ABSTRACT
Mycobacterium tuberculosis successfully subverts the host immune response to promote disease progression. In addition to its known intracellular niche in macrophages, M. tuberculosis interferes with the functions of dendritic cells (DCs), which are the primary antigen-presenting cells of the immune system. We previously showed that M. tuberculosis dampens proinflammatory responses and impairs DC functions through the cell envelope-associated serine protease Hip1. Here we present data showing that M. tuberculosis GroEL2, a substrate of Hip1, modulates DC functions. The full-length GroEL2 protein elicited robust proinflammatory responses from DCs and promoted DC maturation and antigen presentation to T cells. In contrast, the cleaved form of GroEL2, which predominates in M. tuberculosis, was poorly immunostimulatory and was unable to promote DC maturation and antigen presentation. Moreover, DCs exposed to full-length, but not cleaved, GroEL2 induced strong antigen-specific gamma interferon (IFN-γ), interleukin-2 (IL-2), and IL-17A cytokine responses from CD4+ T cells. Moreover, the expression of cleaved GroEL2 in the hip1 mutant restored the robust T cell responses to wild-type levels, suggesting that proteolytic cleavage of GroEL2 allows M. tuberculosis to prevent optimal DC-T cell cross talk during M. tuberculosis infection.
KEYWORDS: Mycobacterium tuberculosis, dendritic cells
INTRODUCTION
Mycobacterium tuberculosis is a highly successful human pathogen that has evolved multiple mechanisms to evade and manipulate host innate and adaptive immunity (1–3). While CD4+ T cell responses are important for mycobacterial control, M. tuberculosis delays the onset of antigen-specific T cell responses, which are unable to effectively eliminate the pathogen from infected hosts. These suboptimal CD4+ T cell responses are in part due to the ability of M. tuberculosis to impair dendritic cell (DC) functions such as the migration of infected DCs from the lung to draining lymph nodes, DC maturation, and antigen presentation to naive CD4+ T cells (4–6). As the primary antigen-presenting cells of the immune system, DCs serve as a bridge between innate and adaptive immunity. By impairing DC functions, M. tuberculosis prevents optimal cross talk between DCs and CD4+ T cells and shapes T cell responses to its benefit. However, the bacterial factors that contribute to the M. tuberculosis-mediated impairment of DCs are poorly defined.
We previously demonstrated that the M. tuberculosis protein GroEL2, which is a chaperone-like immunomodulatory protein, modulates macrophage proinflammatory responses. While those studies focused on the role of the full-length (FL) GroEL2 protein, our data suggest that a cleaved form of GroEL2 [GroEL2(cl)] predominates in wild-type M. tuberculosis and that the cleavage of GroEL2 serves to dampen innate immune responses to M. tuberculosis infection. We showed that the FL GroEL2 protein has a multimeric conformation, is exported to the cell wall of M. tuberculosis, and is secreted extracellularly (7). In wild-type M. tuberculosis, FL GroEL2 is proteolytically cleaved at its N terminus between amino acid residues Arg12 and Gly13 by the serine protease Hip1 into a smaller form, GroEL2(cl), that has a monomeric conformation (7). Cleavage of GroEL2 does not occur in the absence of Hip1 protease activity, and an M. tuberculosis hip1 mutant harbors the FL GroEL2 but not the GroEL2(cl) protein. Moreover, the hip1 mutant induced significantly higher levels of proinflammatory cytokines than did wild-type M. tuberculosis during macrophage infection. This was attributed in part to the enhanced immunostimulatory effect of FL GroEL2 on the hip1 mutant compared to GroEL2(cl), which predominates in wild-type M. tuberculosis. The immunomodulatory effects of GroEL2 on macrophage functions suggest that GroEL2 may also play a role in modulating DCs and downstream T cell responses. Therefore, we sought to investigate whether the cleavage of GroEL2 impacts DC functions. We hypothesized that the FL GroEL2 and GroEL2(cl) proteins would differentially impact DC functions and thereby shape the type of antigen-specific T cell responses elicited during infection with M. tuberculosis. We show that FL GroEL2, but not cleaved GroEL2, induced robust proinflammatory cytokines from DCs and significantly greater expression of the costimulatory molecules CD40 and CD86 on DCs. We also show that FL GroEL2 promoted efficient antigen presentation and polarization of antigen-specific CD4+ T cells into T helper (Th) subsets that secreted gamma interferon (IFN-γ), interleukin-2 (IL-2), and IL-17. In contrast, GroEL2(cl) was poorly stimulatory and unable to promote antigen presentation to T cells. Moreover, the expression of GroEL2(cl) within the hip1 mutant restored T cell responses to levels induced by wild-type M. tuberculosis in DC-T cell coculture assays. Our studies suggest that the Hip1-mediated cleavage of GroEL2 compromises the ability of DCs to initiate optimal antigen-specific T cell responses, thus dampening the host response to infection.
RESULTS
Enhanced maturation of DCs by FL GroEL2 compared to GroEL2(cl).
