Abstract
Background
Although often proposed as a means to reduce the harmful consequences of tumor spill, water lavage has yet to be systematically evaluated in relevant in vitro and in vivo models. This study evaluates the mechanisms and utility of a single water lavage to improve the sequelae of tumor spill during laparotomy.
Methods
Murine colorectal tumor cell susceptibility to water-induced osmotic lysis was characterized in vitro. A reproducible model of tumor spill was established to recapitulate water or saline lavage during laparotomy. Analyses of tumor volumes calculated from noninvasive imaging were performed. The tumor volumes and survival of mice treated with water, normal saline, or sham laparotomy were assessed.
Results
Significant osmotic lysis of cultured murine colorectal cancer cells was observed after a brief exposure to water. Compared to saline or sham laparotomy, water lavage demonstrated superior clinical outcomes with a decrease in tumor burden and concomitant improvement in survival.
Conclusions
The use of water lavage during oncologic surgeries to reduce the sequelae of tumor spill is justified and strongly supported by our study. Data from our study raise several concerns regarding the mechanisms and efficacy of saline lavage. Clinically, the use of water lavage during laparotomy would be anticipated to reduce peritoneal disease burden with minimal toxicity or cost.
The ability of water to induce osmotic lysis of cells was first observed in 1683 by Anton van Leeuwenhoek.1 This simple yet profound property of water has been suggested as a means to combat tumor spillage at the time of surgery.2–4 Advantages of this low-cost, low-toxicity approach include the ease of application and the ability to bypass cancer cell resistance mechanisms.4 Despite a rational basis for the beneficial properties of water lavage, this approach is not universally accepted, and in vivo preclinical data have been conflicting and sparse.3,5 The need for simple measures to eliminate liberated peritoneal tumor cells is significant when considering that 3–28% of peritoneal lavage samples from colorectal cancer (CRC) patients at the time of “curative” surgery may have detectable exfoliated tumor cells.6 In regard to tumor spillage, up to 10% of CRC patients without detectable tumor cells by initial lavage will convert to tumor-positive cytology upon completion of their surgical resection.7 The significance of exfoliated colon carcinoma cells is highlighted by their reported ability to take advantage of local factors and establish growth at sites of surgical trauma.8,9 Additionally, tumor-positive cytology has been clearly correlated with intraperitoneal recurrence and may be associated with decreased disease-free and overall survival.7,10–12 Locoregional control is of paramount importance as the peritoneal cavity may be the only site of active disease in a large number of patients.13 Because CRC is currently the third most common cancer in the United States, with approximately 143,000 new cases reported annually, the number of patients potentially affected by local seeding is significant.14
Rigorous experimentation to support the use of water in addressing tumor spillage during surgery has not been extensively investigated. An early study in breast cancer cells demonstrated that exposure to water in vitro could generate significant cytotoxic effects that were observable after only 1 min.15 In addition, water lavage recovered from human peritoneal cavities maintains the ability to lyse CRC cells in vitro despite being contaminated by peritoneal secretions.4 A reduction in osmolarity of the recovered water lavage was achieved by performing sequential lavages and this was thought to improve cytotoxic effects, but never substantiated in vivo.4 In a small clinical evaluation of high-volume (10 l) water lavage for spontaneously ruptured hepatocellular carcinomas, survival was demonstrably improved compared to controls.2 Collectively, these investigations emphasize the ability of water lavage to lyse tumor cells which are unable to overcome osmotic stress. Contrary to these studies, however, in vivo water lavage was deemed inferior to saline lavage in a mouse model of ovarian cancer.5
Given the paucity of well-designed scientific studies on this topic and conflicting in vivo data, we developed several models to investigate the tumoricidal effects of water on CRC cells both in vitro as well as in vivo using a reproducible animal model and noninvasive magnetic resonance imaging (MRI) to quantify the sequelae of CRC tumor spillage treated with either normal saline or water lavage.5
MATERIALS AND METHODS
Mice
BALB/c age-matched (8–12 weeks of age) female mice were purchased from the National Cancer Institute (Frederick, MD). Mice were maintained in pathogen-free barrier conditions. All animal protocols were approved by the Roswell Park Cancer Institutional Animal Care and Use Committee.
