ABSTRACT
Enterotoxin-producing Clostridium perfringens type A strains cause human gastrointestinal (GI) infections, including a very common food poisoning and 5 to 10% of all cases of antibiotic-associated diarrhea. This bacterium can utilize free sialic acid for growth, but most sialic acids in the GI tract are sequestered on macromolecules, such as the mucin proteins of mucus or glycoconjugates in host cells. However, many C. perfringens strains produce sialidases that might promote growth and survival by generating free sialic acid from those sialyated host macromolecules or by exposing underlying carbohydrates or proteins for digestion by other enzymes. The current study tested that possibility and found that the C. perfringens nonfoodborne human GI disease strain F4969 can use either a mucin preparation or Caco-2 cells, which are human enterocyte-like cells, to support its growth and survival. An isogenic nanI null mutant and complemented strain were used to show that this enhanced growth and survival using mucin or Caco-2 cells involved NanI, which is the major exosialidase of F4969 and many other C. perfringens strains. Experiments also suggested that, at least in part, this growth promotion involves utilization of NanI-generated sialic acid. In addition, a sialidase inhibitor named siastatin B reduced the growth and survival of F4969 growing with either the mucin preparation or Caco-2 cells. These findings suggest that, when produced, NanI may be a significant contributor to C. perfringens human GI infections by promoting the intestinal growth and survival of this bacterium. They also suggest the possibility that sialidase inhibitors might inhibit C. perfringens infections.
KEYWORDS: Clostridium perfringens, sialidase, growth, C. perfringens enterotoxin, sporulation
INTRODUCTION
Clostridium perfringens is a major pathogen of humans and livestock, causing both histotoxic and intestinal infections (1, 2). Toxins are important contributors to all C. perfringens infections (2, 3). This bacterium is capable of producing ∼20 different toxins (2, 4, 5). However, there is considerable variation in toxin production patterns among strains. This diversity is currently used to classify strains into five types (A to E) based upon their ability to produce four typing toxins (alpha, beta, epsilon, and iota toxins) (6).
While not used currently for toxin typing classification, C. perfringens enterotoxin (CPE) ranks among the most biomedically important of all C. perfringens toxins (7, 8). CPE-positive type A strains cause a very common human foodborne disease named C. perfringens type A food poisoning, as well as ∼5 to 10% of all cases of human nonfoodborne gastrointestinal (GI) diseases (8, 9). CPE-associated nonfoodborne diseases include both antibiotic-associated diarrhea and sporadic diarrhea and are thought to involve acquisition of CPE-positive strains from the environment, particularly from hospitals and nursing homes (1, 9). For both CPE-associated foodborne and nonfoodborne human GI diseases, CPE plays a critical role in virulence (8, 10, 11).
CPE-associated foodborne and nonfoodborne human GI diseases are not intoxications but true intestinal infections that involve CPE production following in vivo growth (8, 9). CPE-associated food poisoning is typically an acute disease involving a single round of intestinal growth, sporulation, and CPE production that self-resolves within 24 h (8). As evident by their chronic nature, in which symptoms can persist for several weeks, CPE-associated nonfoodborne GI diseases involve multiple cycles of in vivo growth, sporulation, and CPE production (1, 9).
Intestinal growth and colonization by CPE-positive C. perfringens is poorly understood. However, the major exosialidase NanI is emerging as a potential colonization-promoting factor for several C. perfringens intestinal infections (12). Specifically, while the CPE-positive strains causing type A food poisoning often lack the nanI gene, this gene is typically present in the CPE-positive type A strains causing chronic nonfoodborne human GI diseases (13). In addition, studies have shown that NanI sialidase can facilitate C. perfringens adherence to cultured human enterocyte-like Caco-2 cells (13, 14). That observation suggests that NanI may enhance C. perfringens intestinal adherence, thereby facilitating colonization and contributing to the chronic nature of CPE-associated nonfoodborne human GI diseases.
NanI sialidase may also generate substrates for C. perfringens growth in the GI tract. C. perfringens can use free sialic acids for growth (15–17) via uptake and metabolism that is mediated largely by nan operon-encoded proteins. This may be important for in vivo growth since the human GI tract is rich in sialic acids (18, 19). However, those sialic acids in the GI tract are largely sequestered on host molecules. In particular, mucus is rich in secreted sialic acid-rich mucins, and sialic acids are also located on macromolecules present in host cells, particularly on the cell surface (20–22). Additionally, NanI sialidase might promote C. perfringens growth by removing terminal sialic acids to expose underlying carbohydrates and amino acids for subsequent utilization by this bacterium.
Therefore, the goal of this study was to explore whether, at natural production levels, NanI sialidase can contribute significantly to the growth and survival of a CPE-positive type A C. perfringens nonfoodborne human GI disease strain using either (i) a mucin preparation containing sialyated host macromolecules or (ii) enterocyte-like host cells.
RESULTS
C. perfringens human nonfoodborne human GI disease strain F4969 can use a mucin preparation for growth.
Previous reports demonstrated that C. perfringens strains can use free sialic acid for growth in a semidefined medium (16, 17). However, as mentioned in the introduction, sialic acids in the mammalian GI tract, such as mucins, are sequestered on host macromolecules (19, 20, 23). Therefore, we first tested whether C. perfringens can use GI tract-relevant sialyated host macromolecules for growth and survival by adding an enriched mucin preparation to phosphate-buffered saline (PBS) buffer. The C. perfringens strain used for this analysis was CPE-positive type A strain F4969, a human nonfoodborne human GI disease strain of C. perfringens.
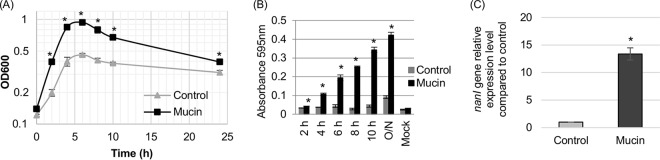
Comparing levels of growth using PBS buffer that was or was not supplemented with the mucin preparation (at a final concentration of 1 mg/ml) revealed that this mucin supplementation significantly increased the growth yield of F4969 at each time point from 2 h to 24 h (Fig. 1A). The limited growth of F4969 observed in PBS without mucin supplementation is likely attributable to two factors. First, cells in the inoculum may have contained sufficient nutrients to support a small amount of growth in this buffer. Second, the addition of 5% Oxyrase solution to all samples used in this study may have facilitated limited C. perfringens growth, since the Oxyrase solution is a complex mixture that may contain some nutrients (L. Stewart, Oxyrase Co., personal communication).
FIG 1.