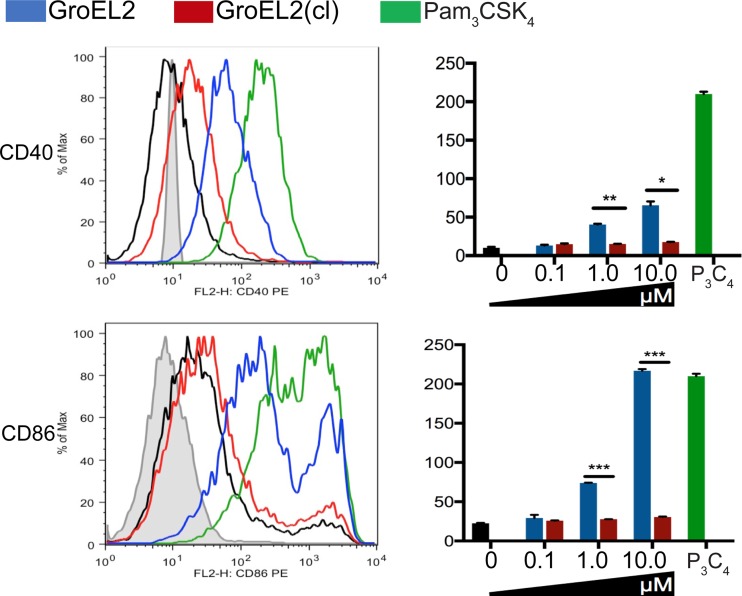
At sites of infection, immature DCs undergo maturation upon contact with antigens; mature DCs are characterized by high surface expression levels of costimulatory molecules such as CD40 and CD86, which interact with ligands on T cells to optimally induce T cell activation. To investigate how proteolytic cleavage alters the immunostimulatory capacity of the GroEL2 protein, we first compared the abilities of the purified recombinant FL GroEL2 and GroEL2(cl) proteins to induce the cell surface expression of key costimulatory molecules on DCs (Fig. 1). Recombinant proteins were generated as described previously (7), and endotoxin levels in these protein preparations were determined to be below detection levels (data not shown). We exposed bone marrow-derived DCs (BMDCs) from C57BL/6 mice to either FL GroEL2 or GroEL2(cl) and measured the expression levels of CD40, CD86, and major histocompatibility complex (MHC) class II on the cell surface by flow cytometry. FL GroEL2 induced the robust expression of CD40 and CD86 (Fig. 1); in contrast, GroEL2(cl) induced significantly lower levels of these two markers. Under these conditions, neither form of GroEL2 induced the further expression of MHC class II above baseline levels (data not shown). Overall, these data indicate that the cleavage of GroEL2 blunts its capacity to induce the maturation of DCs.
FIG 1.
Expression of costimulatory molecules CD40 and CD86 on DCs in response to full-length GroEL2 and GroEL2(cl). We stimulated C57BL/6 BMDCs with recombinant GroEL2 or GroEL2(cl) for 24 h and analyzed the cell surface expression of CD40 and CD86. Representative histograms and mean fluorescence intensity values for the CD11c+ DC subpopulation are shown. Isotype and Pam3CSK4 controls are shown as gray and green outlines, respectively. Data are shown as means ± SD of results of one representative experiment from three independent experiments.
Cleavage of M. tuberculosis GroEL2 attenuates its ability to induce cytokine responses in DCs.
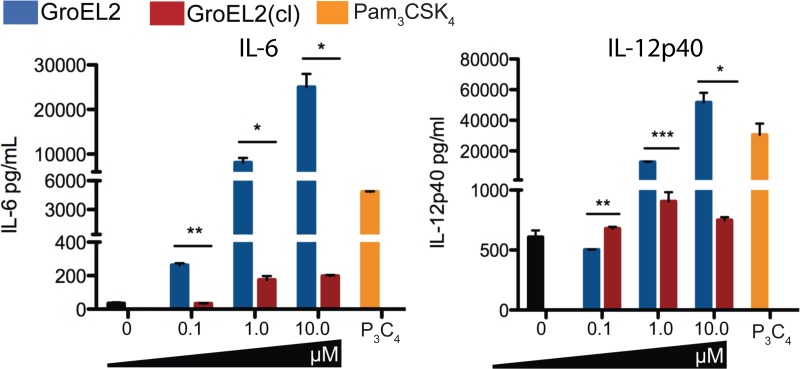
As DCs undergo maturation, they produce key proinflammatory cytokines, such as IL-12 and IL-6, that are important for polarizing naive Th cells into Th subsets such as IFN-γ-producing Th1 cells (8). We therefore compared the levels of IL-12p40 and IL-6 induced by the recombinant GroEL2 and GroEL2(cl) proteins (Fig. 2). We exposed BMDCs to various concentrations of FL GroEL2 and GroEL2(cl) and measured the levels of IL-12p40 and IL-6 in the supernatants after 24 h. FL GroEL2 induced high levels of both IL-12p40 and IL-6 in DCs at each concentration tested. Cytokine levels induced by the FL protein were comparable to those induced by Pam3CysSerLys4 (Pam3CSK4). In contrast, GroEL2(cl) was unable to induce these two cytokines above background levels at all concentrations of the protein tested. We did not detect IL-10, tumor necrosis factor alpha (TNF-α), or IL-1β production under the conditions tested (data not shown). These data indicate that FL GroEL2 has the capacity to induce proinflammatory cytokine production in DCs but that Hip1-mediated cleavage of GroEL2 abrogates its immunostimulatory capacity.
FIG 2.
Differential stimulation of proinflammatory cytokines from dendritic cells by GroEL2 and GroEL2(cl). We measured levels of the IL-6 and IL-12p40 cytokines produced by C57BL/6 BMDCs 24 h after stimulation with various levels of recombinant GroEL2 or GroEL2(cl). Data are shown as means ± SD of results from one representative experiment of three independent experiments.
