Abstract
Our robust visual experience is based on the reliable transfer of information from our photoreceptor cells, the rods and cones, to higher brain centers. At the very first synapse of the visual system information is split into two separate pathways, ON and OFF, which encode increments and decrements in light intensity, respectively. The importance of this segregation is borne out in the fact that receptive fields in higher visual centers maintain a separation between ON and OFF regions. In the past decade the molecular mechanisms underlying the generation of ON signals have been identified, which is unique in its use of a G protein signaling cascade. In this review we consider advances in our understanding of G protein signaling in ON bipolar cell dendrites, and how insights about signaling have emerged from visual deficits, mostly night blindness. Studies of G protein signaling in ON-BCs reveal an intricate mechanism that permits the regulation of visual sensitivity over a wide dynamic range.
Keywords: ON-bipolar neurons, Regulators of G Protein Signaling, Synaptic transmission, Signal transduction, Scaffolding
Retinal circuits and the role of ON-BCs
Bipolar cells in the vertebrate retina are responsible for conveying light-driven signals from rods and cones to the retinal output, the ganglion cells. Although early physiological studies on ON-BCs were performed in amphibian and fish retinas, we will consider mostly the mammalian retina due to the distinct classes of cells that make selective contacts with rods or cones. ON-BCs can be subdivided into approximately 10 classes (Euler et al. 2014, Ghosh et al. 2004), with one class predominantly contacting rods (ON-RBCs;(Dacheux & Raviola 1986) and the remaining classes contacting cones (ON-CBCs). While these cell types utilize a common G protein signaling pathway, their activity can be further tuned by changes in the expression of signaling components that control the time course and efficacy of G protein activity. This selectivity is believed to underlie the ability of ON-RBCs to maintain high sensitivity in darkness, while allowing ON-CBCs to convey signals in bright light with higher temporal resolution.
ON-RBCs in the mammalian retina predominantly contact rods (Tsukamoto et al. 2001)– but see also (Pang et al. 2010) – unlike the mixed ON-BCs of lower vertebrates that contact both rods and cones (Zhang & Wu 2009). The selective contact of ON-RBCs with rods initiates the rod bipolar pathway, or primary rod pathway, which is responsible for conveying responses with the greatest sensitivity to the retinal output. The physiological properties of mGluR6 signaling in ON-RBCs is responsible for the faithful transmission of single-photon responses across the retinal circuitry to define the lowest 10,000-fold of light intensity in our visual experience (reviewed by (Field et al. 2005, Pahlberg & Sampath 2011). The rod-to-ON-RBC synapse is a specialized structure that not only brings these two cells together, but also includes lateral retinal connections among horizontal cells. This ‘triad’ synapse has a structure whereby the dendrites of both ON-RBCs and horizontal cells invaginate within the rod spherule to stereotyped positions with respect to the presynaptic active zone (Rao-Mirotznik et al. 1998, Rao-Mirotznik et al. 1995), which uses a specialized synaptic ribbon to support a high rate of glutamate release.
ON-CBCs share a common structure with ON-RBCs with invaginating triad synapses that also contact horizontal cells. However, a cone pedicle may contain as many as 40 release sites (ribbons) depending on the species, compared to just a single release site for rod spherules. The multiple active zones on the cone pedicle provide the opportunity for the 9+ classes of ON-CBCs to receive input from a single cone. Each ON-CBC in turn takes the cone input and relays it to distinct classes of retinal ganglion cells based on the stratification of their axons in the proximal half of the inner plexiform layer (Wassle 2004). The heterogeneity in ON-CBC class likely reflects differences in the sensitivity and temporal properties of their responses, which will arise from a combination of their dendritic distance from the synaptic ribbon (see (DeVries et al. 2006) for OFF-CBCs) and in modulation of their signaling cascades.
Glutamate released by the photoreceptors is sensed by mGluR6 on ON-BC dendrites
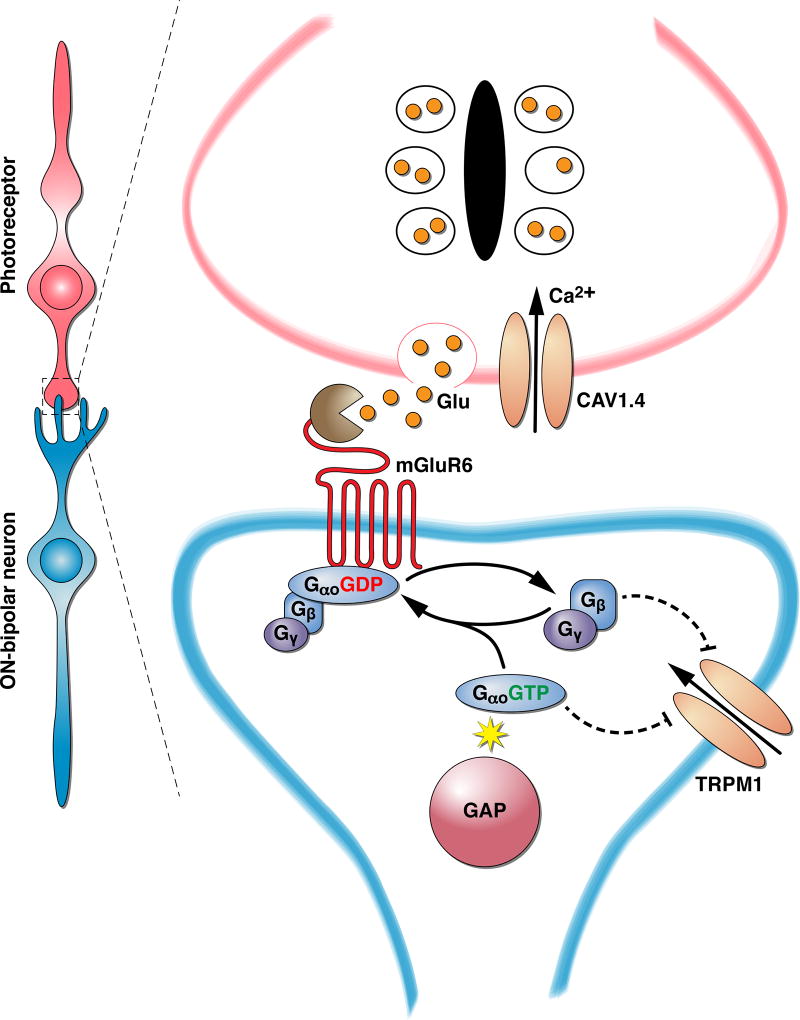
Central excitatory synapses are typically activated when the presynaptic cell is depolarized, leading to the influx of Ca2+ at the active zone, which in turn promotes vesicular fusion and the release of glutamate into the synaptic cleft. Early electrophysiological studies revealed that retinal photoreceptors signal the presence of a stimulus in reverse, with light causing a membrane hyperpolarization (Penn & Hagins 1969, Tomita 1965). The membrane hyperpolarization in turn reduces the rate of glutamate release (Copenhagen & Jahr 1989, Trifonov 1968) constituting the signal that light has been absorbed. The mechanisms underlying the inversion of the hyperpolarizing (or OFF) light-evoked response of photoreceptor cells to the depolarizing response of ON-BCs stumped investigators for many decades. Early recordings from dogfish ON-BCs revealed many of the key characteristics of responses, including high voltage gain and the large number of activation stages (Ashmore & Falk 1980), and a high sensitivity to L-AP4 that eliminates the ON response entirely (Slaughter & Miller 1983). Identification of the postsynaptic receptor underlying the ON response was not complete until its cloning, expression, and functional characterization by Nakanishi and colleagues (Masu et al. 1995) identifying the glutamate receptor as mGluR6 (Figure 1).
Figure 1. Organization of the first visual synapse between photoreceptors and ON-bipolar cells.
Changes in glutamate release by photoreceptors are detected by the signaling cascade initiated by the mGluR6 receptor.
TRPM1 is an essential component of the ON-BC transduction channel
As described, the development of a depolarizing light-evoked response in ON-BCs had been well characterized electrophysiologically, yet the molecular identity of the channel mediating it remained elusive for many years. Studies in amphibians and fish have shown that native transduction channel is a non-selective cation channel that conducts both Na+ and Ca2+ ions and displays prominent outward rectification with a reversal potential close to 0 mV (Kaneko & Tachibana 1985, Lasater 1988, Nawy & Jahr 1990, Nelson 1973, Werblin & Dowling 1969). Furthermore, at negative potentials, the channel showed rapid Ca2+ desensitization (Berntson et al. 2004, Dacheux & Raviola 1986, Kaur & Nawy 2012, Shiells 1999). It should be noted though that interpretation of early studies in fish, are complicated by the presence of glutamate-gated currents mediated by a Cl− transporter present on some ON-BCs (Connaughton & Nelson 2000, Grant & Dowling 1995). Nevertheless, subsequent work in mammalian retinas confirmed much of the same properties of the transduction channel (Shen et al. 2009, Xu et al. 2012).
Initial studies on the molecular identification of the transduction channel were driven by the idea that it belongs to the family of cyclic nucleotide gated channels, stemming from observations that cGMP produced an inward current that was inhibited by the mGluR6 activation when introduced into ON-BCs (Nawy & Jahr 1990, Shiells & Falk 1990). However, subsequent work established that cGMP played a modulatory role rather than gating the channels directly, leaving the identity of the transduction channel unknown (Shiells & Falk 2002, Snellman & Nawy 2004, Wassle et al. 1992). The progress came from two directions. First, genetic profiling of individual retinal neurons identified TRPM1 among transcripts specifically enriched in ON-BCs (Kim et al. 2008). Second, genomic mapping in Appaloosa horses identified mutations in TRPM1 as a cause for the night blindness plaguing this breed (Bellone et al. 2008). Almost simultaneously, the identification of TRPM1 as essential component for the development of the depolarizing response was confirmed in mouse knockout strain with a selective deletion of the TRPM1 gene. Ablation of TRPM1 resulted in complete elimination of ON-BC depolarizing responses to light as evidenced by both ERG recordings, which resulted in the typical no b-wave phenotype, and single cell recordings (Koike et al. 2010b, Morgans et al. 2009, Shen et al. 2009). Similar conclusions were obtained upon examination of human patients with loss-of-function mutations in the TRPM1 gene, which were associated with congenital stationary night blindness of a complete type exhibiting no b-waves upon ERG analysis (Audo et al. 2009, van Genderen et al. 2009). When taking together, the evidence is very strong that TRPM1 is an essential component of the machinery that mediates the depolarizing light-evoked responses of ON-BCs (Figure 1).
