | <0.001 | <0.001 | <0.001 | 3580 |
| Loop diuretics | 3189 (89.1) | 2002 (89.7) | 423 (83.9) | 764 (90.5) | <0.001 | <0.001 | <0.001 | 3580 |
| Digoxin | 959 (27.7) | 675 (30.8) | 106 (21.7) | 178 (22.5) | <0.001 | <0.001 | 0.788 | 3467 |
| ICD | 396 (11.1) | 359 (16.1) | 26 (5.16) | 11 (1.31) | <0.001 | <0.001 | <0.001 | 3578 |
| CRT | 234 (6.54) | 213 (9.54) | 16 (3.17) | 5 (0.59) | <0.001 | <0.001 | 0.001 | 3580 |
| Anticoagulants | 1684 (47.0) | 969 (43.4) | 239 (47.4) | 476 (56.4) | <0.001 | 0.113 | 0.002 | 3580 |
Data are mean±SD deviation, median (IQR) or n (%). Anaemia was defined as a haemoglobin<12 g/dL.
*P values for the comparison of all three groups (null hypothesis: all three groups have the same characteristics).
**P value only applies to the comparison of HFrEF vs HFmrEF.
***P value only applies to the comparison of HFpEF vs HFmrEF.
ACEI, ACE inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy; eGFR, estimated glomerular filtration rate (CKD-EPI equation); HFmrEF, heart failure with mid-range left ventricle ejection fraction; HFpEF, heart failure with preserved left ventricle ejection fraction; HFrEF, heart failure with reduced left ventricle ejection fraction; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
bmjopen-2017-018719supp001.pdf (250.6KB, pdf)
Follow-up events
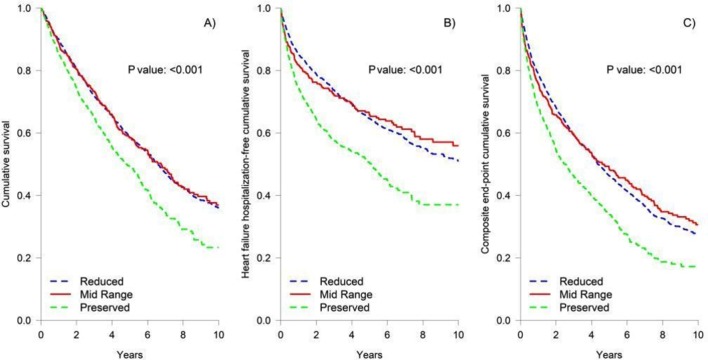
During a median follow-up of 3.66 (1.69–6.04) years, all-cause death, HF hospitalisation and the composite end-point were 47%, 35% and 59%, respectively (table 2). The cause of death was CV in 24.7% of patients, without differences in the three groups (overall P=0.068). Outcomes were worse in HFpEF, without differences between HFrEF and HFmrEF (figure 1). In multivariable Cox regression analyses, no differences in all-cause death and composite end-point were seen between the three groups. HF hospitalisation and CV death were not statistically different between patients with HFmrEF and HFrEF, although a tendency (P=0.068) towards a lower CV mortality in HFmrEF was observed (table 3). On the other hand, patients with HFpEF had significantly higher HF hospitalisation and lower CV death (table 3).
Table 2.
Mortality, cause of death and HF hospitalisation during follow-up
| All n=3580 | HFrEF n=2232 (62%) |
HFmrEF n=504 (14%) |
HFpEF n=844 (24%) |
P value* | P value** | P value*** | N | |
| All-cause death | 1688 (47.2) | 1023 (45.8) | 221 (43.8) | 444 (52.6) | 0.001 | 0.448 | 0.003 | 3580 |
| Cause of death | <0.001 | 0.164 | 0.011 | 3580 | ||||
| HF | 458 (12.8) | 269 (12.1) | 58 (11.5) | 131 (15.5) | ||||
| Sudden death | 126 (3.52) | 101 (4.53) | 13 (2.58) | 12 (1.42) | ||||
| Other cardiovascular | 196 (5.47) | 122 (5.47) | 29 (5.75) | 45 (5.33) | ||||
| Non-cardiovascular | 500 (14.0) | 265 (11.9) | 72 (14.3) | 163 (19.3) | ||||
| Unknown | 408 (11.4) | 266 (11.9) | 49 (9.7) | 93 (11.0) | ||||
| HF hospitalisation | 1259 (35.2) | 724 (32.4) | 157 (31.2) | 378 (44.8) | <0.001 | 0.613 | <0.001 | 3580 |
| Composite end-point | 2113 (59.0) | 1277 (57.2) | 272 (54.0) | 564 (66.8) | <0.001 | 0.201 | <0.001 | 3580 |
Data are n (%).