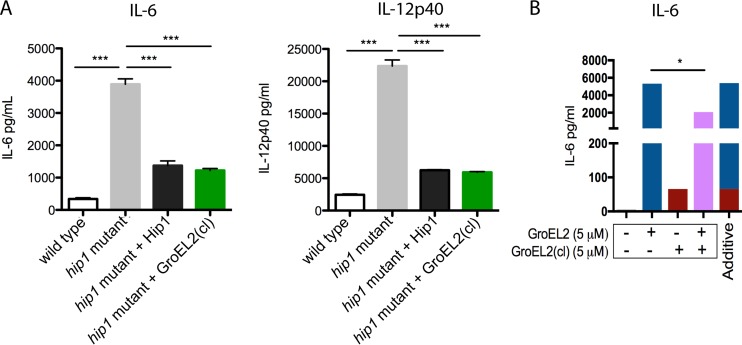
We next investigated whether the Hip1-dependent cleavage of GroEL2 contributes to impaired DC functions during infection in the context of live wild-type and hip1 mutant M. tuberculosis strains. As shown in Fig. 3A, the hip1 mutant, which harbors only FL GroEL2, induced higher levels of the IL-6 and IL-12 cytokines in infected BMDCs than those induced by wild-type M. tuberculosis (Fig. 3) (9). Complementation of the hip1 mutant with Hip1 (hip1 comp) restored wild-type levels of these cytokines (Fig. 3A). To assess the contribution of GroEL2 cleavage to the hip1 mutant phenotype, we used an engineered hip1 mutant strain that ectopically expressed secreted GroEL2(cl). We previously confirmed that the levels of GroEL2(cl) in the supernatant fraction of the hip1 mutant-GroEL2(cl) strain were comparable to those of wild-type M. tuberculosis (7). Importantly, the expression of GroEL2(cl) in the hip1 mutant background restored wild-type levels of IL-12p40 and IL-6 in infected BMDCs, comparable to the levels seen in the hip1 comp strain. These data suggest that the cleavage of GroEL2 in wild-type M. tuberculosis dampens DC cytokine responses. To more directly assess the immunomodulatory capacity of GroEL2(cl), we compared the effects of exposing BMDCs to both GroEL2(cl) and FL GroEL2 together. We found that the levels of cytokines induced by a 1:1 molar ratio of GroEL2 and GroEL2(cl) in combination were lower than those with the additive effect of each individual protein (Fig. 3B). Together, data from these studies suggest that GroEL2(cl) is capable of dampening the stimulatory effect of FL GroEL2.
FIG 3.
GroEL2(cl) restores wild-type levels of proinflammatory cytokine responses in dendritic cells and dampens the stimulatory capacity of full-length GroEL2. (A) We measured levels of the IL-6 and IL-12p40 cytokines produced by C57BL/6 BMDCs 24 h after infection with live M. tuberculosis strains. (B) We incubated C57BL/6 BMDCs with recombinant GroEL2 and GroEL2(cl) either alone (5 μM) or together (5 μM each) for 24 h. The calculated additive effect of GroEL2 and GroEL2(cl) is represented as a sum of the cytokine levels for each protein alone. Data are shown as means ± SD of results of one representative experiment from three independent experiments.
FL GroEL2, but not GroEL2(cl), augments antigen-specific T cell responses.
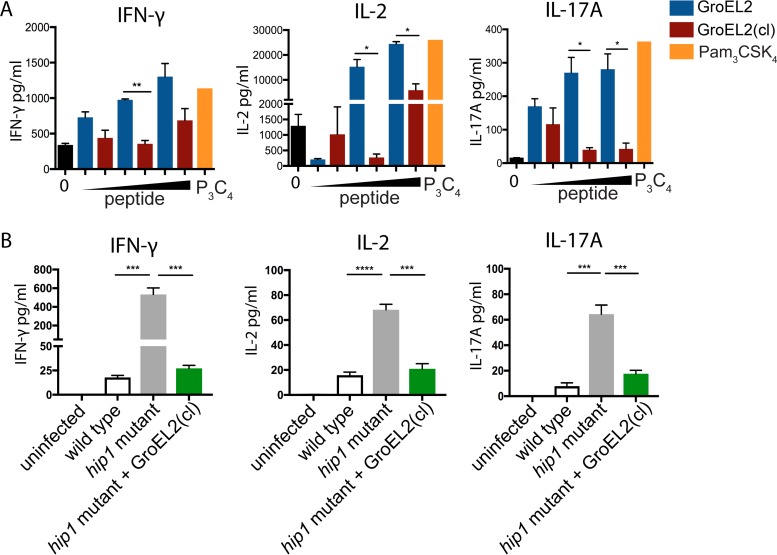
Based on our observation of the differential production of proinflammatory cytokines and expression of costimulatory markers by DCs in response to GroEL2 and GroEL2(cl), we sought to test whether the cleavage of GroEL2 impacted DC antigen presentation to naive antigen-specific CD4+ T cells. We used an in vitro antigen presentation assay involving the coculture of DCs with CD4+ T cells isolated from OT-II mice, which are T cell receptor-transgenic (TCR-Tg) mice, specific for the ovalbumin peptide spanning residues 323 to 339 (OVA323–339 peptide). We first pulsed BMDCs with the OVA323–339 peptide and then exposed DCs to FL GroEL2 or GroEL2(cl) for 24 h. We then cocultured BMDCs with OT-II TCR-Tg CD4+ T cells, collected supernatants 72 h after coculture, and assayed the cells for IFN-γ, IL-2, and IL-17 by an enzyme-linked immunosorbent assay (ELISA) (Fig. 4A). FL GroEL2 but not GroEL2(cl) stimulated the robust presentation of the OVA323–339 peptide, as assessed by the production of IL-2. Furthermore, DCs stimulated with FL GroEL2 induced significantly higher levels of IFN-γ and IL-17 than those for BMDCs stimulated with GroEL(cl). Thus, FL GroEL2 enhanced the capacity of BMDCs both to present antigens to CD4+ T cells and to induce Th1 and Th17 cytokines, consistent with the enhanced levels of IL-12p40 and IL-6 produced by BMDCs exposed to FL GroEL2. In contrast, BMDCs stimulated with GroEL2(cl), which predominates during M. tuberculosis infection, activated CD4+ T cells poorly. Overall, these results suggest that the cleavage of GroEL2 contributes to the modulation of DC-T cell cross talk during M. tuberculosis infection.
