Abstract
Background
The lifetime prevalence of epistaxis is approximately 60%, and 6–10% of the affected persons need medical care. In rare cases, severe bleeding calls for the rapid initiation of effective treatment.
Methods
This review is based on pertinent articles that were retrieved by a selective search PubMed, and on the authors’ clinical experience.
Results
There are no German guidelines for the management of epistaxis. The available evidence consists mainly of retrospective analyses and expert opinions. 65–75% of the patients who require treatment can be adequately cared for by their primary care physician or by an emergency physician with baseline measures. If there is persistent anterior epistaxis, an otorhinolaryngologist can control the bleeding sastisfactorily in 78–88% of cases with chemical or electrical cauterization. Nasal packing is used if this treatment fails, or for posterior epistaxis. In a retrospective study, surgical treatment was found to be more effective than nasal packing in the treatment of posterior epistaxis (97% versus 62% treatment success). Percutaneous embolization is an alternative treatment for patients whom general anesthesia would put at high risk.
Conclusion
The treatment of severe or recurrent epistaxis requires the interdisciplinary collaboration of the primary care physician, the emergency physician, the practice-based otolaryngologist, and the hospital otolaryngology service. Uniform guidelines and epidemiological studies on this topic would be desirable.
Mild episodes of epistaxis stop spontaneously or are treated, often successfully, by the primary care physician or by the emergency physician. Only when nosebleeds are recurrent or severe are patients referred to an otorhinolaryngologist or to an accident and emergency department for further diagnostic assessment and treatment. No guideline exists in Germany today on the treatment of epistaxis. The aim of the present article is to provide an up-to-date overview of knowledge regarding its epidemiology, anatomy, and risk factors. Specific recommendations will be given for the treatment of epistaxis at the primary and secondary levels of care.
Learning goals
After reading this article, the reader should:
Have acquired a general understanding of the epidemiology, anatomy, and causes of epistaxis.
Know the most important basic elements of the treatment of epistaxis.
Be familiar with the diagnostic and therapeutic procedures performed by, respectively, general practitioners and emergency physicians, otorhinolaryngologists, and ear, nose, and throat (ENT) hospital departments.
Method
This article is based on a selective literature search of the PubMed database, searching for the terms “epistaxis,” “epistaxis anticoagulation,” “epistaxis therapy,” “epistaxis packing,” and “epistaxis embolization” in the title of articles published between 1 January 2000 and 1 February 2017. Some older standard publications, textbooks, and our own clinical experience were also included.
Epidemiology
Epidemiology.
The lifetime prevalence of epistaxis is 60%. Only about 6% to 10% of those affected seek medical help.
About 60% of the population experience a nosebleed at least once in their life (1). Precise epidemiological data on incidence are unavailable, because no epidemiological studies have been performed and only about 6% to 10% of the persons affected seek medical help (1, 2). In Germany, the only accurate data are those collected by emergency departments. One retrospective study reported an epistaxis incidence of 121 / 100 000 inhabitants treated in two emergency departments in East Thuringia (3).
According to a retrospective study from the United States, 1 to 2 out of 200 visits to the emergency department were due to epistaxis, and about 5% of the patients had to be admitted for inpatient care (4, 5). In Germany, a total of 19 841 patients (11 733 male and 8108 female) received inpatient treatment for epistaxis in 2015. The average hospital stay was 3.6 days (6). Of those who received treatment as inpatients, 71% were aged 65 or over, 18% were between 45 and 65 years of age, 5% were aged from 15 to 45, and 6% were under the age of 15 (6). No figures for treatment of epistaxis by primary care physicians have been published.
Anatomy
Anatomy.
In 90% to 95% of cases of epistaxis, the source of the bleed is in the area of the anterior part of the nasal septum, the Kiesselbach area (Little’s area).
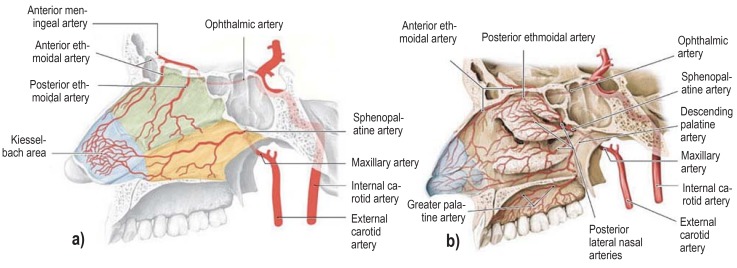
The arterial supply of the nasal cavity is shown in Figure 1. In 90% to 95% of cases, the bleed occurs anteriorly in the area of the anterior part of the nasal septum, the Kiesselbach area (or Little’s area) (7– 10), and in 5% to 10% of cases it occurs posteriorly in the posterior region of the nasal cavity (7, 10, 11).
Figure 1.
Arterial supply of the nasal cavity (e34)
The different territories supplied by the internal carotid artery (green) and the external carotid artery (yellow) are indicated in a). The Kiesselbach area (blue) is supplied by branches of both the main arteries (red).
a) Arteries supplying the nasal septum and
b) the lateral walls of the nasal cavity.
Causes/Etiology
The most frequent cause of epistaxis is trauma due to digital manipulation (nose picking) (12). Other causes are shown in Box 1. In 2014, a systematic review reported that most studies described raised blood pressure at the time the epistaxis occurred. However, these studies were unable to show hypertension to be an immediate cause of epistaxis. Confounding stress and, possibly, “white coat syndrome” may have contributed to raised arterial blood pressure in the setting of epistaxis (13). Several studies have shown a relative increase in epistaxis episodes during cold, dry weather or during periods when there are marked variations in air temperature and pressure (14– 18).
BOX 1.
-
Causes of epistaxis*
-
Traumatic
Digital manipulation
Nasal fracture/contusion
Foreign body in the nose
Iatrogenic (e.g., nasogastric tube, surgical interventions)
-
Neoplastic
Juvenile nasopharyngeal angiofibroma
Tumors of the nasal cavity and paranasal sinuses
-
Hematological
Thrombocytopenia
Hemophilia A and B
Von Willebrand disease
Liver failure
-
Structural
Mucosal dryness
Septal perforation
Osler–Weber–Rendu disease (hereditary hemorrhagic telangiectasia)
-
Drug-related
Anticoagulants and antiplatelet drugs
Glucocorticoid nasal sprays
Nasal consumption of drugs
-
Inflammatory
Allergic rhinitis
Acute infectious diseases
-
*Modified from (29)
Causes.
The most frequent cause of epistaxis is trauma due to digital manipulation.
Ingestion of anticoagulant drugs increases the risk of epistaxis (19). About 24% to 33% of all patients hospitalized for epistaxis take anticoagulants and/or antiplatelet drugs (20, 21). Ingestion of acetylsalicylic acid increases the severity and number of recurrences of epistaxis and the need for surgical intervention (22, 23). A retrospective cohort study in Zurich, Switzerland, showed ingestion of vitamin K antagonists to be an independent and significant risk factor for recurrent epistaxis with an odds ratio (OR) of 11.6 (23). Prescription of direct oral anticoagulants for patients is increasing (24). There is currently a paucity of data concerning this group of drugs in relation to epistaxis.