Tumor Cell Line
The tumor cell line CT26 is a weakly immunogenic colon cancer cell line derived from BALB/c mice and was obtained from the American Type Culture Collection (Rockville, MD). Cells were maintained in RPMI 1640 media (Gibco, Invitrogen, Grand Island, NY) containing 10% fetal bovine serum, 2 mmol/l L-glutamine, 0.15% sodium bicarbonate, and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
In Vitro Assays of Tumor Cell Viability
CT26 cells were cultured in 24-well plates (Costar, Corning, Corning, NY) until they became confluent. Culture media was completely withdrawn and cells were exposed to 2 ml of either sterilized normal saline (NS) or distilled water (H2O) for varying lengths of time (5, 15, and 30 min). Tumoricidal effects as a function of osmolarity were evaluated by exposing CT26 cells to variable osmolarities of clinically common saline solutions (NS = 0.9% NaCl, ½ NS = 0.45% NaCl, or ¼ NS = 0.22% NaCl) or H2O for 15 min. Cultures were then rinsed with media and harvested with 0.25% trypsin-EDTA (Gibco, Invitrogen). Control groups were cultured with media alone. Trypan (0.4%) staining was used to assess cell viability with cell counts performed on a standard hemocytometer (Hausser Scientific, Horsham, PA). Live imaging of cells was obtained by a Leica DMI 6000B camera system with images acquired by a Leica AF6000LX system (Leica Microsystems, Bannockburn, IL) to provide qualitative data regarding cells lysis.
Peritoneal Lavage (In Vivo Tumor Model)
BALB/c mice were injected intraperitoneally with 1 × 104 viable CT26 cells in 50 μl of buffered saline using a 27 gauge needle and 1-ml syringe. The dose of 1 × 104 CT26 was selected because it reliably produced progressive intraperitoneal carcinomatosis and morbidity noted within 30 days of injection in untreated mice. After 15 min to allow for tumor cell distribution, mice were anesthetized by intraperitoneal injection of ketamine/xylazine (80–200/ 10 mg/kg body weight). The abdominal wall was prepared by clipping overlying hair and applying alcohol to the skin. The abdominal wall and peritoneal cavity were opened with scissors and retracted in a fixed coliseum fashion with 4–0 Vicryl suture (Ethicon, Somerville, NJ) anchored to a murine operating table constructed from Lego (Billund, Denmark) building blocks. This murine platform allowed for the simultaneous treatment of up to 5 mice in any given experiment. A total of 3 ml of NS or H2O (5–8 mice/group) was distributed intraperitoneally for 15 min with constant, controlled agitation on a programmable shaker at 160 RPM. To focus on osmolarity as a single variable, the model system was used for a single lavage only and the introduced solutions were not evacuated upon closure of the abdomen. The peritoneum was closed with a 4–0 absorbable monofilament suture and the skin with Vetbond tissue adhesive (3 M Corporation, St. Paul, MN). Control mice underwent tumor injection and sham laparotomy with no peritoneal lavage. All mice recovered on a warming blanket and were provided buprenorphine (0.2 mg/kg body weight) subcutaneously for pain control.
Magnetic Resonance Imaging
MRI studies were carried out using a 4.7-T/33-cm horizontal bore magnet (GE NMR Instruments, Fremont, CA) incorporating AVANCE digital electronics (Bruker Biospec, ParaVision 3.1., Bruker Medical, Billerica, MA), a removable gradient coil insert (G060, Bruker Medical) generating a maximum field strength of 950 mT/m, and a custom-designed 35-mm radiofrequency transmit-receive coil. Induction of anesthesia in animals that underwent imaging was achieved by inhalation of 4% isoflurane (Abbott Laboratories, Chicago, IL). For each experiment, a total of 15 animals (n = 5/group) were imaged 23 days after intraperitoneal inoculation of CT26 tumor cells.