The sufficiency of TRPM1 as the molecular entity constituting transduction channels has proven to be more difficult to answer. The major limitation has been difficulty with reconstituting functional TRPM1 channel upon heterologous expression in cell lines. The first successful reconstitution was reported by Oancea and colleagues who recorded TRPM1 currents expressed in SK-Mel19 melanoma cells (Oancea et al. 2009). The currents were small (<30 pA/pF) and the channels showed extreme outward rectification with essentially no channel activity at negative membrane potentials. This property resembled TRPM1 natively expressed in melanoma cell lines, but deviated substantially from the transduction channels in ON-BCs, which show modest, if any, outward rectification (Nawy & Jahr 1990, Shen et al. 2009, Xu et al. 2012). Indeed, to produce depolarizing responses in ON-BCs, transduction channels must open at fairly negative membrane potentials (resting Vm~−60mV) and displays substantially higher conductances. Similar difficulties were experienced upon reconstitution of functional TRPM1 channels in CHO cells (Koike et al. 2010b), where extremely small (<5pA/pF) currents were observed, and in HEK293 cells (Lambert et al. 2011, Oancea et al. 2009, Shen et al. 2012) where the conductance was negligible unless the channel was mutated or stimulated with pregnolone sulfate (Lambert et al. 2011). These reconstituted channels also did not appear to undergo desensitization as noted for ON-BC transduction channels. Nevertheless, in most of these studies the current was clearly attributable to the ectopic TRPM1.
An unusual property of transduction channels in ON-BCs is their sensitivity to classical pharmacological modulators of TRPV family: agonist capsaicin and antagonist capsazepine. Indeed, application of capsaicin elicited currents from ON-BCs nearly identical to those evoked by mGluR6 inhibition, and resembled the current-voltage relationship of a transduction channel (Morgans et al. 2009, Shen et al. 2009). Interestingly, several studies reported that capsaicin sensitivity was abolished in ON-RBCs in TRPM1 knockout retinas, indicating that it action requires TRPM1 (Shen et al. 2012, Xu et al. 2012). Additionally, other studies reported varying extents of residual capsaicin-evoked currents in ON-BCs lacking TRPM1 (Morgans et al. 2009, Ray et al. 2014). Conversely, capsazepine blocked both capsaicin-activated currents and mGluR6-modulated currents in ON-BCs (Shen et al. 2009), again suggesting the pharmacological similarity of glutamate and capsaisin for the transduction channel. However no consensus has been reached regarding the sufficiency of TRPM1 to impart this sensitivity, as studies in transfected HEK293 cells are in disagreement (Schneider et al. 2015, Shen et al. 2012). Furthermore, none of the closely related channels from the TRPM subfamily show sensitivity to capsaicin or capsazepine, which are believed to be selective modulators of TRPV1 channels.
In sum, the accumulated evidence is clear that native ON-BC transduction channels deviate substantially in key properties (conductance, voltage sensitivity, response to chemical modulators) from the TRPM1 channel studied in isolation. The simplest explanation is that TRPM1 might represent only part of the transduction channel and is insufficient by itself. In this model, association with additional proteins would be required to impart TRPM1 with the salient features characteristic of ON-BC transduction channels. In agreement with this idea, structural studies with a purified TRPM1 protein indicate that it forms a dimer without a classical ionic pore (Agosto et al. 2014), which for TRP channels typically requires tetrameric organization of protomers (Cohen & Moiseenkova-Bell 2014). Since many TRP family members frequently associate with one another, one straightforward possibility could be that TRPM1 forms heteromeric complexes with another TRP channel to build transduction channels. While this possibility remains to be exhaustively tested, neither classical capsaicin activated channel TRPV1 (Shen et al. 2009), nor its closest homolog TRPM3 (Brown et al. 2015), play a role in generating depolarizing responses in ON-BCs. It is also possible that such additional components may be involved in trafficking of the TRPM1 to the cell surface, because the majority of the TRPM1 in both ON-BCs (Xu et al. 2012) and upon heterologous expression in cell lines (Oancea et al. 2009) is intracellular. In fact, these observations initially led to the idea that TRPM1 channels have intracellular functions (Patel & Docampo 2009). Thus, establishing the additional molecular requirements needed in the formation of ON-BC transduction channel complexes will be critical.
Heterotrimeric G proteins transfer light-evoked signals from mGluR6 to TRPM1
Given the clear role of both mGluR6 at the inception point of neurotransmitter action and TRPM1 at the effector output in producing ON-BC depolarizing responses, a critical question has been to elucidate the signaling cascade linking changes in mGluR6 activity to modulation of TRPM1 gating. The key intermediary in this process is the heterotrimeric G protein, Gαo (Figure 1, Figure 2). Early indication of the involvement of Gαo included a demonstration of its selective enrichment in ON-BC dendrites together with mGluR6 (Vardi 1998, Vardi et al. 1993). Acute inactivation of Gαo with antibodies dialyzed into ON-BCs diminished the mGluR6-mediated regulation of the transduction currents, further supporting its involvement (Nawy 1999). The strongest evidence for Gαo came from genetic studies in knockout mice which completely eliminated the depolarizing ON-BC ERG response for both scotopic and photopic flashes while preserving the structural integrity of the synapse and the correct localization of mGluR6 receptors (Dhingra et al. 2000). Deeper investigation by single-cell recordings from ON-RBCs confirmed the requirement of Gαo in generating light-evoked responses (Okawa et al. 2010). Two splice isoforms of Gαo are expressed in ON-RBCs and both are required for establishing the high sensitivity for the detection of single-photon responses, however Gαo1 (also called GαoA) plays the dominant role in this process (Dhingra et al. 2002, Okawa et al. 2010). Because mGluR6 is a GPCR that acts biochemically to stimulate Gαo activation by promoting GTP exchange, the connection of Gαo as a substrate for mGluR6 is intuitive. Indeed, all class III mGluRs (including mGluR6) are Gαi/o-selective nucleotide exchangers and ample biochemical evidence in reconstituted systems indicates that mGluR6 stimulates efficiently GTP binding to Gαo.
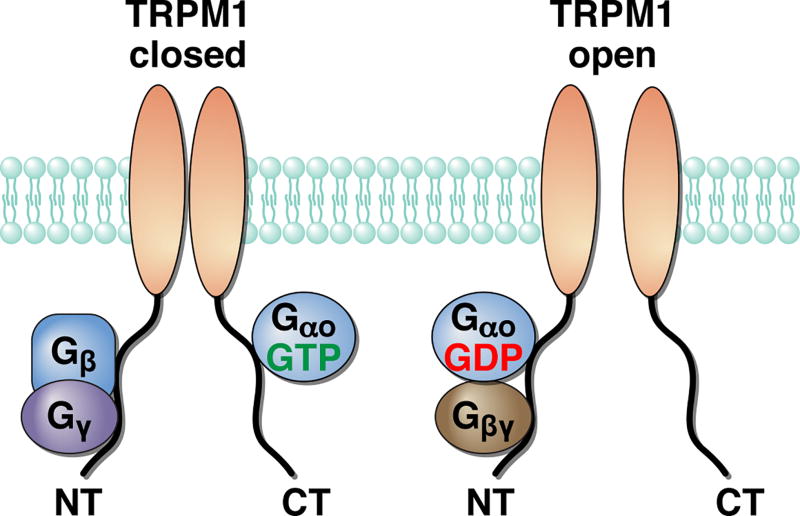
Figure 2. A model for TRPM1 channel gating by G proteins.
Direct binding of both activated Gαo-GTP and Gβγ subunits to the cytoplasmic domains of the TRPM1 channel keep it in closed state. Upon GTP hydrolysis, G proteins rearrange forming inactive heterotrimer allowing the TRPM1 channel to open. Modified from (Xu et al. 2016).
To stimulate GTP binding to Gα subunits, GPCRs typically require that Gα be associated with the Gβγ subunits in the heterotrimeric complex. Given the diversity of the Gβ (4 signal-transducing genes) and Gγ (13 genes) subunits, determining which isoforms are relevant for transmitting mGluR6 signals is critical. A strong body of evidence indicates that Gβ3 constitutes the major isoform involved in ON-BC signal transduction. Gβ3 is expressed prominently and specifically in ON-BCs (Dhingra et al. 2012, Huang et al. 2003, Peng et al. 1992) and its knockout severely reduces the amplitude of depolarizing responses of both ON-RBCs and ON-CBCs (Dhingra et al. 2012). Similarly, Gγ13 was described as the most prominent isoform present in ON-BCs (Dhingra et al. 2008, Huang et al. 2003). However, elimination of Gγ13 in mice only partially affected ON-RBC responses with no appreciable effects in ON-CBC (Ramakrishnan et al. 2015). Thus, while Gβ3γ13 appears to be the major species involved in mGluR6 signaling in ON-RBCs, other Gβγ dimers might play a role in signaling in both ON-RBCs and to a greater extent in ON-CBCs. Indeed, several additional proteins have been identified to be enriched in ON-BCs including Gβ4, γ5, γ10, and γ11 (Huang et al. 2003, Ramakrishnan et al. 2015). It seems possible that this functional heterogeneity of Gβγ combinations may contribute to distinct response properties of ON-BC populations.
The next step in signal transfer bridging Gαo activity to TRPM1 modulation remains poorly defined and rather controversial. The difficulty in pinpointing the molecular mechanism is related to the organizational principles of heterotrimeric G proteins that revolve around the cyclical interplay between Gα and the Gβγ subunits. Complex formation with Gβγ promotes Gα activation by GPCRs, following which both subunits re-arrange or dissociate to transmit a signal through their association with effector molecules. As Gα hydrolyses GTP, Gα-GDP and Gβγ reform the inactive heterotrimer, simultaneously antagonizing each’s actions on their effectors (Gilman 1987). Furthermore, Gβγ and Gα co-assemble together during biogenesis and thus mutually promote stability and trafficking (Krumins & Gilman 2006, Willardson & Tracy 2012). This makes the interpretation of loss-of function studies difficult as elimination of Gβγ would necessarily affect its cognate Gα, and vice versa. This intricate relationship has impeded elucidation of the relative roles of Gβγ vs Gα in TRPM1 inhibition. In the native ON-BCs from salamander retinal slices, introduction of Gαo reduced transduction channel inhibition upon pharmacological stimulation of mGluR6 (Nawy 1999). On the other hand, Gβγ, but not Gαo, inhibited the opening of the transduction channels when mGluR6 activity was antagonized (Shen et al. 2012). The interpretation of these results is not unequivocal; in the first experiment, free Gαo-GDP could either sequester free Gβγ or undergo activation and influence transduction channels directly. Similarly, inhibition of channel opening may be interpreted by either direct inhibitory effects of the Gβγ, or through facilitation of the Gαo activity under the conditions of persistent mGluR6 activity. In cultured cells expressing TRPM1, Gβγ overexpression, or Gβγ liberation by GTPγS, prevented deactivation of TRPM1 (presumably open) upon application of capsazepine, yet the constitutively active Gαo mutant (Q205L) displayed no significant effect (Shen et al. 2009). In conflict with these observations, another study reported that activation of Gαo by the non-hydrolysable analog–GMPPNP, or by a Q205L mutation, but not Gβγ,, inhibited currents of TRPM1 channels co-expressed in the cells (Koike et al. 2010a, Koike et al. 2010b).