One prospective observational study showed a reduction in the number of cases of severe epistaxis in patients taking dabigatran versus vitamin K antagonists. Hospital stay was longer for dabigatran patients, however, because the lack of an easily available coagulation test and persistent oozing after removal of packing made it necessary to keep the patients under continued observation (25). One retrospective study of epistaxis in patients taking rivaroxaban showed a lower percentage of inpatient admissions (10.4% versus 18.0%, p = 0.033) and shorter hospital stay (0.7 ± 2.2 versus 1.5 ± 3.7 days, p = 0.011) in comparison to patients taking vitamin K antagonists (26). Another risk factor identified was alcohol (14– 16). One randomized, controlled, double-blind study showed that steroid nasal sprays increase the risk of epistaxis within 12 months in comparison to placebo from 8% to 20%. The nosebleeds that occurred were slight to moderate; only 1 of 605 patients suffered a severe nosebleed within 12 months (27). In a meta-analysis of randomized, controlled studies, epistaxis was reported to be the most frequent undesired effect of PDE-5 inhibitors, with a relative risk of 4.701 (95% confidence interval [95% CI]: [1.314; 16.812], p = 0.017) (28).
Treatment of epistaxis
No uniform guidelines exist for diagnostic and therapeutic procedures in patients with epistaxis. However, clinically tried and tested treatment paths do emerge in hospitals and doctors’ offices, based largely on retrospective analyses, case series, and expert opinion. Only few prospective or randomized controlled studies are available for some discrete areas of epistaxis treatment.
Epistaxis ranges from light nosebleeds that are easy to manage using simple methods to life-threatening bleedings that require hospital admission and may even need surgical treatment.
Treatment.
The treatment of epistaxis requires a structured interdisciplinary approach by the primary care physician, emergency physician, otorhinolaryngologist, and hospital ENT department.
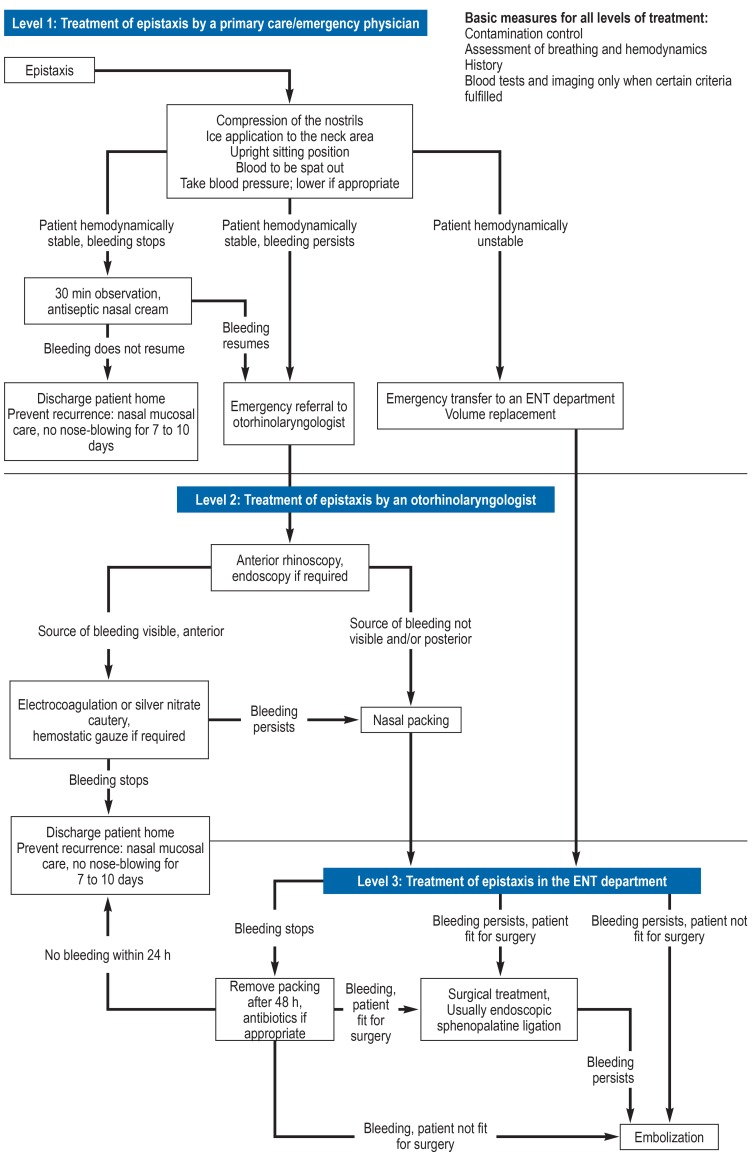
For a structured overview of the interdisciplinary management of epistaxis, in this article treatment recommendations are given separately for level 1 (primary care physician/emergency physician), level 2 (otorhinolaryngologist), and level 3 care (hospital ENT department). Figure 2 shows the treatment algorithm developed by ourselves, which includes treatment recommendations from the international literature as well as our department’s own in-house standard operating procedures. Some steps are relevant at all three levels of care.
Figure 2.
Treatment algorithm
Level 1: By a primary care/emergency physician
Level 2: By an otorhinolaryngologist
Level 3: In the ENT department
Contamination control
Measures to prevent contamination must always be observed. It is recommended that all who have close contact with patients, e.g., in the course of rhinoscopy or endoscopy, should wear protective eye gear, lab coat, gloves, and a face mask (12).
Initial assessment of breathing and hemodynamics
Especially in cases of severe bleeding, following the ABC approach, security of the airway, breathing, and cardiovascular stability should be assessed (29– 31). If symptoms of hypovolemia are found, a peripheral venous access should be placed and volume replacement therapy started. Early blood pressure measurement is an essential part of the diagnostic process.
History taking
The most important parts of the history are first of all the intensity and course over time of the bleed, which allow a judgment to be made about the urgency of treatment (29). The patient should be asked about factors that would predispose to epistaxis (Boxes 1, 2) (12, 29). An important element of the history is what medication the patient is on, especially any anticoagulants or antiplatelet drugs (box 2) (29).
BOX 2. Medical drugs associated with epistaxis*.
Phenprocoumon
Dabigatran
Rivaroxiban
Fondaparinux
Clopidogrel
Acetylsalicylic acid
Glucocorticoid nasal sprays
Phosphodiesterase-5 inhibitors (relative risk: 4.701)
Blood tests
In many cases of uncomplicated epistaxis, no blood tests are required. If the patient is on anticoagulation therapy, however, coagulation testing with International Normalized Ratio (INR) measurement should be carried out.
Imaging
Imaging is not usually necessary. However, in patients with recurrent epistaxis of unknown cause, imaging should be carried out to investigate the possibility of neoplastic disease such as juvenile nasopharyngeal angiofibroma (32).
Management of patients on anticoagulants
Contamination control.
It is recommended that all who have close contact with patients, e.g., in the course of rhinoscopy or endoscopy, should wear protective eye gear, a lab coat, gloves, and a face mask.
In France, guidelines on the management of epistaxis in patients taking anticoagulants have existed since 2016 (33). In acute epistaxis, these recommend screening for overdose and assessment of the risk of thrombosis. Anticoagulation therapy should always be continued so long as the bleeding can be stopped or controlled. Only if bleeding is massive and unstoppable, or if an anticoagulation overdose is found, should adjustment of the anticoagulation therapy be considered in consultation with a hematologist and cardiologist.
Antiplatelet drugs
Patients on anticoagulants.
If the bleeding can be stopped or controlled, anticoagulation therapy should be continued. Only if bleeding is massive and unstoppable, e.g., due to anticoagulation overdose, should adjustment of the anticoagulation therapy be considered.