Comparison of growth yields and supernatant sialidase activities for F4969 in control PBS buffer (Control) and in PBS buffer supplemented with 1 mg/ml mucin (Mucin). (A) F4969 growth yields to 24 h at 37°C in control PBS or PBS with mucin. Every 2 h from 2 to 10 h, as well as at 24 h, the cultures were measured for their OD600. (B) Every 2 h from 2 to 10 h, as well as at 24 h, supernatant sialidase activity was measured in supernatants from F4969 in control PBS versus PBS with mucin. Mock mixtures were uninfected PBS and PBS with mucin. (C) Quantitative RT-PCR analyses of nanI expression levels in F4969 grown in PBS with or without supplementation with mucin (1 mg/ml). Transcript levels were determined with 10 ng of the RNA isolated from 3-h cultures grown at 37°C. Average CT values were normalized to that of the 16S rRNA housekeeping gene, and fold differences were calculated using the comparative CT method (2−ΔΔCT). Values for each bar indicate the calculated fold change from the level in the PBS buffer. Experiments shown in all panels were repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.05 in a comparison with the control as determined by Student's t test.
Supernatant sialidase activities were then compared for F4969 cultures grown from 2 h to 24 h in PBS with or without mucin supplementation (Fig. 1B). Those assays detected, at all time points, significantly higher supernatant sialidase activity when F4969 was grown in PBS with mucin than when it was grown in control PBS. These results showed that the presence of the mucin preparation can increase exosialidase activity for this human enteropathogenic C. perfringens strain.
To assess whether this increase in exosialidase activity involved an induction of nanI expression, nanI expression levels were compared by quantitative reverse transcription-PCR (qRT-PCR) for F4969 cultures grown for 3 h in PBS with and without mucin supplementation (Fig. 1C). Those analyses detected a significant increase in nanI expression in the presence of mucin.
NanI sialidase contributes to C. perfringens strain F4969 growth and survival in mucin-supplemented PBS.
Typical of most CPE-positive type A strains causing nonfoodborne human GI diseases, F4969 produces three sialidases, including two exosialidases (named NanJ and NanI) and a sialidase (named NanH) with an intracellular location in early-log-phase cultures (13). Since (i) NanI is the major exosialidase of F4969 (13), (ii) this strain can use a mucin preparation for growth (Fig. 1), and (iii) addition of the mucin preparation significantly induced F4969 supernatant sialidase activity (Fig. 1), an experiment assessed whether NanI sialidase contributes to the mucin-induced growth and survival of F4969, as well as to the induction of its sialidase activity, observed in Fig. 1.
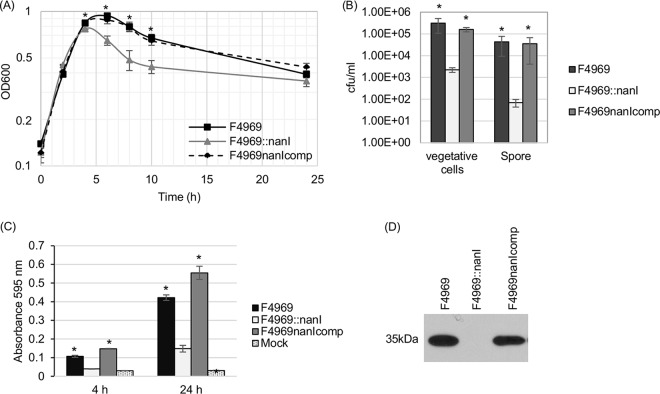
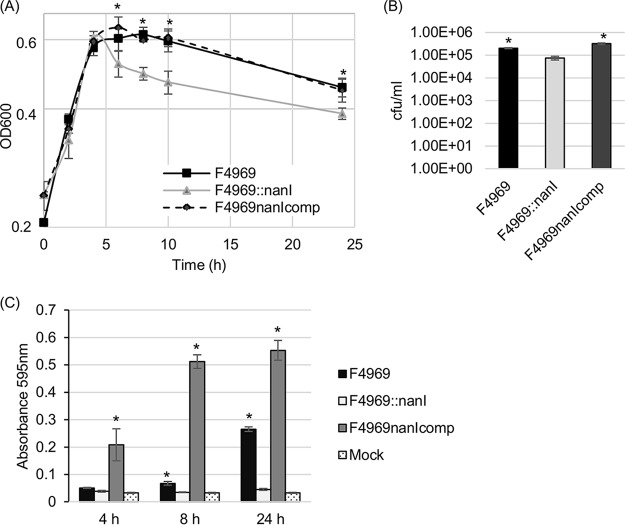
In this experiment, wild-type F4969, a previously prepared F4969 nanI null mutant, and a complemented strain (13) were each cultured in PBS supplemented with mucin, and the culture optical density at 600 nm (OD600) for each culture was then determined at various time points (Fig. 2A). Results demonstrated that, under these conditions, the wild type, complemented strain, and nanI null mutant all grew similarly during early-log-phase growth. However, by 4 h, the nanI null mutant stopped growing, while the wild type and complemented strains continued growing up to 6 h before declining. Over the period from 4 to 10 h of culture, the OD600 values of nanI null mutant cultures were significantly different from the OD600 culture values of the wild type and complemented strains. The OD600 values of all cultures declined between 6 and 10 h. However, over this period, the OD600 culture values for F4969 and the complemented strain remained significantly higher than those of the nanI mutant. Those results strongly suggested that, at natural production levels, NanI not only increases F4969 growth in the presence of mucin but also promotes the survival of this strain under the tested culture conditions.
FIG 2.
Comparison of growth yields, sialidase activities, and levels of vegetative-cell survival, spore formation, and CPE production by F4969, an isogenic nanI null mutant, and a complemented strain cultured in PBS with 1 mg/ml of the mucin preparation (F4969nanIcomp). (A) Culture of F4969, the nanI null mutant, and a complemented strain grown to 24 h at 37°C in PBS buffer with mucin. At designated time points, the OD600s of cultures were determined. (B) Quantification of viable vegetative cells and heat-resistant-spore formation by the wild type, a nanI null mutant, and a complemented strain grown in PBS supplemented with mucin. After the bacteria were cultured for 24 h at 37°C, those cultures were then plated onto BHI agar plates with 10-fold serial dilutions using distilled water. For spore counting, the culture was first heat shocked for 20 min at 70°C. After 10-fold serial dilutions with distilled water were performed, the heat-shocked cultures were plated onto BHI agar plates. These BHI agar cultures were grown anaerobically overnight at 37°C for colony counting (log10 scale). (C) Supernatant sialidase activity was measured in supernatants from F4969, the nanI null mutant, or a complemented strain grown at 37°C for 4 or 24 h in PBS buffer supplemented with mucin. Mock, uninoculated PBS buffer supplemented with mucin. All experiments in panels A to C were repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.05 in a comparison with the nanI null mutant strain by ordinary one-way ANOVA. (D) CPE Western blot performed with the supernatant from the overnight culture supernatant of the wild type, nanI null mutant, or complemented strain grown in PBS supplemented with mucin. The molecular mass is indicated on the left.
By 24 h of culture in mucin-supplemented PBS, the OD600s of all three strains did not statistically differ. Therefore, aliquots of all three 24-h, mucin-supplemented cultures were plate counted to compare levels of vegetative-cell viability. The results (Fig. 2B) showed that, although the OD600s of these three cultures were similar at this time point, ∼100-fold-more viable vegetative cells were present in the 24-h, mucin-supplemented cultures of the wild-type and complemented strains than in the nanI null mutant strain culture, further supporting the ability of mucin to enhance the survival of F4969 bacterial cells.