To visualize and quantitate the extent of tumor burden, coronal and transverse axial multislice, two-dimensional T2-weighted spin echo images incorporating RARE (rapid acquisition with relaxation enhancement) encoding were acquired for each mouse using the following parameters: matrix size 256 × 192, TE/TR = 41/2500 ms, slice thickness 1 mm, field of view (FOV): 4.8 × 3.2 cm (coronal); 3.2 × 3.2 cm (axial), number of slices = 21, acquisition time = 4 min. Tumor volume was calculated by measuring the cross sectional area on each slice and multiplying their sum by the slice thickness.
Indications for Euthanasia
The mice were followed until tumor growth caused a moribund status and/or any of the criteria for euthanasia per approved protocols were observed. Survival was assessed and recorded as the time to euthanasia. Death was not an end point for any of these studies.
Statistical Analysis
In vitro tumor cell viability data were analyzed by one-way analysis of variance (ANOVA). In vivo tumor volume data were analyzed by one-way ANOVA and survival data by Kaplan-Meier log rank tests. For each method, a SigmaStat program was used (Systat Software, version 3.11). P = 0.05 or less was considered to be statistically significant, and all experiments were performed in triplicate to verify reproducibility.
RESULTS
Tumor Cell Lysis In Vitro
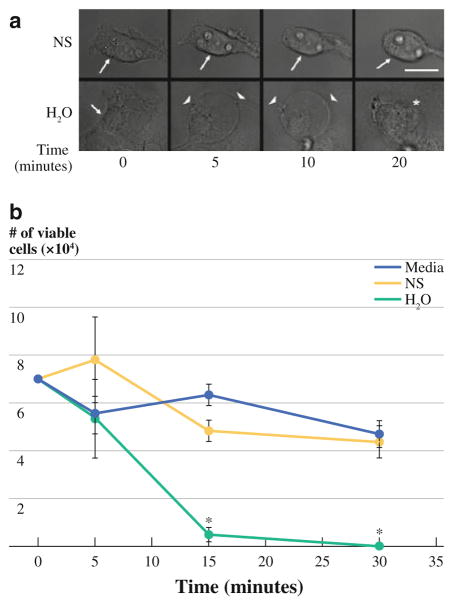
Morphological changes induced by exposure to normal saline or water were recorded by imaging of adherent cultured CT26 cells. At indicated time points, CT26 cells exposed to water demonstrated a rapid loss of both adherence and shape with eventual loss of cellular and nuclear membrane integrity (Fig. 1a). Cytoplasmic changes were noted within 5 min of exposure to water with bleb formation and membrane disruption between the 10- and 20-min time points. In contrast, CT26 tumor cells exposed to normal saline only displayed a loss of adherence with minimal morphological changes and no evidence of either cytoplasmic changes or membrane disruption during the observation period. CT26 tumor cell lysis was quantified by evaluating cell viability using trypan blue exclusion at defined time points (Fig. 1b) and confirmed that >95% of tumor cells were lysed within 15 min of exposure to water. Tumor cells exposed to normal saline or culture media remained viable and did not differ significantly from their baseline cell counts. Although in vitro lysis of tumor cells by water seems intuitive, these experiments defined the 15-min time point as statistically significant, and this was used for each of the subsequent experiments.
FIG. 1.