Because it takes up to 10 days for hemostasis to be restored after cessation of antiplatelet therapy, stopping antiplatelet drugs in a patient with acute epistaxis is not useful. If the bleeding cannot be halted, stopping antiplatelet therapy while at the same time giving platelet transfusions is an option (33).
Vitamin K antagonists
For a patients taking a vitamin K antagonist, the drug should be stopped and an antidote given only if the bleeding is uncontrollable. If the vitamin K antagonist has been overdosed and the bleeding can be controlled, the dosage should be altered (33).
Direct oral anticoagulants
Stopping medication with direct oral anticoagulants is recommended only after consultation with a cardiologist. If bleeding is uncontrolled, dabigatran is the only drug for which an antidote (idarucizumab 5 mg in two consecutive 5– to 10-min intravenous infusions) is currently available (33).
Anticoagulation treatment should not be altered in a patient about to undergo endovascular embolization (expert opinion) (33).
Preventing recurrence
Direct oral anticoagulants.
Stopping medication with these drugs is recommended only after consultation with a cardiologist. If bleeding is uncontrolled, dabigatran is the only drug for which an antidote is currently available.
To prevent recurrences, intensive care of the nasal mucosa using an antiseptic nasal cream is recommended. A prospective, randomized, controlled study in the United Kingdom in children with recurrent epistaxis compared treatment with an antiseptic cream for 4 weeks versus a wait-and-see policy. A significantly lower recurrence rate was seen in the treatment group (45% versus 71% recurrence rate, relative risk reduction 47% with 95% CI [9%; 69%]) (34). In addition, energetic nose blowing should be avoided for 7 to 10 days (29). Bed rest is not necessary. According to a Danish prospective, randomized study, mobilizing the patient does not increase recurrence in comparison to bed rest (35).
Treatment by the primary care physician and/or emergency physician
The first step is to compress both sides of the nose continuously for 15 to 20 min, using two fingers or a nose clip (29, 36, 37). The patient should sit upright and lean slightly forward to prevent the blood from running down the pharynx (12). Local application of ice, e.g., at the back of the neck, is intended to encourage vasoconstriction of the blood vessels of the nose. Its therapeutic value is a matter of debate and has been challenged in the literature (19, 38). No final conclusion can be drawn on the basis of existing publications. In patients with raised blood pressure that is not causing symptoms (>180/120 mmHg, measured several times), the European Society of Hypertension and the European Society of Cardiology recommend oral medication to reduce the blood pressure. The aim is to slowly reduce the blood pressure over a period of 24 to 48 hours (39, 40). In around 65% to 75% of cases, these steps combined with application of a decongestant, oxymetazoline-based nasal spray will succeed in stopping the bleeding (e1, e2). If bleeding does not restart during a 30-min observation period and the patient is hemodynamically stable, emergency specialist ENT treatment is not required.
In the presence of any of the following, we recommend consultation with an otorhinolaryngologist:
Epistaxis uncontrollable by the measures described above
Recurrent epistaxis
Suspected neoplasm as the source of the bleed
Treatment by an otorhinolaryngologist
Anterior rhinoscopy
To locate the source of the bleeding, the first investigation is anterior rhinoscopy with a nasal speculum and headlight (29). Once any clots have been removed by suction or with pincers, the nasal cavity can be inspected, including the Kiesselbach area, where the bleeding often originates. Application of a vasoconstrictor and local anesthetic, e.g., in the form of an impregnated cotton tuft, will enable a better view. Owing to the local anesthetic effect, this step has therapeutic as well as diagnostic value (12, 30, 36).
Endoscopy
Treatment by the primary care physician and/or emergency physician.
Important basic measures are compression of the nostrils, oral medication to reduce blood pressure if appropriate, and use of an oxymetazoline nasal spray.
Especially in cases where the bleeding is from the posterior nasal cavity, locating the source of the bleeding by anterior rhinoscopy is difficult. In such cases, the French guidelines on treating epistaxis recommend as a supplementary procedure rigid endoscopy of the nasal cavity by a physician experienced in endoscopy (30, 36). Two prospective studies have shown that 80% to 94% of bleed sources can be identified by endoscopy (11, e3).
Cauterization
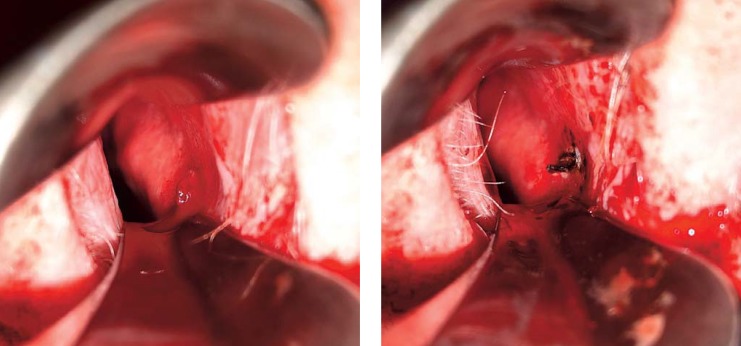
Most cases of epistaxis from an easily visible anterior source can be effectively treated by cauterization with silver nitrate or electrocoagulation. Before starting the procedure, a vasoconstrictor and local anesthetic should be applied (30). Figure 3 shows a bleeding from the Kiesselbach area before and after bipolar coagulation. A Swiss retrospective study showed that in terms of therapeutic success, electrocoagulation was superior to chemical coagulation (88% versus 78%) (failure rate 12% with 95% CI [0.09; 0.16] versus 22% with 95% CI [0.14; 0.33]) (evidence level 2b) (e4). A US study of children treated intraoperatively by these same two methods for recurrent anterior epistaxis also found a lower recurrence rate for electrocoagulation than for chemical cauterization during the 2-year period after the procedure (recurrence events 2% versus 18%) (e5). Chemical cautery is described as simpler to use, cheaper, and more widely available (e6). Complications of cauterization include septal perforation, infection, rhinorrhea, and increased bleeding (12). Bilateral cautery in the area of the nasal septum should be avoided if possible, as this risks septal perforation (e7). There are no published studies on the incidence of septal perforation after cautery (e8, e9).
Figure 3.
Bleeding in the Kiesselbach area (right side) before and after bipolar cautery
Hemostatic gauze
As a supplement to cautery, local application of gauze made of oxidized regenerated cellulose can be used. As a resorbable hemostyptic, it supports physiological hemostasis. Diffuse mucosal bleedings in particular can often be adequately managed by the application of a thin layer of this gauze (e10).
Nasal packing
If cauterization is unsuccessful, the next step in managing epistaxis is nasal packing. Packing takes different forms for anterior and posterior bleeding. Bilateral nasal packing produces a higher intranasal pressure than unilateral packing and its practice is therefore widespread, although there is little evidence to support this (e11)
Treatment by an otorhinolaryngologist.
For anterior epistaxis, the treatment of choice is bipolar coagulation. Where bleeding is persistent or from a posterior source, the first step is nasal packing.