While studies in transfected cells might be similarly open to alternative interpretations regarding manipulations of Gα vs Gβγ in their interaction with the channel, single channel work (Koike et al. 2010b) strongly argues for the involvement of Gαo in gating the TRPM1 channels. A significant limitation of the studies in the reconstituted system, however, is uncertainty about the molecular composition of TRPM1 channels versus native ON-BC transduction channels. In attempt to reconcile these findings a more recent study employed a panel of manipulations, which sequester and activate both Gβγ and Gαo under both dark- and light-adaptated conditions. This study reported evidence that both Gα and Gβγ are involved in regulation of native ON-BC transduction channels (Xu et al. 2016). Furthermore, evidence in transfected cells suggest that both Gα and Gβγ are capable of interacting directly with the TRPM1 protein (Xu et al. 2016). The proposed model (Figure 2) indicates that the interaction of TRPM1 with both free Gβγ and Gαo-GTP is required to close fully transduction channels, and that re-arrangement between the subunits upon their inactivation removes the inhibitory constraint allowing channels to open (Xu et al. 2016). It should be noted, however, that these studies did not use physiologically-relevant combinations of Gβγ, and did not assess the influence of additional components of the ion channel complex. Both of these directions will be important to understand fully the organization and regulation of native ON-BC transduction channels.
Opening of ON-BC transduction channels is driven by GAP complexes
In darkness glutamate released by photoreceptors activates mGluR6, which in turn activates Gαo heterotrimers and keeps TRPM1-containing transduction channels closed. Closed transduction channels allow ON-BCs to maintain a relatively hyperpolarized resting membrane potential. In single-cell recordings from ON-RBCs the dialysis of the non-hydrolysable GTPγS abolished their light responses emphasizing that opening of the transduction channels requires G protein deactivation through GTP hydrolysis (Sampath & Rieke 2004). Although Gαo alone can spontaneously hydrolyze GTP, the speed of this reaction is too slow to account for the fast onset of ON-BC depolarization during light-evoked responses. Thus, the G protein deactivation must be catalyzed to allow fast channel disinhibition on the timescale of changes in synaptic glutamate concentration (Figure 1). It is well established that G protein deactivation in GPCR cascades is catalyzed by a Regulator of G Protein Signaling (RGS) family of proteins containing more than 30 members (Hollinger & Hepler 2002). RGS proteins act as GTPase Activating Proteins (GAP) by interacting with the switch regions of Gα subunits and stabilizing their transition state, thereby accelerating the rate of the GTP hydrolysis (Sprang 2016). There is strong evidence indicating that Go deactivation in ON-BCs is mainly catalyzed by two RGS proteins: RGS7 and RGS11. Both proteins are expressed at approximately stoichiometric levels in the retina and show specific enrichment at the dendritic tips of ON-BCs, where they co-localize with mGluR6 and TRPM1 (Cao et al. 2009, Cao et al. 2008, Mojumder et al. 2009, Mojumder 2008, Morgans et al. 2007, Rao et al. 2007). Knockout of either protein in mice produces only minor changes in ERG waveform (Cao et al. 2012, Chen et al. 2010, Mojumder et al. 2009, Zhang et al. 2010) with no significant effects observed upon recording ON-RBC light responses directly (Cao et al. 2012), indicating that they play a redundant role. Further reduction of RGS levels by combining deletion of RGS11 with a hypomorphic mutation in RGS7 caused a minor delay in the onset of the depolarizing response by ERG, yet leaving it largely intact, suggesting a vast excess of GAP activity in ON-BCs (Chen et al. 2010, Mojumder et al. 2009, Zhang et al. 2010). The exact GAP activity levels needed to sustain fast ON-BC depolarizing responses were determined by titrating RGS7 levels in the absence of RGS11 (Sarria et al. 2015). These studies indicate RGS proteins exist in approximately 4-fold excess over the level where response properties (onset kinetics, sensitivity and amplitude) become influenced by modulation in GAP activity.
The high level of GAP overexpression indicates that timely deactivation of the Gαo is crucial for setting the fast time scale of transduction channel opening. It further suggests that the fast speed of the GTP hydrolysis does not limit the overall rate of the channel activation, which is likely determined by the deactivation of the mGluR6 itself. Interestingly, elimination of both RGS7 and RGS11, does not completely abolish the development of depolarizing response. Although much smaller and much less sensitive, the residual response to a step of bright light is present and exhibits characteristically slow kinetics, reminiscent of behavior of other G protein gated channels upon the loss of RGS control (Cao et al. 2012). Nevertheless, the kinetics of the residual response (t~1 s) is still significantly faster that the rate of the intrinsic GTPase activity of the Gαo (t~30 s). This suggests in the absence of dominant RGS proteins that accelerate Gαo deactivation by a factor of ~15, minor auxiliary GAP proteins in ON-BCs participate in deactivating Gαo. One candidate is RGS20 (RetRGS1), which is expressed by ON-BCs and is capable of speeding deactivation of Gαo-driven signaling in a reconstituted system (Dhingra et al. 2004). Yet, the involvement of other members of the RGS family cannot be excluded.
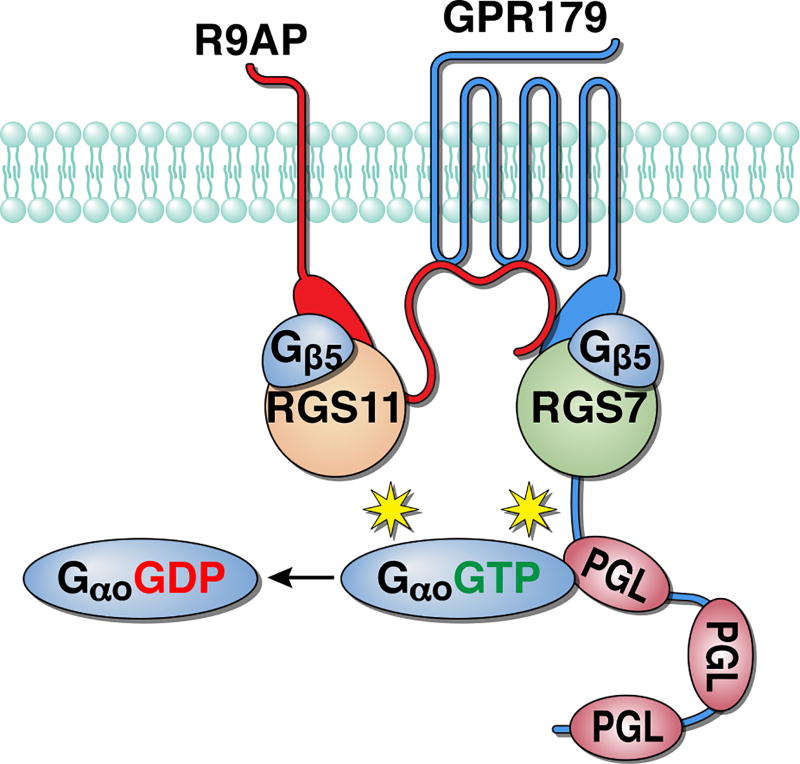
A central organization principle of RGS7 and RGS11 proteins in ON-BCs is their macromolecular integration into single GAP complex (Figure 3). Both proteins share similar domain organization, including N-terminal DEP domain, central G-gamma like region (GGL), and C-terminal RGS homology domain (Anderson et al. 2009). The key role in the structural organization of these proteins belongs to their obligatory subunit, Gβ5, which constitutively binds to the GGL domain and is required for proper folding and stability of these complexes (Cabrera et al. 1998, Chen et al. 2003, Makino et al. 1999, Snow et al. 1999). Knockout of Gβ5 in mice results in the elimination of both RGS7 and RGS11 and phenotypically mimics the dual ablation of RGS7 and RGS11 by abolishing the depolarizing ON-BC responses (Rao et al. 2007). In addition to Gβ5, RGS proteins are bound to two other proteins, which act as their membrane anchors. RGS11 binds to a small, SNARE-like transmembrane protein R9AP and this interaction is required for maintaining RGS11 expression in ON-BCs (Cao et al. 2009, Jeffrey et al. 2010). RGS7 is instead found to be in a complex with the GPCR-like protein, GPR179 (Orlandi et al. 2012). This association is mediated by the R9AP-like domain contained within the cytoplasmic C-terminal region of GPR179 (Sarria et al. 2016). GPR179 also forms complexes with R9AP, independently from RGS proteins, and this binding brings all of the components of the macrocomplex together (Sarria et al. 2016). Consequently, knockout of GPR179 abolishes the postsynaptic targeting of both RGS7-Gβ5 and RGS11-R9AP-Gβ5 complexes resulting in characteristic loss of ON-BC responses with residual slow depolarizing response upon prolonged light stimulation, similar to concurrent ablation of RGS7 and RGS11 (Orlandi et al. 2012, Ray et al. 2014). Therefore, the GAP complex appears to be integrated into a single unit via interdependent interactions between individual subunits (Figure 3). At the top of the hierarchy, GPR179 binds to RGS7 and R9AP and this interaction is needed for the correct targeting of the complex to ON-BC dendritic tips. R9AP in turn recruits RGS11, and both RGS proteins engage Gβ5 to achieve their photolytic stability. The exact reasons for this convergent organization where redundant catalytic RGS subunits are independently scaffolded by a single critical adapter (GPR179) are not clear, but likely related to ensuring the proximity of the GAP complex positioning relative to mGluR6 and TRPM1 (discussed below). Interestingly, the abundance of RGS7 and RGS11 varies across rod and cone synapses (Mojumder et al. 2009). Although these differences in expression had little relevance for ON-RBC function given clear redundancy and excess of RGS activity in this cell type, it seems possible that the varying composition of GAP complex due to differences in expression of individual subunits (RGS proteins or R9AP and GPR179 adapters) may contribute to setting unique properties (e.g. temporal filtering) of the numerous ON-CBCs, a possibility that remains to be determined. Such a possibility is aided by the observations that cone-to-ON-CBC signaling showed sensitivity to the changes in RGS levels in ranges distinct from those for the rod-to-ON-RBC signaling (Sarria et al. 2015).
Figure 3. Organization of the GAP complex of ON-bipolar cells.
Multiple subunits bind together through direct protein-protein interactions to form a single unit that promotes GTP hydrolysis on its G protein substrate, Gαo. PGL, PDEγ-like domain that scaffolds Gαo-GTP.
Overall, the GAP complex provides the biochemical substrate that drives the opening of TRPM1 channels, thus generating ON-BC depolarizing responses (Figure 1). The model that has emerged from these studies posits that in darkness persistent activity of glutamate-bound mGluR6 efficiently activates Gαo. This activity is thresholded by the GAP complex to provide constant negative pressure effectively deactivating the Go. Yet, the balance of the G protein activation and deactivation in darkness is set such that active Gαo is present in excess to ensure nearly complete TRPM1 inhibition (Sampath & Rieke 2004). The light-induced reduction in glutamate concentration changes this equilibrium by reducing the activity of mGluR6. The excess of the GAP activity then permits levels of active Gαo to fall quickly and allow transduction channels to open rapidly on the timescale of changes in synaptic glutamate. Experiments to titrate GAP expression indicate that Gαo deactivation is faster than the reaction rate that limits the speed of the channel opening, leaving the nature of the rate-limiting reaction leading to depolarizing activity undefined.