a Representative photomicrographs obtained from video capture demonstrate the loss of adherence of CT26 cells exposed to saline treatment (NS) (no cell membrane, cytosol, or nuclear changes noted). CT26 cells exposed to H2O show immediate morphological changes with rounding, cell membrane bleb formation, loss of cytosol, and eventual loss of cell and nuclear membrane integrity within 10–20 min (scale bar = 30 μM; arrow = cell membrane, arrowhead = membrane rounding with bleb formation, asterisk = membrane disruption). b CT26 cell viability was minimal after 15 min of exposure to H2O and differed significantly from NS- or media-treated cultures (P <0.05)
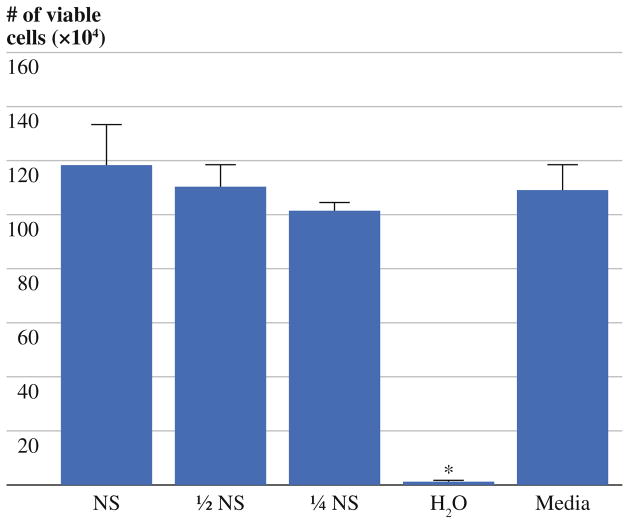
To determine whether other clinically common hypotonic saline solutions could induce similar CT26 cell lysis, ½ NS and ¼ NS were examined. After a 15-min exposure, the hypotonic saline solutions had no significant effect on tumor cell viability as compared to normal saline (Fig. 2). Although a trend was noted towards decreased CT26 cell viability as the hypotonicity of the solutions increased, this was not statistically significant. Collectively, these findings showed the efficiency of water to kill tumor cells in a relatively short time frame (i.e., within 15 min) that could not be matched by hypotonic saline solutions.
FIG. 2.
After 15 min of exposure to treatment solutions in vitro, H2O-treated cultures reproducibly demonstrated a minimal number of viable cells which differed significantly from all other groups (P <0.05). The ability of hypotonic saline solutions to lyse CT26 tumor cells showed a small trend toward improved lysis in proportion to hypotonicity but did not differ significantly from saline-treated (NS)- or media-treated cultures during the 15-min observation period
In Vivo Modeling of Tumor Spillage and Lavage
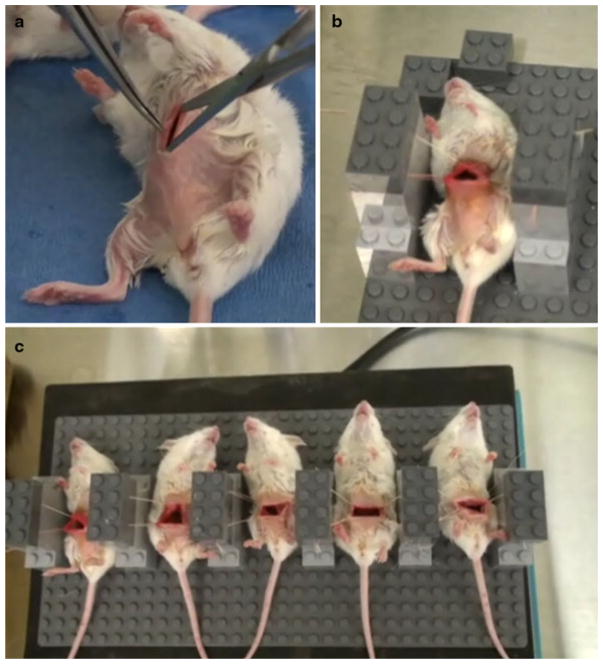
The clinical significance of the ability of water to lyse the colon cancer cell line CT26 was evaluated in our murine model of tumor spillage. The mouse platform was designed to mimic a standard laparotomy and tumor spillage by directly introducing CT26 tumor cells into the peritoneal cavity (Fig. 3). All midline incisions were readily reproducible and safe with no instances of inadvertent injury to underlying viscera (Fig. 3a). In a reliable and feasible manner, 4–0 Vicryl sutures tented and retracted the abdominal wall as Lego blocks firmly locked the sutures and mouse peritoneal cavity in a coliseum configuration (Fig. 3b). The abdominal coliseum accommodated 3 ml of either normal saline or water. In a controlled manner, uniform distribution of the introduced fluids occurred by placing the platform on a programmable shaker (Fig. 3c). The 15-min agitation was well tolerated, and the immediate postoperative survival from this procedure was 100% in each of the experiments performed (data not shown). None of the mice demonstrated evidence of acute morbidity after the completion of the surgical procedure.