*P values for the comparison of all three groups (null hypothesis: all three groups have the same characteristics).
**P value only applies to the comparison of HFrEF vs HFmrEF.
***P value only applies to the comparison of HFpEF vs HFmrEF.
HF, heart failure; HFmrEF, heart failure with mid-range left ventricle ejection fraction; HFpEF, heart failure with preserved left ventricle ejection fraction; HFrEF, heart failure with reduced left ventricle ejection fraction.
Figure 1.
Kaplan-Meier curves for long-term outcome divided by LVEF (A, cumulative survival; B, HF hospitalisation-free cumulative incidence; C, composite end-point cumulative survival). HF, heart failure; LVEF, left ventricular ejection fraction.
Table 3.
Multivariable Cox regression analyses with hospital as strata for all-cause death, HF hospitalisation, composite end-point and cardiovascular death
| All-cause death | HF hospitalisation | Composite end-point (all-cause death+HF hospitalisation) |
CV death | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| HFrEF | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| HFmrEF | 0.93 (0.80 to 1.08) | 0.338 | 1.00 (0.84 to 1.20) | 0.98 | 0.94 (0.82 to 1.07) | 0.358 | 0.80 (0.64 to 1.01) | 0.061 |
| HFpEF | 0.93 (0.81 to 1.06) | 0.265 | 1.18 (1.02 to 1.38) | 0.032 | 0.95 (0.84 to 1.06) | 0.362 | 0.75 (0.62 to 0.92) | 0.006 |
| Female | 0.75 (0.67 to 0.84) | <0.001 | – | 0.85 (0.77 to 0.94) | 0.002 | 0.75 (0.64 to 0.88) | <0.001 | |
| Age | 1.03 (1.03 to 1.04) | <0.001 | – | 1.02 (1.02 to 1.03) | <0.001 | 1.03 (1.02 to 1.04) | <0.001 | |
| Heart rate | 1.00 (1.00 to 1.01) | 0.013 | – | 1.00 (1.00 to 1.01) | 0.008 | – | ||
| DBP | 0.99 (0.99 to 1.00) | 0.002 | 0.99 (0.99 to 1.0) | 0.044 | 0.99 (0.99 to 1.00) | 0.001 | 0.99 (0.98 to 0.99) | <0.001 |
| Dyslipidaemia | 0.86 (0.78 to 0.96) | 0.004 | 1.25 (1.10 to 1.41) | <0.001 | – | – | ||
| DM | 1.30 (1.17 to 1.44) | <0.001 | 1.22 (1.08 to 1.37) | 0.002 | 1.27 (1.16 to 1.39) | <0.001 | 1.27 (1.11 to 1.47) | <0.001 |
| COPD | 1.32 (1.17 to 1.48) | <0.001 | 1.27 (1.10 to 1.47) | <0.001 | 1.30 (1.17 to 1.45) | <0.001 | – | |
| BMI | 0.98 (0.97 to 0.99) | <0.001 | – | – | – | |||
| Sodium | – | – | – | 0.99 (0.97 to 1.00) | 0.024 | |||
| Haemoglobin | 0.93 (0.90 to 0.96) | <0.001 | 0.90 (0.87 to 0.93) | <0.001 | 0.91 (0.89 to 0.93) | <0.001 | 0.93 (0.89 to 0.97) | 0.001 |
| eGFR | 0.99 (0.99 to 1.00) | <0.001 | 0.99 (0.99 to 0.99) | <0.001 | 0.99 (0.99 to 1.00) | <0.001 | 0.99 (0.99 to 0.99) | <0.001 |
| NYHA class III–IV | 1.62 (1.46 to 1.80) | <0.001 | 1.34 (1.18 to 1.51) | <0.001 | 1.54 (1.40 to 1.69) | <0.001 | 1.61 (1.39 to 1.86) | <0.001 |
| ACEI or ARB | 0.70 (0.62 to 0.81) | <0.001 | – | 0.74 (0.65 to 0.83) | <0.001 | – | ||
| Beta-blockers | 0.60 (0.53 to 0.69) | <0.001 | – | 0.70 (0.62 to 0.79) | <0.001 | 0.60 (0.49 to 0.72) | <0.001 | |
| Loop diuretics | 1.28 (1.04 to 1.57) | 0.020 | 2.97 (2.18 to 4.06) | <0.001 | 1.61 (1.33 to 1.94) | <0.001 | 1.88 (1.32 to 2.67) | <0.001 |