Scaffolding of the mGluR6 signaling cascade into the macromolecular “signalosome”
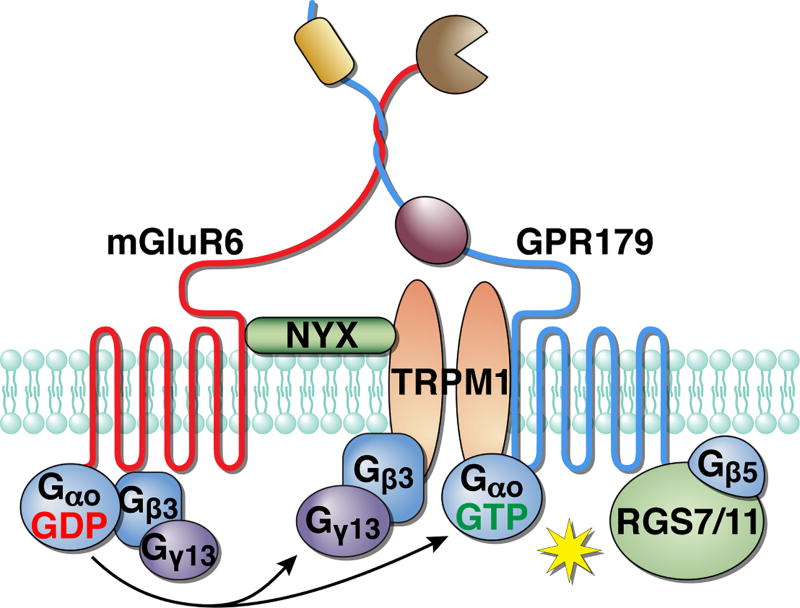
The identification of the principal elements in ON-BC signaling naturally led to studies that examined their physical relationship. What has emerged is the discovery of an intricate web of interactions arranging individual signaling molecules into an interdependent unit. This integrated organization makes intuitive sense when considering the need to generate a depolarizing response on the time scale of tens to hundreds of milliseconds. During the single-photon response, the rod hyperpolarization peaks at ~ 250 ms, causing commensurate changes in changes in the synaptic Ca2+ concentration (Thoreson 2007). Remarkably, ON-BCs generate an even faster depolarizing response, peaking at 50–100 ms after photon absorption, which is comparable to the speed of the OFF-BC ionotropic response (Field & Rieke 2002). Although GPCR signaling cascades are considered as slow modulatory signaling, the depolarization of ON-BCs represents an impressive feat. This ability relies on tight integration of cascade components to minimize diffusional limitations and thus speeding up the rate of the reactions required for signal transfer. Curiously, this organization is reminiscent of phototransduciton in invertebrate photoreceptors, which also use GPCR signaling to provide fast modulation of TRP channel opening (Sanes & Zipursky 2010, Wang & Montell 2007). Bound by similar considerations and constraints, this well-studied cascade also relies on the scaffolding of multiple signaling elements and provides a conceptual framework for understanding the arrangement of the ON-BC transduction pathway (Figure 4).
Figure 4. Scaffolding of multiple components of the ON-BC cascade into a single “signalosome” unit.
The components of the cascade are linked together through physical interactions that promote the efficiency of signal transduction.
Studies with knockout mouse models lacking individual cascade elements have allowed an analysis of the relationship between them. Remarkably, all known elements of ON-BC signaling (mGluR6, Gαo, GAP complex, TRPM1) show a varying extent of interdependence either by controlling the expression level of one another, by subcellular targeting to ON-BC dendrites, or both. A great effort by Ron Gregg and colleagues have provided a meta-analysis of these findings summarizing all such reported effects (Gregg et al. 2014). What emerged from this analysis is that the relationship appears to be hierarchical in nature with a non-reciprocal impact of individual molecules on others. The greatest effects are seen upon elimination of mGluR6 (Cao et al. 2009, Cao et al. 2011, Gregg et al. 2014, Xu et al. 2012) and Gβ3 (Dhingra et al. 2012), which disrupts subcellular targeting of nearly all the signaling molecules examined, and substantially reduces their expression levels. The consequences of Gβ3 deletion are a bit more dramatic as it also affects mGluR6, yet Go heterotrimers are expressed at normal levels in Gβ3 knockout retinas. On the opposite end of the spectrum is TRPM1, whose deletion has not been reported to affect the localization or expression of any ON-BC signaling elements (Gregg et al. 2014, Koike et al. 2010b, Morgans et al. 2009, Pearring et al. 2011, Shen et al. 2009). However, deletion of a majority of the cascade elements produces an intermediate phenotype that selectively influences some proteins. Such a restricted nature is likely related to more confined roles of molecules within the subgroup of partnering proteins. For example, the elements within the GAP complex (RGS7, RGS11, Gβ5, R9AP, and GPR179) show pronounced interdependence within the group, but their elimination has no effects on targeting or localization of other molecules (TPRM1, mGluR6) (Cao et al. 2009, Cao et al. 2012, Chen et al. 2003, Jeffrey et al. 2010, Ray et al. 2014, Shim et al. 2012). Another example of remarkable selectivity is provided by nyctalopin (NYX), a mysterious leucine rich repeat molecule required for the ON-BC depolarization (Gregg et al. 2003, Poopalasundaram et al. 2005). Elimination of NYX has been shown to prevent completely subcellular targeting of TRPM1 with no reported effects on other elements of the cascade (Gregg et al. 2014, Pearring et al. 2011). It should be noted however, that the relationship between individual molecules have not been exhaustively analyzed, and extending such analysis to examine the reciprocal effects for all elements of the cascade for will be needed to complete the picture.
The dependence of expression and/or localization of signaling molecules on one another in the same cellular compartment is often taken as evidence for their association in a macromolecular complex. Indeed, for many ON-BC signaling molecules that show interdependence there is evidence for protein-protein interactions. For example, many Gβ and Gγ subunits are well documented to exist in constitutive complexes and bind to Gα in its inactive GDP bound form as well as with GPCRs for activation (Dupre et al. 2009, Herve 2011, Willardson & Tracy 2012). Furthermore, interactions among the subunits is important for setting the expression levels of G protein heterotrimers and their localization to the plasma membrane (Krumins & Gilman 2006). Thus, although the impact of Gαo and Gγ13 on the stability of mGluR6 and Gβ3 and their association have not been examined, given an extensive theoretical base, a decrease in expression upon elimination of Gβ3 likely reflects disruption of their direct binding within G protein and GPCR complexes. Similarly, GAP complex components are known to engage in direct protein-protein interactions (detailed above) and thus their interdependence of expression and localization is similarly explained by their macromolecular association.
In addition to interactions between proteins within their immediate functional units, many ON-BC signaling molecules are integrated by further associations leading to higher-order scaffolding. One of the central molecules that scaffolds multiple elements of the cascade is the orphan receptor GPR179, which forms physical complexes with all the cascade’s key elements (mGluR6, TRPM1, RGS complex and Gαo) by analogy with its closest homolog, GPR158 (Orlandi et al. 2013, Orlandi et al. 2012, Orlandi et al. 2015, Ray et al. 2014). One critical role of this arrangement may be in bringing GAP complexes in vicinity of both the receptor and the effector, promoting the efficiency of G protein cycle control and its switching between ON and OFF states. Additional scaffolding of Gαo by a virtue of numerous PDE gamma-like (PGL) domains (Orlandi et al. 2015) may serve to increase local RGS concentration for more efficient activation by the receptor, channel gating, and deactivation. Interaction with GPR179 further contributes to of TRPM1 properties independent of GAP complex control because it has been shown to change direct transduction channel gating capsaicin (Ray et al. 2014). Nevertheless, deletion of GPR179 does not affect the expression or localization of either mGluR6 or TRPM1 (Gregg et al. 2014, Ray et al. 2014).
The second protein in the cascade with putative scaffolding functions is NYX (Bech-Hansen et al. 2000, Pusch et al. 2000), which has been reported to interact with both mGluR6 and TRPM1 (Cao et al. 2011, Pearring et al. 2011). While NYX is dispensable for correct localization or expression of mGluR6 (Gregg et al. 2014), it is required for proper trafficking of TRPM1 to the dendritic tips. Thus the mis-localization of TRPM1 seen in NYX loss of function mutant (Pearring et al. 2011) likely results from the loss of a physical association between these proteins. In support of this idea, expressing NYX in ON-BCs of NYX-deficient mice rescues both synaptic signaling and TRPM1 dendritic targeting (Gregg et al. 2007, Scalabrino et al. 2015). How NYX affects TRPM1 trafficking remains unclear. NYX expression in heterologous cells is present in both intracellular sites as well as on the surface of the plasma membrane (Zeitz et al. 2003), while in ON-BCs it is localized mostly to the dendritic tips (Gregg et al. 2007, Morgans et al. 2006). As TRPM1 expressed heterologously is largely intracellular (Oancea et al. 2009) and can only be present on the surface of ON-BC dendrites due to its constitutive activity, one potential model is that NYX may be involved in chaperoning the assembly of transduction channels and their delivery from the ER/Golgi to the cell surface. Alternatively, NYX and TRPM1 may traffic independently with NYX allowing the retention of TRPM1 on the cell surface, possibly by integrating it into mGluR6-containing signaling complexes. The latter model is favored by the observation that NYX targeting to ON-BC dendritic tips does not require TRPM1 (Pearring et al. 2011), and that NYX has an extracellular topology (Bojang & Gregg 2012) making it capable of interacting with other similarly-organized scaffolds in the synapse between photoreceptors and ON-BCs (see below).
In sum the accumulated evidence points to a super-modular organization of the ON-BC cascade; individual elements are first tightly organized into immediate modules, each involved in discrete steps during the signaling transfer, then globally linked together by cross-modular protein-protein interactions through binding to the “hub” scaffolding molecules. This organization leads to the entire ON-BC transduction cascade functioning as a coordinated functional unit, or ‘signalosome’. The biggest gap in our knowledge and the goal for the future efforts would be to obtain structural insight into the organization of this complex by mapping the binding determinants in addition to obtaining a high-resolution structure of the entire assembly.
Modulation of the mGluR6 cascade
While photoreceptor hyperpolarization and the resulting reduction in glutamate release produces a robust response in both ON-RBCs and ON-CBCs, the sensitivity of these responses can be modulated as rods and cones adapt. Adaptation in light-evoked responses in ON-BCs will result from adaptation of the photoreceptors themselves, but also from intrinsic mechanisms in mGluR6 transduction that reduce sensitivity and increase temporal resolution. For example, modulation of sensitivity in ON-RBCs occurs at light intensities where there is little adaptation in the rods themselves (Dunn et al. 2006). Given the complexity of G protein signaling and the large number of control points within a signaling cascade, research over the past 15 years has identified several molecular players that influence adaptation of the ON-BC response.
Perhaps the most robust effector of ON-BC sensitivity is intracellular Ca2+. TRPM1 in ON-BC dendrites are permeable to Ca2+, which allows the photoresponse to adapt. The hyperpolarized resting membrane potential in darkness keeps TRPM1 channels indicates they are in a mostly closed state (Sampath & Rieke 2004), in turn keeping the dendritic Ca2+ low. Work on salamander ON-BCs indicates that dendritic Ca2+ influx during the light response serves to reduce the maximum amplitude of mGluR6 antagonist-based currents, an effect that was eliminated with high intracellular concentrations of the high-affinity Ca2+ chelator, BAPTA (Nawy 2000, Nawy 2004). Further work on mouse ON-RBCs also reveals similar phenomenology, and maps the time constant for these adaptive effects based on paired flash experiments as ~400ms at physiological temperature (Berntson et al. 2004). This effect may occur through the Ca2+-dependent phosphatase, calcineurin (Snellman & Nawy 2002), but the molecular target of the phosphatase remains unknown. The activity-dependence of this effect (Berntson et al. 2004, Nawy 2004) and lack of delay (Nawy 2004), are both suggestive of a direct action on TRMP1 channels.