While only the NanI and NanJ exosialidases are present in supernatants from young (<6-h) cultures of C. perfringens strains, supernatants from >6-h cultures of these strains can also contain some released NanH (24). Therefore, supernatant sialidase activity was assessed at both the 4-h and 24-h time points in cultures (Fig. 2A). Those analyses demonstrated (Fig. 2C) that at both time points, changes in NanI activity levels were responsible for most of the increased supernatant sialidase activity induced by mucin supplementation. However, in the presence of the mucin preparation, there was still some increase in supernatant sialidase activity for the nanI null mutant between 4 h and 24 h, indicating that the presence of other sialidases in F4969 culture supernatants can also be induced by mucin supplementation.
Our previous study showed that, in relatively rich modified Duncan strong (MDS) sporulation medium, the F4969::nanI mutant produced significantly more CPE and sporulated better than wild-type F4969 (13). In contrast, the current study determined that significantly less CPE is produced in mucin-supplemented cultures by the nanI null mutant strain than by the wild-type or complemented strains (Fig. 2D). Since CPE is produced only by sporulating cells (25), spore formation levels were evaluated for F4969, and its isogenic derivatives were cultured in PBS with mucin supplementation. The results (Fig. 2B) showed that, in mucin-supplemented PBS, spore production levels for the F4969::nanI mutant were significantly lower than sporulation levels for the wild-type and complemented strains. Much of this reduction in sporulation levels is related to lower total growth yields. However, NanI also appears to have some direct effect on sporulation levels since, relative to wild-type F4969, the nanI mutant exhibited a 3-fold-stronger reduction in sporulation levels versus total growth.
Given that CPE production is strongly associated with spore formation (25), these reduced sporulation levels explain why CPE production by the nanI null mutant strain was significantly less than that by the wild type or complemented strain when they were grown in mucin-supplemented PBS (Fig. 2D). Considering the poor survival of F4969::nanI mutant vegetative cells observed in Fig. 2B in mucin-supplemented cultures, the reduced amount of F4969::nanI mutant sporulation and CPE production in mucin-supplemented cultures relative to those in MDS medium cultures suggests that many cells of this strain die in the nutrient-poor, mucin-supplemented medium before they can produce CPE and complete sporulation.
Effects of the sialidase inhibitor SB on F4969 growth, survival, sporulation, and CPE production.
Two sialidase inhibitors named siastatin B (SB) and N-acetyl-2,3-dehydro-2-deoxyneuraminic acid (NADNA) were previously shown to inhibit sialidase activity when added to cell-free supernatants of F4969 cultures (13). However, NADNA was unable to inhibit sialidase activity efficiently in the presence of C. perfringens cells (26). Therefore, the current study used only the SB sialidase inhibitor (27) in an attempt to confirm the Fig. 2 results by assessing whether inhibition of sialidase activity can also reduce F4969 growth and CPE production.
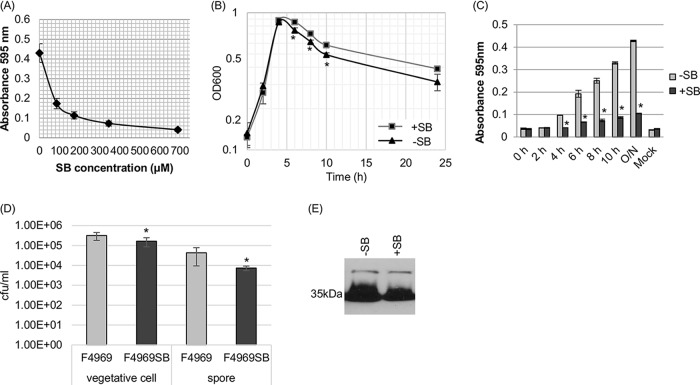
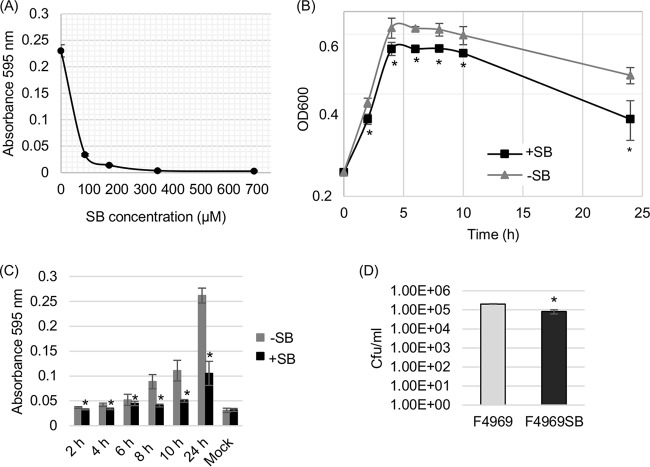
First, the 50% inhibitory concentration (IC50) of the sialidase inhibitor SB was determined for supernatants of overnight F4969 cultures grown in PBS buffer with mucin. The inhibition curve (Fig. 3A) was then used to calculate the IC50 of SB (70 μM) under this culture condition. For comparison against results of our previous study (13), this calculated IC50 is higher than the IC50 dose needed for supernatants from an overnight Todd-Hewitt (TH) culture of F4969 (41 μM). Using this calculated IC50 of SB for mucin-supplemented PBS, the growth of F4969 was then assayed in the presence of PBS containing mucin plus a final 5× IC50 (350 μM) of the SB sialidase inhibitor (Fig. 3B). The result obtained revealed a significant difference between the OD600 values of mucin-supplemented F4969 cultures grown for 6 to 10 h in the presence and the absence of SB.
FIG 3.
Effects of the siastatin B (SB) sialidase inhibitor on growth, sialidase activity, vegetative-cell survival, spore formation, and CPE production by F4969 cultured in PBS with a 1-mg/ml concentration of the mucin preparation. (A) Supernatant sialidase activity of wild-type F4969 grown for 24 h in the absence or presence of various concentrations of the SB sialidase inhibitor. (B) F4969 cultured for 24 h at 37°C in PBS with mucin that did (+SB) or did not (−SB) contain a final 5× IC50 of SB. At designated time points, the OD600s of those cultures were determined. (C) Sialidase activity of wild-type F4969 grown to 24 h (overnight [O/N]) at 37°C in PBS with mucin that did (+SB) or did not (−SB) contain a final 5× IC50 of SB. At designated time points, the sialidase activities of cultures were measured. Mock, an uninfected control. (D) Quantification of vegetative cells and heat-resistant-spore formation by wild-type F4969 grown in PBS with mucin that did or did not contain a final 5× IC50 of SB. After the bacteria were cultured for 24 h at 37°C, those cultures were then plated onto BHI agar plates, using 10-fold serial dilutions in distilled water. For spore counting, the cultures were first heat shocked for 20 min at 70°C. After 10-fold serial dilutions with distilled water, the heat-shocked cultures were plated onto BHI agar plates. These BHI agar plates were then grown anaerobically overnight at 37°C for colony counting (log10 scale). All results in panels A to D are the average results from three repetitions. Error bars depict standard deviations. *, P < 0.05 in a comparison with the wild-type strain by Student's t test. (E) Western blot analysis of CPE in supernatants of F4969 cultures grown for 24 h in PBS with a mucin preparation that did (+SB) or did not (−SB) contain a final 5× IC50 of SB. The molecular mass is indicated on the left.