FIG 4.
GroEL2 proteolysis modulates DC antigen presentation and T cell polarization. (A) We pulsed DCs with various amounts of the OVA323–339 peptide (1 μg/ml, 10 μg/ml, and 50 μg/ml) for 6 h and then stimulated cells with either recombinant GroEL2 or GroEL2(cl) for 24 h. Following coculture with antigen-specific TCR-Tg CD4+ T cells for 72 h, we assayed cells for the cytokines IFN-γ, IL-2, and IL-17A by an ELISA. (B) We infected DCs with live M. tuberculosis strains and then cocultured them with purified ESAT-6-specific TCR-Tg CD4+ T cells. After 80 h, cell-free supernatants were collected and assessed for the cytokines IFN-γ, IL-2, and IL-17A by an ELISA. Data are shown as means ± SD of results of one representative experiment from three independent experiments.
To further investigate whether GroEL2(cl) modulates antigen-specific T cell responses during live M. tuberculosis infection, we used DC-T cell coculture assays to assess the production of Th1 and Th17 cytokines. We infected BMDCs with wild-type, hip1 mutant, and hip1 mutant-GroEL2(cl) M. tuberculosis strains. To assess the ability of DCs infected with each of these strains to polarize naive M. tuberculosis-specific CD4 T cells toward Th1 and Th17 subsets, we cocultured infected BMDCs with purified ESAT-6 TCR-Tg CD4+ T cells (Fig. 4B). Supernatants were harvested 80 h after coculture and assayed for the IL-17A, IL-2, and IFN-γ cytokines by an ELISA. M. tuberculosis hip1 mutant-infected BMDCs induced elevated levels of the IL-17A, IL-2, and IFN-γ cytokines relative to wild-type M. tuberculosis-infected DCs. However, BMDCs infected with the M. tuberculosis hip1 mutant-GroEL2(cl) strain produced significantly lower IL-17A, IL-2, and IFN-γ cytokine levels, comparable to those of BMDCs infected with wild-type M. tuberculosis. Overall, these data suggest that GroEL2(cl) effectively blunts the magnitude of T cell responses during infection and that cleavage of GroEL2 is a strategy employed by M. tuberculosis to modulate DC-T cell cross talk.
DISCUSSION
While it is known that M. tuberculosis impairs DC functions, the underlying bacterial mechanisms are poorly defined. In this study, we characterized the contribution of FL and cleaved forms of the M. tuberculosis GroEL2 protein in modulating the DC-T cell interface. While most studies on GroEL2 have focused on the ability of the FL protein to modulate innate immune responses, our studies are the first to show that the cleavage of GroEL2 prevents robust DC activation and impacts cross talk between DC and CD4+ T cells (10–20). Since GroEL2 is present predominantly as a cleaved monomeric protein in wild-type M. tuberculosis, these studies provide new insights into the way in which the cleavage of GroEL2 impacts DC-T cell cross talk. Our studies also extend previous findings in macrophages showing that recombinant full-length GroEL2 induces proinflammatory cytokines in a Toll-like receptor (TLR)-dependent manner (7, 21).
Using purified recombinant FL and cleaved GroEL2 proteins, we show that the cleavage of GroEL2 abrogates its immunostimulatory capacity toward DCs and significantly limits the production of key cytokine mediators such as IL-12 and IL-6 (Fig. 2). In these in vitro studies, we considered the biological relevance of the amount of GroEL2 used in our experiments. Because the amounts of GroEL2 that are present during infection are not known, we decided to test a range of protein concentrations. Therefore, in our experiments, we show data for molar concentrations of GroEL2 ranging from 0.1 μM to 10 μM. Based on densitometry analysis of Western blot images, we estimate that the concentration of the GroEL2(cl) protein in M. tuberculosis culture supernatants is in the range of 0.1 μM to 1 μM.
To investigate the role of GroEL2 cleavage during M. tuberculosis infection of DCs, we took advantage of M. tuberculosis bacterial strains that predominantly harbored cleaved GroEL2 (wild-type M. tuberculosis and the complemented hip1 mutant), FL GroEL2 (M. tuberculosis hip1 mutant), or a strain that was engineered to express GroEL2(cl) within the hip1 mutant. We found that the ectopic expression of cleaved GroEL2 in the hip1 mutant background significantly diminished the hyperinflammatory phenotype of the M. tuberculosis hip1 mutant (Fig. 3A). These data suggest that the cleavage of GroEL2 in wild-type M. tuberculosis directly contributes to impaired DC functions during infection and is likely to be a major contributor to the hip1 mutant phenotype. Furthermore, we found that the addition of GroEl2(cl) dampens proinflammatory responses of DCs (Fig. 3B). We also generated a hip1 mutant strain expressing full-length GroEL2, which we wanted to include as an important control. Unfortunately, this strain has a significant in vitro growth defect, suggesting that the presence of high levels of full-length GroEL2 in the hip1 mutant strain is not well tolerated. Because of this growth defect, we were unable to include this strain in our in vitro assays.