In addition to intracellular Ca2+, other factors are also known to play a role in the modulation of sensitivity. The most notable of these is cGMP, which as described above was initially thought to be the molecule gating transduction channels (Nawy & Jahr 1990, Shiells & Falk 1990). Further investigation revealed that cGMP was instead a sensitizing factor for mGluR6 transduction (Snellman & Nawy 2004), explaining how response size may have increased during its dialysis for light-adapted ON-BCs. Other factors known to adjust the sensitivity of ON-RBCs include Protein Kinase C α (PKCα; (Ruether et al. 2010, Xiong et al. 2015), and inhibitor of G proteins, PCP2 (Xu et al. 2008). These factors have a modest effect on desensitization imposed by background light, and their major effects occur through acceleration response shutoff.
The high number of control points in mGluR6 transduction ultimately provides an avenue with which to modulate the sensitivity and temporal properties of signaling. The widely held belief is that differences in the physiological properties of ON-RBCs compared to ON-CBCs may simply reflect changes in the relative importance of these modulatory mechanisms. In particular, the temporal filtering of the photoreceptor response is expected to be very different for ON-RBCs versus ON-CBCs due to the slow temporal characteristics of rods versus cone, respectively. Indeed, an analysis of response properties as a function of ON-CBC class reveal that these cells are tuned for different characteristic frequencies (Ichinose et al. 2014) and project to distinct retinal ganglion cells. It is likely that these differences are due to the molecular components of mGluR6 signaling in ON-CBC dendrites, but how these differences are manifested remains to be determined.
Control of mGluR6 cascade via extracellular interactions as an emerging principle
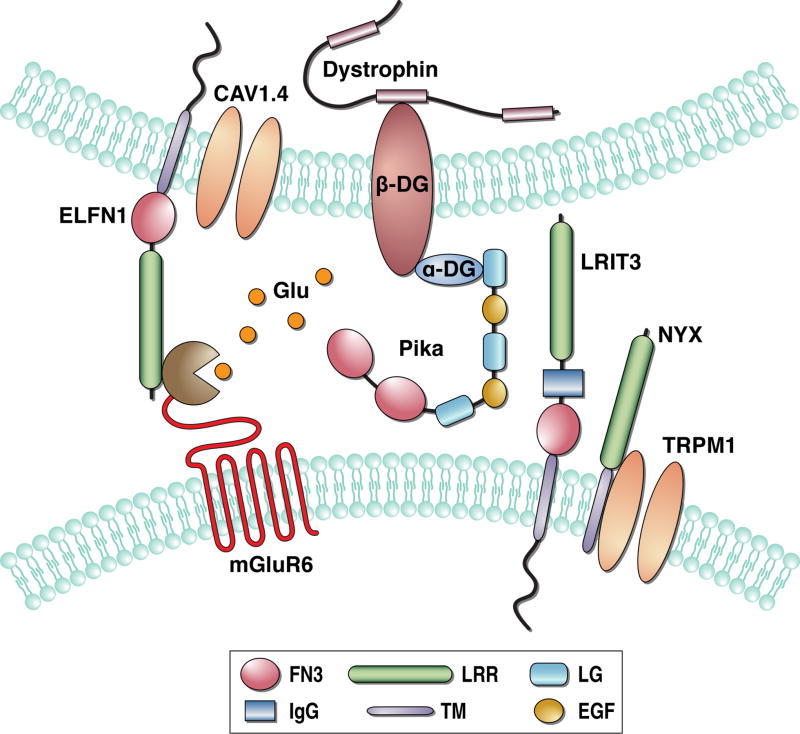
While it is easy to think about ON-BC signaling cascade as an isolated unit, recent evidence increasingly indicates it is subject to integration at the microscopic level for synapse organization. There appears to be bi-directional relationship between the physical assembly of ON-BC synaptic contacts and the functional status of the cascade signaling elements. A growing number of proteins have been implicated in this process and a central theme has evolved in the association of mGluR6 transduction with proteins in the synaptic cleft (Figure 5). In addition to NYX, two other extracellular proteins from the cell-adhesion like family containing leucine-rich repeat domains have been discovered with essential roles in ON-BC signaling. The first is LRIT3, which is expressed by ON-RBCs and ON-CBCs specifically on their dendritic tips (Neuille et al. 2015, Zeitz et al. 2013). Knockout of LRIT3 in mice completely prevents light responsiveness of both rod and cone ON-BCs indicating that it plays essential role in generating the depolarizing response (Neuille et al. 2014, Zeitz et al. 2013). At the cellular level, the loss of LRIT3 was found to prevent postsynaptic targeting of TRPM1 without appreciably affecting other ON-BC signaling components including mGluR6, GAP complex components, or Gαo (Neuille et al. 2015). This specificity bears a striking resemblance to NYX deficient retinas (Gregg et al. 2014, Ray et al. 2014). Given similarities in their structural organization and co-localization at the dendritic tips it appears likely that NYX and LRIT3 function together to regulate TRPM1 trafficking, perhaps though engaging in the same macromolecular complex. Curiously, a knockout of presumably postsynaptic LRIT3 in ON-BCs affected presynaptic organization, at least in cone photoreceptors, as evidenced by the loss of immunoreactivity for peanut agglutinin, a common cone marker (Neuille et al. 2015), which labels an unidentified component of the active zone. This suggests that the ON-BC cascade may be linked physically with the components of the presynaptic release apparatus.
Figure 5. Trans-synaptic integration of ON-bipolar transduction cascade.
Components of mGluR6 interact with multiple cell-adhesion and extracellular matrix proteins present at the synapse.
An illustration how this trans-synaptic integration may be achieved is provided by another leucine rich repeat-containing protein, ELFN1 (Cao et al. 2015). Like NYX and LRIT3, this cell surface protein accumulates specifically at the synaptic puncta. However it is expressed by rod photoreceptors only and is targeted instead to their synaptic terminals. At this site, ELFN1 engages in a trans-synaptic interaction with mGluR6. Knockout of ELFN1 prevents mGluR6 from targeting to the dendritic tips of ON-BCs, consequently disrupting the postsynaptic accumulation of GAP complex components. As a result, synaptic communication between rods and ON-RBCs is abolished and no depolarizing light responses are observed. Curiously, this organization is specific for rods, as mGluR6 targeting in ON-CBCs and synaptic transmission with cones is preserved. This suggests that similar mechanisms relying on yet unidentified molecules present in the cone terminals may be responsible for trans-synaptic integration with signaling in ON-CBCs.
Preceding the discovery of LRIT3 and ELFN1 were observations that disruptions in components of the dystrophin glycoprotein complex (DGC) reduce depolarizing activity during the ON-BC light response, suggesting their role in the synaptic communication between ON-BCs and photoreceptors (Cibis et al. 1993, D'Souza et al. 1995, Lee et al. 2005, Pillers et al. 1993, Pillers et al. 1995). DGC is the membrane specialization that links the cytoskeleton and membrane-associated proteins to the extracellular matrix (Constantin 2014, Waite et al. 2012). Although, DGC is best studied in muscle it plays an important role in the nervous system, particularly in organizing signaling complexes involving ion channels (Connors et al. 2004, Gavillet et al. 2006, Knuesel et al. 1999, Vandebrouck et al. 2007). Several DGC proteins are prominently present in the photoreceptor-to-ON-BC synapse, where they localize either presynaptically in photoreceptors with cytosolic components like dystrophin and membrane associated β-dystroglycan (Schmitz & Drenckhahn 1997b, Ueda et al. 1997, Ueda et al. 1995), or in the synaptic cleft with α-dystroglycan or its extracellular ligand pikachurin, also overlapping with components of the ON-BC signaling (Omori et al. 2012, Sato et al. 2008).
Interestingly, knockout of either dystroglycan or pikachurin caused deficits in the positioning of ON-BC dendrites close to the site of the glutamate release, markedly reducing the ON-BC depolarizing response, while not affect the targeting of mGluR6 or TRPM1 (Omori et al. 2012, Sato et al. 2008). In contrast, deletion of the Gαo, Gβ3, or mGluR6 resulted in reduced accumulation of DCG at the synapse (Tummala et al. 2016). These observations further support the idea of trans-synaptic coordination between the ON-BC signaling cascade and photoreceptors, and highlight that extracellular matrix may play an essential role in this process. How and whether extracellular matrix and DGC components are linked to cell-adhesion like molecules represented by LRIT3, NYX, and ELFN1 is unclear and will be critical to understand the mechanism of trans-synaptic coordination.
Indications are also that ON-BC signaling complexes play a role in the structural organization of synapses, and vice versa that synapse integrity affects the ON-BC signaling cascade. For example, elimination of mGluR6 has been reported to either completely abolish ON-BC synapses with rods and cones (Cao et al. 2009) or cause massive structural deficits (Ishii et al. 2009, Tsukamoto & Omi 2014) resulting in the failure of ON-BC dendrites to invaginate into photoreceptor terminals. Elimination of the GAP complex components causes a variable extent of synapse malformation ranging from intact synapses in GPR179 (Peachey et al. 2012b) and RGS11/RGS7 (exon 4 deletion; (Cao et al. 2012) knockouts to quantitative loss of synapses upon elimination of RGS11/RGS7 (exon 6–8 deletion;(Shim et al. 2012) or Gβ5 (Rao et al. 2007). Similarly, deletion of G protein subunits differentially affected synaptic organization with no morphological defects observed upon elimination of Gαo (Dhingra et al. 2000), yet quantitative loss of synapses upon elimination of Gβ3 (Dhingra et al. 2012). No deficits were observed upon deletion of NYX (Ball et al. 2003, Pardue et al. 2001) or inactivation of TRPM1 by point mutation (Peachey et al. 2012a). The synapse formation deficits are also reported for the deletion of presynaptic molecules. For example, mice lacking alpha subunit of the CaV1.4 channel have no detectable ON-BC dendrites within rod or cone terminals (Mansergh et al. 2005). Similarly, knockout of ELFN1 abolishes synaptic contacts between rods and ON-RBCs, although in this case cone synaptogenesis is preserved (Cao et al. 2015). Curiously, ELFN1 localization is dependent on CaV1.4 suggesting that the entire postsynaptic mGluR6 complex may be coordinated with the presynaptic CaV1.4-ELFN1 containing complexes (Cao et al. 2015). Such trans-synaptic interactions of the mGluR6 signaling cascade may be important for triggering the reactions involved in synaptogenesis and synaptic maintenance, suggesting a role for ON-BC signaling in both synaptic transmission but also in morphogenic signals that lead to formation and/or stabilization of synapses.