Supernatant sialidase activities in mucin-supplemented PBS cultures of F4969 were also evaluated in the presence versus the absence of SB, with measurements taken every 2 h postinoculation from 2 h to 10 h and then at 24 h. The results, shown in Fig. 3C, indicated that sialidase activities in supernatants from SB-treated cultures of F4969 were significantly lower at time points of >4 h than those of supernatants from equivalent F4969 cultures without SB. Both vegetative cells and spores were counted in the overnight cultures, and the results (Fig. 3D) showed that SB can significantly reduce vegetative-cell viability, spore formation, and CPE production (Fig. 3E) in mucin-supplemented cultures.
Caco-2 cells support C. perfringens intestinal strain F4969 growth.
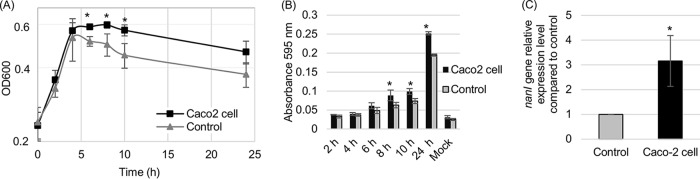
Besides mucus, sialoglycoconjugates are found in host cells of the GI tract (28). Therefore, the current study next evaluated whether human enterocyte-like Caco-2 cells can support the growth yields of F4969. For this purpose, we used a Transwell 0.4-μm-pore-size membrane filter support system (see Materials and Methods) that permits diffusion of proteins (including NanI), but not C. perfringens, between the upper and lower chambers of each Transwell. Those Transwells were prepared so that the lower chamber contained either PBS alone (no host cells) or PBS covering a Caco-2 cell monolayer. When F4969 was added to the upper chamber of this system, the presence of Caco-2 cells significantly promoted the growth of F4969 during the time period from 6 h to 10 h (Fig. 4A).
FIG 4.
Comparison of growth yields and supernatant sialidase activities for F4969 grown in PBS buffer (Control) and grown in PBS buffer supplemented with Caco-2 cells (Caco-2 cell). (A) F4969 growth yields to 24 h at 37°C in PBS buffer (Control) or PBS buffer with Caco-2 cells (Caco-2 cell) in 12-well Transwell culture clusters (see Materials and Methods). Every 2 h from 2 to 10 h, as well as at 24 h, the culture OD600 was measured. (B) Every 2 h from 2 to 10 h, as well as at 24 h, supernatant sialidase activities were measured in supernatants from F4969 in control PBS and in PBS buffer with Caco-2 cells. Mock, uninfected control PBS buffer or PBS buffer with Caco-2 cells. (C) Quantitative RT-PCR analyses of nanI expression levels in F4969 grown in PBS with or without Caco-2 cells. Transcript levels were determined using 10 ng of RNA isolated from 3-h cultures grown at 37°C. Average CT values were normalized to the value for the 16S rRNA housekeeping gene, and fold differences were calculated using the comparative CT method (2−ΔΔCT). The value for each bar is the calculated fold change from the value with PBS buffer. Experiments in all panels were repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.05 in a comparison with the control by Student's t test.
Supernatant sialidase activities in the upper chambers of these Transwells were assayed every 2 h from 2 h to 10 h, as well as at 24 h (Fig. 4B). At time points of >6 h, we detected significantly higher sialidase activities in Transwells whose lower chambers contained both PBS and Caco-2 cells than in control Transwells whose lower chambers contained only PBS (no Caco-2 cells). These results showed that, in addition to suspensions of sialyated host macromolecules (as found in mucus), enterocyte-like host cells can promote growth yields and induce exosialidase activity for this human enteropathogenic strain of C. perfringens.
To assess whether this increase in exosialidase activity noted in Fig. 4 was due to induction of nanI expression, nanI expression levels were compared by qRT-PCR of F4969 cultures grown for 3 h in PBS or PBS with Caco-2 cells (Fig. 4C). Those analyses detected a significant increase in nanI expression in the presence of Caco-2 cells, although this enhanced nanI transcription did not reach the levels detected when F4969 was cultured in PBS with mucin.
NanI sialidase is a significant contributor to C. perfringens F4969 growth and survival in the presence of Caco-2 cells.
For the results shown in Fig. 2, a nanI mutant was used to establish that NanI, the major exosialidase of F4969, is a significant contributor to the growth and survival of this strain when mucin-supplemented PBS is used. Therefore, a similar experiment was performed to assess NanI contributions to F4969 growth and survival in the presence of Caco-2 cells (Fig. 5). The nanI null mutant strain grew similarly to wild-type F4969 and a complemented strain during the early-log-phase growth stage. However, by 6 h of culture in the presence of Caco-2 cells, the OD600 value of nanI null mutant cultures began to decrease, while the OD600 values of the wild-type and complemented-strain cultures continued to increase. In the presence of Caco-2 cells, the OD600 values of the nanI null mutant cultures remained significantly lower from 6 h up to 24 h than those of similar cultures of the wild-type and complemented strains. These results indicated that NanI significantly promotes F4969 growth and survival with Caco-2 cells, as well as with a mucin preparation.
FIG 5.
Comparison of levels of growth, sialidase activity, and vegetative-cell survival by F4969, an isogenic nanI null mutant, or a complemented strain in PBS with Caco2 cells. (A) Cultures of F4969, a nanI null mutant, or a complemented strain were grown to 24 h at 37°C in PBS with Caco-2 cells. At designated time points, the OD600s of cultures were determined. (B) Quantification of vegetative cells in cultures of the wild type, nanI null mutant, or complemented strain cultured in PBS supplemented with Caco-2 cells. After 24 h of growth at 37°C, aliquots of each culture were plated onto BHI agar plates in 10-fold serial dilutions with distilled water (log10 scale). (C) The sialidase activity in the supernatant was measured in F4969, the nanI null mutant, and the complemented strain grown at 37°C for 4, 8, or 24 h in PBS buffer with Caco-2 cells, as indicated. Mock, uninfected PBS buffer with Caco-2 cells. All experiments were repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.05 in a comparison with the nanI mutant by ordinary one-way ANOVA.
Plate counting was used to then compare levels of vegetative-cell viability in 24-h cultures of the wild type, nanI null mutant, and complemented strain when they were grown in the presence of Caco-2 cells. The results (Fig. 5B) showed that there were about three times more viable cells of the wild-type and complemented strains than of the nanI null mutant, and those differences were statistically significant.