| CRT | 0.70 (0.55 to 0.89) | 0.003 | – | – | – | |||
| ICD | – | – | – | 0.77 (0.60 to 0.98) | 0.032 | |||
| MRA | – | 1.18 (1.03 to 1.34) | 0.014 | – | – | |||
| Digoxin | – | 1.48 (1.29 to 1.69) | <0.001 | – | 1.26 (1.08 to 1.47) | 0.004 | ||
| Anticoagulants | – | – | – | – | ||||
| Hypertension | – | – | 1.12 (1.01 to 1.25) | 0.033 | – | |||
ACEI, ACE inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy; CV, cardiovascular; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (CKD-EPI equation); HF, heart failure; HFmrEF, HF with mid-range left ventricle ejection fraction; HFpEF, HF with preserved left ventricle ejection fraction; HFrEF, HF with reduced left ventricle ejection fraction; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association.
Changes in LVEF
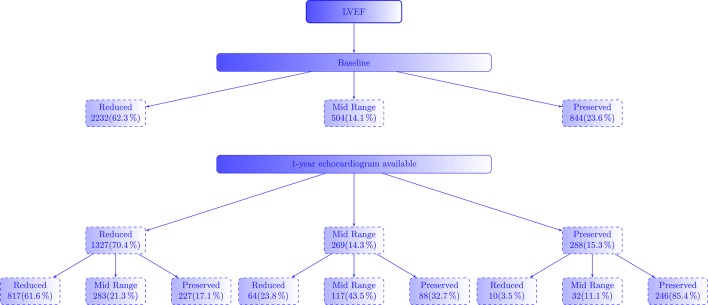
Flowchart of patients according to the change of LVEF is depicted in figure 2. Of the 1971 patients with HFrEF alive at 1 year, 67% had an echocardiogram performed: 62% still had LVEF<40% and 21% had LVEF 40%–50%. In this group, after adjustment for age, sex and baseline EF, HRs for survival for change in LVEF was 0.97 (95% CI 0.96 to 0.98, P<0.001).
Figure 2.
Flowchart of patients according to the change of LVEF. LVEF, left ventricular ejection fraction.
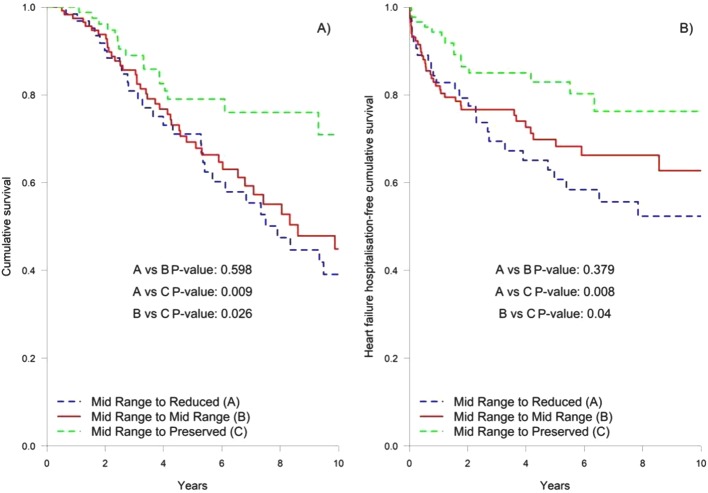
LVEF of patients with HFmrEF (analysed in 61% of the 438 patients alive at 1 year) had the greatest variation: 24% had reduced LVEF, 43% maintained LVEF 40%–49% and 33% had LVEF >50%. Figure 3 shows Kaplan-Meier curves for long-term outcome for changes of LVEF in HFmrEF. There were no differences in mortality between patients who remained in HFmrEF group and those who changed to HFrEF, while survival was significantly higher in those patients who evolved to the HFpEF group (P=0.026). Compared with patients whose LVEF improved enough to move to the HFpEF group, those who remained in the HFmrEF and HFrEF had higher all-cause mortality after adjustment for age, sex and baseline LVEF (HR 1.96 (95% CI 1.08 to 3.54, P=0.027) and HR 2.01 (95% CI 1.04 to 3.86, P=0.037), respectively). As a continuous variable, however, and after adjustment for age, sex and baseline LVEF, HR for survival for change in LVEF was 0.99 (95% CI 0.96 to 1.01, P=0.229). Baseline characteristics of patients who evolved to HFpEF were similar to those who remained in HFmrEF or changed to HFrEF, except for a higher proportion of women (38% vs 23%, P=0.021) and non-ischaemic aetiology (61% vs 38%, P=0.001), higher diastolic blood pressure (76±11 vs 72±12 mm Hg, P=0.009) and estimated glomerular filtration rate (70±24 vs 63±27 mL/min, P=0.028) but lower N-terminal pro-brain natriuretic peptide (NT-proBNP) (901 (450–1690) ng/L vs 1494 (593–4456], P=0.013). Baseline LVEF was higher (44%±3% vs . 42±2%, p<0.001). Interestingly, treatment was similar in both groups, with a high use of beta blockers (95.5% vs 92.3%, P=0.470), ACE inhibitor/angiotensin II receptor blocker (83% vs 83%, P=1.0) and mineralocorticoid receptor antagonists (40.9% vs 51.9%, P=0.117). See online supplementary material (table S2) for comparison among the groups.