Deficits in ON-BC signaling at the nexus of human visual disorders
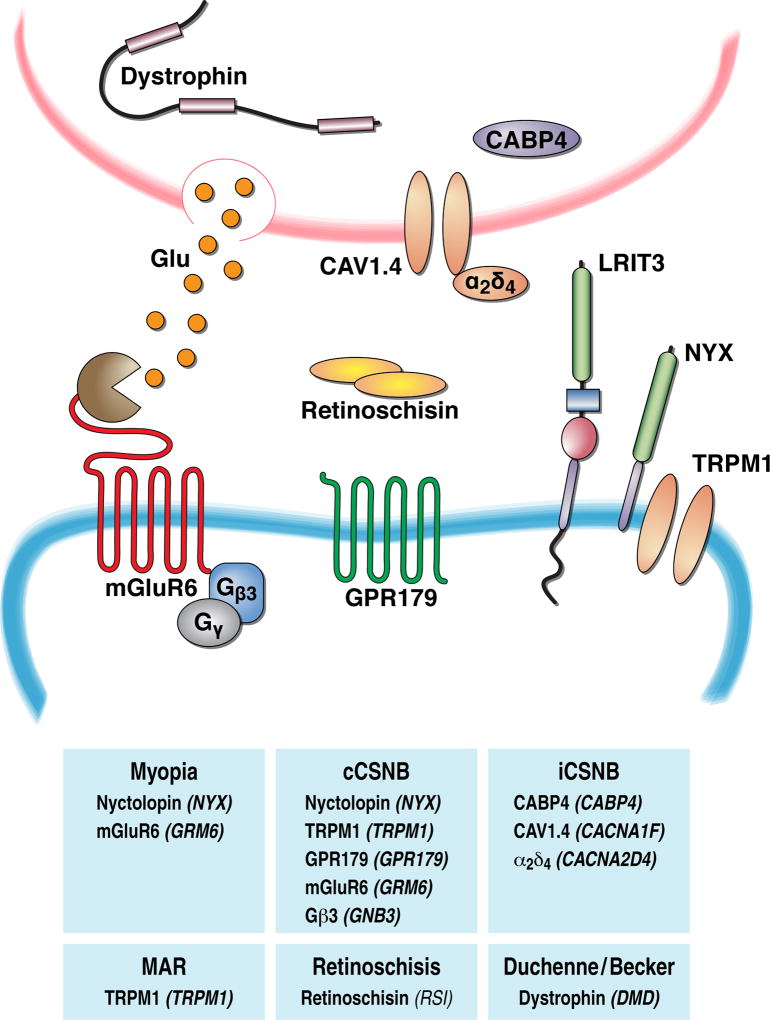
In view of a fundamental role of ON-BCs in encoding light-evoked signals from photoreceptors, it is perhaps not surprising that disruptive mutations in components of ON-BC signaling have been associated with a variety of retinal disorders. As described above, rods rely mainly on ON-RBCs for the transduction of high sensitivity signals. Therefore deficits in signal processing in the ON-RBC cascade most prominently result in inability to see at night, an inherited condition known as congenital stationary night blindness (CSNB) (Zeitz et al. 2015). The basic diagnostic tool for this disease has been ERG, according to which the condition is further subdivided into an “incomplete” form that typically shows involvement of photoreceptors (a-wave changes) and reduction in the ON-BC depolarizing activity (b-wave). In contrast the “complete” form shows intact a-waves amidst totally lacking b-waves, suggesting exclusive involvement of the mGluR6 signaling cascade. This distinction made it possible to isolate these deficits and identify genes specifically responsible for ON-BC signaling dysfunction. The most prevalent form of cCSNB, is brought about by the mutations in NYX (Bech-Hansen et al. 2000, Pusch et al. 2000). In addition, to date mutations in most known components of the ON-BC cascade have been found to result in cCSNB: TRPM1, mGluR6, GPR179, LRIT3, Gβ3 (see (Zeitz et al. 2015) for a comprehensive analysis). Importantly, often identification of mutations in cCSNB patients precedes the knowledge regarding the involvement of a culprit gene in ON-BCs and its functional role, thus providing the discovery platform for the identification of new components of the ON-BC signaling.
In addition to congenital cases of night blindness, loss of night vision is frequently associated with neoplastic conditions, particularly melanoma, a condition commonly referred to as melanoma associated retinopathy (MAR) (Berson & Lessell 1988, Lu et al. 2009). Patients with MAR show a characteristic loss of ERG b-wave, and antibodies derived from their serum specifically stain retinal ON-BCs in the retina and disrupt their depolarizing responses when injected into animal models (Lei et al. 2000, Milam et al. 1993). It was recently determined that the antigen recognized by MAR antibodies is TRPM1 which become inactivated as a result (Dhingra et al. 2011, Kondo et al. 2011, Xiong et al. 2013). Therefore, the underlying cause of MAR appears to be dysfunction in the ON-BC transduction channels. It is possible that other uncharacterized antibodies in a large number of neoplastic retinopathies similarly attack components of the ON-BC transduction cascade with extracellular accessibility to cause visual disturbances in the event they are also expressed by tumor cells and are attacked by autoantibodies.
There is also a long history of clinical research on visual disturbances in patients with Duchenne and Becker muscular dystrophies (Cibis et al. 1993, Pillers et al. 1993, Schmitz & Drenckhahn 1997a). Up to 80% of patients with these diseases show visual disturbances and prominent night blindness. When evaluated by ERG, the main findings appear to be severe reduction in the b-wave and/or an increase in its implicit time. The genetic cause of both of these diseases was found to be loss of function mutations in key DGC component dystrophin. A related group of diseases collectively known as dystroglycanopathies are associated with mutations in genes responsible for glycosylation of dystroglycan (POMT1, POMT2, POMGnT1, fukutin, FKRP, and LARGE). Although ocular phenotypes and ERGs for patients with mutations in these genes are rarely reported (Gerding et al. 1993, Kondo et al. 2010), modeling loss of dystroglycan or its glycosylation in mouse models causes characteristic b-wave deficits (Lee et al. 2005, Liu et al. 2006, Omori et al. 2012). Given the role of DGC in the organization of photoreceptor synapses (described above), dysfunction in ON-BC signaling likely contributes to the symptomatic manifestations of many forms of muscular dystrophies and dystroglycanopathies.
A separate condition associated with dysfunction in the extracellular matrix, where ON-BC signaling deficits significantly contribute to symptomatic manifestations is X-linked retinoschisis (Sikkink et al. 2007, Tantri et al. 2004). In this condition dysfunction in the cell adhesion molecule, retinocshisin, causes splitting of retinal layers and diminishes ON-BC depolarizing activity. A retinoschisin knockout mouse recapitulates human phenotypes and features ERG b-wave deficits associated with progressive loss of GAP complex components and TRPM1 (Ou et al. 2015, Takada et al. 2008, Weber et al. 2002). Remarkably, viral rescue of retinoschisin restores the synaptic function and localizes ON-BC signaling components, suggesting plasticity of the ON-BC cascade interactions with the extracellular matrix (Ou et al. 2015). The molecular links between retinoschisin and ON-BC signalosome are unknown, but likely involve integration with other components of the extracellular matrix.
Finally, dysfunctions in ON-BC signaling are increasingly associated with the development of refractive errors. Indeed, most patients with cCSNB show high co-morbidity with myopia (Lodha et al. 2012, Miyake et al. 1986). Mutations in both mGluR6 (Wang et al. 2016, Xu et al. 2009) and NYX (Yip et al. 2013, Zhang et al. 2007, Zhou et al. 2015) have also been associated with myopia. Interestingly, in some myopic patients NYX mutations occur without night blindness (Zhang et al. 2007, Zhou et al. 2015) suggesting that more subtle alterations of ON-BC signal processing that preserve synaptic transmission may nevertheless cause myopia. The connection of ON-BC dysfunction to myopia is also well supported by the animal models where either pharmacological blockade of ON-BC transmission (Crewther et al. 1996, Smith et al. 1991) or genetic elimination of NYX or mGluR6 (Chakraborty et al. 2015, Pardue et al. 2008) similarly exacerbate development of refractive error in experimental models of induced myopia. The molecular basis underlying the involvement of the ON-BC signaling cascade remains unclear, although it is speculated that changes in the levels of neurotransmitter dopamine and/or inputs provided by ON-BC light-driven activity may be among key factors connecting mGluR6 signaling with pathways that control axial growth (Chakraborty & Pardue 2015).
Figure 6. Mutations in multiple synaptic molecules at the first visual synapse are associated with human disease.
Diagram depicts pre- versus post-synaptic position of signaling molecules and their corresponding genes. Further, the genes implicated in disorders featuring visual deficits are identified.
Summary Points.
Synaptic transmission between photoreceptor cells and ON-bipolar neurons requires a signal transduction cascade originating with mGluR6 receptors on the bipolar cell dendritic tips, which sense the light-induced reduction in glutamate release from photoreceptors.
mGluR6 receptors signal the reduction in glutamate release by coupling with TRPM1-containing transduction channels via a heterotrimeric G protein, Gαo, which inhibit transduction channels.
Transduction channel opening is aided by GTPase Accelerating Protein (GAP) complexes consisting of RGS7, RGS11, Gβ5, R9AP, and GPR179 subunits. The GAP complex is responsible for triggering depolarization of ON-BCs in response to light.
Multiple components of the transduction cascade are scaffolded into a ‘signalosome’ assembly with a coordinated, hierarchical interplay that determines the stability and trafficking of these components.
The transduction cascade is embedded into a web of interactions with extracellular matrix proteins and presynaptic cell adhesion-like molecules in photoreceptors. These interactions are essential for the selective wiring of ON-BCs with their cognate photoreceptor cells.
Mutations in multiple elements of the cascade result in a variety of human diseases characterized by visual impairments that can be modeled in mice.
Future Issues.
Understand the molecular basis by which subunits of the heterotrimeric protein, Go, regulates the activity of TRPM1 channels. Additional components of TRPM1-containing channels will also need to be identified to provide insights into its gating mechanisms.
Determine the structural organization of the mGluR6-TRPM1 signalosome, including precise mapping of structural determinants for complex formation among individual proteins, mechanisms of assembly, and their high-resolution atomic structure.
Delineate the mechanisms that govern coordinated trafficking of signaling elements in the ON-BC cascade and their targeting to the dendritic tips.
Elucidate the integration of the mGluR6 signaling cascade with the network of extracellular proteins, and establish the role of these interactions in selective wiring with photoreceptors.
Determine the relationship between alterations in signaling molecules and disease at the first visual synapse. Define the functional effects of individual mutations.