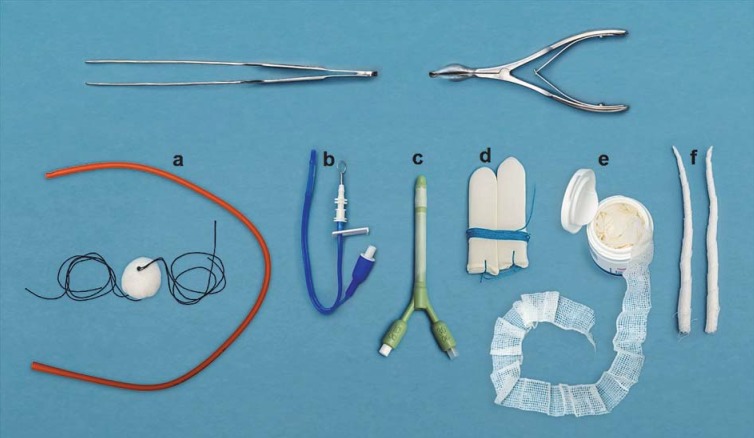
Comprehensive overviews of the features and mechanism of action of the most common forms of nasal packing are presented by Beule et al. in their 2004 publication (e12) and by Weber in his 2009 review article (e10). The eFigure shows a selection of items in common use for nasal packing. The main nasal packing products used in Germany are rubber-coated sponge packs or tampons (Gummifingerlingstamponaden), expandable sponge packs, and ribbon gauze impregnated with a medical cream (e12) (for more details see eBox 1).
eFigure:
Selection of nasal packing products in common use
a) Bellocq posterior nasal pack
b) Nasopharyngeal balloon
c) Xomed catheter
d) Rubber-coated sponge tampons
e) Cotton ribbon gauze
f) Ribbon gauze pledgets for intranasal application of oxymetazoline
eBOX 1. Overview of the most commonly used nasal packing materials.
• (Rubber-coated sponge tampons
Rubber-coated sponge tampons are sponges covered with a layer of rubber that prevents colonization by bacteria or viruses. Effective for hemostasis, simple and relatively atraumatic to apply and remove, and causing little discomfort for the patient, rubber-coated sponge packs are the norm for nasal packing in Germany. Complications to watch out for include damage from pressure to the columella and nasal cartilage, and posterior dislocation (e10).
• (Expandable nasal packs
Expandable nasal packs are usually made of polyvinyl acetal and expand on contact with blood or water. The advantage of this form of pack is that, especially when made of materials with small pores, placement and removal are comparatively less traumatic and unpleasant for the patient.
Rapid Rhino is a special form of this kind of pack. A Rapid Rhino pack consists of a core sponge or balloon covered in a carboxymethyl cellulose fabric intended to promote aggregation of thrombocytes (e10).
• (Cotton ribbon gauze
Pledgets of cotton ribbon gauze are placed intranasally for ongoing packing, usually after impregnation with Vaseline or an antibiotic cream. The advantage is that it is easy to place the gauze exactly where it is wanted for local pressure effect (e12). Disadvantages are that placing and removing the pledgets is relatively painful for the patient, they are less comfortable when in place, bleeding can be caused when they are removed, and paraffinomas may form where they are placed (e10, e12, e37).
• (Balloon packing
The main indication for balloon packing is severe posterior bleeding (e10). Additional anterior packing is always required.
Once the nasopharyngeal balloon has been advanced along the caudal part of the main nasal cavity, it should be filled with up to 10 mL sterile water for injection purposes and then carefully pulled against the choanae (29). If a nasopharyngeal balloon is not available, use of a Foley catheter, which is expanded in the nasopharynx, has been described as a possible alternative (12). Double balloon catheters also exist which, in addition to the posterior balloon for the nasopharynx, also have an anterior balloon that is inflated in the nasal cavity. Because of the relatively high risk of pressure necrosis, balloon packing of the nasal cavity should be used with caution (e10).
• (Bellocq posterior nasal pack
The principle of the Bellocq posterior nasal pack is based on sealing off the nasopharynx with a gauze or sponge pack which is pulled into the nasopharynx via the mouth by means of two transnasally introduced rubber catheters. This is is very uncomfortable for the patient, who should therefore preferably be sedated or under general anesthesia while it is being carried out. Moreover, this method of packing has a markedly higher rate of associated local and general complications than does the nasopharyngeal balloon (e38). Its use is therefore only indicated in patients with posterior epistaxis where anterior packing and balloon catheters fail and in those with nasopharyngeal bleeding, e.g., after cancer surgery (e18).
Nasal packing.
The main nasal packing products used in Germany are rubber-coated sponge packs, expandable sponge packs, and ribbon gauze impregnated with a medical cream.
Complications of nasal packing—The most serious complication of nasal packing is posterior dislocation. Reports have been published of fatal aspiration of nasal packs (e13). Rubber-coated sponge tampons and cotton ribbon gauze packs are liable to dislocate (e10). To prevent this, all nasal packs must be strongly fixed to the patient’s face, e.g., with sticking plaster on the bridge of the nose or the cheek (e7, e12). Additionally, the threads attached to some packs should be tied together in front of the columella. Other reported complications include allergic reaction, mucosal necrosis, foreign body reaction, tube dysfunction, paraffinoma, and decompensation of pre-existing sleep apnea (e7, e10, e12). Nasal packing can also cause discomfort for the patient in the form of pain, obstructed breathing, and a reduced sense of smell (e10). In addition, bilateral nasal packing can result in impaired pressure equalization via the auditory (Eustachian) tube, leading to the patient’s discomfort due to negative pressure in the middle ear (e10). There have been case reports of staphylococcal toxic shock syndrome as a serious complication (e14– e16). The release of toxic shock syndrome toxin 1 (TSST1) causes symptoms such as vomiting, diarrhea, fever, myalgia, diffuse erythema, and even septic shock. Treatment consists of immediate removal of the packing, intravenous antibiotics, and transfer of the patient to an intensive care ward (e10).
Prophylactic antibiotics—The role of prophylactic administration of antibiotics with nasal packing has not been adequately studied. Wide variation in practice has been described in England (e17), e.g., prophylactic antibiotics in patients with cardiac anomalies, especially prosthetic heart valves (30). Like some other authors, with anterior nasal packing we recommend prophylactic antibiotics only after the packing has been in place for more than 48 hours, but with posterior packing we recommend it in all cases, with the aim of preventing migration of infection into the sinuses and middle ear and toxic shock syndrome (e18). Preferred antibiotics are amoxicillin–clavulanic acid, amoxicillin alone, and cephalosporins (e17).
Complications of nasal packing.
Posterior dislocation, allergic reaction, mucosal necrosis, foreign body reaction, tube dysfunction, paraffinoma, decompensation of pre-existing sleep apnea, and staphylococcal toxic shock syndrome.
Removal of packing—When to remove the packing is variously defined in the literature, ranging from 12 or 24 hours to 3 to 5 days after placement (12, 29, 30). For anterior packing alone, we recommend removal after 48 hours. Where a nasopharyngeal balloon has also been placed, this should be at least partially deflated after 24 hours at the latest. If clinically significant bleeding starts again after packing removal, we advise surgical treatment where possible.
Treatment in the ENT department
From the point of view of the ENT department, for both unilateral and bilateral packing, inpatient admission for observation and packing removal are recommended because of the risk of posterior dislocation.
Other indications for inpatient admission are shown in Figure 2.
Surgical treatment
When conservative treatment fails, surgical hemostasis is generally required. A Swiss retrospective cohort study showed surgical intervention to be markedly superior to packing in the management of posterior epistaxis (treatment failure rate 3% [0.00; 0.14] versus 38% [0.30; 0.67]) (e4).
Treatment in an ENT department.
For posterior epistaxis, surgical intervention is markedly superior to packing. The method of choice is endoscopic clipping or coagulation of the sphenopalatine artery, which controls the bleeding in 98% of cases.