Figure 3.
Kaplan-Meier curves for long-term outcome for changes of LVEF in the HFmrEF (A, cumulative survival; B, HF hospitalisation-free cumulative incidence). HF, heart failure; HFmrEF, HF with mid-range left ventricle ejection fraction; LVEF, left ventricular ejection fraction.
Finally, among patients with HFpEF alive at 1 year, only 288 (40%) had LVEF measured at 1-year follow-up, and the majority (85%) of them still had LVEF >50%. After adjustment for age, sex and baseline EF, HR for survival for change in LVEF in this group was 0.99 (95% CI 0.97 to 1.01, P=0.283).
Interestingly, at 1-year follow-up, 22.9% of patients were in the HFmrEF category, irrespective of their initial LVEF.
Discussion
Patients with HFmrEF are a small group in the spectrum of patients with HF and their clinical characteristics did not allow differentiating them from patients with HFpEF or HFrEF. Moreover, all-cause mortality was not different from that of HFpEF or HFrEF. CV mortality, however, tended to be lower in patients with HFmrEF than in patients with HFrEF. Interestingly, change in 1-year LVEF in this group was broader (24% had a decrease in LVEF and 33% had LVEF>50%). While change in LVEF fraction as continuous variable was not associated with better outcomes, those patients who evolve from HFmrEF to HFpEF actually did have a better outcome.
Baseline clinical characteristics
Prevalence of HFmrEF in our study was 14%, similar to other studies carried out in hospitalised (prevalence between 13% and 26%)5–7 14 15 and ambulatory (9% and 21%)9–11 16–18 patients with HF. Consistent with other studies, clinical characteristics of patients with HFmrEF did not allow for a clear clinical differentiation of this group. Age and prevalence of women were higher in HFmrEF than in HFrEF but lower than in HFpEF. This finding is consistent across different studies.5 7 8 10 14 16–19 Some comorbidities such as diabetes mellitus, dyslipidaemia and chronic obstructive pulmonary disease were not different between the three groups. In patients with HFmrEF, the presence of anaemia, chronic kidney disease and New York Heart Association class III–IV was similar to those with HFrEF but was lower than in those with HFpEF. Interestingly, NT-proBNP was not different between HFmrEF and HFpEF but was lower than in HFrEF. Aetiology of HF was also different between the three groups, with a predominant ischaemic cause in HFrEF and HFmrEF and hypertensive in HFpEF. Similar results have been described in other studies and, although some differences can be found in the distribution of comorbidities among studies,5–10 14 16 17 19 20 the overall perception is that it is not possible to identify a clear pattern of clinical characteristics that defines HFmrEF, but differences in aetiology, sex and age would point more to a patient with HFrEF.