FIG. 3.
a Representative photograph shows a shaved, prepared, and anesthetized mouse undergoing a small midline laparotomy incision with care to tent up the abdominal wall and prevent visceral injury. b The abdominal wall is tented upward with 4–0 Vicryl sutures anchored to Lego building blocks to generate a coliseum for instillation of treatment solutions. c A full array of mice (n = 5/group) with their peritoneum instilled with 3 ml of treatment solutions are shown anchored to the assembled Lego platform, which has been placed on a programmable shaker set at 160 RPM
Clinical Impact of Instilled Intraperitoneal Solutions on Tumor Growth
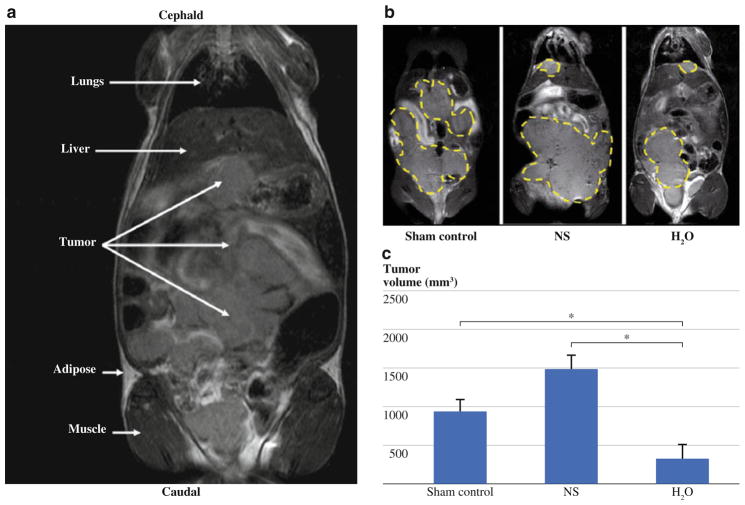
To assess the extent of tumor burden after intraperitoneal instillation of tumor cells, noninvasive MRI was performed on three cohorts of mice: sham-treated control animals, saline treatment, and water lavage (n = 5 per group). Intraperitoneal tumor mass was clearly discernable from surrounding organs and tissues on coronal T2 W MR images on day 23 after treatment (Fig. 4a). Similar to peritoneal carcinomatosis observed clinically in patients with CRC, MR images of animals in the control group revealed extensive distribution of tumor throughout the peritoneal cavity. Animals that received water lavage showed a visible reduction in overall tumor burden (outlined in yellow) as compared to control or saline treatment groups (Fig. 4b). Quantification of tumor burden from multislice T2W MR images (Fig. 4c) confirmed this observation and revealed a statistically significant reduction in tumor volume in mice that received water lavage (316 ± 181 mm3) as compared to control (924 ± 162 mm3, P <0.05) or normal saline treated groups (1477 ± 181 mm3, P <0.05) by day 23 after treatment. Normal saline-treated mice appeared to have a trend toward a greater tumor volume than control mice, but this did not reach statistical significance.
FIG. 4.