The differential functions of GroEL2 and GroEL2(cl) are reminiscent of data from studies on other immunostimulatory heat shock family proteins. Fong et al. reported that heat shock protein 70 (Hsp70), similarly to GroEL2(cl), is secreted from cells and is capable of activating the immunomodulatory Siglec receptors on monocytes and neutrophils (22). Intriguingly, Hsp70 delivers both anti-inflammatory and proinflammatory signals through Siglec activation, pointing to important functional polymorphisms of extracellular Hsp proteins. In the context of these findings, our own data suggest that the two forms of M. tuberculosis GroEL2 are likely to be functionally distinct and that the balance between the amounts of the two forms of GroEL2 during DC infection will influence the type of host immune response generated. We also show that FL GroEL2, but not GroEL2(cl), leads to an upregulation of cell surface-associated costimulatory molecules on DCs such as CD40 and CD86 (Fig. 1) and elicits significantly higher levels of IFN-γ, IL-2, and IL-17 from antigen-specific CD4+ T cells (Fig. 4A). Furthermore, in the context of live infection, during DC-T cell coculture, GroEL2(cl) modulates antigen-specific T cell responses and thus promotes suboptimal immune responses (Fig. 4B). These results are consistent with data from previous reports on the ability of FL M. tuberculosis GroEL2 to enhance antigen cross-presentation during DC-T cell coculture (10). Furthermore, data from biochemical and structural studies indicate that GroEL2 may be involved in promoting antigen presentation. GroEL2 possesses specific protein domains that have the potential to bind peptide substrates, a process that likely facilitates their subsequent association with MHC molecules (23, 24). Together, our results highlight GroEL2 cleavage as a mechanism employed by M. tuberculosis to modulate DC-mediated immunity during infection.
Our studies add insight to a growing body of data implicating GroEL2 in modulating host immune responses to M. tuberculosis infection. Mycobacteria are unusual among bacteria in possessing two GroEL proteins, the cytoplasmic protein GroEL1, which is highly homologous to the Escherichia coli GroEL chaperonin, and GroEL2 (25, 26). While GroEL1 is cytoplasmic, GroEL2 is localized to the cell envelope and secreted extracellularly. Interestingly, GroEL2 has been reported to be among the most abundant M. tuberculosis proteins in vivo and a dominant contributor to the potent immune response elicited by M. tuberculosis purified protein derivative (PPD) (27, 28). Indeed, several studies investigated GroEL2 as a vaccine adjuvant, showing that FL GroEL2 boosts the magnitude of the immune response against infection, thereby improving vaccine-mediated protection against M. tuberculosis (17–20). Those studies show that FL GroEL2 appears to mediate its efficacy via a cellular response dominated by IFN-γ-producing Th1 cells (18). Our results on the functional differences between FL GroEL2 and cleaved GroEL2 add credence to these findings and highlight a potential use for GroEL2 as an immunomodulatory component in strategies aimed at improving vaccine-induced immunity to M. tuberculosis infection.
MATERIALS AND METHODS
Plasmid construction. (i) Plasmids for expression in E. coli.
M. tuberculosis groEL2 was cloned into pACYCDuet-1 (Merck Millipore, Darmstadt, Germany) via the restriction sites EcoRI and KpnI by using the InFusion cloning system according to the manufacturer's protocol. M. tuberculosis groEL2 was amplified by using the primers 5′-GCCAGGATCCGAATTCGATGGCCAAGACAATTGCGTACGAC-3′ and 5′-TTACCAGACTCGAGGGTACCGAAATCCATGCCACCCATGTCGCC-3′, yielding a construct bearing an in-frame N-terminal 6×His tag and a C-terminal S-tag, yielding pACYCDuet-1 GroEL2. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, we previously identified GroEL2 as being cleaved between amino acid positions 12 and 13 at the N terminus to produce the cleaved GroEL2 protein (7). Using this information, we constructed M. tuberculosis groEL2(cl) by using primers 5′-TCCACGGAATTCGGGCCTCGAGCGGGGCTTGAACGCC-3′ and 5′-TCCAGTGGTACCTCAGAAATCCATGCCACCCATGTC-3′, yielding a pACYCDuet-1 GroEL2(cl) construct bearing an in-frame N-terminal 6×His tag and a C-terminal S-tag.
(ii) Plasmids for expression in M. tuberculosis: secreted GroEL2(cl)-Myc.
To express the cleaved form of GroEL2, GroEL2(cl), the groEL2 gene (minus the first 13 amino acids) was amplified from the M. tuberculosis genome by using forward primer 5′-ACGCAGCTGGGCCTCGAGCGGGGCTTGAACGCC-3′ and reverse primer 5′-AGTAAGCTTTCACAGATCTTCTTCAGAAATAAGTTTTTGTTCGAAATCCATGCCACC-3′ and cloned into the PvuII and HindIII sites of pMV762, downstream of the predicted N-terminal signal sequence from M. tuberculosis antigen 85 complex B (NH2-MTDVSRKIRAWGRRLMIGTAAAVVLPGLVGLAGGAATAGA-OH) and an in-frame C-terminal Myc tag.
Expression and preparation of recombinant proteins GroEL2 and GroEL2(cl) from E. coli.