Sialidase activity was evaluated in supernatants from F4969 cultures grown for 4, 8, or 24 h in the presence or absence of Caco-2 cells (Fig. 5C). Unlike the mucin supplementation results shown in Fig. 2, sialidase activities in the 4-h culture supernatant were not significantly different between the wild-type and nanI null mutant strains. However, the sialidase activities in 8-h or 24-h supernatants from wild-type and complemented-strain cultures grown with Caco-2 cells did show a significant increase compared to those in equivalent cultures of the null mutant strain. At 24 h, this resulted in a 23% increase in sialic acid generation from Caco-2 cells for the wild type over that in the nanI null mutant. Those results also revealed that, in 8- or 24-h cultures, NanI activity was responsible for most of the increased supernatant sialidase activity induced by the presence of Caco-2 cells. Furthermore, although sialidase activity of the nanI null mutant strain increased over time in mucin-supplemented medium (Fig. 2), it did not increase in the presence of Caco-2 cells, indicating that NanJ and NanH can be induced by the presence of mucin but not by the presence of Caco-2 cells. Only NanI of F4969 can be induced by exposing it to Caco-2 cells, which is similar to results of a previous report using strain CN3718 (14).
In the cultures of F4969 or its isogenic derivatives grown in the presence of Caco-2 cells, neither spore formation nor CPE production were detected (data not shown).
Effects of the SB sialidase inhibitor on F4969 growth and survival.
To confirm the results shown in Fig. 5, an experiment tested whether the SB sialidase inhibitor also reduces F4969 growth and survival in the presence of Caco-2 cells. First, the IC50 of the SB sialidase inhibitor was determined for supernatants collected from F4969 cultures grown overnight in the presence of Caco-2 cells. The resultant inhibition curve (Fig. 6A) was used to determine the IC50 of SB (47 μM) under this culture condition, which is lower than the IC50 (70 μM) needed for supernatants from overnight cultures of F4969 grown in the presence of mucin. After determining this IC50, the growth of F4969 with Caco-2 cells was assayed in the presence of a final 5× IC50 (235 μM) of the SB sialidase inhibitor (Fig. 6B). The results revealed that SB caused a significant reduction in the culture's OD600 from the >2-h up to the 24-h time points when F4969 was grown in the presence of Caco-2 cells.
FIG 6.
Effects of a sialidase inhibitor on the growth, sialidase activity, and vegetative-cell survival of F4969 grown in PBS with Caco-2 cells. (A) Supernatant sialidase activities of wild-type F4969 grown overnight (24 h) in PBS with Caco-2 cells with or without different doses of the SB sialidase inhibitor. (B) Culture of F4969 grown to 24 h at 37°C in PBS with Caco-2 cells (−SB) or in PBS with Caco-2 cells plus a final 5× IC50 of SB (+SB). At the designated time points, the culture OD600 was determined. (C) Sialidase activity of wild-type F4969 grown to 24 h at 37°C in PBS with Caco-2 cells (−SB) or in PBS with Caco-2 cells containing a final 5× IC50 of SB (+SB). Mock, uninfected Caco-2 cells. At designated time points, the sialidase activities of cultures were measured. (D) Quantification of vegetative cells of the wild type in PBS with Caco-2 cells, with or without the presence of a final 5× IC50 of SB. After the bacteria were cultured for 24 h at 37°C, those cultures were then plated onto BHI agar plates in 10-fold serial dilutions, using distilled water. These BHI agar plates were grown anaerobically overnight at 37°C for colony counting (log10 scale). Shown are the average results from three repetitions. Error bars depict standard deviations. *, P < 0.05 in a comparison with the control (without SB supplied) by Student's t test.
The effects of SB treatment on supernatant sialidase activities when F4969 was grown in the presence of Caco-2 cells was also evaluated every 2 h from 2 h to 10 h, as well as in an overnight 24-h culture. The results, shown in Fig. 6C, indicated that after 2 h of culture, supernatant sialidase activity was significantly lower when SB was supplied.
When viable vegetative cells were counted in those overnight cultures, the results (Fig. 6D) showed that SB had significantly reduced vegetative-cell viability.
Evidence that sialic acid generated by NanI from mucin or Caco-2 cells can impact F4969 growth.
An experiment was then performed to evaluate whether the amount of sialic acid generated by NanI levels present in cultures containing mucin or Caco-2 cells can enhance F4969 growth. For this purpose, 0.11 U/ml (corresponding to the sialidase activity present in F4969 cultures grown for 4 h in the presence of mucin or 8 h in the presence of Caco-2 cells) of NanI was added to PBS containing mucin or Caco-2 cells for 6 h at 37°C. This incubation released a 54 or 24 μM concentration of free sialic acid from mucin or Caco-2 cells, respectively. Therefore, F4969 or F4969::nanI was cultured for 3 h at 37°C in PBS with mucin or in PBS with Caco-2 cells. After that incubation, F4969::nanI growing in the mucin-supplemented or Caco-2 cell-containing culture was or was not fed, respectively, 54 μM or 24 μM sialic acid for 3 more hours of growth. Results shown in Fig. 7 demonstrated that supplementation of the F4969::nanI cultures with these sialic acid concentrations did significantly enhance growth.
FIG 7.

Comparison of 6-h growth yields for F4969, F4969::nanI, and sialic acid-fed F4969::nanI in PBS buffer supplemented with 1 mg/ml mucin or Caco-2 cells. (A) After F4969 or an isogenic nanI null mutant was grown for 3 h at 37°C in PBS with mucin, F4969::nanI was fed a final concentration of 54 μM sialic acid, while control cultures of F4969 or F4969::nanI were fed with equivalent volumes of ddH2O. After 3 h of additional culture, the OD600s of the cultures were determined. (B) After F4969 or an isogenic nanI null mutant were grown for 3 h at 37°C in PBS with Caco-2 cells. F4969::nanI was fed a final concentration of 24 μM sialic acid, while control F4969 and F4969::nanI cultures were fed an equal volume of ddH2O. After 3 h of additional culture, the OD600s of cultures were determined. Shown are the average results from three repetitions. Error bars depict standard deviations. *, P < 0.05 in a comparison with the wild-type control F4969 by ordinary one-way ANOVA.
DISCUSSION
Clostridium perfringens can grow in the intestines, whether as a member of the normal intestinal microbiota or as an important intestinal pathogen of humans and other animals (1, 9, 29). Growth in the intestines is challenging for bacteria. For example, glucose concentrations in the mammalian small intestine vary greatly, spanning from 0.2 mM up to 48 mM (30). In the lower small intestine and large intestine, there is also intense nutrient competition with other intestinal microbiota (31). Also, during intestinal disease, the purging action of diarrhea can reduce luminal nutrient concentrations.