Follow-up events
Given the differences in baseline characteristics and prognosis in the three groups, some authors suggest that HFmrEF has a phenotype closer to HFpEF5–8 whereas other authors consider it closer to HFrEF.9–11 All-cause mortality and HF hospitalisation were similar in HFrEF and HFmrEF (45.8% vs 43.8%, P=0.448) but were lower than in HFpEF (52.6%, overall P=0.002). However, in multivariable Cox regression analyses with competing risk, HFpEF performed worse than those with HFmrEF and HFpEF, relative to HF hospitalisation but had lower CV mortality. While some studies have shown no differences in outcomes among the three groups,5–7 10 14 other authors have found different results, with a higher mortality in HFmrEF compared with HFpEF in patients with ambulatory HF9 and, contrary to our results, a higher HF hospitalisation in HFrEF compared with HFmrEF and HFpEF.8 Interestingly, we found similar all-cause death between patients with HFrEF and HFmrEF, but a trend towards lower CV mortality in the latter group.
Changes in LVEF
At 1-year follow-up, LVEF of patients with HFmrEF had the greatest variation: 24% had reduced LVEF, 43% maintained LVEF 40%–49% and 33% had LVEF >50%. Remarkably, in this group, change in LVEF as continuous variable was not associated with survival but when the improvement in LVEF was enough to move the patient from HFmrEF to HFpEF, survival improved significantly. Women and non-ischaemic aetiology were more frequent in patients who moved to HFpEF and that has been shown in other studies.21 Although treatment has been associated with improvement in LVEF22–24 we did not see any difference in treatment between patients who moved to HFpEF and those who remained in HFmrEF or went to HFrEF. However, this lack of difference might be explained by the high use of Class I medications in our population.
The apparent paradox of absence of a clear difference in long-term prognosis in patients according to the three groups of LVEF can be plausibly disentangled. On the one hand, LVEF measured by echocardiography is an imperfect measure to determine left ventricular systolic function as it only captures one part of the whole biomechanics of cardiac function and exhibits important variability.25 Moreover, impairment of left ventricular systolic function is also present in patients with HFpEF, even though their LVEF might be normal.26 On the other hand, cut-offs used to define the three groups (HFrEF, HFmrEF and HFpEF) are arbitrary.3 Finally, LVEF is a dynamic measure that can vary with treatment22 23 and during follow-up.17 23 In the present study, 437 patients had HFmrEF according to the echocardiogram at 1-year follow-up. This represents an increase from 14% at baseline to 22.9% at 1-year follow-up. Previous studies have shown that depending on the cut-off used (LVEF 40% vs 50%), up to 50% of patients with HFpEF were patients with previously reduced LVEF27 28 and patients with recovered LVEF had a better prognosis compared with those with preserved or reduced LVEF20 21 24 27–29 and with those who did not improve LVEF.12 Although it was less frequent, patients with HFpEF also showed variability in LVEF during follow-up (only 15% of patients with HFpEF had LVEF <50% at 1-year follow-up in our study). Dunlay et al showed that among patients with HFpEF, 21.1% had an EF<50% around 1 year after diagnosis and this increased to 32.5% in those with an echo performed from 4 to 6 years after diagnosis,24 and similar results were seen in other studies.18 22 In the present study, change in LVEF in HFpEF was not independently associated with all-cause mortality. In HFmrEF, patients whose LVEF improved enough to move to HFpEF, outcome was better but when LVEF did not move or worsened, prognosis was worse, consistently with other studies that showed that irrespective of baseline group, the transition to HFrEF was associated with increased all-cause mortality.18
Taken together, the results of the present and previous studies show that the classification of patients in HF with preserved, mid-ranged and reduced LVEF is not static. In other words, many patients with HFmrEF might be either recovered in patients with HFrEF or, probably to a lesser extent, patients with worsened HFpEF and this fact might explain the difficulty in clinically characterising them properly and might explain the lack of differences found in all-cause mortality between the three groups. Irrespective of the limitations, LVEF might have to classify patients with different prognosis, baseline echocardiogram has the crucial role to identify patients in whom disease modifying treatment are useful to improve prognosis. Whether baseline LVEF or follow-up LVEF should be used to classify patients remains unclear.