The method of choice is endoscopic clipping or coagulation of the sphenopalatine artery (e19). A British study reviewed the evidence for endoscopic sphenopalatine artery ligation and compared it to alternate methods. The former proved to be superior to the other treatment methods (monopolar cautery, embolization, etc.), controlling the bleeding in 98% of cases (e20). In retrospective cohort studies, recurrence of bleeding, intranasal dryness with crust formation, sinusitis, impaired nasal and palatal sensitivity, formation of intranasal synechiae, unilateral chronic epiphora, and septal perforation have all been reported as complications. One Brazilian retrospective longitudinal study reported a case of amaurosis after the intervention (e21). Taken together, these studies show endoscopic sphenopalatine artery ligation to have few complications (e21– e25). Clinically significant hypoxia of the territory supplied by this artery has not been described and is not anticipated, given the multiplicity of anastomoses between the sphenopalatine and ethmoidal arteries (9). For this reason, the criteria for surgical treatment can be quite wide: recurrence of bleeding after one attempt at packing and where the source of the bleeding is not evident (e19). Surgical hemostasis (ebox 2) should also be considered early on in patients with persistent bleeding despite packing. Endoscopic ligation of the anterior ethmoidal artery is indicated mostly in the context of revision surgery. In four retrospective studies, approximately 2.9% to 8.6% of all patients undergoing surgery for severe epistaxis had anterior ethmoidal artery ligation (e21– e23, e26).
eBOX 2. Operative procedures.
To give the surgeon a better view of the bleeding area, and to reduce blood loss, local and topical use of vasoconstrictors is recommended, together with raising the patient’s head by 15° to 20° (e25, e39). In addition, care should be taken to keep the patient hypotensive under anesthesia (e25).
• (Sphenopalatine artery ligation
A significant landmark for identifying the sphenopalatine artery is the ethmoid crest. This is exposed either by a vertical incision in front of the posterior attachment of the middle nasal concha or via a middle meatal antrostomy with exposure of the posterior wall of the maxillary cavity and abrasion of the mucosa at the maxilloethmoid angle. When the ethmoid crest has been removed, the arterial branches of the sphenopalatine artery that lie behind it are identified and sealed off with a clip (Ligaclip) or by electrocoagulation (e25).
• (Anterior ethmoidal artery ligation
A less common cause of severe epistaxis is bleeding from the anterior ethmoidal artery (AEA), which may occur spontaneously, postoperatively after surgery of the paranasal sinuses, or after trauma. In cases where anterior epistaxis is difficult to control, clipping of the AEA is a good therapeutic option. The artery can be located via an open approach in the area of the medial canthus (Lynch incision; transcaruncular approach) or endoscopically. In endoscopic procedures, maxillary antrostomy is carried out and the anterior ethmoidal cells are excavated and the lamina papyracea visualized. Angled lenses are used to locate the AEA, which passes through the base of the skull in an anteromedial direction (e25). Apart from recurrent bleeding, complications include injury to orbital structures and the base of the skull (e25).
• (Posterior ethmoidal artery ligation
In rare cases of severe epistaxis, ligation of the posterior ethmoidal artery is indicated. According to a US study, this artery is located an average of 8.1 mm anterior to the anterior wall of the sphenoid sinus (e40).
Where bleeding persists following the usual surgical and neuroradiological procedures, the French guidelines recommend surgical exploration of the paranasal sinuses with elective coagulation of bleeds from side/secondary branches or ethmoidectomy in patients with diffuse bleeding (e31).
Embolization
Another possible method in patients with epistaxis that is difficult to control is percutaneous embolization. This technique has a reported success rate of 87% to 93% (e27– e29). The target vessel is imaged angiographically and then an occluding agent is injected via a percutaneous transarterial catheter (e30). The embolization should be carried out by an experienced interventional neuroradiologist (e31). Because of the potential for complications such as cerebrovascular ischemia, facial nerve paralysis, and soft tissue necrosis, some authors recommend using this technique only in patients who have an increased anesthetic risk because of other comorbidities, or in whom attempted surgical treatment has failed (30). One retrospective cross-sectional study in the US compared embolization with surgical vascular occlusion in terms of morbidity, hospital mortality, and duration of hospital stay. No significant differences were found in relation to blood transfusions (22.8% versus 24.3%), stroke (0.5% versus 0.3%), amaurosis (0.4% versus 0.5%), and hospital mortality. However, surgery is associated with lower hospital costs and a shorter hospital stay (e32).
Treatment of epistaxis in children
An overview of recommended treatment strategies in epistaxis in children is given in a French systematic review by Béquignon et al. (e33). In addition to removal of clots, bidigital compression, and (permissible from the age of 6 onwards) application of a local anesthetic and decongestant, the use of an antiseptic cream is recommended (e33). If bleeding persists, chemical cautery (silver nitrate stick) should be preferred to electrical cautery, as electrical cautery is more painful and would therefore require a general anesthetic (e33).
Conclusions for clinical practice
Embolization.
Where surgical treatment fails or the patient has a high anesthetic risk, percutaneous embolization is a reasonable alternative.
In 65% to 70% of cases of epistaxis, simple first aid measures provided by the primary care physician or emergency physician stop the bleeding. If bleeding -persists, specialist ENT expertise should be urgently consulted. So long as the source of the bleeding is visible, most cases of epistaxis can be successfully treated using electrical or chemical cautery. In cases where the bleeding source is posterior, or where the bleeding remains refractory to packing, surgery should be considered early on and liberally. Because of its high success rate and comparatively low complication rate, endoscopic ligation or coagulation of the sphenopalatine artery is the method of choice. In cases of severe epistaxis, where surgical treatment fails or the patient has a high anesthetic risk, percutaneous embolization is a reasonable alternative.
Treatment of children.
In a child with persistent bleeding, chemical cautery (silver nitrate stick) should be preferred to electrical cautery, as electrical cautery is more painful and would therefore require a general anesthetic.
Further Information about CME.
-
Participation in the CME program is possible exclusively online at: cme.aerzteblatt.de. The last date for submission of answers is 2 April 2018. Answers received by mail, email, or fax cannot be accepted.
-
The following CME units can still be accessed:
„“Impingement syndrome of the shoulder” (issue 45/2017) until 4 February 2018
„“Community-acquired pneumonia in adults” (issue 49/2017) until 4 March 2018
-
-
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Participants in the CME program can manage their CME points with their “uniform CME number” (einheitliche Fortbildungsnummer, EFN).
The EFN must be entered in the appropriate field at our website cme.aerzteblatt.de under „Meine Daten“ („my data“) on registration, and activated by confirming the declaration of consent.The 15-digit EFN is visible on each participant‘s CME certificate (8027XXXXXXXXXXX).
Submit your answers at cme.aerzteblatt.de. Final date for answers is 2 April 2018. Only one answer per question is possible. Please select the answer that is most appropriate.
Question 1
What is the estimated lifetime prevalence of epistaxis?
30%
40%
50%
60%
70%
Question 2
Where is the source of the bleeding most usually found in epistaxis?
In the nasal vestibule
In the area of the anterior part of the nasal septum
In the area of the nasal conchae
In the area of the posterior part of the nasal cavity
In the area of the nasopharynx
Question 3
What is the commonest cause of epistaxis?
Hypertension
Tumor
Iatrogenic manipulation
Digital manipulation
Nasal infection
Question 4
A 37-year-old man presents to his family physician with right-sided epistaxis. His blood pressure is 150/85 mmHg. Apart from self-protection, what is the most important first step?
Ice application to the neck area
Place a peripheral venous access device
Lay the patient flat on his back
Refer to an otorhinolaryngologist immediately
Compress the patient’s nostrils
Question 5
An adult man presents to an otorhinolaryngologist with left-sided epistaxis. The source of the bleed is located in the Kiesselbach area. After basic measures, what should be carried out as the next therapeutic procedure?