One strategy for addressing intestinal nutrient limitations is to produce sialidases (19). This approach is used both by normal microbiota species and by some intestinal pathogens. For nonfoodborne human GI disease C. perfringens strains like F4969, NanI is the major exosialidase (13). The presence of sialidases like NanI in the intestinal lumen then generates sialic acid from sialyated host macromolecules in host secretions like mucus, as well as on the cell surface glycocalyx. If sufficient amounts of sialic acid are generated, this might facilitate the intestinal growth of bacteria, like C. perfringens, that can directly metabolize free sialic acid (16, 17). However, prior to the current study, it had not been determined whether C. perfringens can use its exosialidases at natural production levels to generate sufficient sialic acid for growth or survival. Additionally, sialidases may remove terminal sialic acids and expose underlying carbohydrates or amino acids for release by other C. perfringens glycoside hydrolases or proteases, with subsequent utilization of those nutrients by this bacterium.
This study now provides proof-of-principle results indicating that C. perfringens nonfoodborne human GI disease strain F4969 can use soluble sialyated, GI tract-relevant host macromolecules or mammalian cells to support its growth and survival. Furthermore, using an isogenic nanI mutant, it was established that this enhancement involves, at natural production levels, the major sialidase NanI. Results using the SB inhibitor supported this conclusion, although the growth differences were less prominent than in the nanI mutant, likely because some NanI activity still remained in the SB culture. In addition, it was shown that this growth and survival enhancement by NanI involves, at least in part, the generation and use of free sialic acid from the sialyated macromolecules in the mucin preparation or in host cells. The possibility that removal of terminal sialic acids by NanI also exposes underlying carbohydrates and amino acids for release and utilization cannot be excluded.
Growth curve comparisons between cultures of wild-type F4969 and its isogenic nanI mutant indicated that NanI is not a major contributor to the early-log-phase growth of this strain using the mucin preparation or Caco-2 cells. Presumably, during this period there are other immediately utilizable energy substrates available under these culture conditions so that sialidases are not needed. Consistently with this hypothesis, limited NanI production was detected in the early-log-phase growth of these cultures. However, once these cultures entered late log phase and encountered more-limiting nutrient availability, F4969 apparently sought alternative nutrients, in part by increasing production of sialidases, particularly NanI. This generates free sialic acid and possibly allows other enzymes to generate underlying carbohydrates and amino acids for growth. The involvement of sialic acid release and utilization in late-log-phase growth is supported by the sialic acid supplementation results of Fig. 7. Also consistent with sialic acid release contributing to growth are the greater growth differences observed between F4969 and the wild-type nanI null mutant using mucin versus Caco-2 cells, since >2-fold-more sialic acid was released by NanI with mucin than with Caco-2 cells.
Recent studies (16) provide insights into this regulation of sialidase production in the presence of nutrient-rich versus nutrient-poor environments. Those studies showed that a glucose-rich environment represses NanI production. However, as glucose is consumed, C. perfringens upregulates its production of sialidases, particularly NanI. This probably involves the formation of a metabolic intermediate (likely phosphorylated ManNAc) that removes repression by a regulator named NanR (16, 17). However, once high concentrations of free sialic acid are achieved, C. perfringens again shuts off production of NanI, since that enzyme is no longer required.
Other factors may also work in concert with NanI to enhance C. perfringens growth using sialyated macromolecules present in host cells. It has already been mentioned that other glycoside hydrolases or proteases may free underlying carbohydrates or amino acids after NanI removes terminal sialic acids from sialyated host molecules. In addition, the current study noted (data not shown) destruction of Caco-2 cells after 6 h of infection with cultures of F4969 or its derivatives. This effect is not due to NanI, because it was also noted using the F4969 nanI mutant. Instead, it is likely attributable to the destructive effects of other toxins and exoenzymes made by F4969 and its isogenic derivatives. Destruction of Caco-2 cells in these cultures may facilitate contact of NanI with sialyated host molecules located both on the cell surface and inside these cells; e.g., glycoproteins are synthesized in the Golgi bodies (32).
Extrapolating our previous in vitro observations (16) to the intestines suggests the following model. When readily utilized nutrients like glucose are abundant in the intestinal lumen, small amounts of NanI are produced. However, in a more nutrient-limiting environment, as found in the lower small intestine and large intestine, NanI production increases. This enzyme then enhances the adherence of C. perfringens to the intestinal mucosa. It also permits this bacterium to begin generating sialic acid from host macromolecules in mucin and in host cells. That sialic acid is then metabolized, perhaps along with other free carbohydrates and amino acids, which allows additional growth and survival of C. perfringens in the intestines.
Nonfoodborne human GI disease strains typically cause more-prolonged disease (lasting up to several weeks), while C. perfringens type A food poisoning is usually an acute disease (9). The observations that nonfoodborne GI disease strains typically produce NanI and that most food poisoning strains do not produce this enzyme offer one potential explanation for these differences in disease duration. Specifically, NanI may play an important role in intestinal adherence, as needed for colonization, and may also promote growth and survival when nutrient conditions are poor. This hypothesis now requires in vivo testing using animal models.
MATERIALS AND METHODS
Chemicals and bacterial media.
A partially purified mucin preparation (mucin content, ∼64%) from porcine gastric mucosa was purchased from My BioSource. The sialidase activity substrate 5-bromo-4-chloro-3-indolyl-α-d-N-acetylmuramic acid sodium salt was purchased from Santa Cruz. The sialidase inhibitor siastatin B was purchased from Sigma-Aldrich. Oxyrase for broth (Oxyrase, Inc.), chloramphenicol (Cm), and general chemicals used in this study were purchased from Fisher Scientific Company.
All C. perfringens strains were stored as stock cultures in cooked-meat medium (CMM; Oxoid) at −20°C. Rich media used in this study for culturing C. perfringens included fluid thioglycolate (FTG) medium (Difco Laboratories), TGY medium (3% tryptic soy broth [Becton, Dickinson], 2% glucose [Fisher scientific], 1% yeast extract [Becton, Dickinson], and 0.1% sodium thioglycolate [Sigma-Aldrich]), and brain heart infusion (BHI) agar plates (Becton, Dickinson).
Caco-2 cell culture.
Caco-2 cells were cultured in 10× Eagle minimal essential medium (EMEM; Lonza), supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 1% nonessential amino acids (Sigma-Aldrich), and 100 μg/ml of penicillin-streptomycin (Fisher Scientific Company). The cells were grown at 37°C with 5% atmospheric CO2 in a 12-cluster plate.
C. perfringens strains and growth conditions.
The wild-type C. perfringens strain used in this study was F4969, a type A human nonfoodborne human GI disease strain (13). An isogenic F4969 nanI null mutant strain (F4969::nanI) and a complemented strain (F4969nanIcomp), which carries the nanI gene cloned into the Escherichia coli/C. perfringens shuttle vector pJIR750, was prepared and characterized in a previous study (13).
For the growth of C. perfringens strains, a 0.2-ml aliquot of each CMM stock culture was inoculated into 10 ml of fresh FTG medium or, for F4969nanIcomp, FTG medium supplemented with 15 mg/liter Cm. After overnight culture at 37°C, a 0.2-ml aliquot of this started culture was then transferred into 10 ml of fresh TGY medium (or TGY medium supplemented with Cm for F4969nanIcomp), and those cultures were grown overnight at 37°C.