Limitations
Follow-up echocardiograms were not done at prespecified intervals in all patients. This might have been a source of bias because the decision to perform a follow-up echocardiogram may have been influenced by clinical status, age and baseline LVEF. Table 3 (see online supplemental material) shows the differences between patients who survived 1 year with and without an echocardiogram done at follow-up. We have added a table (see online supplemental material) showing the differences between patients with and without an echocardiogram done at follow-up. Patients without an echocardiogram at follow-up were older and had more comorbidity. This group of patients are more frequently not studied with echocardiogram.24 It might have been thought that no benefit would be derived from serial echocardiograms due to poor predicted outcome or presumed HFpEF. Conversely, patients with 1-year follow-up echocardiogram had lower baseline LVEF and were more frequently on optimal medical therapy. In this group of patients, some recovery of LVEF may be expected and this may influence subsequent decisions regarding implantable cardioverter defibrillator or cardiac resynchronisation therapy suitability, therefore serial echocardiograms are more often done. Finally, Dunlay et al report that their patients had a median of 2 echocardiograms during follow-up and mean time from initial to final EF measurement was around 3 years but these authors did not report how many patients had an echocardiogram done at 1-year follow-up.24 In another study, 43% of patients with a primary hospital discharge diagnosis for HF did not have two or more LVEF tests≥30 days apart during the study period.22 Hence, considering that in the present study two-thirds of patients had an echocardiogram done at 1-year follow-up, we think that results are consistent with common clinical practice.
NT-proBNP was missing in 25% of our patients and therefore this biomarker was not included in the multivariable analysis. Consistently with our results, van Veldhuisen et al showed that BNP levels were lower in patients with HFpEF than in HFrEF. For a given BNP level, that study showed that the prognosis in patients with HFpEF was similar than those with reduced LVEF.30
Finally, the baseline characteristics of the patients included in the four hospitals are remarkably different. Although that may be seen as a limitation, the inclusion of different type of patients with HF allowed us to better characterise this population, combining patients followed up in centres with different degree of specialisation (advanced HF centres and community oriented hospitals), thus including patients that would have been lost if only centres with similar characteristics were analysed.
Conclusion
Patients with HFmrEF have a clinical profile in between HFpEF and HFrEF and there were no differences in all-cause mortality and the composite end-point between the three groups. At 1-year follow-up, patients with HFmrEF had the greatest variability (up and down) in LVEF but change in LVEF was not associated with survival, except when patients actually evolve to HFpEF. The classification of patients in HF with preserved, mid-ranged and reduced LVEF is not static and thus, the only reason to classify patients according to their LVEF would be to identify patients in whom disease-modifying therapies are useful to improve prognosis.
Supplementary Material
Footnotes
Contributors: NF, JC-C, JL, AB-G, JV, SP: substantial contributions to the conception or design of the work. NF, ER, JG-C, MdA, ES-G, CS-E, PM, SR, CE, SM: acquisition of data. NF, JC-C, JL, JV, SP: analysis of data. NF, JC-C, JL, AB-G, JV, SP, ER, JG-C, MA, ES-G, CS-E, PM, SR, CE, SM: interpretation of data. All authors: drafting the work or revising it critically for important intellectual content; final approval of the version published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Each hospital Ethics Committee/Institutional Review Board approved the cohort study (Hospital del Mar (Parc de Salut Mar, Barcelona), Hospital Universitari Germans Trias i Pujol (Badalona), Hospital de Sant Pau (Barcelona) and Hospital de Bellvitge (L’Hospitalet de Llobregat, PR088/16).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional unpublished data might be available to those completing the request for research on acceptance by the GICCAT group.