Plasmids pACYCDuet-1 GroEL2 and pACYCDuet-1 GroEL2(cl) were separately transformed into E. coli BL21 Star(DE3) (Invitrogen, Carlsbad, CA) for protein expression. LB broth (1 liter) containing 34 μg/ml chloramphenicol was inoculated with 5 ml of a culture grown overnight and incubated at 37°C to an optical density at 600 nm (OD600) of 0.6 to 0.8. The cells were cooled to room temperature for 15 to 30 min, after which 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added, and the cells were incubated overnight at 28°C. The cells were then centrifuged at 10,000 rpm for 1 h. The cell pellet containing GroEL2 or GroEL2(cl) was resuspended in binding buffer (20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole [pH 7.9], 200 μg/ml lysozyme, 1.8 μg/μl DNase) plus a protease inhibitor cocktail (Santa Cruz Biotechnology, Dallas, TX), sonicated, and centrifuged at 16,000 × g for 90 min to remove cellular debris and clarify the mixture. The soluble fraction was added to Ni2+-charged beads in a gravity column. The cell lysate in the gravity column was first washed with wash buffer 1 (20 mM Tris-HCl, 500 mM NaCl, 60 mM imidazole [pH 7.9]) and then washed with wash buffer 2 (10 mM Tris-HCl) to remove residual salts from the column. To remove endotoxin, the cell lysate was washed with 0.5% ASB-14 (Millipore, Billerica, MA) in 10 mM Tris-HCl. Finally, the lysate was washed with 10 mM Tris-HCl to remove any excess detergent. The protein was eluted with 1 M imidazole in 10 mM Tris-HCl and dialyzed overnight in 1× phosphate-buffered saline (PBS). The protein was further purified by size exclusion chromatography on a GE Superdex 75 10/300 GL column. The purified protein was then concentrated. The endotoxin levels for each protein were <10 ng−1 ml−1 mg−1, as determined by using a Limulus amebocyte lysate (LAL) chromogenic endotoxin quantitation kit (Thermo Scientific, Rockford, IL). Proteins were subjected to SDS-PAGE and visualized as a single band by staining with 0.05% Coomassie blue R-250. The concentrations of purified proteins were determined by the Bradford method (29), using bovine serum albumin (BSA) as the standard.
Bacterial strains and media.
M. tuberculosis H37Rv, the hip1 mutant strain (described previously) (30, 31), and the M. tuberculosis strain expressing GroEL2(cl) were grown at 37°C in Middlebrook 7H9 broth or 7H10 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) (Becton Dickinson, Franklin Lakes, NJ), 0.02% glycerol, and 0.05% Tween 80 (for broth), with the addition of 25 μg/ml kanamycin (Sigma-Aldrich, St. Louis, MO) for the hip1 mutant, and for complemented strains, 10 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO) or 50 μg/ml hygromycin (Roche Diagnostics, Indianapolis, IN) was added.
Mice.
All mice were housed under specific-pathogen-free conditions in filter-top cages within the vivarium at the Yerkes National Primate Center, Emory University, and provided with sterile water and food ad libitum. C57BL/6 mice were purchased from The Jackson Laboratory. OT-II-Tg mice specific for the OVA323–339 peptide, originally generated in the laboratory of F. Carbone (University of Melbourne, Melbourne, VIC, Australia), were bred at the Yerkes animal facility.
Dendritic cells and cytokine assays.
For generating murine BMDCs, bone marrow cells from C57BL/6 mice were flushed from excised femurs and tibias and grown in RPMI 1640 medium (Lonza, Walkersville, MD) with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 2 mM glutamine, 13 µM 2-mercaptoethanol (2-ME), 10 mM HEPES, 1 mM sodium pyruvate, 13 nonessential amino acids, and 20 ng/ml murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN). Incubations were carried out at 37°C with 5% CO2. Fresh medium with GM-CSF was added on days 3 and 6, and cells were used on day 7 for all experiments. We routinely obtained ∼75% CD11c+ CD11b+ cell purity by flow cytometry. BMDCs were further purified by using magnetic beads coupled to a CD11c+ monoclonal antibody (MAb) and passed through an AutoMACS column according to the manufacturer's instructions, where indicated (Miltenyi Biotec, Auburn, CA). For all experiments, cells were maintained in medium containing GM-CSF.
For infection, BMDCs were plated onto 24-well plates (3 × 105 cells per well). Bacteria were filtered through 5-μm filters, resuspended in complete medium containing 20 ng/ml GM-CSF, and sonicated twice for 5 s each before addition to the adherent monolayers. Each bacterial strain was used for infection (in duplicate or triplicate) at a multiplicity of infection (MOI) of 5 or as indicated. Infection of BMDCs was carried out for 4 h, after which monolayers were washed four times with PBS before replacement with RPMI 1640 medium containing 20 ng/ml GM-CSF. To determine intracellular CFU, one set of DCs was lysed in PBS containing 0.5% Triton X-100 and plated onto 7H10 agar plates containing the appropriate antibiotics.
For stimulation of BMDCs with the recombinant GroEL2 protein, endotoxin-free GroEL2 and GroEL2(cl) in supplemented RPMI 1640 medium (as described above) were added to C57BL/6 BMDCs for 24 h. Cell-free supernatants from DC monolayers were isolated at the indicated points and assayed for cytokines by an ELISA using Duo Set kits for IL-12p40, IL-6, and IL-10 (BD Biosciences, San Jose, CA). Assays were carried out according to the manufacturer's instructions. Uninfected BMDCs were used as controls for each experiment.
DC-T cell coculture assays. (i) Live M. tuberculosis strains.
BMDCs were differentiated and plated as described above. BMDCs were infected (in triplicate) with wild-type, hip1 mutant, or hip1 mutant-GroEL2(cl) M. tuberculosis strains at an MOI of 10. Infection of BMDCs was carried out for 4 h, after which monolayers were incubated with amikacin (200 μg/ml; Sigma-Aldrich) for 45 min to kill extracellular bacteria and then washed four times with PBS before incubation for 24 h in complete medium. Following washing, to determine intracellular CFU, one set of DCs was lysed in PBS containing 0.5% Triton X-100 and plated on 7H10 agar plates containing the appropriate antibiotics. On the following day, BMDCs were cocultured with ESAT-6-specific TCR-Tg CD4+ T cells at a 1:4 DC/T cell ratio for 80 h. CD4+ T cells were purified from single-cell suspensions of spleen and lymph nodes from 6- to 8-week-old transgenic mice by using a MACS Miltenyi CD4+ positive selection kit (L3T4). Purified CD4+ T cells showed ≥99% purity by fluorescence-activated cell sorter (FACS) analysis. Cell-free supernatants collected from cocultured cells were analyzed for IFN-γ (Mabtech, Cincinnati, OH), IL-17A (ELISA Ready-Set-Go; eBioscience), and IL-2 (BD Biosciences) by an ELISA according to the manufacturers' instructions.