A mucin preparation stock solution (5 mg/ml) was prepared with double-distilled water (ddH2O), and the pH was adjusted to 7.0. The solution was filtered on number 1 Whatman filter paper, and the filtered stock solution was autoclaved and then stored at 4°C until use. To test the ability of the mucin preparation to support the growth and survival of the C. perfringens strains, each strain was inoculated into PBS buffer supplemented or not supplemented with 1 mg/ml of the mucin preparation. To prepare those culture media, 5% Oxyrase for broth was added, and before inoculation, the media were preincubated for 30 min at 37°C to create anaerobic conditions. For the inoculation, a 40-μl aliquot of a TGY overnight (∼16-h) culture was added into 1 ml of PBS with or without the mucin preparation. The cultures were then grown at 37°C before growth measurements and sialidase activity measurements were performed as described below.
To assess C. perfringens growth in the presence of Caco-2 cells, 12-well Corning Transwell plates with a polyester membrane containing 0.4-μm pores were used. Monolayers of Caco-2 cells were cultured in the lower Transwell chamber for ∼2 weeks. After two washes of the lower chamber with PBS buffer, a 1.5-ml aliquot of PBS buffer was added. In the upper Transwell insert chamber, an aliquot of 0.6 ml PBS buffer with 5% Oxyrase for broth was added. The Oxyrase-containing medium had been preincubated for 30 min at 37°C to create anaerobic conditions. A 40-μl aliquot of a TGY overnight (∼16-h) culture was added into the 0.6-ml volume of PBS with Oxyrase that was present in the Transwell insert, and the cultures were incubated at 37°C for growth and sialidase activity measurements as described below.
Control experiments (results not shown) were performed to confirm that two washes of Caco-2 monolayers were sufficient to remove any contaminating medium constituents. When the experiments were repeated using up to four washes of the lower chamber, similar results were obtained. Furthermore, little sialic acid was present in the 2nd to 4th washes, and those washes did not support any additional C. perfringens growth beyond that observed using sterile PBS.
Measurement of C. perfringens growth and supernatant sialidase activity.
For growth curve analyses, each inoculated well was used to measure culture density at a single time point, either 0, 2, 4, 6, 8, 10, or 24 h. For this purpose, the cultures were removed for optical density measurement at 600 nm (OD600) using a Bio-Rad SmartSpec microplate reader. Supernatants of these same cultures were also tested for sialidase activity. For this analysis, a 60-μl aliquot of the supernatant was added to a 40-μl aliquot of the substrate (4 mM 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid), and the mixture was then incubated at 37°C for 30 min. After that incubation, the absorbance at 595 nm was measured using a Bio-Rad microplate reader.
Sialidase inhibitor effects on F4969 growth in PBS with the mucin preparation or Caco-2 cells.
A 60-μl aliquot of the supernatant from a 24-h culture of F4969 grown in PBS with the mucin preparation or Caco-2 cells was mixed with PBS buffer that did or did not contain the SB sialidase inhibitor (0, 86.4, 172.7, 345.4, or 690 μM). A 40-μl aliquot of a 4 mM concentration of the 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid sialidase activity substrate was then added to these mixtures, and the total volume was adjusted to 125 μl using 0.05 M Tris buffer (pH 7.2). After these samples were incubated at 37°C for 30 min, sialidase activity was determined by measuring the absorbance at 595 nm using a microplate reader. Based upon sample absorbance at 595 nm, the 50% inhibitory concentration (IC50) for each culture condition was then calculated using the Excel linear-regression method. Those 5× IC50 doses were then used to evaluate whether this sialidase inhibitor can affect F4969 growth under each condition. For this experiment, the same methods described above were used except that the inhibitor was added directly with the mucin preparation or the inhibitor was added to the upper Transwell with F4969.
Quantitative measurement of viable vegetative cells and heat-resistant spores.
To evaluate the presence of viable vegetative cells, overnight cultures of wild-type F4969, the isogenic nanI null mutant, or the complemented strain were serially diluted from 10−2 to 10−7 with sterile water, and aliquots of those dilutions were then plated onto BHI agar plates. For spore counting, the overnight cultures were heated at 70°C for 20 min to kill vegetative cells and promote spore germination. Each heat-shocked suspension was then serially diluted from 10−2 to 10−7 with sterile water, and aliquots of those dilutions were plated onto BHI agar plates.
After an anaerobic incubation overnight at 37°C, colonies appearing on BHI agar plates were counted, and those colony counts were used to calculate the numbers of vegetative cells per milliliter or heat-resistant spores per milliliter.
CPE Western blot analysis.
Supernatants of overnight cultures were collected and mixed with 5× SDS loading buffer. Each sample loading volume was adjusted to the same OD600. Western blotting of those samples was then performed using a CPE anti-rabbit polyclonal antibody, as previously described (33).
qRT-PCR analysis of nanI gene expression levels.
As described previously (16), saturated phenol (Fisher Scientific) was used to extract RNA from pelleted cells collected from 1 ml of F4969 grown for 3 h at 37°C in PBS, PBS with mucin, or PBS with Caco-2 cells. Purified RNA was quantified by determining the absorbance at 260 nm and checked by PCR to ensure that there was no DNA contamination. The purified RNA was aliquoted, and 1-μg samples were synthesized to cDNA using the Thermo Scientific Maxima first-strand synthesis kit for qRT-PCR, per the manufacturer's instructions. Before qRT-PCR, the synthesized cDNA was diluted to 5 ng/μl. The qRT-PCR primers used to amplify 16S RNA and nanI sequences from the synthesized cDNA were published previously (16). Power SYBR green PCR master mix (Thermo Fisher Scientific) and a Step One Plus qRT-PCR instrument (Applied Biosystems) were used to perform qRT-PCR. After qRT-PCR, relative quantification of nanI mRNA expression was normalized to the level of constitutive expression of the 16S RNA housekeeping gene and calculated by the comparative threshold cycle (CT; 2−ΔΔCT) methods (34).
Sialic acid supplementation.
The OD595 sialidase activity value detected at 4 h in the presence of mucin in Fig. 1B or at 8 h in the presence of Caco-2 cell cultures in Fig. 4B was ∼0.1, corresponding to 0.11 U/ml of NanI sialidase. To determine how much sialic acid would be generated by that amount of NanI activity, 0.11 U/ml of purified NanI sialidase (Roche) was added to PBS containing 1 mg/ml of mucin or PBS containing Caco-2 cells for 6 h at 37°C. At that time, the sialic acid concentration was determined using the EnzyChrom neuraminidase assay (Bioassay Systems). Briefly, a 20-μl aliquot of the culture supernatant was added to 80 μl of working reagent (30 μl of assay buffer, 55 μl of ddH2O, 1 μl of cofactors, 1 μl of enzyme, and 0.5 μl of dye reagent, all supplied in the kit). Reaction mixtures were incubated, with protection from light, for 30 min at 37°C, and OD570s were then measured. A standard curve of sialic acid concentrations (supplied in the kit) was then used to determine the sialic acid concentration generated under each incubation condition.