Contributor Information
Collaborators: on behalf of the GICCAT Investigators, J A Smith, and P R Brown
References
- 1.Farré N, Vela E, Clèries M, et al. Real world heart failure epidemiology and outcome: A population-based analysis of 88,195 patients. PLoS One 2017;12:e0172745 10.1371/journal.pone.0172745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farré N, Vela E, Clèries M, et al. Medical resource use and expenditure in patients with chronic heart failure: a population-based analysis of 88 195 patients. Eur J Heart Fail 2016;18:1132–40. 10.1002/ejhf.549 [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 4.Vaduganathan M, Michel A, Hall K, et al. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail 2016;18:54–65. 10.1002/ejhf.442 [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Otero I, Ferrero-Gregori A, Varela Román A, et al. Mid-range ejection fraction does not permit risk stratification among patients hospitalized for heart failure. Rev Esp Cardiol 2017;70:338–46. 10.1016/j.rec.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 6.Farmakis D, Simitsis P, Bistola V, et al. Acute heart failure with mid-range left ventricular ejection fraction: clinical profile, in-hospital management, and short-term outcome. Clin Res Cardiol 2017;106:359–68. 10.1007/s00392-016-1063-0 [DOI] [PubMed] [Google Scholar]
- 7.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168:721–30. 10.1016/j.ahj.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017. (Epub ahead of print). 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, et al. Mid-range left ventricular ejection fraction: Clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–70. 10.1016/j.ijcard.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 10.Rickenbacher P, Kaufmann BA, Maeder MT, et al. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Eur J Heart Fail 2017. (Epub ahead of print). 10.1002/ejhf.798 [DOI] [PubMed] [Google Scholar]
- 11.Vedin O, Lam CSP, Koh AS, et al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 2017;10:e003875 10.1161/CIRCHEARTFAILURE.117.003875 [DOI] [PubMed] [Google Scholar]
- 12.Breathett K, Allen LA, Udelson J, et al. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail 2016;9:e002962 10.1161/CIRCHEARTFAILURE.115.002962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of $K$-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics 1988;16:1141–54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 14.Kapoor JR, Kapoor R, Ju C, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail 2016;4:464–72. 10.1016/j.jchf.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 15.Coles AH, Tisminetzky M, Yarzebski J, et al. Magnitude of and prognostic factors associated with 1-year mortality after hospital discharge for acute decompensated heart failure based on ejection fraction findings. J Am Heart Assoc 2015;4:e002303 10.1161/JAHA.115.002303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghio S, Guazzi M, Scardovi AB, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017;19:873–9. 10.1002/ejhf.664 [DOI] [PubMed] [Google Scholar]
- 17.Allen LA, Magid DJ, Gurwitz JH, et al. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail 2013;6:635–46. 10.1161/CIRCHEARTFAILURE.112.000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji K, Sakata Y, Nochioka K, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail 2017;19:1258–69. 10.1002/ejhf.807 [DOI] [PubMed] [Google Scholar]
- 19.Coles AH, Fisher K, Darling C, et al. Long-term survival for patients with acute decompensated heart failure according to ejection fraction findings. Am J Cardiol 2014;114:862–8. 10.1016/j.amjcard.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadruz W, West E, Santos M, et al. Heart Failure and Midrange Ejection Fraction: Implications of Recovered Ejection Fraction for Exercise Tolerance and Outcomes. Circ Heart Fail 2016;9:e002826 10.1161/CIRCHEARTFAILURE.115.002826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupón J, Díez-López C, de Antonio M, et al. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail 2017. (Epub ahead of print). 10.1002/ejhf.824 [DOI] [PubMed] [Google Scholar]
- 22.Clarke CL, Grunwald GK, Allen LA, et al. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes 2013;6:680–6. 10.1161/CIRCOUTCOMES.111.000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Groote P, Delour P, Mouquet F, et al. The effects of beta-blockers in patients with stable chronic heart failure. Predictors of left ventricular ejection fraction improvement and impact on prognosis. Am Heart J 2007;154:589–95. 10.1016/j.ahj.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 24.Dunlay SM, Roger VL, Weston SA, et al. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012;5:720–6. 10.1161/CIRCHEARTFAILURE.111.966366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 26.DeVore AD, McNulty S, Alenezi F, et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail 2017;19:893–900. 10.1002/ejhf.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punnoose LR, Givertz MM, Lewis EF, et al. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail 2011;17:527–32. 10.1016/j.cardfail.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 28.Basuray A, French B, Ky B, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 2014;129:2380–7. 10.1161/CIRCULATIONAHA.113.006855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, et al. Characteristics and Outcomes of Adult Outpatients With Heart Failure and Improved or Recovered Ejection Fraction. JAMA Cardiol 2016;1:510 10.1001/jamacardio.2016.1325 [DOI] [PubMed] [Google Scholar]
- 30.van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013;61:1498–506. 10.1016/j.jacc.2012.12.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018719supp001.pdf (250.6KB, pdf)