(ii) Recombinant GroEL2 protein.
CD4+ T cells were purified from single-cell suspensions of spleen and lymph nodes from 6- to 8-week-old OTII-Tg mice by using the EasySep naive CD4+ T cell isolation kit (Stemcell Technologies). BMDCs were incubated in 24-well plates (3 × 105 cells/well) with various amounts of the OVA323–339 peptide (1 μg/ml, 10 μg/ml, and 50 μg/ml) for 6 h and then stimulated with either recombinant GroEL2 (10 μM) or GroEL2(cl) (10 μM) for 24 h. BMDCs were then washed twice with PBS and cocultured with OVA323–339-specific TCR-Tg CD4+ T cells at a 1:4 ratio for 72 h. Supernatants collected from these cells were analyzed for IFN-γ (Mabtech), IL-2 (BD Biosciences), and IL-17A (eBioscience) by an ELISA according to the manufacturers' instructions.
Flow cytometry.
Murine anti-CD11c allophycocyanin (clone N418) and anti-CD11b fluorescein isothiocyanate (FITC) (clone M1/70) were obtained from BioLegend, and anti-CD40 phycoerythrin (PE) (clone 3//23), anti-CD86 PE (clone GL1), and anti-MHC class II PE (clone M5/114.15.2) were purchased from BD Biosciences. Staining for cell surface markers was done by resuspending ∼1 × 106 cells in 200 ml PBS with 2% FBS containing the antibody mixture. Cells were incubated at 4°C for 30 min and then washed with PBS containing 2% FBS. Data were immediately acquired by using an LSR flow cytometer (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, San Carlos, CA).
Statistical analysis.
The statistical significance of data was analyzed by using Student's unpaired t test (GraphPad Prism 5.0a) (*, P < 0.05; **, P < 0.01; ***, P < 0.0002; ****, P < 0.0001). Data are shown as means ± standard deviations (SD) of data from one representative experiment from three independent experiments.
ACKNOWLEDGMENTS
This work was supported by grants 5R01AI083366-05 and 2R56AI083366-06A1 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to J.R.); Yerkes National Primate Center Base grant RR000165; and Center for AIDS Research (CFAR) Immunology Core grant P30AI050409 (to Emory University).
J.R., M.G., J.K.S., E.B., and R.M.-L. conceived and designed the experiments. M.G., J.K.S., E.B., and R.M.-L. performed the experiments. J.R., M.G., J.K.S., and E.B. analyzed the data. J.R. contributed reagents/materials/analysis tools. J.R. and M.G. wrote the manuscript.
REFERENCES
- 1.Ehrt S, Schnappinger D. 2009. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philips JA, Ernst JD. 2012. Tuberculosis pathogenesis and immunity. Annu Rev Pathol 7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 3.Russell DG. 2001. Mycobacterium tuberculosis: here today, here tomorrow. Nat Rev Microbiol 2:569–577. doi: 10.1038/35084527. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 5.Hanekom WA, Mendillo M, Manca C, Haslett PAJ, Siddiqui MR, Barry C III, Kaplan G. 2003. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J Infect Dis 188:257–266. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- 6.Marino S, Pawar S, Fuller CL, Reinhart TA, Flynn JL, Kirschner DE. 2004. Dendritic cell trafficking and antigen presentation in the human immune response to Mycobacterium tuberculosis. J Immunol 173:494–506. doi: 10.4049/jimmunol.173.1.494. [DOI] [PubMed] [Google Scholar]
- 7.Naffin-Olivos JL, Georgieva M, Goldfarb N, Madan-Lala R, Dong L, Bizzell E, Valinetz E, Brandt GS, Yu S, Shabashvili DE, Ringe D, Dunn BM, Petsko GA, Rengarajan J. 15 May 2014. Mycobacterium tuberculosis Hip1 modulates macrophage responses through proteolysis of GroEL2. PLoS Pathog doi: 10.1371/journal.ppat.1004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihret A. 2012. The role of dendritic cells in Mycobacterium tuberculosis infection. Virulence 3:654–659. doi: 10.4161/viru.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan-Lala R, Sia JK, King R, Adekambi T, Monin L, Khader SA, Pulendran B, Rengarajan J. 2014. Mycobacterium tuberculosis impairs dendritic cell functions through the serine hydrolase Hip1. J Immunol 192:4263–4272. doi: 10.4049/jimmunol.1303185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Lu J, Wang L, Gan YH. 2004. Mycobacterial heat shock protein 65 enhances antigen cross-presentation in dendritic cells independent of Toll-like receptor 4 signaling. J Leukoc Biol 75:260–266. doi: 10.1189/jlb.0703341. [DOI] [PubMed] [Google Scholar]