Anterior nasal packing
Posterior and anterior nasal packing
Intravenous administration of tranexamic acid
Electrical or chemical cauterization
Surgery
Question 6
When is the use of prophylactic antibiotic treatment recommended in epistaxis?
In all cases requiring cauterization
When treatment is by anterior packing alone, irrespective of how long it is in place
When treatment is by anterior and posterior packing in place for >96 hours
When treatment is by anterior packing in place for >48 h and always when posterior packing is placed
In all patients under the age of 18
Question 7
Which group of antibiotics should be used for first-line prophylactic antibiotic treatment?
Amoxicillin–clavulanic acid, amoxicillin alone, and cephalosporins
Aminoglycosides and lipopeptides
Fluorquinolones and lincosamides
Tetracyclines and glycopeptides
Macrolides and sulfonamides
Question 8
Compared with conservative treatment, what is the treatment method of choice in cases of refractory posterior epistaxis?
Endoscopic clipping or coagulation of the anterior ethmoidal artery
Endoscopic clipping or coagulation of the posterior ethmoidal artery
Endoscopic clipping or coagulation of the sphenopalatine artery
Ligation of the ipsilateral maxillary artery
Ligation of the ipsilateral external carotid artery
Question 9
In which of the following cases is inpatient admission always recommended despite absence of further bleeding after intervention?
Anterior epistaxis persisting after placement of packing impregnated with oxymetazoline
Patient aged <12, anterior epistaxis, hemostasis by bidigital compression and application of a cream
Anterior epistaxis, hemostasis by bipolar coagulation
Anterior epistaxis, hemostasis by chemical cautery
Anterior epistaxis, hemostasis with bilateral rubber-coated sponge packs
Question 10
In which of the following cases is percutaneous embolization indicated?
Patient aged <18, no concomitant disease, posterior epistaxis persisting under packing
Patient aged <18, no concomitant disease, anterior epistaxis persisting under packing
Patient with high anesthetic risk, posterior epistaxis persisting under packing
Patient with recurrent anterior epistaxis
Patient with anterior epistaxis persisting under anterior packing
?The CME program is available exclusively online: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Petruson B, Rudin R. The frequency of epistaxis in a male population sample. Rhinology. 1975;13:129–133. [PubMed] [Google Scholar]
- 2.Small M, Murray JA, Maran AG. A study of patients with epistaxis requiring admission to hospital. Health Bull. 1982;40:20–29. [PubMed] [Google Scholar]
- 3.Weigel K, Volk GF, Müller A, Guntinas-Lichius O. Ein Jahr Epistaxisbehandlung in den Notfallambulanzen der Ostthüringer HNO-Kliniken. Laryngorhinootologie. 2016;95:837–842. doi: 10.1055/s-0042-111013. [DOI] [PubMed] [Google Scholar]
- 4.Smith J, Siddiq S, Dyer C, Rainsbury J, Kim D. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: prospective cohort study. J Laryngol Otol. 2011;125:38–42. doi: 10.1017/S0022215110001921. [DOI] [PubMed] [Google Scholar]
- 5.Pallin DJ, Chng YM, McKay MP, Emond JA, Pelletier AJ, Camargo CA Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77–81. doi: 10.1016/j.annemergmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Statistisches Bundesamt, Krankenhausstatistik. Diagnosedaten der Krankenhäuser ab 2000 (Krankheiten/ Gesundheitsprobleme > Krankheiten allgemein > Tabelle (gestaltbar): Diagnosedaten der Krankenhäuser [Eckdaten der vollstationären Patienten und Patientinnen]) (last accessed on 6 February 2017) www.gbe-bund.de [Google Scholar]
- 7.Viehweg TL, Roberson JB, Hudson JW. Epistaxis: diagnosis and treatment. J Oral Maxillofac Surg. 2006;64:511–518. doi: 10.1016/j.joms.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180–183. doi: 10.1097/MOO.0b013e32814b06ed. [DOI] [PubMed] [Google Scholar]
- 9.Chiu T, Dunn JS. An anatomical study of the arteries of the anterior nasal septum. Otolaryngol Head Neck Surg. 2006;134:33–36. doi: 10.1016/j.otohns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Viducich RA, Blanda MP, Gerson LW. Posterior epistaxis: clinical features and acute complications. Ann Emerg Med. 1995;25:592–596. doi: 10.1016/s0196-0644(95)70169-9. [DOI] [PubMed] [Google Scholar]
- 11.Thornton MA, Mahesh BN, Lang J. Posterior epistaxis: identification of common bleeding sites. Laryngoscope. 2005;115:588–590. doi: 10.1097/01.mlg.0000161365.96685.6c. [DOI] [PubMed] [Google Scholar]
- 12.Morgan DJ, Kellerman R. Epistaxis: evaluation and treatment. Primary Care. 2014;41:63–73. doi: 10.1016/j.pop.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Kikidis D, Tsioufis K, Papanikolaou V, Zerva K, Hantzakos A. Is epistaxis associated with arterial hypertension? A systematic review of the literature. Eur Arch Otorhinolaryngol. 2014;271:237–243. doi: 10.1007/s00405-013-2450-z. [DOI] [PubMed] [Google Scholar]
- 14.Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: an investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32:361–365. doi: 10.1111/j.1749-4486.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 15.McGarry GW, Gatehouse S, Hinnie J. Relation between alcohol and nose bleeds. BMJ. 1994;309 doi: 10.1136/bmj.309.6955.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarry GW, Gatehouse S, Vernham G. Idiopathic epistaxis, haemostasis and alcohol. Clin Otolaryngol. 1995;20:174–177. doi: 10.1111/j.1365-2273.1995.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 17.Danielides V, Kontogiannis N, Bartzokas A, Lolis CJ, Skevas A. The influence of meteorological factors on the frequency of epistaxis. Clin Otolaryngol Allied Sci. 2002;27:84–88. doi: 10.1046/j.1365-2273.2002.00535.x. [DOI] [PubMed] [Google Scholar]
- 18.Stopa R, Schonweiler R. Ursachen von Nasenbluten in Abhängigkeit von Jahreszeit und Wetterlage. HNO. 1989;37:198–202. [PubMed] [Google Scholar]
- 19.Folz BJ, Kanne M, Werner JA. Aktuelle Aspekte zur Epistaxis. HNO. 2008;56 doi: 10.1007/s00106-008-1838-3. [DOI] [PubMed] [Google Scholar]
- 20.Pollice PA, Yoder MG. Epistaxis: a retrospective review of hospitalized patients. Otolaryngol Head Neck Surg. 1997;117:49–53. doi: 10.1016/S0194-59989770205-5. [DOI] [PubMed] [Google Scholar]
- 21.Simmen D, Heinz B. Epistaxis-Strategie - Erfahrungen der letzten 360 Hospitalisationen. Laryngo-Rhino-Otologie. 1998;77:100–106. doi: 10.1055/s-2007-996941. [DOI] [PubMed] [Google Scholar]