a Coronal T2-weighted (T2W) MR image of mouse showing differences in tissue contrast between peritoneal tumor implants and surrounding normal anatomic structures. b Representative T2W MR images of a sham-treated control, saline-treated (NS), and water-treated (H2O) mouse showing extent of tumor burden (outlined in yellow) on day 23 after treatment. c Bar graph shows tumor volumes for animals in all 3 groups (n = 5/group) calculated from T2W MR images. A significant reduction in tumor volume in H2O-treated animals compared to NS- or sham-treated control groups (P <0.05). NS-treated groups are noted to have a larger average tumor volume compared to sham control groups, but statistical significance was not reached
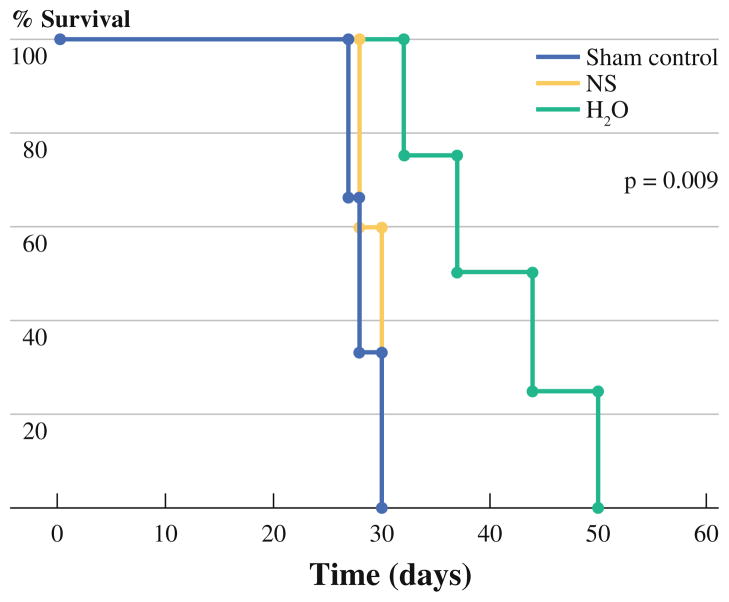
Consistent with the observed reduction in tumor burden, survival was significantly improved in mice treated with water lavage as compared to control or normal saline groups (Fig. 5). All of the control group and normal saline treated mice required euthanasia by day 30 after intraperitoneal tumor seeding as a result of observed morbidities. In contrast, water lavage treated mice had a mean survival of 41 days with the longest survivor out to day 50 before any morbidity was noted. None of the mice were cured in any group, and all appeared to have progressive peritoneal disease as the main determinant for the observed morbidity.
FIG. 5.
Representative Kaplan-Meier graph shows an improvement in survival (time to observation of any morbidity) for H2O-treated mice compared to saline-treated (NS)- or sham-treated control groups (n = 5/group) and was statistically significant (P = 0.009). All sham control and NS-treated mice required euthanasia by day 30 as a result of observed morbidities. There were no cures noted within any of the treatment groups
DISCUSSION
Previously, only anecdotal evidence has supported the use of water lavage during oncologic surgeries. The results of the present study demonstrate the potential utility of water lavage at the time of abdominal surgery to attenuate the effects of tumor spillage. The in vitro data demonstrate the rapidity with which a single 15-min exposure to water can mediate tumor cell lysis. This time point was then applied to an in vivo tumor spillage model and was associated with a lower peritoneal tumor burden and a significantly improved survival as compared to either no lavage or normal saline. The clonogenic CT26 tumor cells that were used are highly aggressive and capable of sustained peritoneal spread, rapid growth, and life-limiting animal morbidity within 30 days. In contrast to clinical scenarios of CRC tumor spillage, the current model may actually represent a more aggressive tumor biology and actually underestimate the true effects of water lavage if applied clinically.