- 11.Cehovin A, Coates AR, Hu Y, Riffo-Vasquez Y, Tormay P, Botanch C, Altare F, Henderson B. 2010. Comparison of the moonlighting actions of the two highly homologous chaperonin 60 proteins of Mycobacterium tuberculosis. Infect Immun 78:3196–3206. doi: 10.1128/IAI.01379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey TB, Thorson LM, Speert DP, Daffe M, Stokes RW. 2009. Mycobacterium tuberculosis Cpn60.2 and DnaK are located on the bacterial surface, where Cpn60.2 facilitates efficient bacterial association with macrophages. Infect Immun 77:3389–3401. doi: 10.1128/IAI.00143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey TB, Ziltener HJ, Speert DP, Stokes RW. 2010. Mycobacterium tuberculosis employs Cpn60.2 as an adhesin that binds CD43 on the macrophage surface. Cell Microbiol 12:1634–1647. doi: 10.1111/j.1462-5822.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Alam K, Mande SC, Valluri VL, Hasnain SE, Mukhopadhyay S. 2008. Mycobacterium tuberculosis heat shock protein 60 modulates immune response to PPD by manipulating the surface expression of TLR2 on macrophages. Cell Microbiol 10:1711–1722. doi: 10.1111/j.1462-5822.2008.01161.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewthwaite JC, Clarkin CE, Coates AR, Poole S, Lawrence RA, Wheeler-Jones CP, Pitsillides AA, Singh M, Henderson B. 2007. Highly homologous Mycobacterium tuberculosis chaperonin 60 proteins with differential CD14 dependencies stimulate cytokine production by human monocytes through cooperative activation of p38 and ERK1/2 mitogen-activated protein kinases. Int Immunopharmacol 7:230–240. doi: 10.1016/j.intimp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Lewthwaite JC, Coates AR, Tormay P, Singh M, Mascagni P, Poole S, Roberts M, Sharp L, Henderson B. 2001. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect Immun 69:7349–7355. doi: 10.1128/IAI.69.12.7349-7355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima KM, Santos SA, Lima VM, Coelho-Castelo AA, Rodrigues JM Jr, Silva CL. 2003. Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther 10:678–685. doi: 10.1038/sj.gt.3301908. [DOI] [PubMed] [Google Scholar]
- 18.Silva CL, Bonato VL, Coelho-Castelo AA, De Souza AO, Santos SA, Lima KM, Faccioli LH, Rodrigues JM. 2005. Immunotherapy with plasmid DNA encoding mycobacterial hsp65 in association with chemotherapy is a more rapid and efficient form of treatment for tuberculosis in mice. Gene Ther 12:281–287. doi: 10.1038/sj.gt.3302418. [DOI] [PubMed] [Google Scholar]
- 19.Silva CL, Lowrie DB. 1994. A single mycobacterial protein (hsp65) expressed by a transgenic antigen-presenting cell vaccinates mice against tuberculosis. Immunology 82:244–248. [PMC free article] [PubMed] [Google Scholar]
- 20.Silva CLP, Pietro RL, Januario A, Bonato VL, Lima VM, da Silva MF, Lowrie DB. 1995. Protection against tuberculosis by bone marrow expressing mycobacterial hsp65. Immunology 86:519–524. [PMC free article] [PubMed] [Google Scholar]
- 21.Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. 2005. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem 280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- 22.Fong JJ, Sreedhara K, Deng L, Varki NM, Angata T, Liu Q, Nizet V, Varki A. 2015. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J 34:2775–2788. doi: 10.15252/embj.201591407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chilukoti N, Kumar CM, Mande SC. 2015. GroEL2 of Mycobacterium tuberculosis reveals the importance of structural pliability in chaperonin function. J Bacteriol 198:486–497. doi: 10.1128/JB.00844-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahar A, Melamed-Frank M, Kashi Y, Shimon L, Adir N. 2011. The dimeric structure of the Cpn60.2 chaperonin of Mycobacterium tuberculosis at 2.8 A reveals possible modes of function. J Mol Biol 412:192–203. doi: 10.1016/j.jmb.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Goyal K, Qamra R, Mande SC. 2006. Multiple gene duplication and rapid evolution in the groEL gene: functional implications. J Mol Evol 63:781–787. doi: 10.1007/s00239-006-0037-7. [DOI] [PubMed] [Google Scholar]
- 26.Dekker C, Willison KR, Taylor WR. 2011. On the evolutionary origin of the chaperonins. Proteins 79:1172–1192. doi: 10.1002/prot.22952. [DOI] [PubMed] [Google Scholar]
- 27.Cho YS, Dobos KM, Prenni J, Yang H, Hess A, Rosenkrands I, Andersen P, Ryoo SW, Bai GH, Brennan MJ, Izzo A, Bielefeldt-Ohmann H, Belisle JT. 2012. Deciphering the proteome of the in vivo diagnostic reagent “purified protein derivative” from Mycobacterium tuberculosis. Proteomics 12:979–991. doi: 10.1002/pmic.201100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Troudt J, Grover A, Arnett K, Lucas M, Cho YS, Bielefeldt-Ohmann H, Taylor J, Izzo A, Dobos KM. 2011. Three protein cocktails mediate delayed-type hypersensitivity responses indistinguishable from that elicited by purified protein derivative in the guinea pig model of Mycobacterium tuberculosis infection. Infect Immun 79:716–723. doi: 10.1128/IAI.00486-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Rengarajan J, Murphy E, Park A, Krone CL, Hett EC, Bloom BR, Glimcher LH, Rubin EJ. 2008. Mycobacterium tuberculosis Rv2224c modulates innate immune responses. Proc Natl Acad Sci U S A 105:264–269. doi: 10.1073/pnas.0710601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan-Lala R, Peixoto KV, Re F, Rengarajan J. 2011. Mycobacterium tuberculosis Hip1 dampens macrophage proinflammatory responses by limiting Toll-like receptor 2 activation. Infect Immun 79:4828–4838. doi: 10.1128/IAI.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]