Overnight FTG cultures of F4969 or F4969::nanI were inoculated into 10 ml of TGY broth for overnight culture at 37°C. This process was then repeated the following day. A 40-ml aliquot of the 2nd overnight TGY culture was then inoculated into 1 ml of PBS, PBS with mucin (1 mg/ml), or PBS with Caco-2 cells, and those cultures were grown for 3 h at 37°C. F4969::nanI was then fed or not fed 54 mM sialic acid in ddH2O (to supplement the mucin culture) or 24 mM sialic acid (to supplement the infected Caco-2 cell culture). As a comparison, identically grown F4969 or F4969::nanI cultures were fed the same volume of ddH2O. All cultures were then cultured for 3 more hours at 37°C, and the OD600 was then detected for each culture.
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 6 software. For comparison of two group samples, Student's unpaired t test was used. For more than two groups, the ordinary one-way analysis of variance (ANOVA) was applied with post hoc analysis using Dunnett's test.
ACKNOWLEDGMENTS
This work was generously supported by grant R21 AI125796-2 (to B.A.M., J.L., and Francisco Uzal) from the National Institute of Allergy and Infectious Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic clostridia, p 688–752. In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E (ed), The prokaryotes, 3rd ed Springer, New York, NY. [Google Scholar]
- 2.Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. 2014. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClane BA, Rood JI. 2001. Clostridial toxins involved in human enteric and histotoxic infections, p 169–209. In Bahl H, Duerre P (ed), Clostridia: biotechnology and medical applications. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 4.Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Uzal FA, Rood JI, McClane BA. 2013. Toxin plasmids of Clostridium perfringens. Microbiol Mol Biol Rev 77:208–233. doi: 10.1128/MMBR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehdizadeh Gohari I, Kropinski AM, Weese SJ, Parreira VR, Whitehead AE, Boerlin P, Prescott JF. 2016. Plasmid characterization and chromosome analysis of two netF+ Clostridium perfringens isolates associated with foal and canine necrotizing enteritis. PLoS One 11:e0148344. doi: 10.1371/journal.pone.0148344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petit L, Gilbert M, Popoff M. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol 7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 7.CDC. 2011. CDC estimates of foodborne illness in the United States: Clostridium perfringens. CDC, Atlanta, GA: http://www.cdc.gov/foodsafety/diseases/clostridium-perfringens.html. [Google Scholar]
- 8.McClane BA, Robertson SL, Li J. 2013. Clostridium perfringens, p 465–489. In Doyle MP, Buchanan RL (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 9.Carman RJ. 1997. Clostridium perfringens in spontaneous and antibiotic-associated diarrhoea of man and other animals. Rev Med Microbiol 8(Suppl 1):S43–S45. doi: 10.1097/00013542-199712001-00023. [DOI] [Google Scholar]
- 10.Shrestha A, Uzal FA, McClane BA. 2016. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe 41:18–26. doi: 10.1016/j.anaerobe.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarker MR, Carman RJ, McClane BA. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol 33:946–958. doi: 10.1046/j.1365-2958.1999.01534.x. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Uzal FA, Mcclane BA. 2016. Clostridium perfringens sialidases: potential contributors to intestinal pathogenesis and therapeutic targets. Toxins (Basel) 8:341. doi: 10.3390/toxins8110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, McClane BA. 2014. Contributions of NanI sialidase to Caco-2 cell adherence by Clostridium perfringens type A and C strains causing human intestinal disease. Infect Immun 82:4620–4630. doi: 10.1128/IAI.02322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Sayeed S, Robertson S, Chen J, McClane BA. 2011. Sialidases affect the host cell adherence and epsilon toxin-induced cytotoxicity of Clostridium perfringens type D strain CN3718. PLoS Pathog 7:e1002429. doi: 10.1371/journal.ppat.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nees S, Schauer R. 1974. Induction of neuraminidase from Clostridium perfringens and the cooperation of this enzyme with acylneuraminate pyruvate lyses. Behring Inst Mitt 55:68–78. [Google Scholar]
- 16.Li J, Evans DR, Freedman JC, McClane BA. 2017. NanR regulates nanI sialidase expression by Clostridium perfringens F4969, a human enteropathogenic strain. Infect Immun 85:e00241-17. doi: 10.1128/IAI.00241-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therit B, Cheung JK, Rood JI, Melville SB. 2015. NanR, a transcriptional regulator that binds to the promoters of genes involved in sialic acid metabolism in the anaerobic pathogen Clostridium perfringens. PLoS One 10:e0133217. doi: 10.1371/journal.pone.0133217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almagro-Moreno S, Boyd EF. 2010. Bacterial catabolism of nonulosonic (sialic) acid and fitness in the gut. Gut Microbes 1:45–50. doi: 10.4161/gmic.1.1.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severi E, Hood DW, Thomas GH. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 20.McGuckin MA, Linden SK, Sutton P, Florin TH. 2011. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 21.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol 1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, Dekker J. 2010. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vimr ER, Kalivada KA, Deszo EL, Steenbergen SM. 2004. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev 68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, McClane BA. 2014. The sialidases of Clostridium perfringens type D strain CN3718 differ in their properties and sensitivities to inhibitors. Appl Environ Microbiol 80:1701–1709. doi: 10.1128/AEM.03440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Paredes-Sabja D, Sarker M, McClane B. 2016. Clostridium perfringens sporulation and sporulation-associated toxin production. Microbiol Spectr 4:TBS-0022-2015. doi: 10.1128/microbiolspec.TBS-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Freedman JC, McClane BA. 2015. NanI sialidase, CcpA, and CodY work together to regulate epsilon toxin production by Clostridium perfringens type D strain CN3718. J Bacteriol 197:3339–3353. doi: 10.1128/JB.00349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp S, Zhao D. 2000. Synthesis of the sialidase inhibitor siastatin B. Org Lett 2:4037–4040. doi: 10.1021/ol0066680. [DOI] [PubMed] [Google Scholar]
- 28.Lewis AL, Lewis WG. 2012. Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol 14:1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x. [DOI] [PubMed] [Google Scholar]
- 29.Carman RJ, Sayeed S, Li J, Genheimer CW, Hiltonsmith MF, Wilkins TD, McClane BA. 2008. Clostridium perfringens toxin genotypes in the feces of healthy North Americans. Anaerobe 14:102–108. doi: 10.1016/j.anaerobe.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. 1990. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol 259:G822–G837. [DOI] [PubMed] [Google Scholar]
- 31.Tailford LE, Crost EH, Kavanaugh D, Juge N. 2015. Mucin glycan foraging in the human gut microbiome. Front Genet 6:81. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dick G, Akslen-Hoel LK, Grondahl F, Kjos I, Prydz K. 2012. Proteoglycan synthesis and Golgi organization in polarized epithelial cells. J Histochem Cytochem 60:926–935. doi: 10.1369/0022155412461256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, McClane BA. 2010. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect Immun 78:4286–4293. doi: 10.1128/IAI.00528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. 2005. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci U S A 102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]