Earlier clinical studies have suggested that the osmolarity of water might be contaminated by existing peritoneal secretions and that multiple high-volume lavages may be necessary to overcome this effect.4 The results of this study do not support this notion. A single lavage of water with a brief 15-min dwell time and agitation seemed to be effective and would also avoid the theoretical issues of electrolyte abnormalities or osmotic damage to normal tissues associated with repeated high-volume water lavages. Clinically, irrigation of the abdominal cavity at the time of laparotomy in patients is almost always performed with normal saline. Not only did the in vitro data fail to show that normal saline or hypotonic saline could induce significant tumor cell lysis, but also normal saline was observed to enhance detachment of adherent CT26 tumor cells in vitro and to show a trend toward increased tumor burden in an in vivo model system. These findings raise potential concern for actually increasing peritoneal tumor cell dissemination with saline lavage. Finally, there are also reports of povidone-iodine or chlorhexidine use for tumor spillage, but water lavage would be cost-effective and more readily available, and now it has demonstrated efficacy in a reproducible in vivo animal model.16–19
The beneficial effects of water lavage on tumor burden and survival in our murine model are clearly measurable, but limitations of this experimental approach must be recognized. First, the experiments utilized a model where the intraperitoneal solutions were allowed to dwell with agitation but were not evacuated. In patients, saline lavage is often repeated and removed before abdominal closure.20,21 Perhaps multiple water lavages, as has been previously proposed, would have demonstrated even greater efficacy. The current investigation, however, purposely focused on a single lavage to eliminate confounding variables associated with lavage volume delivery, recovery, and loss. Regardless, the results suggest that any benefit to the clinical practice of saline lavage would be mechanical because normal saline was unable to induce tumor cell lysis in our model. Second, the absolute number of tumor cells liberated during CRC surgery may be significantly greater than the 1 × 104 cells that were introduced into the peritoneum. Although the results of this study suggest a potential benefit, it is possible that the utility of a single water lavage in the presence of millions of exfoliated cells may prove clinically futile. Third, the model was clinically equivalent to an inadvertent tumor spillage scenario as the tumor cells were injected prior to the lavage. It did not address the presence of preestablished microscopic tumor dissemination (such as a positive peritoneal cytology). Therefore, one cannot extrapolate to the potential benefits of water lavage in patients with subclinical carcinomatosis as the biologic aggressiveness may be different as a result of the variable ability of the tumor cells to attach, grow, and replicate on free peritoneal surfaces.22 Finally, the effects of a single water lavage on the healing of intestinal anastomoses, return of bowel function, and adhesion formation were not evaluated by this model. It is possible that normal cells, such as peritoneal macrophages, may also be lysed by a single water lavage, but any potential detrimental effects are anticipated to be minimal.
In conclusion, the findings of the current study could have significant clinical implications not only for tumor spillage at the time of CRC surgery, but in any operation where tumor cell liberation is suspected. For example, during a pancreaticoduodenectomy where additional pancreas tissue is resected for a positive margin, water lavage could potentially reduce the risk for peritoneal recurrence. The current study would suggest that water lavage, while not curative, may delay peritoneal outgrowth and recurrence that may prolong survival. Although water lavage is unlikely to ever be evaluated in randomized, prospective clinical trials, the simplicity, low cost, and provocative findings of this study would favor the use of a single water lavage during oncologic procedures with tumor spillage. Because it is increasingly recognized that the perioperative period of CRC surgery is critical to successful outcomes, water lavage represents an opportunity to quickly and reproducibly eradicate liberated colon carcinoma cells at the time of surgery and potentially improve long-term outcome.23
Acknowledgments
The authors would like to thank Ree Dolnick, Daniel Fisher, and Jason Muhitch for their outstanding technical assistance. Supported by the U.S. National Institutes of Health (CA16056 to Roswell Park Cancer Institute).
Footnotes
CONFLICT OF INTEREST The authors report no commercial interests or conflicts of interest regarding the contents of this manuscript.