References
- Agosto MA, Zhang Z, He F, Anastassov IA, Wright SJ, et al. Oligomeric state of purified transient receptor potential melastatin-1 (TRPM1), a protein essential for dim light vision. J Biol Chem. 2014;289:27019–33. doi: 10.1074/jbc.M114.593780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54:33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Falk G. The single-photon signal in rod bipolar cells of the dogfish retina. J Physiol. 1980;300:151–66. doi: 10.1113/jphysiol.1980.sp013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85:720–9. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci. 2003;20:267–72. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–23. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, et al. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–70. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–24. doi: 10.1017/S095252380421611X. [DOI] [PubMed] [Google Scholar]
- Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106:307–11. doi: 10.1016/0002-9394(88)90366-2. [DOI] [PubMed] [Google Scholar]
- Bojang P, Jr, Gregg RG. Topological analysis of small leucine-rich repeat proteoglycan nyctalopin. PLoS One. 2012;7:e33137. doi: 10.1371/journal.pone.0033137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Xiong WH, Peters JH, Tekmen-Clark M, Strycharska-Orczyk I, et al. TRPM3 expression in mouse retina. PLoS One. 2015;10:e0117615. doi: 10.1371/journal.pone.0117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera JL, De Freitas F, Satpaev DK, Slepak VZ. Identification of the G*5-RGS7 protein complex in the retina. Biochemical and Biophysical Research Communications. 1998;249:898–902. doi: 10.1006/bbrc.1998.9218. [DOI] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, et al. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–13. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7905–10. doi: 10.1073/pnas.1202332109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11521–6. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sarria I, Fehlhaber KE, Kamasawa N, Orlandi C, et al. Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron. 2015;87:1248–60. doi: 10.1016/j.neuron.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Song H, Okawa H, Sampath AP, Sokolov M, Martemyanov KA. Targeting of RGS7/Gbeta5 to the dendritic tips of ON-bipolar cells is independent of its association with membrane anchor R7BP. J Neurosci. 2008;28:10443–9. doi: 10.1523/JNEUROSCI.3282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Pardue MT. Molecular and Biochemical Aspects of the Retina on Refraction. Prog Mol Biol Transl Sci. 2015;134:249–67. doi: 10.1016/bs.pmbts.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015;137:79–83. doi: 10.1016/j.exer.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Eversole-Cire P, Zhang HK, Mancino V, Chen YJ, et al. Instability of GGL domain-containing RGS proteins in mice lacking the G protein *-subunit G*5. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6604–09. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Shim H, Morhardt D, Dallman R, Krahn E, et al. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest Ophthalmol Vis Sci. 2010;51:686–93. doi: 10.1167/iovs.09-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibis GW, Fitzgerald KM, Harris DJ, Rothberg PG, Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Invest Ophthalmol Vis Sci. 1993;34:3646–52. [PubMed] [Google Scholar]
- Cohen MR, Moiseenkova-Bell VY. Structure of thermally activated TRP channels. Curr Top Membr. 2014;74:181–211. doi: 10.1016/B978-0-12-800181-3.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol. 2000;524(Pt 1):135–46. doi: 10.1111/j.1469-7793.2000.t01-1-00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors NC, Adams ME, Froehner SC, Kofuji P. The potassium channel Kir4.1 associates with the dystrophin-glycoprotein complex via alpha-syntrophin in glia. J Biol Chem. 2004;279:28387–92. doi: 10.1074/jbc.M402604200. [DOI] [PubMed] [Google Scholar]
- Constantin B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim Biophys Acta. 2014;1838:635–42. doi: 10.1016/j.bbamem.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–9. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG, Xie RZ. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. J Ocul Pharmacol Ther. 1996;12:193–208. doi: 10.1089/jop.1996.12.193. [DOI] [PubMed] [Google Scholar]
- D'Souza VN, Nguyen TM, Morris GE, Karges W, Pillers DA, Ray PN. A novel dystrophin isoform is required for normal retinal electrophysiology. Hum Mol Genet. 1995;4:837–42. doi: 10.1093/hmg/4.5.837. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:331–45. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–48. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004;24:5684–93. doi: 10.1523/JNEUROSCI.0492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Fina ME, Neinstein A, Ramsey DJ, Xu Y, et al. Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci. 2011;31:3962–7. doi: 10.1523/JNEUROSCI.6007-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, et al. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–84. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang MS, Pugh EN, Jr, Birnbaumer L, et al. The light response of ON bipolar neurons requires G*o. Journal of Neuroscience. 2000;20:9053–58. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, et al. Gbeta3 is required for normal light ON responses and synaptic maintenance. J Neurosci. 2012;32:11343–55. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, Vardi N. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008;510:484–96. doi: 10.1002/cne.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci. 2006;26:3959–70. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci. 2014;15:507–19. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–85. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annual review of physiology. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, et al. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res. 2006;99:407–14. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- Gerding H, Gullotta F, Kuchelmeister K, Busse H. Ocular findings in Walker-Warburg syndrome. Childs Nerv Syst. 1993;9:418–20. doi: 10.1007/BF00306196. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annual review of biochemistry. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J Neurosci. 1995;15:3852–62. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, et al. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol. 2007;98:3023–33. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, et al. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003;44:378–84. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Ray TA, Hasan N, McCall MA, Peachey NS. Interdependence Among Members of the mGluR6 G-protein Mediated Signalplex of Retinal Depolarizing Bipolar Cells. In: KAaS Martemyanov, A.P., editor. G Protein Signaling Mechanisms in the Retina. Springer; 2014. pp. 67–79. [Google Scholar]
- Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Frontiers in neuroanatomy. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacological Reviews. 2002;54:527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Fyk-Kolodziej B, Cohn J. Roles of ON cone bipolar cell subtypes in temporal coding in the mouse retina. J Neurosci. 2014;34:8761–71. doi: 10.1523/JNEUROSCI.3965-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Morigiwa K, Takao M, Nakanishi S, Fukuda Y, et al. Ectopic synaptic ribbons in dendrites of mouse retinal ON- and OFF-bipolar cells. Cell Tissue Res. 2009;338:355–75. doi: 10.1007/s00441-009-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey BG, Morgans CW, Puthussery T, Wensel TG, Burke NS, et al. R9AP stabilizes RGS11-G beta5 and accelerates the early light response of ON-bipolar cells. Vis Neurosci. 2010;27:9–17. doi: 10.1017/S0952523809990319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985;358:131–52. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Nawy S. Characterization of Trpm1 desensitization in ON bipolar cells and its role in downstream signalling. J Physiol. 2012;590:179–92. doi: 10.1113/jphysiol.2011.218974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–62. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Koike C, Numata T, Ueda H, Mori Y, Furukawa T. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell calcium. 2010a;48:95–101. doi: 10.1016/j.ceca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010b;107:332–7. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saito K, Urano M, Sagara Y, Uchio E, Kondo M. A case of Fukuyama congenital muscular dystrophy associated with negative electroretinograms. Jpn J Ophthalmol. 2010;54:622–4. doi: 10.1007/s10384-010-0875-0. [DOI] [PubMed] [Google Scholar]
- Kondo M, Sanuki R, Ueno S, Nishizawa Y, Hashimoto N, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6:e19911. doi: 10.1371/journal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281:10250–62. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- Lambert S, Drews A, Rizun O, Wagner TF, Lis A, et al. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J Biol Chem. 2011 doi: 10.1074/jbc.M110.202945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM. Membrane currents of retinal bipolar cells in culture. J Neurophysiol. 1988;60:1460–80. doi: 10.1152/jn.1988.60.4.1460. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, et al. Ocular abnormalities in Large(myd) and Large(vls) mice, spontaneous models for muscle, eye, and brain diseases. Mol Cell Neurosci. 2005;30:160–72. doi: 10.1016/j.mcn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–6. [PubMed] [Google Scholar]
- Liu J, Ball SL, Yang Y, Mei P, Zhang L, et al. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1) Mech Dev. 2006;123:228–40. doi: 10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lodha N, Loucks CM, Beaulieu C, Parboosingh JS, Bech-Hansen NT. Congenital stationary night blindness: mutation update and clinical variability. Adv Exp Med Biol. 2012;723:371–9. doi: 10.1007/978-1-4614-0631-0_48. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jia L, He S, Hurley MC, Leys MJ, et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmol. 2009;127:1572–80. doi: 10.1001/archophthalmol.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino ER, Handy JW, Li TS, Arshavsky VY. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein * subunit. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1947–52. doi: 10.1073/pnas.96.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, et al. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–46. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–65. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Milam AH, Saari JC, Jacobson SG, Lubinski WP, Feun LG, Alexander KR. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:91–100. [PubMed] [Google Scholar]
- Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–20. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–65. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumder DKaW TG. Variable Distribution of RGS7 and RGS11 in the Dendritic-Tips of ON-Bipolar Cells in the Murine Retina. Invest. Ophthalmol. Vis. Sci. 2008;49:762. [Google Scholar]
- Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci. 2006;23:1163–71. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, Duvoisin RM. Gbeta5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. Eur J Neurosci. 2007;26:2899–905. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–8. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–44. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–9. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Desensitization of the mGluR6 transduction current in tiger salamander On bipolar cells. J Physiol. 2004;558:137–46. doi: 10.1113/jphysiol.2004.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–71. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973;36:519–35. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Neuille M, El Shamieh S, Orhan E, Michiels C, Antonio A, et al. Lrit3 deficient mouse (nob6): a novel model of complete congenital stationary night blindness (cCSNB) PLoS One. 2014;9:e90342. doi: 10.1371/journal.pone.0090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuille M, Morgans CW, Cao Y, Orhan E, Michiels C, et al. LRIT3 is essential to localize TRPM1 to the dendritic tips of depolarizing bipolar cells and may play a role in cone synapse formation. Eur J Neurosci. 2015;42:1966–75. doi: 10.1111/ejn.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Pahlberg J, Rieke F, Birnbaumer L, Sampath AP. Coordinated control of sensitivity by two splice variants of Galpha(o) in retinal ON bipolar cells. The Journal of general physiology. 2010;136:443–54. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Araki F, Chaya T, Kajimura N, Irie S, et al. Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J Neurosci. 2012;32:6126–37. doi: 10.1523/JNEUROSCI.0322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Cao Y, Martemyanov KA. Orphan receptor GPR179 forms macromolecular complexes with components of metabotropic signaling cascade in retina ON-bipolar neurons. Investigative ophthalmology & visual science. 2013;54:7153–61. doi: 10.1167/iovs.13-12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, et al. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. The Journal of cell biology. 2012;197:711–9. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Martemyanov KA. Orphan Receptor GPR158 Is an Allosteric Modulator of RGS7 Catalytic Activity with an Essential Role in Dictating Its Expression and Localization in the Brain. J Biol Chem. 2015;290:13622–39. doi: 10.1074/jbc.M115.645374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J, Vijayasarathy C, Ziccardi L, Chen S, Zeng Y, et al. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. J Clin Invest. 2015;125:2891–903. doi: 10.1172/JCI81380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlberg J, Sampath AP. Visual threshold is set by linear and nonlinear mechanisms in the retina that mitigate noise: how neural circuits in the retina improve the signal-to-noise ratio of the single-photon response. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:438–47. doi: 10.1002/bies.201100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Lem J, Bramblett DE, Paul DL, Wu SM. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc Natl Acad Sci U S A. 2010;107:395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Ball SL, Candille SI, McCall MA, G GR, Peachey NS. nob: a mouse model of CSNB1. In: ea Anderson, editor. New Insights Into Retinal Degenerative Diseases. Kluwer Academic/Plenum Publishers; 2001. pp. 319–28. [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–12. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Docampo R. In with the TRP channels: intracellular functions for TRPM1 and TRPM2. Sci Signal. 2009;2:pe69. doi: 10.1126/scisignal.295pe69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey NS, Pearring JN, Bojang P, Jr, Hirschtritt ME, Sturgill-Short G, et al. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J Neurophysiol. 2012a;108:2442–51. doi: 10.1152/jn.00137.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, et al. GPR179 Is Required for Depolarizing Bipolar Cell Function and Is Mutated in Autosomal-Recessive Complete Congenital Stationary Night Blindness. American journal of human genetics. 2012b;90:331–9. doi: 10.1016/j.ajhg.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Bojang P, Jr, Shen Y, Koike C, Furukawa T, et al. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10060–6. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-W, Robishaw JD, Levine MA, Yau K-W. Retinal rods and cones have distinct G protein * and gamma subunits. 1992;89:10882–86. doi: 10.1073/pnas.89.22.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–4. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Pillers DA, Bulman DE, Weleber RG, Sigesmund DA, Musarella MA, et al. Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nat Genet. 1993;4:82–6. doi: 10.1038/ng0593-82. [DOI] [PubMed] [Google Scholar]
- Pillers DA, Weleber RG, Woodward WR, Green DG, Chapman VM, Ray PN. mdxCv3 mouse is a model for electroretinography of Duchenne/Becker muscular dystrophy. Invest Ophthalmol Vis Sci. 1995;36:462–6. [PubMed] [Google Scholar]
- Poopalasundaram S, Erskine L, Cheetham ME, Hardcastle AJ. Focus on molecules: nyctalopin. Exp Eye Res. 2005;81:627–8. doi: 10.1016/j.exer.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–7. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan H, Dhingra A, Tummala SR, Fina ME, Li JJ, et al. Differential function of Ggamma13 in rod bipolar and ON cone bipolar cells. J Physiol. 2015;593:1531–50. doi: 10.1113/jphysiol.2014.281196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007;27:14199–204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Buchsbaum G, Sterling P. Transmitter concentration at a three-dimensional synapse. J Neurophysiol. 1998;80:3163–72. doi: 10.1152/jn.1998.80.6.3163. [DOI] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal: architecture of a binary synapse. Neuron. 1995;14:561–9. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Ray TA, Heath KM, Hasan N, Noel JM, Samuels IS, et al. GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells. J Neurosci. 2014;34:6334–43. doi: 10.1523/JNEUROSCI.4044-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruether K, Feigenspan A, Pirngruber J, Leitges M, Baehr W, Strauss O. PKC{alpha} is essential for the proper activation and termination of rod bipolar cell response. Invest Ophthalmol Vis Sci. 2010;51:6051–8. doi: 10.1167/iovs.09-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–43. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria I, Orlandi C, McCall MA, Gregg RG, Martemyanov KA. Intermolecular interaction between anchoring subunits specify subcellular targeting and function of RGS proteins in retina. Journal of Neuroscience. 2016;36 doi: 10.1523/JNEUROSCI.3833-15.2016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria I, Pahlberg J, Cao Y, Kolesnikov AV, Kefalov VJ, et al. Sensitivity and kinetics of signal transmission at the first visual synapse differentially impact visually-guided behavior. Elife. 2015;4 doi: 10.7554/eLife.06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–31. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Scalabrino ML, Boye SL, Fransen KM, Noel JM, Dyka FM, et al. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum Mol Genet. 2015;24:6229–39. doi: 10.1093/hmg/ddv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Drenckhahn D. Dystrophin in the retina. Prog Neurobiol. 1997a;53:547–60. doi: 10.1016/s0301-0082(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Drenckhahn D. Localization of dystrophin and beta-dystroglycan in bovine retinal photoreceptor processes extending into the postsynaptic dendritic complex. Histochem Cell Biol. 1997b;108:249–55. doi: 10.1007/s004180050165. [DOI] [PubMed] [Google Scholar]
- Schneider FM, Mohr F, Behrendt M, Oberwinkler J. Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells. Eur J Cell Biol. 2015;94:420–7. doi: 10.1016/j.ejcb.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–93. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Rampino MA, Carroll RC, Nawy S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gbetagamma dimer. Proc Natl Acad Sci U S A. 2012;109:8752–7. doi: 10.1073/pnas.1117433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA. Ca(2+)-induced light adaptation in retinal ON-bipolar cells. Keio J Med. 1999;48:140–6. doi: 10.2302/kjm.48.140. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242:91–4. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Potentiation of 'on' bipolar cell flash responses by dim background light and cGMP in dogfish retinal slices. J Physiol. 2002;542:211–20. doi: 10.1113/jphysiol.2002.019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Wang CT, Chen YL, Chau VQ, Fu KG, et al. Defective retinal depolarizing bipolar cells (DBCs) in Regulators of G-protein Signaling (RGS) 7 and 11 double null mice. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.345751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkink SK, Biswas S, Parry NR, Stanga PE, Trump D. X-linked retinoschisis: an update. J Med Genet. 2007;44:225–32. doi: 10.1136/jmg.2006.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983;303:537–8. doi: 10.1038/303537a0. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Fox DA, Duncan GC. Refractive-error changes in kitten eyes produced by chronic on-channel blockade. Vision Res. 1991;31:833–44. doi: 10.1016/0042-6989(91)90150-4. [DOI] [PubMed] [Google Scholar]
- Snellman J, Nawy S. Regulation of the retinal bipolar cell mGluR6 pathway by calcineurin. J Neurophysiol. 2002;88:1088–96. doi: 10.1152/jn.2002.88.3.1088. [DOI] [PubMed] [Google Scholar]
- Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6621–8. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Fidelity of G protein *-subunit association by the G protein gamma-subunit-like domains of RGS6, RGS7, and RGS11. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6489–94. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang SR. Invited review: Activation of G proteins by GTP and the mechanism of Galpha-catalyzed GTP hydrolysis. Biopolymers. 2016;105:449–62. doi: 10.1002/bip.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci. 2008;49:3677–86. doi: 10.1167/iovs.07-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Jr, Donoso LA. X-linked retinoschisis: a clinical and molecular genetic review. Surv Ophthalmol. 2004;49:214–30. doi: 10.1016/j.survophthal.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Thoreson WB. Kinetics of synaptic transmission at ribbon synapses of rods and cones. Mol Neurobiol. 2007;36:205–23. doi: 10.1007/s12035-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–66. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Trifonov IU. Study of synaptic transmission between photoreceptor and horizontal cell by electric stimulations of the retina. Biofizika. 1968;13:809–17. [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–23. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Omi N. Effects of mGluR6-deficiency on photoreceptor ribbon synapse formation: comparison of electron microscopic analysis of serial sections with random sections. Vis Neurosci. 2014;31:39–46. doi: 10.1017/S0952523813000473. [DOI] [PubMed] [Google Scholar]
- Tummala SR, Dhingra A, Fina ME, Li JJ, Ramakrishnan H, Vardi N. Lack of mGluR6-related cascade elements leads to retrograde trans-synaptic effects on rod photoreceptor synapses via matrix-associated proteins. Eur J Neurosci. 2016;43:1509–22. doi: 10.1111/ejn.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Baba T, Terada N, Kato Y, Tsukahara S, Ohno S. Dystrophin in rod spherules; submembranous dense regions facing bipolar cell processes. Histochem Cell Biol. 1997;108:243–8. doi: 10.1007/s004180050164. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kobayashi T, Mitsui K, Tsurugi K, Tsukahara S, Ohno S. Dystrophin localization at presynapse in rat retina revealed by immunoelectron microscopy. Invest Ophthalmol Vis Sci. 1995;36:2318–22. [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–6. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, et al. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21:608–17. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Vardi N, Matesic DF, Manning DR, Liebman PA, Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci. 1993;10:473–8. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- Waite A, Brown SC, Blake DJ. The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci. 2012;35:487–96. doi: 10.1016/j.tins.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Su S, Yang M, Hu N, Yao Y, et al. Association of ZNF644, GRM6, and CTNND2 genes with high myopia in the Han Chinese population: Jiangsu Eye Study. Eye (Lond) 2016;30:1017–22. doi: 10.1038/eye.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–47. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–57. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H, Grunert U, Cook NJ, Molday RS. The cGMP-gated channel of rod outer segments is not localized in bipolar cells of the mammalian retina. Neurosci Lett. 1992;134:199–202. doi: 10.1016/0304-3940(92)90516-a. [DOI] [PubMed] [Google Scholar]
- Weber BHF, Schrewe H, Molday LL, Gehrig A, White KL, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6222–27. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–55. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Willardson BM, Tracy CM. Chaperone-mediated assembly of G protein complexes. Subcell Biochem. 2012;63:131–53. doi: 10.1007/978-94-007-4765-4_8. [DOI] [PubMed] [Google Scholar]
- Xiong WH, Duvoisin RM, Adamus G, Jeffrey BG, Gellman C, Morgans CW. Serum TRPM1 autoantibodies from melanoma associated retinopathy patients enter retinal on-bipolar cells and attenuate the electroretinogram in mice. PLoS One. 2013;8:e69506. doi: 10.1371/journal.pone.0069506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WH, Pang JJ, Pennesi ME, Duvoisin RM, Wu SM, Morgans CW. The Effect of PKCalpha on the Light Response of Rod Bipolar Cells in the Mouse Retina. Invest Ophthalmol Vis Sci. 2015;56:4961–74. doi: 10.1167/iovs.15-16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li S, Xiao X, Wang P, Guo X, Zhang Q. Sequence variations of GRM6 in patients with high myopia. Mol Vis. 2009;15:2094–100. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T, Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012;107:948–57. doi: 10.1152/jn.00933.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Orlandi C, Cao Y, Yang S, Choi CI, et al. The TRPM1 channel in ON-bipolar cells is gated by both the alpha and the betagamma subunits of the G-protein Go. Sci Rep. 2016;6:20940. doi: 10.1038/srep20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sulaiman P, Feddersen RM, Liu J, Smith RG, Vardi N. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J Neurosci. 2008;28:8873–84. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SP, Li CC, Yiu WC, Hung WH, Lam WW, et al. A novel missense mutation in the NYX gene associated with high myopia. Ophthalmic Physiol Opt. 2013;33:346–53. doi: 10.1111/opo.12036. [DOI] [PubMed] [Google Scholar]
- Zeitz C, Jacobson SG, Hamel CP, Bujakowska K, Neuille M, et al. Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2013;92:67–75. doi: 10.1016/j.ajhg.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015;45:58–110. doi: 10.1016/j.preteyeres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Zeitz C, Scherthan H, Freier S, Feil S, Suckow V, et al. NYX (nyctalopin on chromosome X), the gene mutated in congenital stationary night blindness, encodes a cell surface protein. Invest Ophthalmol Vis Sci. 2003;44:4184–91. doi: 10.1167/iovs.03-0251. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–97. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jeffrey BG, Morgans CW, Burke NS, Haley TL, et al. RGS7 and-11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010;51:1121–9. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Xiao X, Li S, Jia X, Yang Z, et al. Mutations in NYX of individuals with high myopia, but without night blindness. Mol Vis. 2007;13:330–6. [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li T, Song X, Li Y, Li H, Dan H. NYX mutations in four families with high myopia with or without CSNB1. Mol Vis. 2015;21:213–23. [PMC free article] [PubMed] [Google Scholar]