- 22.Soyka MB, Rufibach K, Huber A, Holzmann D. Is severe epistaxis associated with acetylsalicylic acid intake? Laryngoscope. 2010;120:200–207. doi: 10.1002/lary.20695. [DOI] [PubMed] [Google Scholar]
- 23.Stadler RR, Kindler R, Holzmann D, Soyka MB. The long-term fate of epistaxis patients with exposure to antithrombotic medication. Eur Arch Otorhinolaryngol. 2016;273:2561–2567. doi: 10.1007/s00405-016-3913-9. [DOI] [PubMed] [Google Scholar]
- 24.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med. 2014;127:1075–1082e1. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Garcia CFJ, Becares MC, Calvo GJ, Martinez BP, Marco SM, Marco AJ. Epistaxis and dabigatran, a new non-vitamin K antagonist oral anticoagulant. Acta Otorrinolaringol Esp. 2014;65:346–354. doi: 10.1016/j.otorri.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Sauter TC, Hegazy K, Hautz WE, et al. Epistaxis in anticoagulated patients: Fewer hospital admissions and shorter hospital stays on rivaroxaban compared to phenprocoumon. Clin Otolaryngol. 2017 [Epub ahead of print] doi: 10.1111/coa.12904. [DOI] [PubMed] [Google Scholar]
- 27.Rosenblut A, Bardin PG, Muller B, et al. Long-term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitis. Allergy. 2007;62:1071–1077. doi: 10.1111/j.1398-9995.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 28.Giannetta E, Feola T, Gianfrilli D, et al. Is chronic inhibition of phosphodiesterase type 5 cardioprotective and safe? A meta-analysis of randomized controlled trials. BMC Med. 2014;12 doi: 10.1186/s12916-014-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond L. Managing epistaxis. JAAPA. 2014;27:35–39. doi: 10.1097/01.JAA.0000455643.58683.26. [DOI] [PubMed] [Google Scholar]
- 30.Daudia A, Jaiswal V, Jones NS. Guidelines for the management of idiopathic epistaxis in adults: how we do it. Clin Otolaryngol. 2008;33:618–620. doi: 10.1111/j.1749-4486.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- 31.Nikoyan L, Matthews S. Epistaxis and hemostatic devices. Oral Maxillofac Surg Clin North Am. 2012;24:219–228. doi: 10.1016/j.coms.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Szymanska A, Golabek W, Siwiec H, Pietura R, Szczerbo-Trojanowska M. Juvenile angiofibroma: the value of CT and MRI for treatment planning and follow-up. Otolaryngol Pol. 2005;59:85–90. [PubMed] [Google Scholar]
- 33.Escabasse V, Bequignon E, Verillaud B, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL) Managing epistaxis under coagulation disorder due to antithrombotic therapy. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;134:195–199. doi: 10.1016/j.anorl.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Kubba H, MacAndie C, Botma M, et al. A prospective, single-blind, randomized controlled trial of antiseptic cream for recurrent epistaxis in childhood. Clin Otolaryngol Allied Sci. 2001;26:465–468. doi: 10.1046/j.1365-2273.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 35.Kristensen VG, Nielsen AL, Gaihede M, Boll B, Delmar C. Mobilisation of epistaxis patients—a prospective, randomised study documenting a safe patient care regime. J Clin Nurs. 2011;20:1598–1605. doi: 10.1111/j.1365-2702.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 36.Bequignon E, Vérillaud B, Robard L, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL) First-line treatment of epistaxis in adults. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:185–189. doi: 10.1016/j.anorl.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Kucik CJ, Clenney T. Management of epistaxis. Am Fam Physician. 2005;71:305–311. [PubMed] [Google Scholar]
- 38.Teymoortash A, Sesterhenn A, Kress R, Sapundzhiev N, Werner JA. Efficacy of ice packs in the management of epistaxis. Clin Otolaryngol. 2003;28:545–547. doi: 10.1046/j.1365-2273.2003.00773.x. [DOI] [PubMed] [Google Scholar]
- 39.Henny-Fullin K, Buess D, Handschin A, Leuppi J, Dieterle T. Hypertensive Krise. Ther Umsch. 2015;72:405–411. doi: 10.1024/0040-5930/a000693. [DOI] [PubMed] [Google Scholar]
- 40.Muiesan ML, Salvetti M, Amadoro V, et al. An update on hypertensive emergencies and urgencies. J Cardiovasc Med. 2015;16:372–382. doi: 10.2459/JCM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- E1.Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104:704–706. doi: 10.1177/000348949510400906. [DOI] [PubMed] [Google Scholar]
- E2.Doo G, Johnson DS. Oxymetazoline in the treatment of posterior epistaxis. ¬Hawaii Med J. 1999;58:210–212. [PubMed] [Google Scholar]
- E3.Chiu TW, McGarry GW. Prospective clinical study of bleeding sites in idiopathic adult posterior epistaxis. Otolaryngol Head Neck Surg. 2007;137:390–393. doi: 10.1016/j.otohns.2006.10.035. [DOI] [PubMed] [Google Scholar]
- E4.Soyka MB, Nikolaou G, Rufibach K, Holzmann D. On the effectiveness of treatment options in epistaxis: an analysis of 678 interventions. Rhinology. 2011;49:474–478. doi: 10.4193/Rhino10.313. [DOI] [PubMed] [Google Scholar]
- E5.Johnson N, Faria J, Behar P. A comparison of bipolar electrocautery and ¬chemical cautery for control of pediatric recurrent anterior epistaxis. Otolaryngol Head Neck Surg. 2015;153:851–856. doi: 10.1177/0194599815589583. [DOI] [PubMed] [Google Scholar]
- E6.Traboulsi H, Alam E, Hadi U. Changing trends in the management of epistaxis. Int J Otolaryngol. 2015;2015 doi: 10.1155/2015/263987. 263987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Murer K, Soyka MB. Die Behandlung des Nasenblutens [The treatment of ¬epistaxis] Praxis (Bern 1994) 2015;104:953–958. doi: 10.1024/1661-8157/a002112. [DOI] [PubMed] [Google Scholar]
- E8.Kridel RW. Considerations in the etiology, treatment, and repair of septal ¬perforations. Facial Plast Surg Clin North Am. 2004;12:435–450, vi. doi: 10.1016/j.fsc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- E9.Lanier B, Kai G, Marple B, Wall GM. Pathophysiology and progression of nasal septal perforation. Ann Allergy Asthma Immunol. 2007;99:473–480. doi: 10.1016/S1081-1206(10)60373-0. [DOI] [PubMed] [Google Scholar]
- E10.Weber RK. Nasal packing and stenting. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009;8 doi: 10.3205/cto000054. Doc02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Hettige R, Mackeith S, Falzon A, Draper M. A study to determine the benefits of bilateral versus unilateral nasal packing with Rapid Rhino packs. Eur Arch Otorhinolaryngol. 2014;271:519–523. doi: 10.1007/s00405-013-2590-1. [DOI] [PubMed] [Google Scholar]
- E12.Beule AG, Weber RK, Kaftan H, Hosemann W. Übersicht: Art und Wirkung ¬geläufiger Nasentamponaden [Review: pathophysiology and methodology of nasal packing] Laryngorhinootologie. 2004;83:534–551;. doi: 10.1055/s-2004-825695. quiz 553-6. [DOI] [PubMed] [Google Scholar]