References
- 1.BookRags Biographies. http://www.bookrags.com/biography/anton-van-leeuwenhoek/
- 2.Lin CH, Hsieh HF, Yu JC, et al. Peritoneal lavage with distilled water during liver resection in patients with spontaneously ruptured hepatocellular carcinomas. J Surg Oncol. 2006;94:255–6. doi: 10.1002/jso.20596. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside OJ, Tytherleigh MG, Thrush S, et al. Intra-operative peritoneal lavage—who does it and why? Ann R Coll Surg Engl. 2005;87:255–8. doi: 10.1308/1478708051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huguet EL, Keeling NJ. Distilled water peritoneal lavage after colorectal cancer surgery. Dis Colon Rectum. 2004;47:2114–9. doi: 10.1007/s10350-004-0788-4. [DOI] [PubMed] [Google Scholar]
- 5.Morris PC, Scholten V. Osmotic lysis of tumor spill in ovarian cancer: a murine model. Am J Obstet Gynecol. 1996;175:1489–92. doi: 10.1016/s0002-9378(96)70095-9. [DOI] [PubMed] [Google Scholar]
- 6.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–22. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guller U, Zajac P, Schnider A, et al. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg. 2002;236:768–75. doi: 10.1097/00000658-200212000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oosterling SJ, van der Bij GJ, van Egmond M, van der Sijp JR. Surgical trauma and peritoneal recurrence of colorectal carcinoma. Eur J Surg Oncol. 2005;31:29–37. doi: 10.1016/j.ejso.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Raa ST, Oosterling SJ, van der Kaaij NP, et al. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. J Surg Oncol. 2005;92:124–9. doi: 10.1002/jso.20273. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Akasu T, Fujita S, Moriya Y. Long-term prognostic value of conventional peritoneal cytology after curative resection for colorectal carcinoma. Jpn J Clin Oncol. 2003;33:33–7. doi: 10.1093/jjco/hyg007. [DOI] [PubMed] [Google Scholar]
- 11.Kanellos I, Demetriades H, Zintzaras E, et al. Incidence and prognostic value of positive peritoneal cytology in colorectal cancer. Dis Colon Rectum. 2003;46:535–9. doi: 10.1007/s10350-004-6595-0. [DOI] [PubMed] [Google Scholar]
- 12.Wind P, Norklinger B, Roger V, et al. Long-term prognostic value of positive peritoneal washing in colon cancer. Scand J Gastroenterol. 1999;34:606–10. doi: 10.1080/003655299750026074. [DOI] [PubMed] [Google Scholar]
- 13.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–50. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 14.Altekruse SF, Kosary CL, Krapcho M, Neyman N, et al., editors. SEER cancer statistics review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 15.Park KG, Chetty U, Scott W, Miller W. The activity of locally applied cytotoxics to breast cancer cells in vitro. Ann R Coll Surg Engl. 1991;73:96–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Pattana-arun J, Wolff BG. Benefits of povidone-iodine solution in colorectal operations: science or legend. Dis Colon Rectum. 2008;51:966–71. doi: 10.1007/s10350-008-9213-8. [DOI] [PubMed] [Google Scholar]
- 17.Basha G, Ghirardi M, Geboes K, et al. Limitations of peritoneal lavage with antiseptics in prevention of recurrent colorectal cancer caused by tumor-cell seeding: experimental study in rats. Dis Colon Rectum. 2000;43:1713–8. doi: 10.1007/BF02236856. [DOI] [PubMed] [Google Scholar]
- 18.Basha G, Penninckx F, Yap P. Influence of blood components and faeces on the in vitro cancericidal activity of povidone-iodine. Br J Surg. 1998;85:534–7. doi: 10.1046/j.1365-2168.1998.00631.x. [DOI] [PubMed] [Google Scholar]
- 19.Stuntz M, Wilmoth G, Ong J, et al. Use of intraperitoneal 5-fluorouracil and chlorhexidine for prevention of recurrence of perforated colorectal carcinoma in a rat model. Dis Colon Rectum. 1997;40:1085–8. doi: 10.1007/BF02050934. [DOI] [PubMed] [Google Scholar]
- 20.Brundell S, Tucker K, Chatterton B, Hewett P. Influence of lavage timing on tumor cell burden during colonic resection. Dis Colon Rectum. 2003;46:460–6. doi: 10.1007/s10350-004-6583-4. [DOI] [PubMed] [Google Scholar]
- 21.Brundell SM, Tucker K, Chatterton B, Hewett PJ. The effect of lavage on intraabdominal cell burden. Surg Endosc. 2002;16:1064–7. doi: 10.1007/s00464-001-9111-9. [DOI] [PubMed] [Google Scholar]
- 22.Fermor B, Umpleby HC, Lever JV, et al. Proliferative and metastatic potential of exfoliated colorectal cancer cells. J Natl Cancer Inst. 1986;76:347–9. [PubMed] [Google Scholar]
- 23.van der Bij GJ, Oosterling SJ, Beelen RH, et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249:727–34. doi: 10.1097/SLA.0b013e3181a3ddbd. [DOI] [PubMed] [Google Scholar]