- E13.Spillmann D. Aspiration von Nasentamponaden mit Todesfolge. Laryngorhino¬otologie. 1981;60 [PubMed] [Google Scholar]
- E14.Hull HF, Mann JM, Sands CJ, Gregg SH, Kaufman PW. Toxic shock syndrome related to nasal packing. Arch Otolaryngol. 1983;109:624–626. doi: 10.1001/archotol.1983.00800230060015. [DOI] [PubMed] [Google Scholar]
- E15.Allen ST, Liland JB, Nichols CG, Glew RH. Toxic shock syndrome associated with use of latex nasal packing. Arch Intern Med. 1990;150:2587–2588. [PubMed] [Google Scholar]
- E16.Márquez Moyano JA, Jiménez Luque JM, Sánchez Gutiérrez R, et al. Shock tóxico estafilocócico asociado a cirugía nasal [Toxic shock syndrome associated with nasal packing] Acta Otorrinolaringol Esp. 2005;56:376–378. doi: 10.1016/s0001-6519(05)78633-7. [DOI] [PubMed] [Google Scholar]
- E17.Biswas D, Wilson H, Mal R. Use of systemic prophylactic antibiot¬ics with ¬anterior nasal packing in England, UK. Clin Otolaryngol. 2006;31:566–567. doi: 10.1111/j.1365-2273.2006.01336.x. [DOI] [PubMed] [Google Scholar]
- E18.Lenarz T, Boenninghaus HG. Hals-Nasen-Ohren-Heilkunde Berlin, Heidelberg. Springer Berlin Heidelberg. 2012 [Google Scholar]
- E19.Loughran S, Hilmi O, McGarry GW. Endoscopic sphenopalatine artery liga¬tion—when, why and how to do it An on-line video tutorial. Clin Otolaryngol. 2005;30:539–543. doi: 10.1111/j.1749-4486.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- E20.Kumar S, Shetty A, Rockey J, Nilssen E. Contemporary surgical treatment of epistaxis What is the evidence for sphenopalatine artery ligation? Clin Otolaryngol Allied Sci. 2003;28:360–363. doi: 10.1046/j.1365-2273.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- E21.Saraceni Neto P, Nunes LMA, Gregório LC, Santos RdP, Kosugi EM. Surgical treatment of severe epistaxis: an eleven-year experience. Braz J Otorhinolaryngol. 2013;79:59–64. doi: 10.5935/1808-8694.20130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Agreda B, Urpegui A, Ignacio Alfonso J, Valles H. Ligadura de la arteria ¬esfenopalatina en la epistaxis recidivante posterior. Estudio retrospectivo de 50 pacientes [Ligation of the sphenopalatine artery in posterior epistaxis. Retrospective study of 50 patients] Acta Otorrinolaringol Esp. 2011;62:194–198. doi: 10.1016/j.otorri.2010.11.005. [DOI] [PubMed] [Google Scholar]
- E23.Nouraei SAR, Maani T, Hajioff D, Saleh HA, Mackay IS. Outcome of endoscopic sphenopalatine artery occlusion for intractable epistaxis: a 10-year experience. Laryngoscope. 2007;117:1452–1456. doi: 10.1097/MLG.0b013e318065b86f. [DOI] [PubMed] [Google Scholar]
- E24.Gede LL, Aanaes K, Collatz H, Larsen PL, von Buchwald C. Nation¬al long-lasting effect of endonasal endoscopic sphenopalatine artery clipping for epistaxis. Acta Otolaryngol. 2013;133:744–748. doi: 10.3109/00016489.2013.773596. [DOI] [PubMed] [Google Scholar]
- E25.Lin G, Bleier B. Surgical management of severe Epistaxis. Otolaryngol Clin North Am. 2016;49:627–637. doi: 10.1016/j.otc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- E26.Bermüller C, Bender M, Brögger C, Petereit F, Schulz M. Epistaxis bei ¬Antikoagulation - eine klinische und ökonomische Herausforderung? Laryngo-Rhino-Otologie. 2014;93:249–255. doi: 10.1055/s-0033-1355400. [DOI] [PubMed] [Google Scholar]
- E27.Andersen PJ, Kjeldsen AD, Nepper-Rasmussen J. Selective embolization in the treatment of intractable epistaxis. Acta Otolaryngol. 2005;125:293–297. doi: 10.1080/00016480410023029. [DOI] [PubMed] [Google Scholar]
- E28.Gurney TA, Dowd CF, Murr AH. Embolization for the treatment of idiopathic posterior epistaxis. Am J Rhinol. 2004;18:335–339. [PubMed] [Google Scholar]
- E29.Riemann R. Tipps & Tricks - Epistaxis Management unter Berücksichtigung der Kontamination. Laryngo-Rhino-Otologie. 2016;95:11–14. doi: 10.1055/s-0035-1570368. [DOI] [PubMed] [Google Scholar]
- E30.Gary L, Ferneini AM. Interventional radiology and bleeding disorders: what the oral and maxillofacial surgeon needs to know. Oral Maxillofac Surg Clin North Am. 2016;28:533–542. doi: 10.1016/j.coms.2016.06.012. [DOI] [PubMed] [Google Scholar]
- E31.Verillaud B, Robard L, Michel J, et al. Guidelines of the French Society of ¬Otorhinolaryngology (SFORL) Second-line treatment of epistaxis in adults. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:191–193. doi: 10.1016/j.anorl.2016.09.009. [DOI] [PubMed] [Google Scholar]
- E32.Sylvester MJ, Chung SY, Guinand LA, Govindan A, Baredes S, Eloy JA. Arterial ligation versus embolization in epistaxis management: Counterintuitive national trends. Laryngoscope. 2017;127:1017–1020. doi: 10.1002/lary.26452. [DOI] [PubMed] [Google Scholar]
- E33.Béquignon E, Teissier N, Gauthier A, et al. Emergency department care of childhood epistaxis. Emerg Med J. 2017;34:543–548. doi: 10.1136/emermed-2015-205528. [DOI] [PubMed] [Google Scholar]
- E34.Aumüller G, editor. Thieme. 3. Stuttgart: 2014. Anatomie. [Google Scholar]
- E35.Yamazaki H, Kobayashi N, Taketsuna M, Tajima K, Murakami M. Safety and ¬effectiveness of tadalafil in patients with pulmonary arterial hypertension: Japanese post-marketing surveillance data. Curr Med Res Opin. 2017;33:963–971. doi: 10.1080/03007995.2017.1297931. [DOI] [PubMed] [Google Scholar]
- E36.Gokdogan O, Akyildiz I, Sayin BY, Okutucu S, Tanalp AC, Arslan N. The ¬rate of ¬epistaxis incidence in new-generation anticoagulants and perioperative ¬approach in otorhinolaryngological practices. J Craniofac Surg. 2017;28:e178–e182. doi: 10.1097/SCS.0000000000003135. [DOI] [PubMed] [Google Scholar]
- E37.Illum P, Grymer L, Hilberg O. Nasal packing after septoplasty. Clin Otolaryngol Allied Sci. 1992;17:158–162. doi: 10.1111/j.1365-2273.1992.tb01065.x. [DOI] [PubMed] [Google Scholar]
- E38.Martin F. Ballonkatheter als Alternative zur Bellocq-Tamponade (Foley catheter technique as an alternative to bellocq pack [author‘s transl]) Laryngol Rhinol Otol. 1979;58:336–339. [PubMed] [Google Scholar]
- E39.Gan EC, Habib ARR, Rajwani A, Javer AR. Five-degree, 10-degree, and ¬20-degree reverse Trendelenburg position during functional endoscopic sinus surgery: a double-blind randomized controlled trial. Int Forum Allergy Rhinol. 2014;4:61–68. doi: 10.1002/alr.21249. [DOI] [PubMed] [Google Scholar]
- E40.Han JK, Becker SS, Bomeli SR, Gross CW. Endoscopic localization of the ¬anterior and posterior ethmoid arteries. Ann Otol Rhinol Laryngol. 2008;117:931–935. doi: 10.1177/000348940811701212. [DOI] [PubMed] [Google Scholar]